Abstract
Premise of the Study
The Macrofungi Collection Consortium (MaCC) is a digitization project funded by the National Science Foundation's Advancing Digitization of Biodiversity Collections program. The main scientific objective of the MaCC project was to provide baseline data for determining the extent and distribution of macrofungal diversity.
Methods and Results
Between 2012 and 2017, 39 participating institutions digitized approximately 1,250,000 specimens of macrofungi from U.S. herbaria. These newly digitized data, combined with existing data and contributions from the Microfungi Collections Consortium, have created a database of approximately 3.4 million specimen records that are shared online through MyCoPortal, a Symbiota‐based data portal. In addition to the digitized herbarium specimen data, MyCoPortal also contains descriptions, illustrations, and observational records.
Discussion
The database of digitized specimen data created through this project is a resource for both amateur and professional mycologists. The data provided through MyCoPortal will provide a foundation for a comprehensive Mycoflora of North America. Such a project is now under development as a collaboration between the professional and amateur mycological communities, with the goal of documenting the macrofungi of North America with gene sequences as well as phenotypic descriptions and images.
Keywords: citizen science, fungi, macrofungi, MyCoPortal, specimen digitization
The National Science Foundation's Advancing Digitization of Biodiversity Collections (ADBC) program funded the Macrofungi Collection Consortium (MaCC) for the period of 2012–2017. Macrofungi are those with conspicuous spore‐bearing structures commonly known as mushrooms, boletes, puffballs, club fungi, morels, stinkhorns, truffles, and cup fungi. As defined here (Table 1), this is a phylogenetically heterogeneous assemblage, united by the presence of a macroscopic sporocarp (spore‐bearing body) and the key roles these organisms play in plant and animal life and the global carbon cycle. Most of these fungi belong to the Agaricomycetes, but some ascomycetous groups are also included. Ectomycorrhizal fungi belong to this group as do many forest pathogens, wood‐decay fungi, and invertebrate predators such as nematode‐trapping fungi. Many animals, including humans, use these organisms as food, and the spore‐bearing structures serve as homes and incubation sites for many arthropod groups. The economic value of the global wild mushroom market is growing on an annual basis and currently exceeds US$2 billion (Boa, 2004). Because macrofungi change dramatically upon drying, photographs and descriptions of the living organism must supplement dried specimens to permit evaluation of key taxonomic features such as color, odor, taste, color change, or production of latex or mucilage‐like liquids upon bruising of the sporocarp surface texture, all of which can be observed only in the fresh condition. Although fungi represent an evolutionary lineage separate from plants (and possibly are more closely related to animals), fungi have traditionally been maintained in herbaria and are preserved and documented in a manner most similar to plants.
Table 1.
Primary groups of macrofungi included in this project (Kirk et al., 2008) and common names of some widely known members. The numbers may vary somewhat because many of these groups are undergoing reclassification as molecular phylogenetic studies are completed
| Phylum/Class | Order | Groups included | Common names (if available) | Families | Genera | Species |
|---|---|---|---|---|---|---|
| Ascomycota | ||||||
| Pezizomycetes | Pezizales | Macroscopic genera, e.g., Morchella, Helvella, Geoglossum, Tuber | Morels, false morels, earth tongues, truffles | 4 | 22 | 340 |
| Leotiomycetes | Leotiales | Macroscopic genera, e.g., Leotia, Chlorociboria | Chicken lips, green elf cup | 2 | 11 | 41 |
| Basidiomycota | ||||||
| Agaricomycetes | ||||||
| Agaricales | All | True mushrooms | 32 | 410 | >13,000 | |
| Atheliales | All | 1 | 22 | 106 | ||
| Boletales | All | Porcinis | 16 | >95 | 1300 | |
| Geastrales | All | Earth stars | 1 | 8 | 62 | |
| Gomphales | All | Club corals, pigs ears | 3 | 18 | 336 | |
| Hysterangiales | All | False truffles | 5 | 18 | 114 | |
| Phallales | All | Stink horns | 2 | 26 | 88 | |
| Cantherellales | All | Coral fungi | 7 | 38 | 544 | |
| Corticiales | All | Crust fungi | 3 | >30 | Unknown | |
| Hymenochaetales | All | Crust fungi | 3 | >50 | 600 | |
| Polyporales | All | Polypores or conks | 9 | 200 | 1800 | |
| Russulales | All | Brittle gills, milkcaps | 12 | >80 | 1760 | |
| Thelephorales | All | Earth fans | 2 | 18 | 250 | |
| Trechisporales | All | 1 | 15 | 105 | ||
The perspective on macrofungal biodiversity afforded by the digitization of specimens is critical not only for professional mycologists, but also for the large and dedicated group of citizen mycologists in North America. Citizen mycologists are represented by the North American Mycological Association (NAMA, 2017), whose affiliated regional clubs have approximately 10,000 members. Citizen mycologists are the conduit between professional mycologists and the general public, providing significant outreach about fungi through training courses, internet resources, lectures, fungus fairs, and field trips. Citizen mycologists are active collectors, helping to document the diversity and distribution of macrofungi, often in thoroughly documented publications (e.g., Evenson, 1997). In addition to the digitization of fungarium specimens, a goal of the project was to provide opportunities to share knowledge about macrofungi with a broader audience, including citizen mycologists, high school teachers, university students, and the general public. These interactions were designed to raise the profile of macrofungi as organisms of great interest and ecological importance, and of collections and digitization efforts that provide value for science, conservation, and recreation.
