Abstract
Despite their frequent occurrence and strong impacts on native biota, biological invasions can long remain undetected. One reason for this is that an invasive species can be morphologically similar to either native species or introduced species previously established in the same region, and thus be subject to mistaken identification. One recent case involves congeneric invasive parasites, copepods that now infect bivalve hosts along European Atlantic coasts, after having been introduced independently first from the Mediterranean Sea (Mytilicola intestinalis Steuer, 1902) and later from Japan (Mytilicola orientalis Mori, 1935). At least one report on M. intestinalis may have actually concerned M. orientalis, and M. orientalis thus qualifies as a “cryptic invader”. Because these two parasitic copepods are morphologically similar, knowledge about their distribution, impact and interactions depends crucially on reliable species identification. In this study, we evaluated the reliability of morphological identification of these two species in parts of their invasive range in Europe (Dutch Delta and Wadden Sea) in comparison with molecular methods of well-established accuracy based on COI gene sequences and ITS1 restriction fragment length polymorphism. Based on seven easily measured or scored macro-morphological variables that were recorded for 182 individual copepods isolated from blue mussels (Mytilus edulis Linnaeus, 1758), principal component analysis showed two relatively distinct but overlapping morphological species groups for females, but no clear separation in males. Discriminant function analysis showed that the females can be discriminated reasonably well based on some of the morphological characteristics (identification error rate of 7%) while males cannot (error rate of 25%). The direction of the dorsolateral thoracic protuberances was identified as the most important trait for species discrimination, but among the morphological features checked, none could flawlessly discriminate between both species. We recommend the use of molecular techniques in future studies of invasive Mytilicola to reliably discriminate between the species. The morphological similarity of these two invaders suggests a more general problem of cryptic invasions and compromised identification of parasites in invaded ecosystems. This problem should be borne in mind whenever invasive parasites are investigated.
Introduction
Biological invasions represent a well-known threat to ecological communities worldwide [1–4], yet it is not uncommon for the presence of an invasive species to remain undetected for longer periods of time [5]. One reason for this is that because of morphological similarity, invasive species might be mistaken for native species or other introduced species that have invaded the same localities [5–9]. While such cryptic invasions have occasionally been revealed for free-living species [5–9], cryptic invasions of internal parasites are more problematic to detect as the organisms are hidden inside their hosts.
The invasion of two congeneric parasites infecting the intestines of marine bivalve species along the northwestern European coast might represent such a case of a cryptic invasion of parasites. The parasitic copepod Mytilicola intestinalis Steuer, 1902 was first described in the mussel Mytilus galloprovincialis Lamarck, 1819 in the Adriatic Sea [10] and presumably spread with infected mussels as fouling on ships’ hulls to the Atlantic coast of Europe, where it started to infect native bivalve species [11–14]. The congeneric Mytilicola orientalis Mori, 1935 was first documented in Pacific oysters (Magallana (previously Crassostrea) gigas (Thunberg, 1793)) from Japan [15] and was introduced to British Columbia in 1938 in shipments of Pacific oysters [16]. Via subsequent oyster transports to France in the 1970s [17], M. orientalis spread northward with its host to the Dutch Delta [18] and the Dutch, German and Danish Wadden Sea [19, 20]. The two copepod species have accumulated a minimum interspecific genetic difference of 16.08% while showing very low intraspecific diversity for COI (cytochrome-c-oxidase I, a mitochondrial gene) [19]. Their divergence time could thus be in the order of seven to eleven million years ago based on a molecular clock commonly used for crustaceans [21, 22], and their status as distinct species by molecular criteria is not in dispute.
The first morphological species description for M. intestinalis by Steuer in 1902 [10] was later followed by a more detailed characterization by the same author [23]. When M. orientalis was later discovered in Japan, its investigator Mori was aware of the existence of M. intestinalis and included a list of differences between the taxa in the original species description [15]. These differences were: 1) more prominent dorsolateral thoracic protuberances in the Japanese species, 2) no dorsolateral protuberances on the first thoracic body segment (males only), and 3) upper lip triangular with a small notch on the tip instead of being rounded with a wavy edge. This last feature has not been mentioned again in later literature, presumably because it required a magnification of 270× and the complicated removal of other mouth parts to be visible [15], and is thus unsuitable in practice for screening large numbers of individuals. In a later study, Ho and Kim in 1992 [24] mention other features that discriminate between the species: 4) longer ovisacs in M. orientalis (females only), and 5) parallel or nearly parallel caudal rami in M. orientalis versus diverging caudal rami in M. intestinalis. Perhaps because two of the five adduced discriminated characters are sex-specific, and one requires intensive microscopy preparation, subsequent researchers have typically focussed on the dorsolateral thoracic protuberances and caudal rami for species identification [18, 19]. Since Ho and Kim [24] no new morphological research has been published on these two taxa to the best of our knowledge.
