Abstract
Background
No study has previously analyzed aggressiveness, homicide, and Lyme disease (LD).
Materials and methods
Retrospective LD chart reviews analyzed aggressiveness, compared 50 homicidal with 50 non-homicidal patients, and analyzed homicides.
Results
Most aggression with LD was impulsive, sometimes provoked by intrusive symptoms, sensory stimulation or frustration and was invariably bizarre and senseless. About 9.6% of LD patients were homicidal with the average diagnosis delay of 9 years. Postinfection findings associated with homicidality that separated from the non-homicidal group within the 95% confidence interval included suicidality, sudden abrupt mood swings, explosive anger, paranoia, anhedonia, hypervigilance, exaggerated startle, disinhibition, nightmares, depersonalization, intrusive aggressive images, dissociative episodes, derealization, intrusive sexual images, marital/family problems, legal problems, substance abuse, depression, panic disorder, memory impairments, neuropathy, cranial nerve symptoms, and decreased libido. Seven LD homicides included predatory aggression, poor impulse control, and psychosis. Some patients have selective hyperacusis to mouth sounds, which I propose may be the result of brain dysfunction causing a disinhibition of a primitive fear of oral predation.
Conclusion
LD and the immune, biochemical, neurotransmitter, and the neural circuit reactions to it can cause impairments associated with violence. Many LD patients have no aggressiveness tendencies or only mild degrees of low frustration tolerance and irritability and pose no danger; however, a lesser number experience explosive anger, a lesser number experience homicidal thoughts and impulses, and much lesser number commit homicides. Since such large numbers are affected by LD, this small percent can be highly significant. Much of the violence associated with LD can be avoided with better prevention, diagnosis, and treatment of LD.
Keywords: Borrelia burgdorferi, impulsive, tick-borne, rage, suicide, immune
Video abstract
Plain language summary
This study was performed because a recent study on Lyme disease (LD) and suicide showed 26% of suicidal patients with late stage LD were also homicidal. Violence and homicide rarely have a single cause, instead there are many contributors, deterrents, and acute triggers. Many infections and the immune reactions to them can be associated with violence, including those transmitted by ticks, lice, mosquitos, and so on. The evidence supporting the association between infections and violence is from evolutionary concepts, epidemiology, historical perspectives, case reports, animal studies, brain chemistry, brain immunology, brain circuits, and medical literature review. In this study, inactive charts of Lyme patients were reviewed and analyzed for aggressiveness, homicidality, and homicide. Most aggression in these patients was associated with poor impulse control, sometimes triggered by intrusive images, thoughts and emotions; sound or other stimulation and frustration, and the resulting aggression was invariably bizarre and senseless. In this study of homicidal Lyme patients, it was found that the average patient diagnosis was delayed by 9 years. Lyme infections caused a broad spectrum of clinical findings identified in this study that can collectively be associated with homicidality. A large number of patients with LD have no aggressive tendencies or only mild degrees of low frustration tolerance and irritability, pose no danger to society, and should not be stigmatized. This study can help explain and prevent some previously unexplainable violence and homicide and has broader implications. Understanding the role of Lyme and other infections in causing violence, and prevention, diagnosis, and treatment can help reduce some violence and promote peace.
Introduction
Background
Lyme disease (LD) is the most common tick-borne disease, which is a spirochete, and LD has been compared to syphilis and labeled the New Great Imitator since it may present as many different medical conditions.1,2 The Lyme Borrelia has been found in 15 million-year-old fossilized ticks and in the 5,300-year-old Iceman in the Italian Alps.3,4 LD is transmitted by Ixodes ticks and is the most common tick-borne disease.5 Although Ixodes ticks and Borrelia are present throughout the world, there are regional differences in Borrelia species, tick populations, tick-borne coinfections, and infection rates. In the past 30 years, there has been an expanding awareness of a broad spectrum of neuropsychiatric symptoms that invariably evolve in the later stages of these infections and may include cognitive, emotional, and behavioral symptoms. The diagnosis of LD is often overlooked, and it is increasingly common for psychiatrists to make the initial diagnosis.6–11
LD, or Lyme borreliosis, is a tick-borne infectious disease caused by Borrelia burgdorferi, Borrelia garinii, or Borrelia afzelii while the term Lyme borreliosis/tick-borne diseases recognize that there may also be other tick-borne pathogens and secondary opportunistic infections causing a complex pathophysiological process.
No study has ever before attempted to examine the association between aggressiveness, violence, homicidality, homicide, and LD. A recent article on suicide and Lyme and associated diseases revealed that 68% of LD patients acquired some form of aggressiveness, including 11% who became homicidal after infection, and 26% of the suicidal patients were also homicidal.12 Based upon this finding, a further assessment of aggression and homicidality in LD patients was indicated.
Currently there is a tendency to deal with aggressiveness more through punitive approaches rather than scientific understanding and proactive prevention. Weapon technology is more advanced than our capability to understand and prevent violence, and this limitation is a serious concern when atomic and other weapons of mass destruction may be in the hands of individuals or groups at risk for violent behavior. Therefore, every contributor to violence needs to be better understood and addressed.
Defining aggression and violence
The word aggression may refer to either healthy adaptive emotional effort or more commonly violent maladaptive behavior. Healthy adaptive aggression exists throughout the animal kingdom and is an integral part of human behavior. It includes the pursuit of life, liberty, and happiness and the drives for territoriality, social integration, and reproduction. Social cooperation and respectful competition are an integral part of adaptive aggression and healthy human and societal functioning. A healthy individual in a healthy society values social cooperation and respectful competition over violence as a means of achieving goals.
Anger is an emotion characterized by antagonism toward someone, oneself, or something. Violence can be defined as the use of physical force, power, or threat with the intent to harm or injure another person, oneself, or a group or community, or the destruction of property. Rage is violent, uncontrolled anger. Characteristics of violent acts include self-directed violence (suicidal behavior and self-abuse), interpersonal violence (family – child, partner, elder, or community – acquaintance, and stranger), and collective violence (social, political, and economic). The nature of violent acts can include physical, sexual, psychological, and involving deprivation or neglect.13 Sometimes aggression is used as a synonym for violence. Homicide is the act of one human killing another.
Contributors, deterrents, and triggers of violence
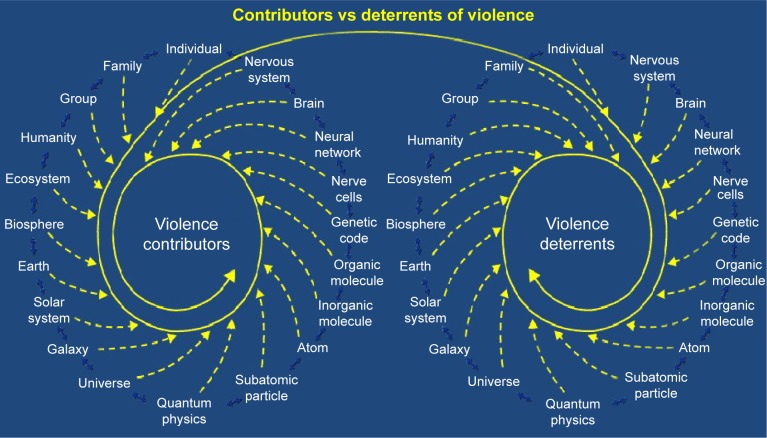
It can rarely be stated that there is only one single cause in any given violent act. Violent behavior is the result of an interaction of multiple contributors, deterrents, acute triggers, and many different sequential events, some of which are both known and unknown (Figure 1).
Figure 1.
The presence or absence of violence is the interactive result of all the multisystem acute and chronic contributors and deterrents to violence.
Some of the well-recognized contributors to violence include homelessness, social isolation, being single, living alone, gang membership, low education, unemployment, incarceration, history of violent behavior, fascination with weapons, being a victim of violence, opposing beliefs (religious, political, ethnic, and lifestyle), exposure to violence, exposure to media and computer games with violent themes, younger age, intoxication of potential victim, response of the potential victim, gender, genes (monoamine oxidase A, catechol-O-methyltransferase, serotonin transporter gene, FK506 binding protein s, XYY), threat sensitivity, grudge holding, victim blaming, childhood abuse, child conduct disorder, multiple psychiatric diagnoses (intermittent explosive disorder, narcissistic personality disorder, antisocial personality disorder, psychopathy, bipolar illness, depression, social communication disorder, and other conditions), longer duration of untreated psychosis, cognitive dysfunction, treatment non-adherence, substance abuse, cannabis use, intoxication, head injuries, toxicity, endocrine disorders, limbic encephalitis, Wilson’s disease, brain infections, brain tumors, neurological impairments, and sensory and motor impairments.14–18 One hundred percent of death row inmates had both neurological impairments and a history of abuse.19–21
Deterrents to violence include empathy, frustration tolerance, cognitive abilities, restraint capabilities, higher education, coping skills, supportive relationships, community and other social connections, ethical and religious beliefs, access to social services and psychiatric and medical care, and social structure.22
Triggering events can include acute stress, apperception of threat, jealousy, obsession, psychotic delusions, post-traumatic flashbacks, hopelessness, competition, exposure to violence, traumatic brain injury, unintentional injury, being suicidal, self-harm, impairment from substance use, intoxication, and losing a parent.23,24
While acknowledging the significance of all of these other components to violence, the scope of this article shall specifically focus upon violence and LD.
Epidemiology of violence and infections
Globally, ~1.6 million people die each year because of violence.13,25 Currently violence is not equally distributed throughout the world.26 Peace correlates strongly with education, health, and economic opportunity, and health correlates with a lower prevalence of infectious disease.27 Cognitive impairments contribute to violence, and higher cognitive functioning is a deterrent to violence. There is a strong correlation between national intelligence quotient and lower parasite stress. As countries overcome infectious diseases, the intelligence of their citizens increases; this helps explain the Flynn Effect.28 One study sampled 20 European nations and demonstrated that the prevalence of the brain parasite Toxoplasma gondii was positively associated with national homicide rates.29,30
Violence and infections: historical perspective
Violence is a significant part of history. Death due to violence was much more common in prehistoric societies, in which it was estimated that 10% died because of homicide. Throughout history, violence has decreased as civilization has advanced with increases in literacy, education, democratic governments, and health.31 Different parts of the world at different times in history had different rates of violence in individuals and groups. Poor hygiene correlates with a higher rate of infectious diseases, which in turn correlates with a greater prevalence of violence. The greater prevalence of violence in a critical mass of individuals and influential individuals may contribute to collective violence and wars at different times in history.32
Influential leaders considered to have mental symptoms from syphilis impacting their behavior include Joseph Stalin, Adolf Hitler, Ivan the Terrible, Idi Amin Dada, Benito Mussolini, Henry VIII, Al Capone, Napoleon Bonaparte, Vladimir Lenin, Pope Alexander VI, Charles VIII of France, Peter the Great, and Catherine the Great.33–36
Types of violence
Two basic types of violence are individual violence and collective or group violence. Individual violence involves one person directing violence against others. Collective or group violence is acts committed by normal people and includes situational collective violence which is unplanned and spontaneous, organized collective violence that is unofficial and lacking government approval, and institutional collective violence which is directed by legally constituted officials.