The main scientific objective of the MaCC project was to provide baseline data for determining the extent and distribution of macrofungal diversity, a fundamental task that is a long ongoing challenge (Mueller and Schmit, 2007; Schmit and Mueller, 2007). Fungal distributions are affected by the plant species that they are dependent on, either as mycorrhizal symbionts or as saprobes, and it is expected that areas of high plant diversity will be reflected in high fungal endemism. Some studies suggest, however, that macrofungal biodiversity significantly exceeds plant biodiversity. The coniferous forests of northern latitudes, for example, may have more than 1000 species of ectomycorrhizal fungi where only a few ectomycorrhizal plant species dominate (Allen et al., 1995). A similar situation has been documented in the montane oak forests of Costa Rica (Mueller and Halling, 1995). If these fungi were widely distributed and had broad host specificity, we might expect that a few widely dispersed fungi could occupy most niches, but increasingly, putative intercontinental and intracontinental distributions have been shown to comprise different but related species (Taylor et al., 2007; Dentinger et al., 2010; Justo et al., 2014). This is true for macrofungi with both symbiotic and saprobic lifestyles. The volume of authoritative data that will be available through this project will help identify disjunct populations under the same species epithet for further comparison and possible disambiguation using gene sequence analysis. Ultimately, these data will increase estimates of species diversity and identify areas of unusual species richness for protection and conservation.
Additionally, despite centuries of collecting macrofungi, we know surprisingly little regarding the phenology of macrofungi across the seasons and how climate may affect sporocarp production. However, several recently published studies have documented changes in patterns of macrofungal fruiting over the past 50 years (Gange et al., 2007, 2011; Kauserud et al., 2008, 2010). These studies have pointed to global climate change and specifically increased temperature as a likely source of changes in fruiting phenology. The foundation of all of these studies was digitized and georeferenced fungarium collections.
Methods
The MaCC project unites established and nascent collections of macrofungi through the digitization of label data from essentially all the macrofungal collections deposited in U.S. herbaria during the past 150 years. The data include images of specimen labels, transcribed label data, images of fungal specimens, and critical ancillary items such as photographs, drawings, paintings, field notes, and field book pages. Members of the Consortium include 39 institutions, including two botanical gardens, two natural history museums, and 35 large and small universities from 23 states (Appendix 1). The largest collections have broad temporal, geographic, and taxonomic coverage; the smaller ones generally emphasize the regional mycota and a few areas of deep taxonomic specialization, reflecting the research of past mycologists and their students. The collections mostly consist of specimens amassed by professional mycologists, although significant authoritative collections made by dedicated citizen (amateur) mycologists dominate the holdings of herbaria such as the Denver Botanic Gardens (DBG) and College of the Atlantic (HCOA). Codes used for collections are from Index Herbariorum (Thiers, 2017).