In practice, these small interspecific differences between the two copepods make a correct morphological species assessment difficult, and this likely led researchers to draw erroneous conclusions about the distribution and host range of both Mytilicola species in the past [19]. For instance, a study in the Exe estuary in the UK reported the presence of M. intestinalis in Pacific oysters [25], even though this parasite-host relationship has never been reported again after the existence of M. orientalis in these waters became known and it may have been an error [19, 26]. The lack of successful artificial infections of Pacific oysters with M. intestinalis supports this [19] (personal communications of M. E. Feis). Potential misidentifications may also underlie the often contradictory results of studies on the impacts of the two parasite species on ecologically or commercially important bivalve hosts e.g., [27, 28]. When the presence of only a single species of parasite continues to be assumed by researchers, the introduction of a second copepod species in northwestern Europe qualifies as a cryptic invasion sensu [4].
Currently, the two copepod species do not only co-occur along the Atlantic coasts of northwestern Europe, but they also have overlapping host ranges, both reportedly being capable of infecting blue mussels (M. edulis) [18–20, 26, 29], common cockles (Cerastoderma edule (Linnaeus, 1758)) [26, 27, 30] and Pacific oysters (M. gigas) [25, 31, 32]. However, whether the latter species actually serves as a host for M. intestinalis is debatable, as was discussed above [19, 26]. The sympatric occurrence of both copepods in the invaded region while sharing at least two host species increases the chance of misidentification even more.
The aim of this study was to assess the reliability of morphology-based identification of M. intestinalis and M. orientalis in co-invaded ecosystems. We approached this in three ways. First, we made quantitative morphological measurements of a large pool of Mytilicola spp. from blue mussel and Pacific oyster hosts originating from different locations in the Dutch and German Wadden Sea and the Dutch Delta, where the geographical ranges of the two parasite species overlap. In this invaded region, prevalences and mean intensities (± SE) of M. intestinalis (3–72%, 2.4 ± 0.3) and M. orientalis (3–63%, 2.1 ± 0.2) in blue mussels and M. orientalis in oysters (2–43%, 4.1 ± 0.6) can be high [26]. Second, we verified the species identity of every copepod molecularly by applying a diagnostic RFLP assay (Restriction Fragment Length Polymorphism) [26]. And third, we applied multivariate morphometric analyses to investigate the accuracy of morphological identification and to identify reliable features for species discrimination. In addition, as various methods of preservation and storage are known to modify or destroy morphological characteristics [33, 34], we investigated the effect of several preservation and storage methods on the morphology of both species.
Materials and methods
Parasite sampling
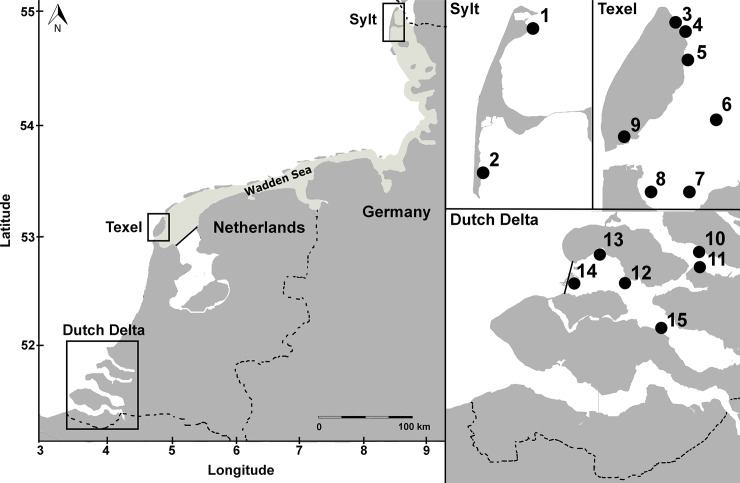
To ascertain whether morphological identification based on macro-characteristics is reliable for all Mytilicola individuals found in the introduced region, we used a large pool of specimens originating from different locations and different host species. Copepods were collected in two regions that were important invasion pathways for both parasites: the Dutch Delta (6 locations; May 2012) and the Wadden Sea (9 locations; different months in 2010–2012; Fig 1; S1 Table). A Natuurbeschermingswet (Nature Conservation Law) license was obtained for the field work in the Netherlands. For the German Wadden Sea samples, permission came from Nationalparkamt Schleswig-Holsteinisches Wattenmeer, Tönning. The field work did not involve endangered or protected species and complied with local legislation.