Individual violence can be categorized into three major types – impulsive (or affective), psychotic, and predatory (or psychopathic). Some individual violent acts may be a combination of more than one of these categories. Impulsive violence, also called disorders of impulse control or affective violence is reactive, emotional, rapid and unplanned in response to a real or perceived perception of threat with intense autonomic arousal without regard for long-term consequences. Psychotic violence is motivated by a persecution belief, and there may be an attack against imagined enemies. Paranoia, paranoid schizophrenia, and drug-induced paranoid states are included in this category. Predatory violence is planned, purposeful, and emotionless without autonomic arousal. Impaired functioning contributes to violence in all the three categories, which include impairments of cognition, threat perception, autonomic arousal, impulse control, empathy, and reality testing.14,37
The characteristics of violent acts include self-directed violence and interpersonal violence. Self-directed violence can include suicidal behavior and self-abuse. Interpersonal violence can include family and intimate partner violence (child, partner, and elder), community violence (acquaintance and stranger), and collective violence (social, political, and economic). The nature of violent acts includes physical, sexual, psychological, and/or involving deprivation or neglect.13,38
Impaired and violent individuals or groups of impaired and violent individuals may also contribute to causing collective violence on others who are normal healthy individuals if these impaired individuals are in positions of leadership or influence upon other normal healthy individuals. Leaders or influential individuals prone to predatory violence are particularly dangerous which may result in genocide.32
Neural circuitry of violence
Although a detailed review of all the known neural circuits and transmitters involved in violence is beyond the scope of this article, some general concepts deserve attention. In a state of health, higher level cortical and limbic functioning has sufficient control over more primitive limbic and hypothalamic functioning. Higher level brain functioning integrates many feedforward and feedback pathways that are both stimulatory and inhibitory. Injury to a higher center can result in a dysfunction or a loss of a function leading to a decline in adaptive abilities. Injury to an inhibiting pathway will cause a decline or an inability to inhibit that function. In individuals prone to violence, higher level neural circuits associated with civilized behavior have insufficient control over more primitive mammalian and reptilian neural circuits.39
The right ventromedial prefrontal cortex integrates cognition and affect which contributes to mediating the empathic response.40 The orbital frontal cortex contributes to constraining impulsive outbursts, while the anterior cingulate cortex recruits other brain regions in the response to conflict. Parts of the amygdala are involved in the production of a fear response and other negative emotions. Other parts of the amygdala recognize fear, social emotions, and multiple other emotions in facial expressions as demonstrated by impaired recognition of emotions of social significance following amygdala damage.41,42
In impulsive violence, cortical functioning generally has insufficient control over lower level functioning. This failure of the prefrontal cortex to modulate aggressive acts triggered by anger-provoking stimuli combined with a hyper-responsive amygdala and other limbic regions that are part of affective reactivity contributes to impulsive violence.43 Brain activity in the orbital and anterior regions (impulse control) were blunted or entirely absent in many violent offenders, while the amygdala (fear) showed normal or heightened activity. The inability of the two brain regions to effectively balance the response of the amygdala may help explain how threatening situations can become explosive in some people.44 Brain imaging in murderers supports this model and demonstrates reduced activity in the prefrontal cortex as well as the superior parietal cortex, left angular gyrus, and corpus callosum.45
In psychotic aggression, there is also a hypoactive prefrontal cortex but in contrast to impulsive violence there is invariably normal limbic drive and striatal hyperactivity.14,46 In predatory aggression, there is also a hypoactive prefrontal cortex, but instead a hypoactive limbic system and amygdala.14,46
Particularly violence is correlated with intrusive symptoms, which is relevant to impulsive, predatory, and psychotic aggression. The neural circuit relevant to these symptoms are associated with the activation of basolateral amygdala and ventral hippocampus.47–50 Activation of this circuit also seems to be associated with chronic inflammation with elevated inflammatory markers (tumor necrosis factor-alpha, interleuken-6, interleukin-1β, and C-reactive protein).51–53 LD is associated with chronic inflammation and activation of this circuit.54
Neurochemistry of violence
The neurotransmitters serotonin, dopamine, norepinephrine, acetylcholine, glutamate, and gamma-aminobutyric acid are thought to be involved in violent behavior.14 Particularly serotonin is significant.55–57 Impulsively violent individuals have decreased serotonin activity as demonstrated by significantly lower concentrations of the major metabolite of serotonin, 5-hydroxyindoleacetic acid, in their cerebrospinal fluid.58 Serotonin serves an important inhibitory function in the amygdala, anterior cingulate cortex, dorsolateral prefrontal cortex, and orbitofrontal cortex.59
Inflammatory markers are elevated in individuals with intermittent explosive disorder. Elevations of both plasma C-reactive protein and interleukin-6 levels were significantly elevated in individuals with intermittent explosive disorder compared with psychiatric or normal controls. In addition, these inflammatory markers were directly correlated with a composite measure of aggression and with measures reflecting history of actual aggressive behavior in all individuals.60
Persistence of inflammation is associated with persistent elevations of proinflammatory cytokines, which results in the dysregulation of tryptophan metabolism, that increases quinolinic acid, which is a N-methyl-D-aspartate receptor agonist and this can result in glutamate dysfunction and violent behavior.12
Violence and infections
Violent behavior has been associated with a number of infectious diseases including Spanish flu, encephalitis lethargica, Western equine encephalitis, viral encephalitis, herpes simplex encephalitis, neurosyphilis, cerebral malaria, sepsis, and toxoplasmosis.61–76 In a review of 108 cases of acute viral encephalitis, 23% experienced violence or aggression.77 Criminal behavior as a result of neurological injury has been documented in both acute and chronic infections including neurosyphilis, encephalitis lethargica, herpes simplex encephalitis, AIDS dementia complex, infections causing dementia, and various other viral encephalitides.78–81
T. gondii seropositive individuals have higher aggression and impulsivity scores, which suggests T. gondii changes brain chemistry in a fashion that increases the risk of aggressive behavior. The personality of men infected with T. gondii showed higher apprehension compared to uninfected controls, lower superego strength, and higher vigilance, they were more likely to disregard rules, and were more expedient, suspicious, jealous, and dogmatic. Possible mechanisms by which T. gondii may affect human behavior include its effect on dopamine and on testosterone.82
Parasitic infections have adverse effects throughout life, but are particularly traumatic to the young. Newborn children need 87% of their metabolic energy for brain functioning, while the brains of 5-year-old children consume 87% of their metabolic energy and an adult brain requires approximately a quarter of the body’s energy. In addition to the pathogenic effects of parasites, they also compete for energy which can harm brain development and functioning, which can in turn contribute to violent behavior.28
Violence and infectious diseases in animals
Parasites sometimes manipulate the behavior of hosts in a manner that facilitates their survival and reproduction.83 Sometimes this manipulation includes fostering aggressive behavior.84,85 Historically, rabies has been the main model for infections causing violence in animals.86 In addition, aggression associated with tick-borne infections in animals has been documented with Babesia microti in mice,87 Babesia gibsoni in dogs,88 B. burgdorferi in dogs,89,90 Bartonella henselae in dogs,91 B. henselae in horses,92 and B. burgdorferi in chimpanzees.93,94
In order to study the neuroendocrine effects, work has been done with prairie voles and dogs to understand aggressiveness in animals with selective aggression versus affiliation and bonding in the context of arginine-vasopressin versus oxytocin.95–97 Both the neuropeptides are significant in social behavior, but oxytocin is more associated with affiliation while arginine-vasopressin is more associated with aggression and male behavior.98 In sheep, increased oxytocin results in maternal acceptance while lower levels may result in violent rejection and indifference toward a newborn.99 In humans, low oxytocin levels are associated with alexithymia and anorexia nervosa.100 Arginine-vasopressin and oxytocin are released from the posterior pituitary gland, a part of the brain lacking a blood–brain barrier and more vulnerable to penetration by B. burgdorferi which has been demonstrated in the vasculature in a murine model.101–104
Murine and primate models demonstrated infection of the trigeminal nerve is a transneuronal entry route for brain infections involving temporal lobe and limbic structures.22,105
Violence and LD
More serious cases often involve a complex interactive infection with Lyme borreliosis and other tick-borne coinfections and sometimes the activation of opportunistic viruses and other pathogens. A survey of over 3,000 chronic LD patients demonstrated that over 50% had laboratory-confirmed coinfections and 30% had two or more coinfections, including Babesia (32%), Bartonella (28%), Ehrlichia (15%), Mycoplasma (15%), Rickettsia rickettsia (6%), Anaplasma (5%), and Francisella tularemia (1%). A very similar study of chronic Lyme patients in Canada demonstrated similar coinfection rates.106,107 Although some tick studies show that coinfection rates are relatively low, these findings can be explained since chronically ill patients often report exposure to multiple tick bites.
The first human reference to aggressiveness with tick-borne diseases that could be located was a 1990 study by Logigian et al demonstrating that 26% of chronic Lyme patients had extreme irritability.108 In 1991, Harvey et al described a patient who became aggressive and combative following a Bartonella infection.109 The first reference to homicide associated with LD was a meatpacker who killed his supervisor at a meat-packing plant in Michigan in 1992 and used LD as part of the defense. His identical twin who did not have LD had no neurological deficits or history of violence.110 In 1992, Fallon et al described a man acutely sensitive to sound who was so intensely bothered by the noise of his 3-year-old son that he picked him up and shook him in a sudden and unprecedented fit of violence.111 Sherr described LD patients having sudden personality changes, in people of known character after acquiring LD, including outbursts of anger, breaking doors, cursing at other drivers, and road rage.112 In a publication, a LD patient described an incident where someone else pulled into a parking space that he wanted; he responded by jumping out of his car and knocking the other driver unconscious. Another LD patient was described lunging out of his car after a motorist beeped the horn and he began pounding on the windshield of their car, then suddenly stopped in bewilderment because he did not recall or understand why he was behaving in this manner. Another LD patient was arrested for shoplifting during a state of confusion, and another patient was accused of pedophilia.113
Other media reports include: a LD patient who stalked, threatened to rape, and made repeated threats against multiple political candidates;114 a LD patient committed sexual acts with his 9-year-old son;115 a LD patient shot at police after a 911 call;116 a LD patient was charged with assault associated with marital conflicts;117 and there have been multiple additional references to aggressiveness, violence, homicidality, and homicide associated with LD.6,9,54,113,118–136
In one presentation, 26 different individuals with LD committing multiple violent acts including multiple assaults on police officers and 11 homicides were described.137 Violent behavior seen with LD included homicide; suicide; combined homicide and suicide; assaults with a gun, knife, sword, axe, hammer, rocks, and darts; assaulting a sibling’s head with a rock; pedophilia; stalking; child abuse; assault toward teachers and other students; assaults at summer camp; self-inflicted injuries; road rage incidents; domestic violence; torturing household pets; killing pets; armed robbery; fire setting; shoplifting; destroying property with total amnesia of the event afterwards; breaking and attempting to break automobile windshields; and various forms of public lewdness.137
Antibiotic treatment has on occasion provoked aggressive behavior as a result of the Jarisch–Herxheimer reaction.111,138 There were a number of homicides associated with LD in the press. A LD patient strangled his fiancé with a dog leash. He was considered psychotic at the time of the homicide by the examining forensic psychiatrist.139,140 A LD patient bludgeoned his mother to death while she was sleeping.141 There was a murder, suicide of a couple with advanced LD who also killed the pet dog.142 There was a LD homicide in a Shelter Island in which a Lyme patient killed a longtime friend. The news release states, “Norstrom stated ‘in my own research in the past dozen years we’ve seen a lot of this.’”143–145
In a further communication with Norstrom, he gave consent to publish the following information. He has worked with a very large number of LD patients; he described a significant number of patients with aggressive behavior, such as women who had told him they wanted to hurt their children and did not know why; an easygoing man is arrested for a brawl who had no history of abuse; a teenager who became involved in physical altercations with peers, parents, and teachers; people who never said hell or damn going into a rage; children breaking the bones of their sibling, throwing a TV set through a window, destroying their bedroom, picking fights with classmates; and a teenager who went to a neighbor’s house and attacked him with a hammer because he thought the neighbor was going into his house. He stated he could list hundreds of other similar examples. Noise was magnified and certain pitches, a whiney voice, a squeak in a gate hinge, a barking dog, a crowd of people’s voices seem to bring on irrational behavior (Nostrom SJ, founder and director of Lyme borrelia Out-Reach Foundation, personal communication, August, 2008).
There were indications the shooter at the Sandy Hook Elementary School massacre, Adam Lanza, may have had LD, however the medical examiner failed to pursue LD assessment.146–148 LD has been a valid defense in the legal system.149 LD has been used as a defense in assaults, such as detonating a fertilizer bomb and an axe attack.150,151
When discussing homicide associated with LD, it is more common to think of a LD patient with a mental impairment who kills another person; however, there are other homicides associated with LD. In one case, the wife of a debilitated LD patient assisted in the planning and implementation of his suicide and pleaded guilty to felony murder.152 In reviewing other legal cases involving fatalities, LD patients who were inadequately diagnosed and treated earlier in their illness became debilitated with chronic pain, developed opioid dependence and abuse, and died from opioid overdoses.153
In a prior study analyzing the prevalence of findings, patients who were both homicidal and suicidal demonstrated low frustration tolerance (100%), cognitive symptoms (100%), sudden mood swings (93%), generalized anxiety (90%), explosive anger (83%), disinhibition (80%), panic disorder (80%), paranoia (76%), depersonalization (76%), hypervigilance (72%), anhedonia (72%), intrusive images (48%), obsessive compulsive disorder (48%), hallucinations (45%), substance abuse (28%), posttraumatic stress disorder (PTSD) (24%), and dissociative episodes (18%).12 In another presentation evaluating children with LD, 53% had anger/irritability, 25% had rages/explosive behavior, 54% had panic attacks, and 50% had mood swings.154
Neural circuitry of violence and infections
Infections and the associated immune provocation can sometimes alter the functioning of cortical, limbic, and brainstem functioning. Brain regions and associated circuits particularly significant to aggressive potential include the amygdala, the right ventromedial prefrontal cortex, the orbitofrontal cortex, the anterior cingulate, and the ventral hippocampus.53,155,156 The amygdala is involved in fear responsiveness and other negative emotions, the right ventromedial prefrontal cortex mediates empathic responsiveness, and the orbital frontal cortex plays a crucial role in constraining impulsive outbursts, while the anterior cingulate cortex recruits other brain regions in response to conflict, and the basolateral amygdala and ventral hippocampus are significant with traumatic memory.
Infectious encephalopathies are most commonly associated with impulse control disorders and predominately white matter encephalopathies with dysfunction of the prefrontal cortex and limbic structures. LD is commonly associated with white matter dysfunction, and many patients have explosive anger. Intermittent explosive disorder is associated with lower white matter integrity of the superior longitudinal fasciculus and density and lower white matter integrity in long-range connections between the frontal and temporoparietal regions.157
A certain type of temporal lobe and amygdala damage is strongly associated with predatory aggression in which there is impaired recognition of social emotions. This type of aggression has many similarities to Kluver–Bucy syndrome, which is a rare condition associated with temporal lobe injury and dysfunction. It includes visual agnosia, excessive oral tendencies, hypermetamorphosis, placidity, altered sexual behavior, and changes in dietary habits.158 Psychopathic and serial killers display visual agnosia and consistently lack normal empathetic human interpersonal recognition, and humans are not human to them.159 Kluver–Bucy syndrome is caused by a number of conditions including head injuries and a number of infections that injure the temporal lobes. These infections include Mycoplasma pneumoniae, tubercular meningitis, herpes simplex encephalitis, herpes meningoencephalitis, tick-borne infections, Epstein–Barr virus, and neurocysticercosis.160–170
Infections and aggression: psychoimmunology and neurochemistry
From an evolutionary medicine perspective, zoonotic parasites that manipulate the aggressive and social behavior of hosts achieve this by altering the actions of serotonin, dopamine, and norepinephrine, but in particular serotonin.171 Persistent infections and the immune reactions to them are associated with chronic inflammation and sometime autoimmune reactions.
Persistent inflammation is associated with increased interleukin-6 and interleukin-1 beta, reduced serotonin as demonstrated by reduced 5 hydroxy-indoleacetic acid, and increased quinolinic acid which is a N-methyl-D-aspartate receptor agonist that contributes to glutamate dysregulation and can result in both aggressive and self-destructive behavior (the death formula).12,172,173 These immune and associated biochemical findings have been seen in a number of infections associated with violence, including LD, cerebral malaria, and HIV.53,54,174 Other evidence supporting the association between inflammation and aggression includes hepatitis C treatment with interferon alpha, which can be associated with irritability, impulsiveness, hostility, aggression, anger, emotional lability, and relapse of substance abuse.175–177
Autoimmune reactions are also associated with aggression, and they have been seen in cases of pediatric autoimmune diseases associated with Streptococcal infections, M. pneumoniae and human herpesvirus 7 encephalitis, limbic encephalitis, and anti-N-methyl-D-aspartate receptor encephalitis.178–182
Materials and methods
The author specializes in treating treatment-resistant psychiatric illnesses and some of the patient charts include patients with LD. A total of 1,000 inactive charts of patients with LD from the author’s practice were retrospectively reviewed. Three groups of charts were reviewed, and aggregate data were collected – of those involving LD and aggressiveness, 50 homicidal LD subjects were compared with 50 who were not homicidal, and charts involving homicide were analyzed in more detail.
In the first part of the study, the charts of patients who had aggressive thoughts, impulses, and/or behaviors were selected and reviewed. These charts were reviewed for documentation or statements giving insight into the pathophysiology. All but two were from the United States.