The foundation for the structure and procedures for the MaCC project derives from projects such as the North American Lichens and Bryophytes Thematic Collections Network (EF‐1115116) and the Tri‐trophic Thematic Collection Network (focusing on plants, herbivores, and parasitoids) (EF‐1115080) funded in 2011, the first year of the ADBC program. The imaging protocol for the MaCC project, however, was novel, reflecting the particular biology of these organisms. Most macrofungi change drastically in size, shape, and color upon drying. Therefore, images of the dried specimens themselves are often not particularly useful. As they collect, mycologists will photograph the living organism to be collected and will take extensive notes on the characteristics that will be lost upon drying. The MaCC project called for imaging of all specimen labels and a selection (20%) of all actual specimens, but also included imaging all ancillary data such as images of the living organism, notes taken on the fresh specimen, and spore prints (captured by placing the spore‐bearing surface on a piece of white paper), as well as the measurements of spores and other microscopic structures. The virtual association of these data with the specimen record and label image make the full documentation of each specimen available to all online.
The MaCC project also innovated the approach to training and project management. During the first year and a half of the project, all participants traveled to the New York Botanical Garden, in groups of three to five, for hands‐on training in all steps of the project, from barcoding to imaging and transcription to georeferencing and data upload. To support the training and for reference afterward, we distributed a 125‐page procedures manual that contained step‐by‐step procedures for all aspects of the project, as well as sources for ordering supplies and services. This manual was updated several times during the course of the project. Our goal with this intensive training was not only to teach basic techniques, but also to teach efficient digitization so that participants could meet the ambitious goals of the project. Experience has taught us that without careful oversight it is nearly impossible to digitize specimens at a rate that would keep the per‐specimen cost between US$1 and $2, the rate generally considered ideal.
Like the Lichen‐Bryophyte and the Tri‐Trophic digitization projects, the MaCC project protocol called for transcription of specimen label data from specimen images. In the Lichen‐Bryophyte project, the plan was for all transcription to be done by volunteers. The MaCC project followed the protocol established by the Tri‐Trophic project of having the lead institution do data transcription for all institutions, with a limited number of transcriptions by volunteers. But whereas the Tri‐Trophic project also centralized the georeferencing effort, in the MaCC project, participants were responsible for their own georeferencing.
The data generated through this project, combined with previously digitized data from macrofungal collections, are served through the Mycology Collections Portal (2017), or MyCoPortal, as well as through the iDigBio database. MyCoPortal uses the Symbiota software, which also powers a variety of other collaborative projects (e.g., SEINet, 2017; Consortium of North American Bryophyte Herbaria, 2017; Consortium of North American Lichen Herbaria, 2017). The basic function of MyCoPortal is similar to those of other Symbiota sites: users can search for records based on taxon name or other criteria across all institutions, or they can search any desired combination of institutions for specimens of a given species or collections from a particular area, collector, or range of dates. The records can be viewed as a text report or points on a map (for specimens that have geocoordinates). An underlying taxonomic database that relates synonyms to accepted names ensures that searches return all specimens of the desired species regardless of the name on the specimen. MyCoPortal uses two taxonomic thesauri: Index Fungorum (Kirk, 2017) and MycoBank (Bensch, 2017).
Results
Data from approximately 1,250,000 specimens were newly digitized through the MaCC project, approximately 130,000 more than estimated in the original proposal. All of the data are served through MyCoPortal (2017). Completion of this project required 275 participants: 40 principal investigators and senior personnel, 35 salaried digitization staff, 200 undergraduate and graduate student workers, and nine volunteers.
The project exceeded expected deliverables mostly because some participants underestimated their holdings of macrofungi, but nonetheless completed the digitization of all specimens. By completing additional specimens, these institutions demonstrated that they not only mastered the project workflow, but also managed to make it even more efficient than originally projected. Some institutions, however, fell short of expectations and did not deliver as many specimens as promised. In most cases, this was because they did not follow the digitization protocol, or because they had difficulty sustaining a well‐trained workforce. These problems are actually related—often the person who trained with us at the outset of a project left before the end of the project, and the knowledge transfer to new staff was not complete. If we had been able to budget for multiple trainings per institution, we could have made sure that all workers understood the workflow, and if institutions could have offered higher pay for digitization staff, then turnover would likely have been less. Better communication would have alerted us to problems sooner and might have avoided some under‐performance. However, given the magnitude of the project to digitize all U.S. natural history specimens, project costs have to be kept as low as possible, and funds for travel and administration are sometimes considered unnecessary or excessive by reviewers.