Fig 1. Sampling locations of blue mussel (Mytilus edulis) and Pacific oyster (Magallana (previously Crassostrea) gigas) hosts.
Left: The sampled regions in the Dutch Delta and the Wadden Sea (shaded area), with the islands Sylt (north) and Texel (south). Above right: Sampling locations around the islands of Sylt and Texel in the Wadden Sea. Below right: Sampling locations in the Dutch Delta. For exact coordinates see S1 Table.
Before dissecting specimens of the two main hosts, Pacific oysters (Magallana gigas; n = 109, mean shell length ± SE: 120.3 mm ± 2.8 mm) and blue mussels (Mytilus edulis; mean shell length ± SE: n = 198, 47.3 mm ± 0.6 mm), we measured the maximum length of each shell with Vernier calipers to the nearest 0.1 mm. Subsequently, the shells were opened, and the tissue was searched for the presence of Mytilicola individuals by using a magnification glass (magnification 3–8×). All Mytilicola individuals were removed from their hosts and stored in 96% ethanol. Individual copepods were then randomly drawn from each region/host species combination until, when possible, all host-sex categories (i.e., males and females of M. intestinalis and males and females of M. orientalis; see ‘Morphological scoring and identification’ section below) were filled with at least 20 individuals for the Dutch Delta and 25 individuals for the Wadden Sea, where we sampled more locations (Fig 1, S1 Table). However, as expected, M. intestinalis was not found in Pacific oysters and therefore this host/parasite category remained empty.
Morphological scoring and identification
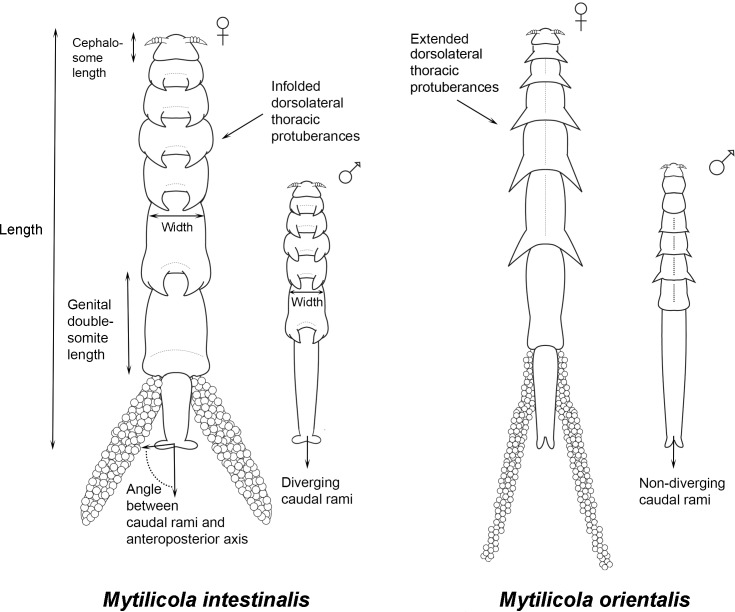
Morphological species identification of individual copepods (performed by MAG) was based on the direction of the dorsolateral thoracic protuberances (folded inwards in M. intestinalis and extended outwards for M. orientalis [15, 19] and on the shape of the caudal rami (thick and diverging in M. intestinalis and narrow and non-diverging in M. orientalis, Fig 2) [19]. Regarding sex determination, individuals were considered to be females when egg sacs were present and, if not, when the cephalosome was trapezoidally shaped [24] and the maxilliped (referred to as the second maxilliped in early descriptions [15, 23]) was missing (too small to be shown in Fig 2) [10]. Additionally, only in females the genital double-somite was trapezoidal (Fig 2) (MAG, unpublished observations).
Fig 2. Schematic representation (not to scale) of both sexes of both introduced Mytilicola species.
On the left both sexes of M. intestinalis and on the right both sexes of M. orientalis, all viewed from the ventral side, with indications of the body size measurements that were taken. Note the dorsolateral thoracic protuberances which are folded inwards in M. intestinalis and extended outwards in M. orientalis. For detailed drawings see original species descriptions, including views from other sides and close-ups [15, 23]. Drawings with courtesy of Felipe Ribas.