In the second part of the study, the charts were screened until 50 charts of LD patients with homicidality with completed neuropsychiatric assessments were identified. Fifty randomly selected charts of LD patients who were not homicidal were selected as a control group. None of these control charts were used in the previous study on suicidality, and all were from the United States.12 These patients were evaluated with a comprehensive assessment including premorbid assessment, history of tick exposure, history of the presence of erythema migrans rash, early tick-borne disease symptoms, symptoms in the course of illness, current status with a comprehensive neuropsychiatric and general medical assessment, review of systems using the 280-item Neuropsychiatric Lyme Assessment with pattern recognition, and a review of laboratory assessment, which may include enzyme-linked immunosorbent assay, immunofluorescent assay, Western blot, DNA-based testing, coinfection testing, single-photon emission tomography, magnetic resonance imaging, or other diagnostic testing to determine the diagnosis in compliance with the Institute of Medicine–recognized guidelines.183–187 In addition to diagnostic criteria, the patients who met Center for Disease and Prevention surveillance criteria were noted, which is more stringent criteria and captureŝ10% of LD cases.188
Only testing from laboratories validated by the Clinical Laboratory Improvement Amendments of the US Department of Health and Human Services Centers for Medicare and Medicaid Services was considered valid. This is sometimes confused with the US FDA clearance for mass marketed test kits which does not validate the fundamental accuracy of the test but instead demonstrates it is comparable to other similar tests that may or may not have been properly validated in the first place. Charts in which the diagnosis of LD was unclear were excluded from the study. Names were converted to an identifier before entering the findings into a database of individual participant data. The charts were then reviewed with their identity protected to extract aggregate data, including findings relevant to homicidality and aggression, and were analyzed. The baseline health status of each of these two groups before infection was compared with the postinfection findings and the clinical findings of the subjects who were homicidal were compared with the subjects who were not homicidal. Symptoms associated with risk were ranked by comparing the prevalence of different findings among the two different groups.
Data collection included age, sex, diagnosed by comprehensive criteria, Center for Disease Control and Prevention surveillance criteria, years before diagnosis and antibiotic treatment, coinfections, comorbid conditions, pre-existing symptoms before infection, misdiagnoses, number of homicides, attention deficits, auditory hyperacusis, other sensory hyperacusis, memory impairments, processing impairments, dyslexia symptoms, facial memory, depersonalization, derealization, intrusive aggressive images, intrusive sexual images, nightmares, hallucinations, executive functioning impairments, decreased frustration tolerance, sudden abrupt mood swings, hypervigilance, paranoia, disinhibition, exaggerated startle reflex, explosive anger, suicidal tendencies, homicidal tendencies, decline in social relationships, decline in school or work productivity, marital and/or family problems, substance abuse, legal problems, dissociative episodes, depression, rapid cycling bipolar disorder episodes, panic disorder, obsessive compulsive disorder, social anxiety disorder, generalized anxiety disorder, PTSD, sleep disorders, anhedonia, cranial nerve symptoms, seizures, neuropathy, incontinence, decreased libido, irritable bladder, genital pain, chronic pain, and intolerance to alcohol.
The data of the impairments were charted and calculated to the 95% confidence interval (CI). The total impairments of all the 100 patients analyzed was charted preinfection and postinfection, and the 95% CI was calculated for each impairment. The incidence of each psychiatric symptom was calculated for the homicidal versus the non-homicidal groups.
In addition, homicides involving LD were reviewed in greater detail and categorized in regard to the type of homicide (impulsive, psychotic, or predatorial), and additional information was extracted. All the patients were from the United States.
The data were then abstracted to assess the association between aggression, violence, and homicide and LD to create a model for aggressiveness, violence, and homicidality in LD patients to possibly explain and ultimately prevent such an association, if it is found.
Ethical considerations
The Meridian Health Institutional Review Board, Neptune, NJ, USA, approved this study (IRB # 201704192J). Patient consent to review their medical records was not required by the Institutional Review Board as there was minimal risk to subjects, no subject identifiers or links to identifiers were used or collected, and it was a retrospective chart review of already existing data.
Results
Retrospective chart review: aggressiveness
A total of 1000 charts of LD patients were screened to identify charts documenting aggression. All but two were from the US. These charts were then reviewed to identify patterns of aggressiveness. This part of the study was not a statistical analysis of frequency, dominance, or general occurrence of the behaviors discussed.
The most commonly reported aggression with LD was impulsive, sometimes provoked by intrusive symptoms, sensory stimulation or frustration and was invariably bizarre and senseless. Aggression occurred both in homes and in public settings. Some of the aggressive patients had temporal lobe dysfunction, sometimes accompanied with partial seizures. Acute episodes of rage, sometimes referred to as “Lyme rage,” were reported and were witnessed on multiple occasions. The anger during these episodes had a very abrupt onset and was extremely intense and often with minimal cognitive control.
Patients described horrific intrusive symptoms. At night, they would take the form of vivid nightmares. During the day, these intrusive symptoms could be intrusive images, thoughts, and/or impulses. A typical description is that these intrusive symptoms include descriptions of frightening, stabbing, horrific images, usually of death, dying, or pain and suffering. They could be gory and unreal as in a horror story with faces with blood or terror exaggerated, awful expressions. They are described as people in the worse possible situation, perhaps close to death. These images can then evolve into visions of stabbing or killing, most commonly of people close or familiar to the person. A number of patients have described intrusive thoughts of killing multiple family members and themselves. Representative cases include a young adolescent with seizures who had repeated urges to kill their younger sibling. Another patient who had frequent temporal lobe seizures had intrusive thoughts of killing with an axe or knife any female he had a relationship with and described a mechanism in their head that would say kill her, kill her. These thoughts were invariably ego dystonic. The same patient also had thoughts of killing infant and adult female family members. In some cases, the aggression can be particularly toward women. Some patients described intrusive bizarre sexual and pedophilic thoughts and impulses. Others also described sudden and unprovoked urges to harm family members, and sometimes these episodes would be preceded by feeling a sense of derealization. Patients also describe intrusive and unprovoked episodes of anger with an urge to destroy. One patient described sudden urges to rip the room apart and kill everyone and every animal in the house. The intrusive episodes are often described as being episodic and not continuous. The images do not seem to necessarily be associated with a particular trigger, and one patient described them as invading the privacy of their mind.
Patients described fear of their suicidal and homicidal rage. It is described as rage that comes out of nowhere and takes over their whole body, with no control over it. In some, it is worse when they are driving. Some patients are scared for the people who come in contact with them and are afraid the thoughts will become even worse. Patients have described episodes of anger that would occur suddenly and totally unprovoked. A patient described looking at someone and feeling anger for no reason whatsoever, for example, looking at someone in a restaurant and having an urge to hurt the person. A patient described road rage out of nowhere and fantasies of beating and killing people. Patients have also described obsessions where they wished they had an excuse to go after someone. Another patient had an intrusive urge to throw a baby off of a balcony. He was particularly frightened when he had these urges in the presence of children and considered suicide to prevent himself from killing anyone.
One patient described that his pain was so bad, and he wanted to take it out on everyone else. Another patient who developed an impairment of empathy and was highly sensitive to perceived injury expressed, “if someone hurts me, I want to hurt them back. I want to make them cry. I want them to feel my pain.” Others responded to the intrusive homicidal and harmful impulses by becoming suicidal or self-destructive in an effort to prevent themselves from harming others. One patient accused his family members of being imposters.
Frequently, the thoughts or impulses to harm are preceded by being overstimulated, often by sound. For example, a normally passive individual who is an animal lover has hyperacusis and has intrusive hostile imagery of killing the dog by bludgeoning, suffocation, stabbing, or poisoning when it howled. Another patient responded to the neighbor’s dog barking by opening the window and screaming death threats to the dog. After acting in that manner, the patient began crying uncontrollably and wanted to die. Another patient described intrusive, overpowering very violent thoughts which sometimes would be triggered by stimulation; such as a dog barking, a bird chirping, a strobe light, or the presence of other people. Another patient described that the noise of her neighbors playing sports was unbearable. She had an urge to go to the neighbors and start screaming wildly. Sound is not the only trigger. A patient described sitting at a table with guests and her father behind her. When the father called for the dog, the patient became startled, punched her father and screamed at him with a total lack of control.
Not all aggressiveness is described as being provoked by stimulation. Some LD patients are intolerant to alcohol and/or react strongly to low doses of psychotropics or have idiosyncratic reactions to them. Some episodes are associated with the combination of the effects of LD and exposure to a number of medications and/or alcohol. A number of legal cases have involved bizarre and aggressive behavior while under the effects of alcohol and/or medications.
A few patients who had acquired LD as a congenital infection or were infected at a very young age had significantly impaired recognition of emotions of social significance and had difficulty processing interpersonal cues. They would sometimes take offense when normal limits were set on them. This occasionally resulted in a perceived injury, a rapid escalation of agitation, and because of their impaired capacity for empathy, it would sometimes result in assaultive behavior. Violent behavior seemed more problematic when the illness began in young children who have no memory to serve as a reference point for healthy functioning. There were a number of cases involving children infected at a young age that were quite severe. In these cases, psychopathic traits are evident, and the patients may repeatedly assault their family members, damage their house, knock over furniture, and kick and punch holes through walls and doors. Frequent police intervention was needed with these patients. In these cases, there is often a lack of empathy and a lack of remorse for their behavior. With antibiotic and psychotropic treatment, the impulse control disorder can improve, but sometimes lack of empathy and lack of remorse can persist.
The oldest legal case that could be found involving LD was in 1993, in which the patient was treated and the legal charges were dismissed. A number of cases were managed in a similar manner over a span of years. There were a number of shoplifting cases, mostly involving females. There were a few cases involving fire setting. In one case, the patient burned down his house as a result of a paranoid delusion. Some individuals with psychotic symptoms acted bizarrely and were repeatedly drawing the attention of the legal system and occasionally SWAT (special weapons attack team) teams. A few patients attacked multiple police officers in a bizarre and senseless manner.
Retrospective chart review: homicidality
Inactive charts were reviewed until 50 LD subjects with homicidal tendencies were identified, that is, 520 charts were screened until 50 cases could be identified (9.6%). A few of these charts had a few incomplete data points. There were 98% (N=49) of the subjects who were also suicidal. In this group of 50 homicidal patients, there were five homicides. The subjects who committed homicides were incarcerated and evaluated in forensic settings. Forty nine of the 50 homicidal patients were not homicidal before being infected with LD. It is possible one patient may have been homicidal before being infected with LD. The average age was 35 years (youngest 11 years and oldest 63 years). The average age of onset of illness was 26 years. There were 52% male and 48% female subjects, and 10% were diagnosed immediately after infection, but had inadequate initial treatment. The average subject experienced a delay of 9 years before diagnosis and treatment. There were 22% of subjects that were diagnosed in ≤2 years. The longest length of time before diagnosis was 40 years. There were 66% that met Center for Disease and Prevention Surveillance Criteria for Lyme disease. All who committed homicides met the Surveillance Criteria. There were 84% of subjects misdiagnosed at some point, and the misdiagnoses included “all in your head,” “just nuts,” “laziness,” “nervousness,” “hyper,” “a witch in another life,” “psychiatric,” “everything,” bipolar depression, depression, major depression, panic disorder, anxiety attacks, alcoholism, attention deficit disorder, pervasive developmental disorder, PTSD, anxiety, intermittent explosive disorder, adjustment reaction, obsessive compulsive disorder, oppositional defiant disorder, multiple sclerosis, brain tumor, Alzheimer’s disease, visual labyrinthitis, headaches, sensory motor dysfunction syndrome, migraine syndrome, sciatica, bulging disc, dysautonomia, orthostatic intolerance, seizure disorder, amyotrophic lateral sclerosis, vertigo, Meniere’s disease, chronic fatigue/immune deficiency syndrome, fibro-myalgia, lupus, arthritis, rheumatoid arthritis, mononucleosis, bronchitis, asthma, fever of unknown origin, erythromelalgia, systemic candidiasis, food allergies, and flu.
Coinfections in this group in order of frequency included Babesia, Anaplasma, Bartonella, Ehrlichia chaffeensis, Epstein–Barr virus, cytomegalovirus, mycoplasma, Rickettsia rickettsii, and Hepatitis C.
An additional 50 charts of LD patients who were not homicidal were randomly selected, and the prevalence of symptoms was compared with the prevalence of the same symptoms in LD patients who were homicidal. In this group, the average age was 42 years (youngest 8 years and oldest 67 years). There were 44% of male and 56% of female subjects. About 10% were diagnosed immediately after infection, but had inadequate initial treatment. The average subject experienced a delay of 8.1 years before diagnosis and treatment. There were 32% who were diagnosed in ≤2 years. The greatest length of time before diagnosis was 39 years. There were 74% meeting Center for Disease and Prevention Surveillance Criteria. Misdiagnosis had occurred in 92% of these subjects.
Both the groups of patients in this study were generally quite healthy compared with the general population, preinfection; however, postinfection, both the groups developed significant cognitive symptoms, emotional symptoms, vegetative symptoms, behavioral symptoms, neurological symptoms, musculoskeletal symptoms, cardiovascular symptoms, gastrointestinal symptoms, urogenital symptoms, and other symptoms. Coinfections in this group in order of frequency included Babesia, Bartonella, E. chaffeensis, Anaplasma, mycoplasma, R. rickettsii, Epstein–Barr virus, Chlamydia pneumoniae, and Coxiella burnetii.
Comparing the two groups, it was found that there were significant differences in the prevalence of psychiatric symptoms, behavioral symptoms, psychiatric syndromes, cognitive symptoms, neurological symptoms, and urogenital symptoms. Not all symptoms that were studied showed a statistically significant separation between the two groups that might explain homicidality or appeared relevant to violence. These symptoms included attention deficits, auditory hyperacusis, other sensory hyperacusis, facial memory impairments, executive functioning impairments, decreased frustration tolerance, fatigue, sleep disorder, decline in social relationships, decline in school or work productivity and chronic pain. A number of signs, symptoms and syndromes associated with violent risk were increased in the homicidal group and these findings were further analyzed. The 95% CI was calculated for each data point (Table 1).
Table 1.