In the resulting data set, approximately 73% of the specimens are from North America; 18% are from Central America, South America, and the Caribbean region; 5% are from Europe; 2% are from Asia and the Pacific region; 1% are from Australia and New Zealand; and 1% are from Africa. Within North America, the greatest concentrations of specimens are unsurprisingly in states where there is a history of mycology at the states' research institutions (e.g., California, Florida, Illinois, Michigan, New York, and North Carolina), as shown in Fig. 1. With regard to taxonomic groups represented, the 20 largest families represented in MyCoPortal are shown in Table 2. The relative sizes of these groups roughly mirror the abundance and diversity of macrofungi, although the Polyporaceae are likely over‐represented because these fungi are large and persistent for long periods of time, meaning that many species are encountered more often than species of other groups.
Figure 1.
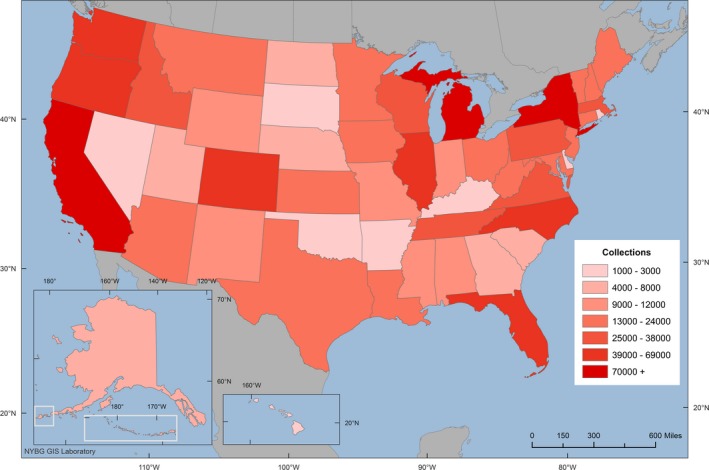
Map showing the density of macrofungal collections in the United States.
Table 2.
The 20 largest families of macrofungi represented in MyCoPortal
| Family | No. of specimens |
|---|---|
| Polyporaceae | 202,069 |
| Agaricaceae | 100,416 |
| Russulaceae | 85,127 |
| Tricholomataceae | 77,078 |
| Strophariaceae | 65,111 |
| Boletaceae | 59,781 |
| Cortinariaceae | 55,833 |
| Hymenochaetaceae | 40,564 |
| Stereaceae | 37,607 |
| Amanitaceae | 37,105 |
| Mycenaceae | 36,376 |
| Meruliaceae | 35,599 |
| Inocybaceae | 33,042 |
| Hygrophoraceae | 28,253 |
| Fomitopsidaceae | 26,479 |
| Corticiaceae | 25,137 |
| Entolomataceae | 24,826 |
| Gomphaceae | 20,373 |
| Psathyrellaceae | 18,558 |
| Peniophoraceae | 17,585 |
Discussion
MyCoPortal has been cited in approximately 30 articles, as revealed though a Google Scholar search on 17 October 2017. These include genus‐ or species‐level treatments (Kuo and Matheny, 2015; Medina‐Ortiz et al., 2017), as well as some large‐scale phylogenetic (Hibbett et al., 2016) and ecological studies in native versus invasive range comparisons of ectomycorrhizal fungi (e.g., Nuñez and Dickie, 2014). Data from MyCoPortal have been used to verify species distributions for field guides of the Rocky Mountain region (Evenson, 2015) and northeastern North America (Baroni, 2017). MaCC was also instrumental in the rehabilitation of the mycological collections of the University of North Carolina Fungarium (NCU) (McCormick, 2017).
MaCC will play a foundational role in a long‐term project: a Mycoflora of North America. Despite their ecological and economic importance, and the long history of documenting macrofungi through collections, there are no comprehensive treatments or floras (mycofloras) for the country as a whole, let alone the continent. An inaugural meeting to explore how a Mycoflora of North America might be organized was held at Yale University in 2012, attended by 75 professional and amateur mycologists (Bruns, 2012). The digitization efforts of the MaCC project were only just beginning at that time, but the funding of that project was an important catalyst for the 2012 meeting.