After pre-sorting the copepods into the four species-sex categories the same observer (MAG) made quantitative morphological observations of them. See Fig 2 for more details on body measurements of both sexes of both parasite species. With a camera (AxioCam ICc3) attached to a stereo microscope (Zeiss V8 discovery), pictures were taken of each individual copepod, filling the field of vision view as much as possible. The magnification used was 10× for females and 30× for males. Body size measurements were done using the software package AxioVision. Cephalosome length (maximum), body width (males: thinnest part of the animal; females: between the 4th and 5th metasomal body segments) and body length were measured, and the error rate (± SE) based on ten repeated length measurements was 0.40 ± 0.09 μm for males and 0.50 ± 0.13 μm for females. The divergence of the caudal rami [19] was measured as the angle between the caudal rami and the anteroposterior axis (see Fig 2). In addition, the number of body segments carrying dorsolateral thoracic protuberances was counted and the direction of the protuberances was noted (folded inwards or extended outwards). For females, the length of the genital double-somite was measured. With this set of macro-characters, we covered all the previously published traits that are typically used to distinguish between the two species [10, 15, 18, 19] as well as features related to their general shape characters. Shape of the upper lip was not observed in this study, although it supposedly differs between the species [15], because to do so would require detailed microscopical work, which is unpractical (and has, to our knowledge, never been applied) in large field studies. We ignored other microscopic details, such as the number of body segments carrying dorsolateral thoracic protuberances and the shape of the first and second antennae, because no variation or difference was originally [10, 15] nor subsequently noted for these characters.
Molecular identification
For the molecular identification of each copepod individual we used two assays, one targeting the mitochondrial cytochrome-c-oxidase I gene (COI) and one for the nuclear internal transcribed spacer 1 (ITS1). The combination of both assays potentially allowed us to identify hybrids with the ITS assay, and the direction of hybridisation with the COI assay.
For the COI assay, we newly developed two taxon-specific primers. They were designed on the basis of previously published Mytilicola intestinalis and M. orientalis sequences (Genbank accession numbers HM775191-HM775197) [19]. The new primers (MOICOIf 5'-CTTAATTACAGGGGTMTGATCGG-3' and MOICOIr 5'-TCGATCTGTTAAAAGCATAGTAATYG-3') amplify a 534 bp fragment yielding a PCR product of 583 bp (base pairs) in length.
A diagnostic RFLP (restriction fragment length polymorphism) assay was developed that could distinguish between M. intestinalis and M. orientalis. The restriction enzyme NciI recognizes and cuts the sequence CC/SGG (where S = C or G and / indicates the cut site). The PCR product of M. intestinalis is cut once by NciI, and that of M. orientalis is cut twice. In M. intestinalis this results in two restriction fragments that are 366 and 217 bp long. In M. orientalis the resulting pattern consists of three fragments, respectively 286, 222 and 75 bp long. This difference between the species was visualized on 2% agarose gels, which were run for 120 min. at 60 V, followed by staining with ethidium bromide. The COI assay was performed on all morphologically identified individuals (n = 307).
For the ITS assay we developed a TaqMan assay [29] targeting a species-discriminating single nucleotide polymorphism. We amplified the target region with the primers IntOri_ITS_F 5'-GCGTGTCGGAATGTGAACTG-3' and IntOri_ITS_R 5'-CCTGAGCCAGACATGGACAGA-3' and discriminated the species with the probes Myt_ITS_intP 5'FAM-ACATATGAGCATCGACAATTTGAACGC-3'BHQ1 and Myt_ITS_oriP 5'HEX-AGCATCGACACTTTGAACGCATATTGC-3'BHQ1. Each 20 μl reaction consisted of 10 μl of TaqMan Fast PCR Master Mix (Applied Biosystems, Darmstadt, Germany), 2 μl of each 5μM primer, 1 μl of each 5μM probe, 3 μl of nuclease-free water and 1 μl of sample DNA. An ABI 7500 fast system (Applied Biosystems, Darmstadt, Germany) was used for amplification and end point detection by applying the following cycling program of 60 s at 60°C and 20 s initial denaturation at 95°C followed by 40 cycles of 3 s denaturation at 95°C and 30 s combined annealing and extension at 60°C before a final post-PCR stage of 60 s at 60°C. This assay could successfully discriminate between the pure species and also detect potential hybrids (simulated by mixed DNA) and was performed on a subset of 75 individuals originating from Texel.