The homicidal group had more impairments postinfection
| 50 homicidal and 50 non-homicidal Lyme disease patients
| ||||
|---|---|---|---|---|
| Findings | Homicidal group
|
Non-homicidal group
|
||
| Preinfection, % (95% Cl) | Postinfection, % (95% CI) | Preinfection, % (95% Cl) | Postinfection, % (95% Cl) | |
| Psychiatric symptoms | ||||
| Suicidal | 2 (0–6) | 98 (94–100) | 0 (0–0) | 46 (32–60) |
| Sudden abrupt mood swings | 4 (0–9) | 94 (87–100) | 0 (0–0) | 66 (53–79) |
| Explosive anger | 4 (0–9) | 91 (83–99) | 0 (0–0) | 52 (38–66) |
| Paranoia | 0 (0–0) | 88 (79–97) | 0 (0–0) | 36 (23–49) |
| Ahedonia | 2 (0–6) | 86 (76–96) | 0 (0–0) | 56 (42–70) |
| Hypervigilance | 2 (0–6) | 84 (74–94) | 0 (0–0) | 54 (40–68) |
| Exaggerated startle | 2 (0–6) | 84 (74–94) | 0 (0–0) | 66 (53–79) |
| Disinhibition | 2 (0–6) | 84 (74–94) | 0 (0–0) | 32 (19–45) |
| Nightmares | 2 (0–6) | 82 (71–93) | 0 (0–0) | 58 (44–72) |
| Depersonalization | 0 (0–0) | 71 (58–84) | 0 (0–0) | 52 (38–66) |
| Intrusive images aggressive | 4 (0–9) | 62 (49–75) | 0 (0–0) | 16 (6–26) |
| Hallucinations | 2 (0–6) | 47 (33–61) | 0 (0–0) | 42 (28–56) |
| Dissociative episodes | 0 (0–0) | 38 (25–51) | 0 (0–0) | 12 (3–21) |
| Derealization | 0 (0–0) | 37 (24–50) | 0 (0–0) | 24 (12–36) |
| Intrusive images sexual | 2 (0–6) | 26 (14–38) | 0 (0–0) | 6 (0–13) |
| Behavioral symptoms | ||||
| Marital/family problems | 0 (0–0) | 80 (69–91) | 0 (0–0) | 48 (34–62) |
| Legal problems | 0 (0–0) | 42 (28–56) | 0 (0–0) | 4 (0–9) |
| Substance abuse | 6 (0–13) | 33 (20–46) | 0 (0–0) | 10 (2–18) |
| Psychiatric syndromes | ||||
| Depression | 6 (0–13) | 98 (94–100) | 0 (0–0) | 76 (64–88) |
| Panic disorder | 2 (0–6) | 82 (71–93) | 2 (0–5.9) | 50 (36–64) |
| Social anxiety disorder | 4 (0–9) | 66 (53–79) | 4 (0–9.4) | 70 (57–83) |
| OCD | 2 (0–6) | 51 (37–65) | 2 (0–5.9) | 32 (19–45) |
| PTSD | 6 (0–13) | 36 (23–49) | 2 (0–5.9) | 24 (12–36) |
| Rapid bipolar | 0 (0–0) | 28 (16–40) | 0 (0–0) | 10 (2–18) |
| Cognitive symptoms | ||||
| Memory impairments | 0 (0–0) | 98 (94–100) | 0 (0–0) | 76 (64–88) |
| Processing impairments | 0 (0–0) | 94 (87–100) | 0 (0–0) | 78 (67–89) |
| Dyslexia symptoms | 4 (0–9) | 78 (67–89) | 0 (0–0) | 68 (55–81) |
| Neurological symptoms | ||||
| Neuropathy | 0 (0–0) | 92 (84–100) | 0 (0–0) | 70 (57–83) |
| Cranial nerve symptoms | 0 (0–0) | 92 (84–100) | 0 (0–0) | 66 (53–79) |
| Seizures | 0 (0–0) | 20 (9–31) | 0 (0–0) | 24 (12–36) |
| Urogential | ||||
| Decreased libido | 0 (0–0) | 80 (69–91) | 4 (0–9.4) | 44 (30–58) |
| Irritable bladder | 4 (0–9) | 56 (42–70) | 2 (0–5.9) | 44 (30–58) |
| Intolerance to alcohol | 0 (0–0) | 44 (30–58) | 0 (0–0) | 24 (12–36) |
| Incontinence | 2 (0–6) | 38 (25–51) | 2 (0–5.9) | 18 (7–29) |
| Genital pain | 0 (0–0) | 32 (19–45) | 0 (0–0) | 24 (12–36) |
Abbreviations: CI, confidence interval; OCD, obsessive compulsive disorder; PTSD, posttraumatic stress disorder.
Both the homicidal and non-homicidal groups showed an increase of all the impairments studied preinfection versus postinfection (95% CI) (Table 2).
Table 2.
All impairments were increased postinfection
| Total patient group (n=100) pre vs postinfection
| ||
|---|---|---|
| Findings | Preinfection, % (95% Cl) | Postinfection, % (95% CI) |
| Psychiatric symptoms | ||
| Sudden abrupt mood swings | 2 (0–6) | 80 (69–91) |
| Exaggerated startle | 1 (0–4) | 75 (63–87) |
| Suicidal | 1 (0–4) | 72 (60–84) |
| Explosive anger | 2 (0–6) | 72 (59–84) |
| Ahedonia | 1 (0–4) | 71 (58–84) |
| Nightmares | 1 (0–4) | 70 (57–83) |
| Hypervigilance | 1 (0–4) | 69 (56–82) |
| Paranoia | 0 (0–0) | 62 (49–75) |
| Depersonalization | 0 (0–0) | 62 (48–75) |
| Disinhibition | 1 (0–4) | 58 (44–72) |
| Hallucinations | 1 (0–4) | 45 (31–58) |
| Intrusive images aggressive | 2 (0–5) | 39 (29–49) |
| Derealization | 0 (0–0) | 31 (18–43) |
| Dissociative episodes | 0 (0–0) | 25 (13–37) |
| Intrusive images sexual | 1 (0–4) | 16 (6–26) |
| Behavioral symptoms | ||
| Marital/family problems | 0 (0–0) | 64 (51–77) |
| Legal problems | 0 (0–0) | 23 (11–35) |
| Substance abuse | 3 (0–8) | 22 (10–33) |
| Psychiatric syndromes | 0 (0–0) | 0 (0–0) |
| Depression | 3 (0–8) | 87 (78–96) |
| Social anxiety disorder | 4 (0–9) | 68 (55–81) |
| Panic disorder | 2 (0–6) | 66 (53–79) |
| OCD | 2 (0–6) | 42 (28–55) |
| PTSD | 4 (0–9) | 30 (17–43) |
| Rapid bipolar | 0 (0–0) | 19 (8–30) |
| Cognitive symptoms | ||
| Memory impairments | 0 (0–0) | 87 (78–96) |
| Processing impairments | 0 (0–0) | 86 (76–96) |
| Dyslexia symptoms | 2 (0–6) | 73 (61–85) |
| Neurological symptoms | ||
| Neuropathy | 0 (0–0) | 81 (70–92) |
| Cranial nerve symptoms | 0 (0–0) | 79 (68–90) |
| Seizures | 0 (0–0) | 22 (11–33) |
| Urogential | ||
| Decreased libido | 2 (0–6) | 62 (49–75) |
| Irritable bladder | 3 (0–8) | 50 (36–64) |
| Intolerance to alcohol | 0 (0–0) | 34 (21–47) |
| Incontinence | 2 (0–6) | 28 (16–40) |
| Genital pain | 0 (0–0) | 28 (16–40) |
Abbreviations: CI, confidence interval; OCD, obsessive compulsive disorder; PTSD, posttraumatic stress disorder.
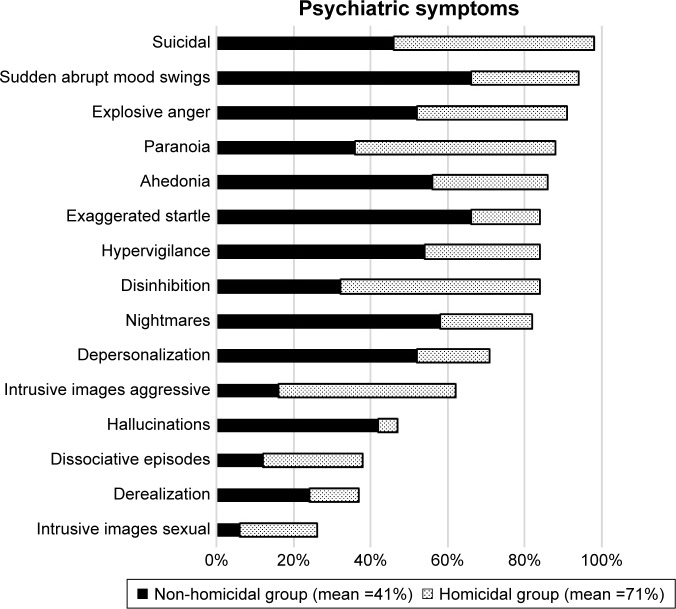
The homicidal group had a greater incidence of all the psychiatric symptoms studied when compared to the non-homicidal group. The mean of the psychiatric symptoms was 71% in the homicidal versus 41% in the non-homicidal group (Figure 2).
Figure 2.
All the psychiatric symptoms were greater in the homicidal versus the non-homicidal group.
Retrospective chart review: homicide
In reviewing the LD charts involving both LD and homicide, 10 homicides were identified. None of these homicides were cases discussed when reviewing media reports in the “Background” section. Three of the 10 homicides involved suspected LD that was not confirmed, and these cases were not included in the study. The other seven homicides were associated with well-confirmed cases of LD. All seven victims had a prior personal relationship with the perpetrators, either real or imagined by the perpetrator. Two of these cases did not have completed neuropsychiatric assessments and were included in the retrospective chart review of homicides but were not included in the retrospective data analysis of homicidal LD patients.
Of the seven homicides, one was a combined homicide, dog killing, and suicide, which seemed to have been related to impulsivity. Also there were other homicide, dog killing, and suicide cases which occurred that were not evaluated by the author.189,190 Another homicide was associated with psychosis, and the other five had predatory features. In a predatory case, the Lyme enzyme-linked immunoassay was 6.83 (positive ≥ 1.10). Whenever these cases involving homicide were tested for coinfections, there was positive reactivity to coinfections, which included Bartonella, Babesia, mycoplasma, Anaplasma phagocytophilum, E. chaffeensis, and viral coinfections. All cases involving predatory aggression also included a history of reported abuse. Findings also included head injuries, social communication impairments, an impaired capacity to bond and empathize with others, social isolation, a fascination with knives, less than a normal amount of anxiety, fire setting, and torturing animals prior to the homicides. In all of the LD cases involving homicide, there were obstacles preventing both adequate antibiotic and psychiatric treatments.
Discussion
Upon reviewing the existing literature and the retrospective chart reviews, LD patients postinfection may acquire a number of impairments that are collectively associated with an increased risk of violence and homicidality, but some acquired impairments are more likely to increase risk than others. In spite of having impairments, the majority of LD patients are able to compensate and may only demonstrate low frustration tolerance and irritability. Some, however, demonstrate more severe symptoms. To better understand why many LD patients are not aggressive, while others can be highly aggressive, it is necessary to look at the different symptoms seen in these two groups.
For organizational purposes, the data are presented as cognitive impairments, emotional impairments, behavioral symptoms, psychiatric syndromes (multiple brain regions), neurological impairments, and somatic impairments.
Individuals with cognitive impairments have less coping skills and in general can have increased risk for violence. Among the homicidal subjects in this study, 98% had impaired capacity for sustained and/or selective attention; 88% had auditory hyperacusis (misophonia); 86% had sensory hyperacusis to light, touch, and/or smell; 98% had memory impairments (most commonly working memory and short-term memory); 94% had processing impairments; 78% had dyslexia symptoms; and 98% had executive functioning memory impairments. Sensory hyperacusis is associated with an impaired capacity to filter incoming stimulation, resulting in a sensory overload, and contributes to making a patient more reclusive and socially isolated and also contributes to them being more emotionally reactive to stimulation that average people can easily tolerate. Some patients have selective hyperacusis to mouth sounds, which I propose may possibly be the result of a disinhibition of a primitive fear of oral predation from brain dysfunction. Facial memory impairments were similar in both the homicidal and non-homicidal groups and seems to be non-contributory. However, patients with more violent potential had an impairment of emotional recognition when they saw a familiar person, and this impairment would sometimes result in their accusations that family and friends were imposters (Capgras’ syndrome).191
Individuals with certain emotional impairments can have greater risk for aggressiveness. Among the homicidal subjects in this study, 71% had depersonalization, 37% had derealization, 62% had intrusive aggressive images, 26% had intrusive sexual images, 82% had vivid nightmares, 47% had hallucinations, 98% had decreased frustration tolerance, 94% had sudden abrupt mood swings, 84% had hypervigilance, 88% had paranoia, 84% had disinhibition, 84% had exaggerated startle reactions, 91% had explosive anger, 38% had dissociative episodes, and 86% had anhedonia.
Individuals with certain behavioral symptoms can have greater risk for aggressiveness. Among the homicidal subjects in this study, 98% were suicidal, 91% had a decline in social functioning, 90% had a decline in school or work productivity, 80% had marital and/or family problems, 33% had substance abuse, and 42% had legal problems.
Individuals with certain psychiatric syndromes can have greater risk for aggressiveness. Among the homicidal subjects in this study, 98% had depression, 28% had rapid cycling bipolar illness, 82% had panic disorder, 51% had obsessive compulsive disorder, 66% had social anxiety disorder, 86% had generalized anxiety disorder, and 36% had PTSD.
Individuals with certain urological symptoms, particularly urinary incontinence (along with torturing animals and fire setting), are considered by some to be at greater risk for predatory aggression. Among the homicidal subjects in this study, 38% had urinary incontinence, 56% had irritable bladder, and 32% had genital pain.
Among the homicidal subjects in this study, 44% had intolerance to alcohol with a more exaggerated response to alcohol consumption. Alcohol intoxication is a well recognized risk for violence and homicide.192
Among the homicidal subjects in this study, other symptoms that were present but may or may not correlate with homicidal risk included 96% with sleep disorders, 38% with decreased libido, 92% with cranial nerve symptoms, 92% with neuropathy, 20% with seizures (mostly complex partial), 48% with chronic pain, and 92% with fatigue.
Violent behavior was more problematic when the illness began in young children who had no memory to serve as a reference point for healthy functioning.
Although data are limited in two of the homicide cases, it seems in the cases involving homicides that there were other contributors to violence; however, it also seems that these homicides would not have occurred if the perpetrators had not had neurological deterioration as a result of LD.