The 2012 meeting did not result in the self‐organization of the community to advance the planning of the structure or content of the Mycoflora. The meeting did, however, stimulate considerable interest within mushroom clubs in vouchering and DNA sequencing (Sheehan, 2017). A survey conducted in 2016 sought to determine the extent to which the approximately 80 mushroom clubs (approximately 10,000 citizens in total) have engaged in activities relevant to a Mycoflora, such as specimen vouchering and DNA sequencing. The results, based on 38 club responses, indicated that 53% of clubs engage in some vouchering of specimens; some keep their vouchers at home, but more deposit them in established herbaria. Approximately half of the responding clubs have engaged in DNA sequencing, and 63% are interested in participating in this activity (Sheehan, 2017). In one club, the Fungus Federation of Santa Cruz, the vouchering and sequencing activities are highly focused, with the objective of documenting, through specimens and DNA sequences, all known regional fungi through the Santa Cruz Mycoflora Project (2017). Sheehan's assessment of Mycoflora‐related activities since 2012 was that a greater level of participation by the professional mycological community was needed to guide the project, and that the sequencing effort by clubs needed more coordination and funding. Consequently, a workshop was held in July 2017 at the University of Georgia in Athens, entitled, “Mycoflora 2.0.” As suggested by the name of the workshop, the purpose was to reassess the concept and progress of the Mycoflora since the original 2012 meeting, with the goal of developing a more coordinated approach. The completion of the digitization of specimens held in U.S. herbaria is a critical tool for the Mycoflora, and use of MyCoPortal software to compile and share data gathered for the Mycoflora has gained broader acceptance. The Symbiota software that underlies MyCoPortal provides interactive key‐ and description‐writing functions, which can help with the synthesis and assembly of taxon‐level data, as it has for the Sonoran Desert Region Flora (2017). A series of joint meetings between the professional and citizen mycological communities are planned for the next five years to further refine the methods and objectives of the Mycoflora, and the Mycological Society of America has recently offered significant funds to the project for DNA sequencing by citizen mycology groups. It is too early to predict whether the Mycoflora project will be successful and how it will proceed, but it finds a firm foundation in the data amassed through the MaCC project.
Even though specimen digitization for the MaCC project is now complete, there is still a great deal of work to be done to standardize and update the resulting data. It will require effort on the part of collection managers as well as users to improve the data. Therefore, keeping the mycological community engaged with MyCoPortal is key to ensuring that these data can reach their full potential. We hope the following actions will help to sustain the resource.
Expand the project to include all fungi
The ultimate goal of the digitization effort was to include all fungal collections, but the magnitude of that project was too great to contemplate in one funded project. Fortunately, Andrew Miller (Illinois Natural History Survey, University of Illinois at Urbana‐Champaign, Champaign, Illinois, USA) spearheaded a complementary project to digitize microfungi (the Microfungi Collections Consortium [MiCC]), and this effort, which involved many of the same institutions that participated in the MaCC project, was funded in 2015. The microfungi project has greatly expanded the taxonomic depth and volume of data in MyCoPortal, and has brought a number of technical advancements to the Symbiota software that underlies MyCoPortal, including tools to expedite the digitization and organization of specimens published as exsiccati.
Internationalize the project
Since the beginning of the project, we have encouraged herbaria outside the United States to contribute their data. Mostly through the efforts of the MiCC project, we now have 10 foreign herbaria contributing to the project, and preliminary discussions are underway with the Australasian mycological community to consider joining the fungal records in MyCoPortal with those in the Atlas of Living Australia (2017).
Continue to grow the collections
In order for specimens of macrofungi to serve the purpose of documenting changes in diversity over time, we must continue to make collections and deposit those specimens in established herbaria. In order to realize the full scientific value of their holdings, MaCC herbaria should be willing to accept new properly documented collections within the areas of their geographic and/or taxonomic interest, and to make their collections available for study. Collections will only be relevant for documenting changes in biodiversity over time if their holdings reflect the current mycota. The Mycoflora effort will generate many voucher specimens, and ideally these should be deposited in herbaria that are geographically as near as possible to the person who created the voucher. As the Mycoflora project becomes more organized internally, we, as principal investigators on the MaCC project, will try to serve as an intermediary between mushroom clubs and established collections to make sure that new collections can be properly and efficiently accessioned, digitized, and made available online and as physical specimens for study.
Acknowledgments
We greatly appreciate funding from the National Science Foundation for support of the Macrofungi Collections Consortium, through grant DBI‐1206197.
Appendix 1.
Institutions participating in the Macrofungi Collection Consortium, indicating digitization work done at each. Index Herbariorum codes are given in parentheses. Names with a single asterisk (*) indicate those institutions that contributed specimen data but were not funded by the NSF grant that created the Macrofungi Collection Consortium. Names with two asterisks (**) indicate institutions that joined the project through PEN awards.