Effects of preservation and storage method on morphology
To investigate the effects of preservation and storage method on copepod macro-morphology, we sampled males and females of both Mytilicola species from blue mussel hosts (mean ± SD: 50.0 ± 6.3 mm) which were collected at two locations at the island of Texel: Vlakte van Kerken and Mokbaai (locations 5 and 9, respectively, in Fig 1). These samples were randomly assigned to two groups: 1) samples taken fresh from the host, measured, stored in 96% ethanol and measured again after two weeks (n = 33); and 2) samples taken fresh from the host, measured, frozen (-20°C) for six weeks, defrosted, measured, stored in 96% ethanol and measured again after two weeks (n = 29; for descriptions of morphological measurements see the ‘Morphological scoring and identification’ section above).
Statistical analysis
All statistical analyses were performed using the statistical software package R [35]. Homogeneity and normality assumptions were checked by using boxplots, histograms and qqplots [36].
Effects of origin
In order to rule out any potential biases of the origin of the parasite on body measurements, we used linear models to investigate the relationships between parasite body size and 1) host species (only for M. orientalis which infects both host species), 2) host size (for each of three parasite-host combinations) and 3) region (for each of the two host species). Sex of the parasite was used as an additional factor in all models. Parasite intensity was included as covariate to the host size models. In the region models, we corrected parasite body size for host size by using the residuals of a linear regression of host size and parasite size as response variable, and the molecular identity of the parasite species (only for the mussel model) and region as explanatory variables. We used parasite body length for these investigations, because this variable was correlated with other size variables (cephalosome length, body width and length of the genital double-somite, the latter only for females; see S1 Fig).
Multivariate morphometric analyses
As both parasite species only co-occur in blue mussels in the investigated region, only parasites originating from this shared host were used in the multivariate analyses. The morphology of each individual parasite was characterized as described above via seven morphometric parameters: body length, cephalosome length, body width, angle between caudal rami and anteroposterior axis, number of body segments and direction of dorsolateral thoracic protuberances (see ‘Morphological scoring and identification’ section above), in which the latter was a dummy-coded nominal variable. The variable ‘genital double-somite’ is a feature only of females, resulting in a total of seven different morphological variables for females and six for males. For this reason and the fact that sex was only assessed morphologically, the multivariate morphometric analyses were executed for each sex separately.
These analyses were performed in two steps. First, a principal component analysis (PCA) was conducted to illustrate the relative contribution of each of the morphological variables for each parasite species. Prior to this analysis, all the morphometric variables were checked for outliers, which were removed when the value was larger than the mean plus three times the standard deviation, and subsequently all morphometric variables were log-transformed. Second, a discriminant function analysis (DFA) was performed to investigate the accuracy of the morphological species identifications. With this analysis, the hypothesis was tested that the a priori defined groups of parasites (molecular species identity) would differ significantly in their combination of morphometric variables. In the DFA, untransformed variables (excluding outliers) were standardized by using the decostand function in the vegan package in R [37]. The DFA was executed by the lda function of the MASS package [38], in which the Wilks lambda statistic was used to test for an overall group effect. To determine the most important variables in the discrimination between groups, a stepwise discriminant function analysis was employed. Based on the total sum of Mahalanobis distances, this backward selection procedure removed variables to produce a discriminant function with only important variables. In addition to the analysis of the full morphological data set, we did several straightforward calculations on the proportions of correct identifications when using only the two morphological features that are the easiest to assess (angle between caudal rami and anteroposterior axis; and direction of the dorsolateral thoracic protuberances).
Effects of preservation and storage method
Differences between body size measurements within group 1 (fresh → ethanol samples) were tested with (paired) Welch’s t-tests when variables were normally distributed and with non-parametric Wilcoxon signed-rank tests when this assumption was violated. Differences between measurements within group 2 (fresh → frozen → ethanol samples) were tested with a repeated-measures ANOVA. To test for differences between groups for both fresh and ethanol treatments, Welch’s t-tests or non-parametric Wilcoxon signed-rank tests were used.
Results
Molecular results
Of the 307 morphologically identified Mytilicola specimens (Table 1), most individuals displayed one of the two expected banding patterns in the PCR product and could accordingly be assigned to one of the two Mytilicola species (for examples of banding patterns see S2 Fig). Nevertheless, in 15 cases, banding patterns deviated from expectations in three different ways. The PCR fragment of one individual remained uncut after the restriction reaction, eight individuals displayed an ambiguous pattern consisting of bands from both species (e.g., lane 15 in S2 Fig) and six individuals showed incompletely digested patterns (e.g., lanes 1–8 and lane 16 in S2 Fig). All these individuals were omitted from subsequent analyses. This resulted in a pool of 292 molecularly identified individual copepods of both sexes that originated from three different regions and two different host species, as follows: M. intestinalis female (n = 40), M. intestinalis male (n = 47), M. orientalis female (n = 109) and M. orientalis male (n = 96). The TaqMan assay was performed on a subset of 75 individuals all originating from Texel. In all 68 cases for which both the TaqMan assay and the COI assay successfully amplified, the species identification was identical, and no hybrids were detected.