Multiple patients described intrusive thoughts, images, and impulses to kill everything, including themselves. These descriptions have similarities to the episodes in which the individuals attempt to kill everyone and everything, including house pets and themselves. The pathophysiology behind this process may be the result of the previously described death formula (persistent infection→persistent proinflammatory cytokines→dysregulation of tryptophan metabolism→quinolinic acid→N-methyl-D-aspartate receptor agonism→glutamate dysregulation→neural circuit dysfunction→psychiatric dysfunction→suicidal and/or homicidal tendencies) which may be a pathophysiological explanation of the death drive (Thantos) concept that was first proposed by Spielrein and further advanced by Freud.12,193 It is unclear why a few LD patients develop the collective risk contributors for violence, but most do not. Possible explanations include the following – there is a genetic vulnerability that is triggered in only some who become infected, some other pathogen or pathogens other than Borrelia (either tick-borne or opportunistic within the host microbiome) may be responsible for causing the aggressiveness or it may be more the immune reaction to the infection rather than the infection itself that is causative, and this immune reaction may have multiple other causes. In this study, there was no clear difference in the coinfections in the homicidal versus the non-homicidal group.
In synthesizing these findings, the following model for violence and LD can be constructed (Figures 3 and 4).
Figure 3.
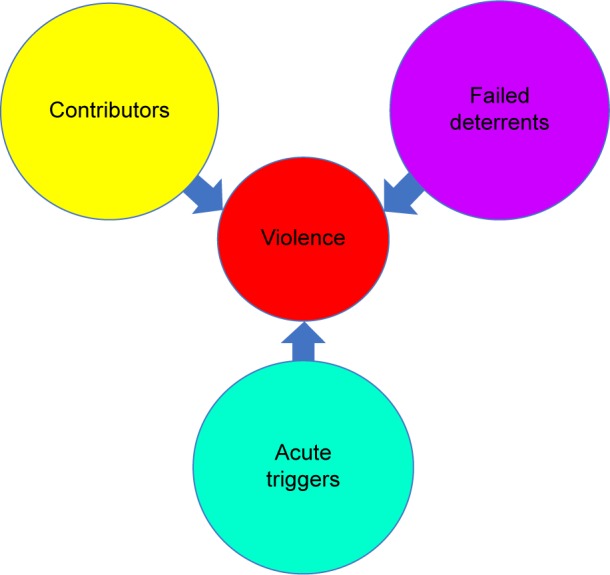
Violence results from the interactive effects of contributors, failed deterrents, and acute triggers.
Figure 4.
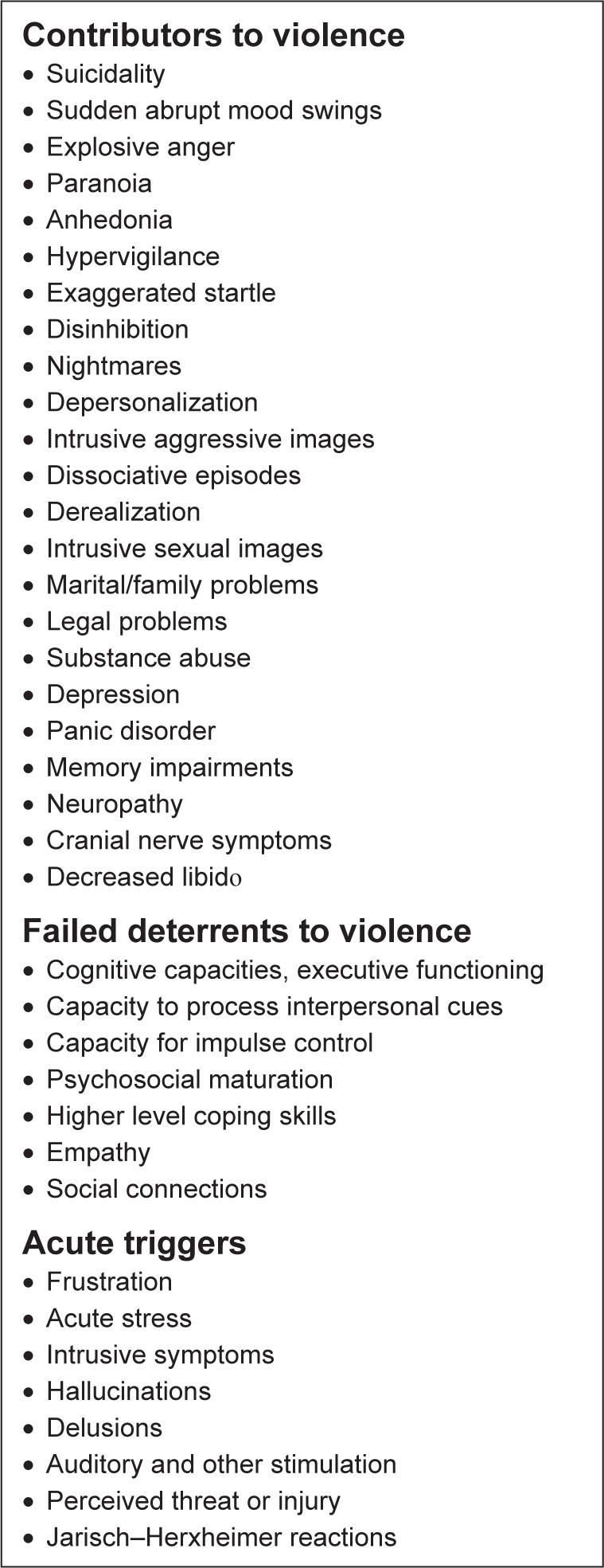
Violence is more likely to occur in Lyme disease patients when there is a greater number and severity of the contributors to violence combined with compromised deterrents and acute triggers.
There are multiple mechanisms through which infection may contribute to violence, and many seem to be immune mediated. Impairments associated with infectious disease that can contribute to violent behavior include decreased frustration tolerance; decreased impulse control; cognitive impairments; sensory hyperacusis; hypervigilance; paranoia; increased startle reflex, particularly increased acoustic startle; sudden abrupt mood swings; impairment in the ability to regulate emotional arousal levels; social anxiety disorder when contributing to social isolation; obsessive compulsive disorder combined with intrusive thoughts, images, and compulsions that are of aggressive nature and/or sexually aggressive nature; hyposexuality and hypersexuality, both of which cause increased interpersonal frustration; suicidal and homicidal preoccupations, intrusive images, and urges; decreased bonding capacity; decreased empathy; decreased emotional insight; depersonalization; derealization; dissociative states; illusions; delusions; hallucinations and decreased tolerance to the effects of drugs and alcohol.
Conclusion
Violent behavior is the result of a complex interaction of many contributors, failures of deterrents, and acute triggering events. From evolutionary, geographical, epidemiological, and historical perspectives, multiple studies, case reports, and animal studies, some infections can be associated with violence. These infections have psychoimmune and pathophysiological implications through multiple mechanisms that include proinflammatory and autoimmune mechanisms that can cause multiple impairments impacting brain neurochemistry and neural circuit functioning which can cause different types of violence. LD can be one of the many infectious contributors associated with aggressiveness, violence, homicidality, and homicide. There is support for this from journal citations, presentations, animal studies, psychoimmunology, neurochemistry, neural circuity, reports, and observations. Delays in diagnosing and treating LD may result in an increased risk of aggressiveness that can result in impulsive, psychotic, and/or predatory aggression. Most violence seen with LD seems to be impulsive, bizarre, and senseless.
The most common case is a young male with impairments during development who lacks a reference point of mature healthy functioning before the onset of impairment who may be at greater risk for violence if he was infected at a younger age and has impaired recognition of emotions of social significance and difficulty processing interpersonal cues.
LD causes a number of impairments that collectively increase the risk of homicidality. Postinfection findings associated with homicidality that separated from the non-homicidal group within the 95% CI included suicidality, sudden abrupt mood swings, explosive anger, paranoia, anhedonia, hypervigilance, exaggerated startle, disinhibition, nightmares, depersonalization, intrusive aggressive images, dissociative episodes, derealization, intrusive sexual images, marital/family problems, legal problems, substance abuse, depression, panic disorder, memory impairments, neuropathy, cranial nerve symptoms, and decreased libido. Although predatory aggression with LD seems to be an uncommon presentation, it accounts for the greatest number of homicides. A patient who acquires LD and is inadequately diagnosed and treated may be at risk for committing homicide if they have a history of reported abuse, head injuries, social communication impairments, an impaired capacity to bond and empathize with others, social isolation, a fascination with knives, less than a normal amount of anxiety, fire setting, torturing animals, and obstacles preventing adequate antibiotic and psychiatric treatment.
Some patients have selective hyperacusis to mouth sounds, which I propose may possibly be the result of brain dysfunction causing a disinhibition of a primitive fear of oral predation.
As with the vast majority of patients with mental illness, many patients with LD have no aggressive tendencies or only mild degrees of low frustration tolerance and irritability and pose no danger to society. A lesser number experience marital and family conflicts, a lesser number experience explosive anger, a lesser number experience homicidal thoughts and impulses, and a much lesser number commit homicides. Since such a large number of individuals are affected by LD, this small percent of LD cases can be significant and is a cause for concern.
Many of the patients who became aggressive and homicidal responded to effective treatment, and much of the violence associated with LD can be avoided with better prevention, diagnosis, and treatment of LD. Although this article focused upon violence associated with LD, violence associated with other infectious diseases are also significant, and greater research into the association between infections and violence is strongly recommended. Human history is also a history of violence. Understanding violence and recognizing the role of microbes and psychoimmunology opens new opportunities to prevent violent behavior, suffering, crime, war, and social discord and protect peace. We need a multidisciplinary mobilization of our scientific community to better understand all the facets of violence to prevent future tragedies similar to the approach used to understand and prevent airplane crashes.
Acknowledgments
Thanks to Michael J Cook for statistical assistance and Rhiannon Woolwich-Holzman, VMD; Ed Breitschwerdt, DVM, DACVIM; Barbara Rosenthal; Courtney Bransfield; Douglas Bransfield; Phillis Chrampanis and Willison Reed for assistance.
Footnotes
Disclosure
The author has treated, evaluated, and been an expert witness in cases involving homicide and other violent behaviors over a span of 45 years. The author has worked in correctional systems. The author has worked with patients with LD over a span of 30 years and has been an expert witness in cases involving LD and in cases involving LD and homicide, multiple homicides, violent behavior, and other legal cases. The author reports no conflicts of interest in this work.
References
- 1.Dupuis MJ. Multiple neurologic manifestations of Borrelia burgdorferi infection. Rev Neurol (Paris) 1988;144(12):765–775. French. [PubMed] [Google Scholar]
- 2.Pachner AR. Borrelia burgdorferi in the nervous system: the new “great imitator”. Ann N Y Acad Sci. 1988;539:56–64. doi: 10.1111/j.1749-6632.1988.tb31838.x. [DOI] [PubMed] [Google Scholar]
- 3.Gannon M. Ancient Lyme Disease Bacteria Found in 15-Million-Year-Old Tick Fossils. Live Science. 2014. [Accessed Jan 6, 2018]. Available from: https://www.livescience.com/46007-lyme-disease-ancient-amber-tick.html.
- 4.Kean WF, Tocchio S, Kean M, Rainsford KD. The musculoskeletal abnormalities of the Similaun Iceman (“ÖTZI”): clues to chronic pain and possible treatments. Inflammopharmacology. 2013;21(1):11–20. doi: 10.1007/s10787-012-0153-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lyme disease. Center for Disease Control and Prevention; [Accessed Jan 6, 2018]. Available from: https://www.cdc.gov/lyme/index.html. [Google Scholar]
- 6.Fallon BA, Nields JA. Lyme disease: a neuropsychiatric illness. Am J Psychiatry. 1994;151(11):1571–1583. doi: 10.1176/ajp.151.11.1571. [DOI] [PubMed] [Google Scholar]
- 7.Fallon BA, Kochevar JM, Gaito A, Nields JA. The underdiagnosis of neuropsychiatric Lyme disease in children and adults. Psychiatr Clin North Am. 1998;21(3):693–703. viii. doi: 10.1016/s0193-953x(05)70032-0. [DOI] [PubMed] [Google Scholar]
- 8.Tager FA, Fallon BA, Keilp J, Rissenberg M, Jones CR, Liebowitz MR. A controlled study of cognitive deficits in children with chronic Lyme disease. J Neuropsychiatry Clin Neurosci. 2001;13(4):500–507. doi: 10.1176/jnp.13.4.500. [DOI] [PubMed] [Google Scholar]
- 9.Greenberg R. The role of infection and immune responsiveness in a case of treatment-resistant pediatric bipolar disorder. Front Psychiatry. 2017;8:78. doi: 10.3389/fpsyt.2017.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hájek T, Pasková B, Janovská D, et al. Higher prevalence of antibodies to Borrelia burgdorferi in psychiatric patients than in healthy subjects. Am J Psychiatry. 2002;159(2):297–301. doi: 10.1176/appi.ajp.159.2.297. [DOI] [PubMed] [Google Scholar]
- 11.Banerjee R, Liu JJ, Minhas HM. Lyme neuroborreliosis presenting with alexithymia and suicide attempts. J Clin Psychiatry. 2013;74(10):981. doi: 10.4088/JCP.13cr08493. [DOI] [PubMed] [Google Scholar]
- 12.Bransfield RC. Suicide and Lyme and associated diseases. Neuropsychiatr Dis Treat. 2017;13:1575–1587. doi: 10.2147/NDT.S136137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krug EG, Mercy JA, Dahlberg LL, Zwi AB. The world report on violence and health. Lancet. 2002;360(9339):1083–1088. doi: 10.1016/S0140-6736(02)11133-0. [DOI] [PubMed] [Google Scholar]
- 14.Stahl SM, Morrissette DA, Munter N. Stahl’s Illustrated Violence. Cambridge UK: Cambridge University Press; 2014. [Google Scholar]
- 15.Lidz CW, Mulvey EP, Gardner W. The accuracy of predictions of violence to others. JAMA. 1993;269(8):1007–1011. [PubMed] [Google Scholar]
- 16.Beck JC, White KA, Gage B. Emergency psychiatric assessment of violence. Am J Psychiatry. 1991;148(11):1562–1565. doi: 10.1176/ajp.148.11.1562. [DOI] [PubMed] [Google Scholar]
- 17.Tardiff K. Unusual diagnoses among violent patients. Psychiatr Clin North Am. 1998;21(3):567–576. doi: 10.1016/s0193-953x(05)70023-x. [DOI] [PubMed] [Google Scholar]
- 18.Dugré JR, Dellazizzo L, Giguère CÉ, Potvin S, Dumais A. Persistency of cannabis use predicts violence following acute psychiatric discharge. Front Psychiatry. 2017;8:176. doi: 10.3389/fpsyt.2017.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewis DO, Pincus JH, Feldman M, Jackson L, Bard B. Psychiatric, neurological, and psychoeducational characteristics of 15 death row inmates in the United States. Am J Psychiatry. 1986;143(7):838–845. doi: 10.1176/ajp.143.7.838. [DOI] [PubMed] [Google Scholar]
- 20.Feldman M, Mallouh K, Lewis DO. Filicidal abuse in the histories of 15 condemned murderers. Bull Am Acad Psychiatry Law. 1986;14(4):345–352. [PubMed] [Google Scholar]
- 21.Freedman D, Hemenway D. Precursors of lethal violence: a death row sample. Soc Sci Med. 2000;50(12):1757–1770. doi: 10.1016/s0277-9536(99)00417-7. [DOI] [PubMed] [Google Scholar]
- 22.Bransfield RC. Why Violence? 1999. [Accessed October 20, 2017]. Available from: http://www.mentalhealthandillness.com/may1999.htm.