College of the Atlantic (HCOA)
Cornell University (CUP)
Davis and Elkins College Herbarium (DEWV)*
Denver Botanic Gardens (DBG)
Duke University (DUKE)
Eastern Illinois University (EIU)
Field Museum of Natural History (F)
Fort Lewis College Herbarium (FLD)*
Harvard University Farlow Herbarium (FH)
Illinois Natural History Survey, Illinois Department of Natural Resources (ILLS)
Louisiana State University (LSUM)
Miami University (MU)
The New York Botanical Garden (NY)
New York State Museum (NYS)
North Carolina State University Larry F. Grand Mycological Herbarium (NCLSG)
Oregon State University (OSC)
Purdue University (PUL)
San Francisco State University (SFSU)
State University of New York College at Cortland (CORT)
State University of New York Syracuse (SYRF)
U.S. National Fungus Collections, USDA‐APHIS (BPI)*
University of Arizona (ARIZ)
University of California, Berkeley (UC)
University of California Santa Cruz Fungal Herbarium (UCSC)*
University of Central Oklahoma (CSU)
University of Florida (FLAS)
University of Illinois (ILL)
University of Maine (MAINE)**
University of Michigan (MICH)
University of Minnesota (MINN)
University of Montana (MONTU)
University of North Carolina (NCU)
University of South Alabama (USAM)
University of Tennessee (TENN)
University of Vermont (VT)**
University of Washington (WTU)
University of Wyoming (RMS)
Virginia Polytechnic Institute and State University (VPI)
Thiers, B. M. , and Halling R. E.. 2018. The Macrofungi Collection Consortium. Applications in Plant Sciences 6(2): e1021.
Literature cited
- Allen, E. B. , Allen M. F., Helm D. J., Trappe J. M., Molina R., and Rincon E.. 1995. Patterns and regulation of mycorrhizal plant and fungal diversity. Plant and Soil 170: 47–62. [Google Scholar]
- Atlas of Living Australia . 2017. Website http://www.ala.org.au [accessed 22 January 2018].
- Baroni, T. 2017. Mushrooms of the northeastern United States and eastern Canada. Timber Press, Portland, Oregon, USA. [Google Scholar]
- Bensch, K. 2017. MycoBank. Website http://www.mycobank.org/ [accessed 22 January 2018].
- Boa, E. 2004. Wild edible fungi: A global overview of their use and importance to people Non‐wood forest products, 17. Food and Agriculture Organization of the United Nations, Rome, Italy. [Google Scholar]
- Bruns, T. 2012. The North American Mycoflora project—the first steps on a long journey. New Phytologist 196: 972–974. [DOI] [PubMed] [Google Scholar]
- Consortium of North American Bryophyte Herbaria . 2017. Website http://bryophyteportal.org/ [accessed 22 January 2018].
- Consortium of North American Lichen Herbaria . 2017. (updated continuously). Website http://lichenportal.org/portal/ [accessed 23 January 2018].
- Dentinger, B. T. , Ammirati J. F., Both E. E., Desjardin D. E., Halling R. E., Henkel T. W., Moreau P.‐A., et al. 2010. Molecular phylogenetics of porcini mushrooms (Boletus section Boletus). Molecular Phylogenetics and Evolution 57: 1276–1292. [DOI] [PubMed] [Google Scholar]
- Evenson, V. S. 1997. Mushrooms of Colorado and the southern Rocky Mountains. Westcliffe Publishers Inc., Denver, Colorado, USA. [Google Scholar]
- Evenson, V. S. 2015. Mushrooms of the Rocky Mountain Region: Colorado, New Mexico, Utah, Wyoming. Timber Press, Portland, Oregon, USA. [Google Scholar]
- Gange, A. C. , Gange E. G., Sparks T. H., and Boddy L.. 2007. Rapid and recent changes in fungal fruiting patterns. Science 316: 71. [DOI] [PubMed] [Google Scholar]
- Gange, A. C. , Gange E. G., Mohammad A. B., and Boddy L.. 2011. Host shifts in fungi caused by climate change? Fungal Ecology 4: 184–190. [Google Scholar]
- Hibbett, D. , Abarenkov K., Kõljalg U., Öpik M., Chai B., Cole J., Wang Q., et al. 2016. Sequence‐based classification and identification of Fungi. Mycologia 108: 1049–1068. [DOI] [PubMed] [Google Scholar]
- Justo, A. , Malysheva E., Bulyonkova T., Vellinga E. C., Cobian G., Nguyen N., Minnis A. M., and Hibbett D. S.. 2014. Molecular phylogeny and phylogeography of Holarctic species of Pluteus section Pluteus (Agaricales: Pluteaceae), with description of twelve new species. Phytotaxa 180: 1–85. [Google Scholar]
- Kauserud, H. , Stige L. C., Vik J. O., Okland R. H., Hoiland K., and Stenseth N. C.. 2008. Mushroom fruiting and climate change. Proceedings of the National Academy of Sciences USA 105: 3811–3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauserud, H. , Heegaard E., Semenov M. A., Boddy L., Halvorsen R., Stige L. C., and Sparks T. H., et al. 2010. Climate change and spring‐fruiting fungi. Proceedings of the Royal Society B. Biological Sciences 277: 1169–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk, P. 2017. Index Fungorum. Website http://www.indexfungorum.org [accessed 22 January 2018].