- 23.Sariaslan A, Lichtenstein P, Larsson H, Fazel S. Triggers for violent criminality in patients with psychotic disorders. JAMA Psychiatry. 2016;73(8):796–803. doi: 10.1001/jamapsychiatry.2016.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Volavka J. Triggering violence in psychosis. JAMA Psychiatry. 2016;73(8):769–770. doi: 10.1001/jamapsychiatry.2016.1348. [DOI] [PubMed] [Google Scholar]
- 25.Mathers C. The Global Burden of Disease, 2004 Update. Geneva, Switzerland: World Health Organization; 2008. [Google Scholar]
- 26. Homicide-world.png Wikimedia Commons. 2009. [Accessed October 16, 2017]. [updated June 8, 2014]. Available from: https://en.wikipedia.org/wiki/File:Homicide-world.png.
- 27.US Peace Index [Accessed October 16, 2017]. Available from: http://peacealliance.org/cms/assets/uploads/2013/05/U.S.-Peace-Index-Overview.pdf.
- 28.Eppig C, Fincher CL, Thornhill R. Parasite prevalence and the worldwide distribution of cognitive ability. Proc Biol Sci. 2010;277(1701):3801–3808. doi: 10.1098/rspb.2010.0973. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Lester D. Toxoplasma gondii and homicide. Psychol Rep. 2012;111(1):196–197. doi: 10.2466/12.15.16.PR0.111.4.196-197. [DOI] [PubMed] [Google Scholar]
- 30.Thornhill R, Fincher CL. Parasite stress promotes homicide and child maltreatment. Philos Trans R Soc Lond B Biol Sci. 2011;366(1583):3466–3477. doi: 10.1098/rstb.2011.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roser M. The Visual History of Decreasing War and Violence – Our World in Data. 2011. [Accessed October 16, 2017]. Available from: https://ourworldindata.org/slides/war-and-violence/
- 32.Bransfield RC. Could a Pandemic Causing Mental Dysfunction Contribute to Global Instability?. Paper presented at: International Lyme and Associated Diseases 7th European Conference; May 19, 2017; Paris, France. [Google Scholar]
- 33.Retief FP, Wessels A. Did Adolf Hitler have syphilis? S Afr Med J. 2005;95(10):750, 752, 754, 756. [PubMed] [Google Scholar]
- 34.Sacks O. Awakenings. New York: Harper Perennial; 1973. [Google Scholar]
- 35.Morton RS. Did Catherine the Great have syphilis? Genitourin Med. 1991;67:498–502. doi: 10.1136/sti.67.6.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nevins M. Meanderings in Medical History. Book Four. Bloomington, IN: iUniverse; 2016. [Google Scholar]
- 37.Dietz PE. Mentally disordered offenders. Patterns in the relationship between mental disorder and crime. Psychiatr Clin North Am. 1992;15(3):539–551. [PubMed] [Google Scholar]
- 38.Gómez JM, Verdú M, González-Megías A, Méndez M. The phylogenetic roots of human lethal violence. Nature. 2016;538(7624):233–237. doi: 10.1038/nature19758. [DOI] [PubMed] [Google Scholar]
- 39.Bransfield R. Are There Psychoimmune and Infectious Contributors to Violence?. Paper presented at: New Jersey Psychiatric Association Meeting; September 21, 2016; Princeton, NJ. [Accessed Oct 1, 2017]. Available from: https://www.researchgate.net/publication/308549616_Are_There_Psychoimmune_and_Infectious_Contributors_to_Violence. [Google Scholar]
- 40.Shamay-Tsoory SG, Tomer R, Berger BD, Aharon-Peretz J. Characterization of empathy deficits following prefrontal brain damage: the role of the right ventromedial prefrontal cortex. J Cogn Neurosci. 2003;15(3):324–337. doi: 10.1162/089892903321593063. [DOI] [PubMed] [Google Scholar]
- 41.Adolphs R, Tranel D, Damasio H, Damasio A. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature. 1994;372(6507):669–672. doi: 10.1038/372669a0. [DOI] [PubMed] [Google Scholar]
- 42.Adolphs R, Baron-Cohen S, Tranel D. Impaired recognition of social emotions following amygdala damage. J Cogn Neurosci. 2002;14(8):1264–1274. doi: 10.1162/089892902760807258. [DOI] [PubMed] [Google Scholar]
- 43.Siever LJ. Neurobiology of aggression and violence. Am J Psychiatry. 2008;165(4):429–442. doi: 10.1176/appi.ajp.2008.07111774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation – a possible prelude to violence. Science. 2000;289(5479):591–594. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- 45.Raine A, Buchsbaum M, LaCasse L. Brain abnormalities in murderers indicated by positron emission tomography. Biol Psychiatry. 1997;42(6):495–508. doi: 10.1016/S0006-3223(96)00362-9. [DOI] [PubMed] [Google Scholar]
- 46.Stahl SM. Deconstructing violence as a medical syndrome: mapping psychotic, impulsive, and predatory subtypes to malfunctioning brain circuits. CNS Spectr. 2014;19(5):357–365. doi: 10.1017/S1092852914000522. [DOI] [PubMed] [Google Scholar]
- 47.Ritov G, Ardi Z, Richter-Levin G. Differential activation of amygdala, dorsal and ventral hippocampus following an exposure to a reminder of underwater trauma. Front Behav Neurosci. 2014;8:18. doi: 10.3389/fnbeh.2014.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heath NM, Chesney SA, Gerhart JI, et al. Interpersonal violence, PTSD, and inflammation: potential psychogenic pathways to higher C-reactive protein levels. Cytokine. 2013;63(2):172–178. doi: 10.1016/j.cyto.2013.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eraly SA, Nievergelt CM, Maihofer AX, et al. Marine Resiliency Study Team Assessment of plasma C-reactive protein as a biomarker of posttraumatic stress disorder risk. JAMA Psychiatry. 2014;71(4):423–431. doi: 10.1001/jamapsychiatry.2013.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zimmerman G, Shaltiel G, Barbash S, et al. Post-traumatic anxiety associates with failure of the innate immune receptor TLR9 to evade the pro-inflammatory NFκB pathway. Transl Psychiatry. 2012;2:e78. doi: 10.1038/tp.2012.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Copeland WE, Wolke D, Lereya ST, Shanahan L, Worthman C, Costello EJ. Childhood bullying involvement predicts low-grade systemic inflammation into adulthood. Proc Natl Acad Sci U S A. 2014;111(21):7570–7575. doi: 10.1073/pnas.1323641111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bob P, Raboch J, Maes M, et al. Depression, traumatic stress and interleukin-6. J Affect Disord. 2010;120(1–3):231–234. doi: 10.1016/j.jad.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 53.Bransfield RC. Intrusive symptoms and infectious encephalopathies. Neurol Psychiatry Brain Res. 2016;22:3–4. [Google Scholar]
- 54.Bransfield RC. The psychoimmunology of Lyme/tick-borne diseases and its association with neuropsychiatric symptoms. Open Neurol J. 2012;6:88–93. doi: 10.2174/1874205X01206010088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rosell DR, Siever LJ. The neurobiology of aggression and violence. CNS Spectr. 2015;20(3):254–279. doi: 10.1017/S109285291500019X. [DOI] [PubMed] [Google Scholar]
- 56.Lidberg L, Belfrage H, Bertilsson L, Evenden MM, Asberg M. Suicide attempts and impulse control disorder are related to low cerebrospinal fluid 5-HIAA in mentally disordered violent offenders. Acta Psychiatr Scand. 2000;101(5):395–402. doi: 10.1034/j.1600-0447.2000.101005395.x. [DOI] [PubMed] [Google Scholar]
- 57.Pucilowski O, Kostowski W. Aggressive behaviour and the central serotonergic systems. Behav Brain Res. 1983;9(1):33–48. doi: 10.1016/0166-4328(83)90012-8. [DOI] [PubMed] [Google Scholar]
- 58.Virkkunen M, Goldman D, Nielsen DA, Linnoila M. Low brain serotonin turnover rate (low CSF 5-HIAA) and impulsive violence. J Psychiatry Neurosci. 1995;20(4):271–275. [PMC free article] [PubMed] [Google Scholar]
- 59.de Boer SF, Caramaschi D, Natarajan D, Koolhaas JM. The vicious cycle towards violence: focus on the negative feedback mechanisms of brain serotonin neurotransmission. Front Behav Neurosci. 2009;3:52. doi: 10.3389/neuro.08.052.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Coccaro EF, Lee R, Coussons-Read M. Elevated plasma inflammatory markers in individuals with intermittent explosive disorder and correlation with aggression in humans. JAMA Psychiatry. 2014;71(2):158–165. doi: 10.1001/jamapsychiatry.2013.3297. [DOI] [PubMed] [Google Scholar]
- 61.Hall A. Epidemic Encephalitis. (Encephalitis Lethargica) Bristol, UK: John Wright and Sons; 1924. [Google Scholar]
- 62.Cheyette SR, Cummings JL. Encephalitis lethargica: lessons for contemporary neuropsychiatry. J Neuropsychiatry Clin Neurosci. 1995;7(2):125–134. doi: 10.1176/jnp.7.2.125. [DOI] [PubMed] [Google Scholar]
- 63.Mulder D, Parrott M, Thaler M. Sequelae of western equine encephalitis. Neurology. 1951;1:318–327. doi: 10.1212/wnl.1.7-8.318. [DOI] [PubMed] [Google Scholar]
- 64.McMillan TM, Papadopoulos H, Cornall C, Greenwood RJ. Modification of severe behaviour problems following herpes simplex encephalitis. Brain Inj. 1990;4(4):399–406. doi: 10.3109/02699059009026193. [DOI] [PubMed] [Google Scholar]
- 65.Ahokas A, Rimon R, Koskiniemi M, Vaheri A, Julkunen I, Sarna S. Viral antibodies and interferon in acute psychiatric disorders. J Clin Psychiatry. 1987;48(5):194–196. [PubMed] [Google Scholar]
- 66.Anstett RE, Wood L. The patient exhibiting episodic violent behavior. J Fam Pract. 1983;16(3):605–609. [PubMed] [Google Scholar]
- 67.Gamache FW, Jr, Ducker TB. Alterations in neurological function in head-injured patients experiencing major episodes of sepsis. Neurosurgery. 1982;10(4):468–472. doi: 10.1227/00006123-198204000-00009. [DOI] [PubMed] [Google Scholar]
- 68.Rayel MG, Land WB, Gutheil TG. Dementia as a risk factor for homicide. J Forensic Sci. 1999;44(3):565–567. [PubMed] [Google Scholar]
- 69.Hoffman BF. Reversible neurosyphilis presenting as chronic mania. J Clin Psychiatry. 1982;43(8):338–339. [PubMed] [Google Scholar]
- 70.Varney NR, Roberts RJ, Springer JA, Connell SK, Wood PS. Neuropsychiatric sequelae of cerebral malaria in Vietnam veterans. J Nerv Ment Dis. 1997;185(11):695–703. doi: 10.1097/00005053-199711000-00008. [DOI] [PubMed] [Google Scholar]
- 71.Sowunmi AS. Psychosis after cerebral malaria in children. J Natl Med Assoc. 1993;85(9):695–696. [PMC free article] [PubMed] [Google Scholar]
- 72.Bransfield RC. Did Infections Caused by World War I Contribute to Causing World War II? Contagion Live. Jan 5, 2018. [Accessed January 6, 2018]. Available from: http://www.contagionlive.com/news/did-infections-caused-by-world-war-i-contribute-to-causing-world-war-ii.
- 73.Sobin A, Ozer M. Mental disorders in acute encephalitis. J Mt Sinai Hosp. 1966;33:73–82. [PubMed] [Google Scholar]
- 74.Flegr J, Hrdy I. Influence of chronic toxoplasmosis on some human personality factors. Folia Parasitol (Praha) 1994;42(2):122–126. [PubMed] [Google Scholar]
- 75.Flegr J, Zitkova S, Kodym P, Frynta D. Induction of changes in human behaviour by the parasitic protozoan Toxoplasma gondii. Parasitology. 1996;113(Pt 1):49–54. doi: 10.1017/s0031182000066269. [DOI] [PubMed] [Google Scholar]
- 76.Piekarski G. Behavioral alterations caused by parasitic infection in case of latent toxoplasma infection. Zentralbl Bakteriol Mikrobiol Hgy [A] 1981;250(3):403–406. [PubMed] [Google Scholar]
- 77.Caroff S, Mann S, Gliatto M, Sullivan K, Campbell E. Psychiatric manifestations of acute viral encephalitis. Psychiatr Ann. 2001;31:193–204. [Google Scholar]
- 78.Schlesinger LB. Sexual Murder: Catathymic and Compulsive Homicides. Boca Raton, FL: CRC Press; 2004. [Google Scholar]
- 79.Tselis A, Booss J. Behavioral consequences of infections of the central nervous system: with emphasis on viral infections. J Am Acad Psychiatry Law. 2003;31(3):289–298. [PubMed] [Google Scholar]
- 80.Rayel MG, Land WB, Gutheil TG. Dementia as a risk factor for homicide. J Forensic Sci. 1999;44(3):565–567. [PubMed] [Google Scholar]
- 81.Bachet M. The concept of encephaloses criminogenic (criminogenic encephalosis) Am J Orthopsychiatry. 1951;21:794–799. doi: 10.1111/j.1939-0025.1951.tb00029.x. [DOI] [PubMed] [Google Scholar]
- 82.Coccaro EF, Lee R, Groer MW, Can A, Coussons-Read M, Postolache TT. Toxoplasma gondii infection: relationship with aggression in psychiatric subjects. J Clin Psychiatry. 2016;77(3):334–341. doi: 10.4088/JCP.14m09621. [DOI] [PubMed] [Google Scholar]
- 83.Zimmer C. Parasite Rex: Inside the Bizarre World of Nature’s Most Dangerous Creatures New Ed Edition. New York, NY: Atria Publishing Group; 2000. [Google Scholar]
- 84.Sergiev VP. Change of host’s behavior including man under the influence of parasites. Zh Mikrobiol Epidemiol Immunobiol. 2010;(3):108–114. [PubMed] [Google Scholar]
- 85.Whitfield J. Worm turns fish antisocial. Nature. 2000. Jul 9, [Accessed October 21, 2017]. Available from: http://www.nature.com/news/1998/020708/full/news020708-3.html.
- 86.Winkler WG, Schneider NJ, Jennings WL. Experimental rabies infection in wild rodents. J Wildl Dis. 1972;8(1):99–103. doi: 10.7589/0090-3558-8.1.99. [DOI] [PubMed] [Google Scholar]
- 87.Barnard CJ, Behnke JM, Sewell J. Environmental enrichment, immunocompetence, and resistance to Babesia microti in male mice. Physiol Behav. 1996;60(5):1223–1231. doi: 10.1016/s0031-9384(96)00174-6. [DOI] [PubMed] [Google Scholar]
- 88.Birkenheuer AJ, Correa MT, Levy MG, Breitschwerdt EB. Geographic distribution of babesiosis among dogs in the United States and association with dog bites: 150 cases (2000–2003) J Am Vet Med Assoc. 2005;227(6):942–947. doi: 10.2460/javma.2005.227.942. [DOI] [PubMed] [Google Scholar]
- 89.Mitchell C. Dog aggression link-deer tick Lyme disease: dogs that are too aggressive to people. Leerburg Webboard. 2007. [Accessed October 16, 2017]. Available from: http://leerburg.com/webboard/thread.php?topic_id=20140.
- 90.Goswani N. Tour of Duty Ends for Dog. Bennington Banner. 2006. Nov 28, [Accessed October 16, 2017]. Available from: http://www.benningtonbanner.com/stories/tour-of-duty-ends-for-dog,257240.
- 91.Bradley JM, Mascarelli PE, Trull CL, Maggi RG, Breitschwerdt EB. Bartonella henselae infections in an owner and two Papillon dogs exposed to tropical rat mites (Ornithonyssus bacoti) Vector Borne Zoonotic Dis. 2014;14(10):703–709. doi: 10.1089/vbz.2013.1492. [DOI] [PubMed] [Google Scholar]
- 92.Bishop BL, Cherry NA, Hegarty BC, Breitschwerdt EB. Enrichment blood culture isolation of Bartonella henselae from horses with chronic circulatory, musculoskeletal and/or neurologic deficits. Adv Biotech Micro. 2017;4(5) [Google Scholar]
- 93.CBSNews CBS/AP Chimp Shot Dead After Attacking Woman. 2009. Feb 17, [Accessed October 16, 2017]. Available from: www.cbsnews.com/news/chimp-shot-dead-after-attacking-woman.
- 94.Christoffersen J, Easton Robb P. Huge chimp shot dead after mauling woman in Conn. Associated Press; Feb 17, 2007. [Accessed October 16, 2017]. Available from: https://www.nhregister.com/news/article/Huge-chimp-shot-dead-after-mauling-woman-11635518.php. [Google Scholar]
- 95.Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365(6446):545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- 96.Tabbaa M, Paedae B, Liu Y, Wang Z. Neuropeptide regulation of social attachment: the prairie vole model. Compr Physiol. 2016;7(1):81–104. doi: 10.1002/cphy.c150055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.MacLean EL, Gesquiere LR, Gruen ME, Sherman BL, Martin WL, Carter CS. Endogenous oxytocin, vasopressin, and aggression in domestic dogs. Front Psychol. 2017;8:161327. doi: 10.3389/fpsyg.2017.01613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Heinrichs M, Domes G. Neuropeptides and social behaviour: effects of oxytocin and vasopressin in humans. Prog Brain Res. 2008;170:337–350. doi: 10.1016/S0079-6123(08)00428-7. [DOI] [PubMed] [Google Scholar]
- 99.Keverne EB, Kendrick KM. Maternal behaviour in sheep and its neuroendocrine regulation. Acta Paediatr Suppl. 1994;397:47–56. doi: 10.1111/j.1651-2227.1994.tb13265.x. [DOI] [PubMed] [Google Scholar]
- 100.Schmelkin C, Plessow F, Thomas JJ, et al. Low oxytocin levels are related to alexithymia in anorexia nervosa. Int J Eat Disord. 2017;50(11):1332–1338. doi: 10.1002/eat.22784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Norman MU, Moriarty TJ, Dresser AR, Millen B, Kubes P, Chaconas G. Molecular mechanisms involved in vascular interactions of the Lyme disease pathogen in a living host. PLoS Pathog. 2008;4(10):e1000169. doi: 10.1371/journal.ppat.1000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Moriarty TJ, Norman MU, Colarusso P, Bankhead T, Kubes P, Chaconas G. Real-time high resolution 3D imaging of the Lyme disease spirochete adhering to and escaping from the vasculature of a living host. PLoS Pathog. 2008;4(6):e1000090. doi: 10.1371/journal.ppat.1000090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kandel ER, Schwartz JH, Jessell TM. Principles of Neural Science. 4th ed. New York: McGraw-Hill Health Professions Division; 2000. [Google Scholar]
- 104.Rhee H, Cameron DJ. Lyme disease and pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS): an overview. In J Gen Med. 2012;5:163–174. doi: 10.2147/IJGM.S24212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Barnett EM, Jacobsen G, Evans G, Cassell M, Perlman S. Herpes simplex encephalitis in the temporal cortex and limbic system after trigeminal nerve inoculation. J Infect Dis. 1994;169(4):782–786. doi: 10.1093/infdis/169.4.782. [DOI] [PubMed] [Google Scholar]
- 106.Johnson L, Wilcox S, Mankoff J, Stricker RB. Severity of chronic Lyme disease compared to other chronic conditions: a quality of life survey. PeerJ. 2014;2:e322. doi: 10.7717/peerj.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sperling J, Middelveen M, Klein D, Sperling F. Evolving perspectives on Lyme borreliosis in Canada. Open Neurol J. 2012;6:94–103. doi: 10.2174/1874205X01206010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Logigian EL, Kaplan RF, Steere AC. Chronic neurologic manifestations of Lyme disease. N Engl J Med. 1990;323(21):1438–1444. doi: 10.1056/NEJM199011223232102. [DOI] [PubMed] [Google Scholar]
- 109.Harvey R, Misselbeck W, Uphold R. Cat-scratch disease: an unusual cause of combative behavior. Am J Emerg Med. 1991;9:52–53. doi: 10.1016/0735-6757(91)90016-d. [DOI] [PubMed] [Google Scholar]
- 110.Dee JE. Ax attacker using Lyme disease as defense case intensifies debate over ailment’s effects on brain. Courant. 1999 Jan 17; [Google Scholar]
- 111.Fallon BA, Nields JA, Burrascano JJ, Liegner K, DelBene D, Liebowitz MR. The neuropsychiatric manifestations of Lyme borreliosis. Psychiatr Q. 1992;63(1):95–117. doi: 10.1007/BF01064684. [DOI] [PubMed] [Google Scholar]
- 112.Sherr VT. Spotlight on Lyme. Lyme Alliance Inc; Concord, MI, USA: Jun, 1998. Letter to Editor. [Google Scholar]
- 113.Bransfield RC. Aggression and Lyme disease. Spotlight. 1998. Jun, [Accessed October 21, 2017]. Available from: http://www.thedogplace.org/health/aggression-lyme-disease-1608-bansfield-md.asp.
- 114.Staff “I fear for my friends’ and for my family’s safety”: Palin wins restraining order against teenage stalker (and his dad) Daily Mail. 2012. Dec 12, [Accessed October 21, 2017]. Available from: http://www.dailymail.co.uk/news/article-1385553/Sarah-Palin-wins-restraining-order-teenage-stalker-Shawn-Christy-dad.html#ixzz4wAYLzGST.
- 115.Howell T. Depraved’ mom gets five years. New Jersey Herald. 2009. Apr 16, [Accessed October 21, 2017]. Available from: http://www.njherald.com/article/20090416/ARTICLE/304169997#.
- 116.Cutroni L. Tusten resident surrenders after shooting at police. The River Reporter. 2006. Feb 2, [Accessed October 21, 2017]. Available from: http://www.riverreporter.com/issues/06-02-02/news-tusten.html.
- 117.Greenstein T. Down for the count. Chicago Tribune. 2000. Feb 13, [Accessed October 21, 2017]. Available from: http://articles.chicagotribune.com/2000-02-13/sports/0002130275_1_casino-industry-art-collection-afterglow/2.
- 118.Fallon BD, Schwartzenberg M, Bransfield R, et al. Late stage neuropsychiatric Lyme borreliosis. Psychosomatics. 1995;36(3):295–300. doi: 10.1016/S0033-3182(95)71669-3. [DOI] [PubMed] [Google Scholar]
- 119.Bransfield RC. The Many Faces of Lyme: Neuropsychiatric Lyme Disease Case Presentations. Paper presented at: Neuropsychiatric Lyme Disease Symposium, 149th Annual Meeting; May 9, 1996; New York, NY. American Psychiatric Association; [Google Scholar]
- 120.Bransfield RC. Diagnosis, treatment, and prevention of Lyme disease. JAMA. 1998;280(12):1049. Author reply 1051. [PubMed] [Google Scholar]
- 121.Bransfield RC, Fallon BA, Raxlen BD, Shepler LT, Sherr VT. A modest proposal. Psychiatric News. 1998;33:18(18):16. [Google Scholar]
- 122.Bransfield RC, Fallon BA. Microbes and Mental Illness, Neuropsychiatric Features of Lyme Disease. Papers presented at: Microbes and Mental Illness Symposium, American Psychiatric Association Institute on Psychiatric Services Meeting; October 25, 2000; Philadelphia, PA. [Google Scholar]
- 123.Bransfield R. Lyme neuroborreliosis and aggression. Paper presented at: 14th International Scientific Conference on Lyme Disease and Other Tick-Borne Disorder; April 22; 2001; Hartford, CT. [Accessed October 21, 2017]. Available from: https://sites.google.com/site/marylandlyme/symptoms-information/mental-health-issues/lyme-neuroborreliosis-agression-dr-bransfield. [Google Scholar]
- 124.Bransfield RC. Posttraumatic stress disorder and infectious encephalopathies. Spotlight. 2001. Nov-Dec. [Accessed October 21, 2017]. Available from: http://www.mentalhealthandillness.com/Articles/PosttraumaticStressDisorder.htm.
- 125.Bär KJ, Jochum T, Häger F, Meissner W, Sauer H. Painful hallucinations and somatic delusions in a patient with the possible diagnosis of neuroborreliosis. Clin J Pain. 2005;21(4):362–363. doi: 10.1097/01.ajp.0000120791.69705.12. [DOI] [PubMed] [Google Scholar]
- 126.Schaller JL, Burkland GA, Langhoff PJ. Do bartonella infections cause agitation, panic disorder, and treatment-resistant depression? MedGenMed. 2007;9(3):54. [PMC free article] [PubMed] [Google Scholar]
- 127.Bransfield R. Can infections and immune reactions to them cause violent behavior?. Paper presented at: International Lyme and Associated Diseases Society Neurological/Psychiatric Lyme Disease Conference; October 29, 2011; Toronto, Canada. [Accessed October 19, 2017]. Available from: https://www.youtube.com/watch?v=UI3Yap1Kgmo. [Google Scholar]
- 128.Bransfield RC. Can infections and immune reactions to them cause violent behavior? Neurol Psychiatry Brain Res. 2012;18(3):42. [Google Scholar]
- 129.Bransfield RC. The role of brain mechanisms in intrusive symptoms and post-traumatic stress disorders. Paper presented at: International Lyme and Associated Diseases Annual Conference; October 10, 2014; Washington, DC. [Google Scholar]
- 130.Bransfield RC. Chronic infections as aetiological factors in psychiatric disorders. Paper presented at: 21st Annual International Integrative Medicine Conference; July 19, 2015; Melbourne, Australia. [Google Scholar]
- 131.Breitschwerdt EB, Mascarelli PE, Schweickert LA, et al. Hallucinations, sensory neuropathy, and peripheral visual deficits in a young woman infected with Bartonella koehlerae. J Clin Microbiol. 2011;49(9):3415–3417. doi: 10.1128/JCM.00833-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Munir A, Aadil M, Rehan Khan A. Suicidal and homicidal tendencies after Lyme disease: an ignored problem. Neuropsychiatr Dis Treat. 2017;13:2069–2071. doi: 10.2147/NDT.S145359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bransfield RC. Response to Letter to Editor Suicidal and homicidal tendencies after Lyme disease: an ignored problem. Neuropsychiatr Dis Treat. 2017;13:2071. doi: 10.2147/NDT.S145359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Fink PJ. Another great imposter. Philadelphia Medicine. 1999 Jun [Google Scholar]
- 135.Fink PJ. Another great imposter. Clinical Psychiatry News. 1999 Mar [Google Scholar]
- 136.Langhoff PJ. Recognizing the face of rage in tick-borne illness. [Accessed October 20, 2017];Public Health Alert. 2007 2(8):4–9. Available from: http://www.betterhealthguy.com/images/stories/PDF/PHA/2007_08.pdf. [Google Scholar]
- 137.Bransfield R. Can infections and immune reactions to them cause violent behavior?. Paper presented at: International Lyme and Associated Diseases Society Neurological/Psychiatric Lyme Disease Conference; October 29, 2011; Toronto, Canada. [Accessed October 19, 2017]. Available from: https://www.youtube.com/watch?v=UI3Yap1Kgmo. [Google Scholar]
- 138.Bransfield RC. Antimicrobials in psychiatry. Psychiatric Times. 1999 Jul [Google Scholar]
- 139.Singley P. Lawyer: Southbury strangling sparked by Lyme disease or drugs. Republican-American. 2006. Feb 9, [Accessed October 20, 2017]. Available from: http://www.lymeblog.com/modules.php?name=News&file=article&sid=390.