- Kirk, P. , Cannon P., Stalpers J. A., and Minter D. W.. 2008. Ainsworth and Bisby's dictionary of the fungi, 10th ed. CAB International, Wallingford, United Kingdom. [Google Scholar]
- Kuo, M. , and Matheny P. B.. 2015. Contemporary documentation of the rare eastern North American species Inocybe insignis (Inocybaceae, Agaricales). MycoKeys 11: 23–31. [Google Scholar]
- McCormick, C. 2017. Featured Herbarium: NCU – The University of North Carolina Chapel Hill Herbarium. The Vasculum 12: 6–7. [Google Scholar]
- Medina‐Ortiz, J. K. , Herrera T., Vásquez‐Dávila M., Raja H. A., and Figueroa M.. 2017. The genus Podaxis in arid regions of Mexico: Preliminary ITS phylogeny and ethnomycological use. MycoKeys 20: 17–36. [Google Scholar]
- Mueller, G. M. , and Halling R. E.. 1995. Evidence for high biodiversity of Agaricales (Fungi) in neotropical montane Quercus forests In Churchill S. P., Balsev H., Forero E., and Luteyn J. L. [eds.], Biodiversity and conservation of neotropical montane forests, 303–312. New York Botanical Garden Press, Bronx, New York, USA. [Google Scholar]
- Mueller, G. M. , and Schmit J. P.. 2007. Fungal biodiversity: What do we know? What can we predict? Biodiversity and Conservation 16: 1–5. [Google Scholar]
- Mycology Collections data Portal (MyCoPortal) . 2017. Website http://mycoportal.org/ [accessed 22 January 2018].
- North American Mycological Association (NAMA) . 2017. (continuously updated). Website https://namyco.org/ [accessed 23 January 2018].
- Nuñez, M. , and Dickie I. A.. 2014. Invasive belowground mutualists of woody plants. Biological Invasions 16: 645–661. [Google Scholar]
- Santa Cruz Mycoflora Project . 2017. (updated continuously). Website http://www.scmycoflora.org/projects.php [accessed 23 January 2018].
- Schmit, J. P. , and Mueller G. M.. 2007. An estimate of the lower limit of global fungal diversity. Biodiversity Conservation 16: 99–111. [Google Scholar]
- SEINet . 2017. (updated continuously). Website http://swbiodiversity.org/seinet/ [accessed 23 January 2018].
- Sheehan, B. 2017. Mushroom citizen science in the USA: From species lists to Mycoflora 2.0. Fungi Magazine 101: 28–36. [Google Scholar]
- Sonoran Desert Region Flora . 2017. (updated continuously). Website http://www.desertmuseum.org/center/swbiodiversity.php [accessed 23 January 2018].
- Taylor, A. F. , Hills A. E., Simonini G., Muñoz J. A., and Eberhardt U.. 2007. Xerocomus silwoodensis sp. nov., a new species within the European X. subtomentosus complex. Mycological Research 111: 403–408. [DOI] [PubMed] [Google Scholar]
- Thiers, B. M. 2017. (continuously updated). Index Herbariorum: A global directory of public herbaria and associated staff. New York Botanical Garden's Virtual Herbarium. New York Botanical Garden, Bronx, New York, USA. Website http://sweetgum.nybg.org/science/ih/ [accessed 22 January 2018].