- 140.Appellate Court of Connecticut Gregg Madigosky v. Commissioner of Correction (AC 38962) Decided: 2017 April 11. [Accessed October 20, 2017]. Available from: http://caselaw.findlaw.com/ct-court-of-appeals/1855564.html.
- 141.Weir R. Librarian savagely beaten while sleeping in her West Islip home. Son held in slay of Mom. New York Daily News. 2006. Oct 10, [Accessed October 20, 2017]. Available from: http://www.nydailynews.com/archives/boroughs/librarian-savagely-beaten-sleeping-west-islip-home-son-held-slay-mom-article-1.645217.
- 142.Miller AL. Two dead in murder-suicide had advanced Lyme disease. Ocala Star Banner. 2010. Jul 10, [Accessed October 20, 2017]. Available from: http://www.ocala.com/article/LK/20100710/News/604190440/OS/
- 143.McQuiston JT. Man Pleads Not Guilty in Slaying on Shelter I. The New York Times. 2008. May 7, [Accessed October 20, 2017]. Available from: http://www.nytimes.com/1998/05/07/nyregion/man-pleads-not-guilty-in-slaying-on-shelter-i.html?mcubz=3.
- 144.McQuiston JT. Fear, Not Anger, Led to Shelter Island Slaying, Defense Says. The New York Times. 2000. May 23, [Accessed October 20, 2017]. Available from: http://www.nytimes.com/2000/05/23/nyregion/fear-not-anger-led-to-shelter-island-slaying-defense-says.html?mcubz=3.
- 145.Wacker T. Lyme made him do it? Lyme psychosis eyed as defense in S.I. Shooting. The Suffolk Times. 1998 May 14; [Google Scholar]
- 146.Bernstein J. Did Adam Lanza have Lyme disease? Counterpunch. 2013. Jan 11, [Accessed October 20, 2017]. Available from: https://www.counterpunch.org/2013/01/11/did-adam-lanza-have-lyme-disease/
- 147.Abrahams Wilson A. Under Our Skin 2: Emergence. [Accessed October 20, 2017]. Available from: http://underourskin.com/emergence/
- 148.Altimari D. Full Report Confirms No Drugs, Alcohol in Lanza’s System. The Hartford Courant. Oct 29, 2013. [Accessed October 20, 2017]. Available from: http://articles.courant.com/2013-10-29/news/hc-sandy-hook-lanza-toxicology-20131029_1_peter-lanza-adam-lanza-toxicology-report.
- 149.Staff The tick defense. New Jersey Law Journal. 1999;155NJLJ(1331):3. [Google Scholar]
- 150.Fritz M. Foreboding at Ground Zero of Lyme Disease. LA Times. 1998. Jun 7, [Accessed October 21, 2017]. Available from: http://articles.latimes.com/1998/jun/07/news/mn-57486/2.
- 151.Viviano J. Defense blames Lyme disease in ax attack. Register. 2001. Jan 26, [Accessed October 20, 2017]. Available from: https://groups.google.com/forum/#!topic/sci.med.diseases.lyme/9Q3813YOx70.
- 152.Staff Woman pleads guilty to fatal fire. Times Free Press. 2010. Dec 11, [Accessed October 20, 2017]. Available from: http://www.timesfreepress.com/news/news/story/2010/dec/11/woman-pleads-guilty-to-fatal-fire/36780/
- 153.Staff NY follow-up letter, Dr. Bransfield connects Lyme to opioid crisis 2017 Sept 6. LymeDisease.org. [Accessed October 20, 2017]. Available from: https://www.lymedisease.org/bransfield-lyme-opioid-crisis/?utm_source=sept+9-sleeper&utm_campaign=sept+5-sleeper+cells&utm_medium=email.
- 154.Shea LJ. The Neuropsychology of Lyme Disease. Paper presented at: Challenges and Controversy in Lyme Disease and Tick-Borne Illness Care Symposium; November 9, 2013; Boston, MA. [Google Scholar]
- 155.Blair RJ. The amygdala and ventromedial prefrontal cortex: functional contributions and dysfunction in psychopathy. Philos Trans R Soc Lond B Biol Sci. 2008;363(1503):2557–2565. doi: 10.1098/rstb.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Blair RJ. The Neurobiology of Impulsive Aggression. J Child Adolesc Psychopharmacol. 2016;26(1):4–9. doi: 10.1089/cap.2015.0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lee R, Arfanakis K, Evia AM, Fanning J, Keedy S, Coccaro EF. White matter integrity reductions in intermittent explosive disorder. Neuropsychopharmacology. 2016;41(11):2697–2703. doi: 10.1038/npp.2016.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Shraberg D, Weisberg L. The Klüver-Bucy syndrome in man. J Nerv Ment Dis. 1978;166(2):130–134. doi: 10.1097/00005053-197802000-00008. [DOI] [PubMed] [Google Scholar]
- 159.Morana HC, Stone MH, Abdalla-Filho E. Transtornos de person-alidade, psicopatia e serial killers. [Personality disorders, psychopathy and serial killers] Rev Bras Psiquiatr. 2006;28(2):74–79. doi: 10.1590/s1516-44462006000600005. Portuguese. [DOI] [PubMed] [Google Scholar]
- 160.Jha KK, Singh SK, Kumar P, Arora CD. Partial Kluver-Bucy syndrome secondary to tubercular meningitis. BMJ Case Rep. 2016;16:2016. doi: 10.1136/bcr-2016-215926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Oumerzouk J, Mouhadi KK, Hssaini Y, et al. Klüver-Bucy syndrome complicating herpes meningoencephalitis in a pregnant woman. Presse Med. 2014;43(7–8):871–874. doi: 10.1016/j.lpm.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 162.Eikmeier G, Forquignon I, Honig H. Klüver-Bucy syndrome after herpes simplex encephalitis. Psychiatr Prax. 2013;40(7):392–393. doi: 10.1055/s-0033-1343274. [DOI] [PubMed] [Google Scholar]
- 163.Bransfield RC, Wulfman JS, Harvey WT, Usman AI. The association between tick-borne infections, Lyme borreliosis and autism spectrum disorders. Med Hypotheses. 2008;70(5):967–974. doi: 10.1016/j.mehy.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 164.Daxboeck F. Mycoplasma pneumoniae central nervous system infections. Curr Opin Neurol. 2006;19(4):374–378. doi: 10.1097/01.wco.0000236617.04958.60. [DOI] [PubMed] [Google Scholar]
- 165.Auvichayapat N, Auvichayapat P, Watanatorn J, Thamaroj J, Jitpimolmard S. Kluver-Bucy syndrome after mycoplasmal bronchitis. Epilepsy Behav. 2006;8(1):320–322. doi: 10.1016/j.yebeh.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 166.Patel R, Jha S, Yadav RK. Pleomorphism of the clinical manifestations of neurocysticercosis. Trans R Soc Trop Med Hyg. 2006;100(2):134–141. doi: 10.1016/j.trstmh.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 167.De Tiège X, De Laet C, Mazoin N. Postinfectious immune-mediated encephalitis after pediatric herpes simplex encephalitis. Brain Dev. 2005;27(4):304–307. doi: 10.1016/j.braindev.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 168.Locatelli C, Vergine G, Ciambra R. Kluver Bucy syndrome and central diabetes insipidus: two uncommon complications of herpes simplex encephalitis. Pediatr Med Chir. 2003;25(6):442–446. [PubMed] [Google Scholar]
- 169.Mancini J, Chabrol B, Livet MO, Pinsard N. Résultat de l’encéphalite herpétique. À propos de 10 cas. [Outcome of herpetic encephalitis. Apropos of 10 cases] Ann Pediatr (Paris) 1991;38(3):143–149. French. [PubMed] [Google Scholar]
- 170.Hart RP, Kwentus JA, Frazier RB, Hormel TL. Natural history of Klüver-Bucy syndrome after treated herpes encephalitis. South Med J. 1986;79(11):1376–1378. doi: 10.1097/00007611-198611000-00014. [DOI] [PubMed] [Google Scholar]
- 171.Webster J, Lamberton PH, Donnelly C, Torrey E. Parasites as causative agents of human affective disorders? The impact of anti-psychotic, mood-stabilizer and anti-parasite medication on Toxoplasma gondii’s ability to alter host behaviour. Proc Roy Soc B Biol Sc. 2006;273(1589):1023–1030. doi: 10.1098/rspb.2005.3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Carroll JE, Low CA, Prather AA, et al. Negative affective responses to a speech task predict changes in interleukin (IL)-6. Brain Behav Immun. 2011;25:232–238. doi: 10.1016/j.bbi.2010.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Westling S, Ahrén B, Träskman-Bendz L, Brundin L. Increased IL-1β reactivity upon a glucose challenge in patients with deliberate self-harm. Acta Psychiatr Scand. 2011;124(4):301–306. doi: 10.1111/j.1600-0447.2011.01734.x. [DOI] [PubMed] [Google Scholar]
- 174.Sergiev VP. Change of host’s behavior including man under the influence of parasites. Zh Mikrobiol Epidemiol Immunobiol. 2010;(3):108–114. Russian. [PubMed] [Google Scholar]
- 175.Constant A, Castera L, Dantzer R, et al. Mood alterations during interferon-alfa therapy in patients with chronic hepatitis C: evidence for an overlap between manic/hypomanic and depressive symptoms. J Clin Psychiatry. 2005;66(8):1050–1057. doi: 10.4088/jcp.v66n0814. [DOI] [PubMed] [Google Scholar]
- 176.Lotrich FE, Sears B, McNamara RK. Anger induced by interferon-alpha is moderated by ratio of arachidonic acid to omega-3 fatty acids. J Psychosom Res. 2013;75(5):475–483. doi: 10.1016/j.jpsychores.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chantal Henry C, Laurent Castéra L, Demotes-Mainard J. Hepatitis C and interferon: Watch for hostility, impulsivity. Curr Psychiatr. 2006;5(8):71–77. [Google Scholar]
- 178.Kleinsasser BJ, Misra LK, Bhatara VS, Sanchez JD. Risperidone in the treatment of choreiform movements and aggressiveness in a child with “PANDAS”. S D J Med. 1999;52(9):345–347. [PubMed] [Google Scholar]
- 179.Tillman JA, Raff GJ. Paraneoplastic limbic encephalitis cured with resection of an adnexal mass. JSLS. 2009;13(3):433435. [PMC free article] [PubMed] [Google Scholar]
- 180.Khan N, Wieser HG. Limbic encephalitis: a case report. Epilepsy Res. 1994;17(2):175–181. doi: 10.1016/0920-1211(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 181.Venâncio P, Brito MJ, Pereira G, Vieira JP. Anti-N-methyl-D-aspartate receptor encephalitis with positive serum antithyroid antibodies, IgM antibodies against mycoplasma pneumoniae and human herpesvirus 7 PCR in the CSF. Pediatr Infect Dis J. 2014;33(8):882–883. doi: 10.1097/INF.0000000000000408. [DOI] [PubMed] [Google Scholar]
- 182.Frohman EM, Frohman TC, Moreault AM. Acquired sexual paraphilia in patients with multiple sclerosis. Arch Neurol. 2002;59(6):1006–1010. doi: 10.1001/archneur.59.6.1006. [DOI] [PubMed] [Google Scholar]
- 183.Bransfield RC. A structured clinical interview when neuropsychiatric Lyme disease is a possibility. Paper presented at: LDF’s 10th Annual International Scientific Conference on Lyme Borrelosis and Other Tick-borne Disorders; April 28, 1997; Bethesda, MD. [Google Scholar]
- 184.Citera M, Freeman PR, Horowitz RI. Empirical validation of the Horowitz Multiple Systemic Infectious Disease Syndrome Questionnaire for suspected Lyme disease. Int J Gen Med. 2017;10:249–273. doi: 10.2147/IJGM.S140224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.AHRQ National Guideline Clearinghouse. 2016. [Accessed April 30, 2017]. Available from: https://www.guideline.gov.
- 186.Cameron DJ, Johnson LB, Maloney EL. Evidence assessments and guideline recommendations in Lyme disease: the clinical management of known tick bites, erythema migrans rashes and persistent disease. Expert Rev Anti Infect Ther. 2014;12(9):1103–1135. doi: 10.1586/14787210.2014.940900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.The American Psychiatric Association practice guidelines for the psychiatric evaluation of adults. Third edition. 2016. [Accessed March 3, 2017]. Available from: http://psychiatryonline.org/doi/pdf/10.1176/appi.books.9780890426760.
- 188.U.S. Centers for Disease Control and Prevention How many people get Lyme disease? 2015. [Accessed October 20, 2017]. Available from: https://www.cdc.gov/lyme/stats/humancases.html.
- 189.Glista K, Moran D. Murder-suicide of Durham couple shakes community. Hartford Courant. 2015. [Accessed October 20, 2017]. Available from: http://www.courant.com/breaking-news/hc-durham-murder-suicide-0319-20150318-story.html.
- 190.Enchassi NJ. Tribune Media Wire and CNN Wire. DA: Pennsylvania dad of heart-transplant toddler killed family, self. 2016. [Accessed October 21, 2017]. Available from http://kfor.com/2016/08/15/pennsylvania-dad-of-heart-transplant-toddler-killed-family-self-da/
- 191.Salvatore P, Bhuvaneswar C, Tohen M, Khalsa HM, Maggini C, Baldessarini RJ. Capgras’ syndrome in first-episode psychotic disorders. Psychopathology. 2014;47(4):261–269. doi: 10.1159/000357813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Goodman RA, Mercy JA, Loya F, et al. Alcohol use and interpersonal violence: alcohol detected in homicide victims. Am J Public Health. 1986;76(2):144–149. doi: 10.2105/ajph.76.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Frank C. On the reception of the concept of the death drive in Germany: expressing and resisting an ‘evil principle’? Int J Psychoanal. 2015;96(2):425–444. doi: 10.1111/1745-8315.12213. [DOI] [PubMed] [Google Scholar]