Table 1. Sample sizes of introduced Mytilicola species in different sub-categories.
| Host species | Parasite species | Sex | Dutch Delta | Wadden Sea | Total |
|---|---|---|---|---|---|
| Magallana gigas | M. orientalis | Males | 22 | 31 | 53 |
| Females | 24 | 32 | 56 | ||
| Mytilus edulis | M. orientalis | Males | 20 | 27 | 47 |
| Females | 21 | 35 | 56 | ||
| M. intestinalis | Males | 20 | 30 | 50 | |
| Females | 19 | 26 | 45 | ||
| Total | 126 | 181 | 307 |
Effects of origin
Host species and size
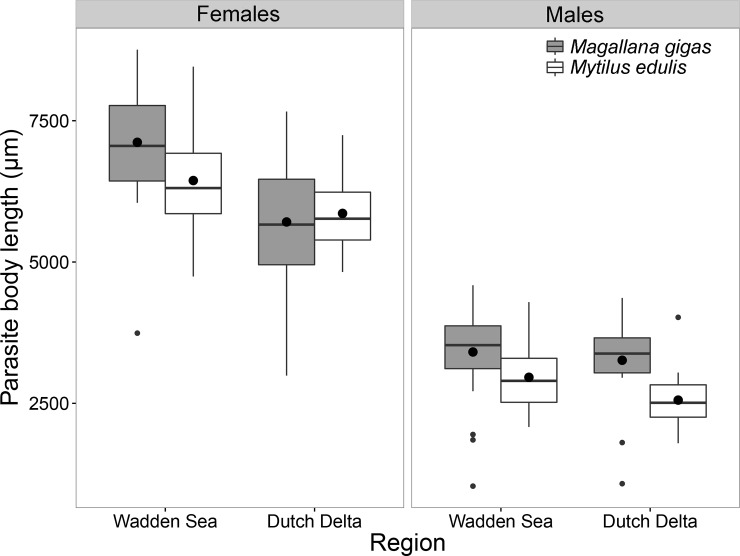
For Mytilicola orientalis, which uses both blue mussels (Mytilus edulis) and Pacific oysters (Magallana gigas) as hosts, host species identity was an important determinant for parasite body length as the copepods were significantly larger in oysters (linear model; F1,193 = 6.721, p < 0.05; Fig 3). Additionally, in both host species, females of M. orientalis larger than males (F1,193 = 599.470, p < 0.001).
Fig 3. Boxplots of Mytilicola orientalis body length (μm) in both host species.
Female (left) and male (right) copepods originating from oysters (Magallana gigas) in grey and from mussel (Mytilus edulis) hosts in white, from the Dutch Delta and Wadden Sea. The boxes represent the interquartile range, the whiskers denote the lowest and highest values within the 1.5 interquartile range, the black line in each box denotes the median, the large black dots represent the mean and the smaller dots outside the boxes are outliers.
Parasite body length was positively related to host size in mussels (linear regression; M. orientalis R2 = 0.87, p < 0.001; M. intestinalis R2 = 0.85, p < 0.01), but not in oysters (p = 0.149; Fig 4). In addition, parasite intensity was positively related to body length of M. intestinalis in mussels (linear regression, F1,86 = 59.754, p < 0.01), but not for M. orientalis in either mussels (linear regression, p = 0.846) or oysters (p = 0.181). For all combinations of parasite species and hosts in this model, there was a significant difference between the parasite sexes regarding the relationship between host size and parasite body length (M. orientalis in mussels F1,91 = 598.653, p < 0.001; M. intestinalis in mussels F1,86 = 453.474, p < 0.001, M. orientalis in oysters F1,96 = 201.696, p < 0.001). No significant interaction between sex and host size was found for any of the parasite-host combinations (M. orientalis in mussels p = 0.366; M. intestinalis in mussels p = 0.787; M. orientalis in oysters p = 0.376).
Fig 4. Relationship between host shell length (mm) and parasite body length (μm) per sex for each Mytilicola-host species combination.
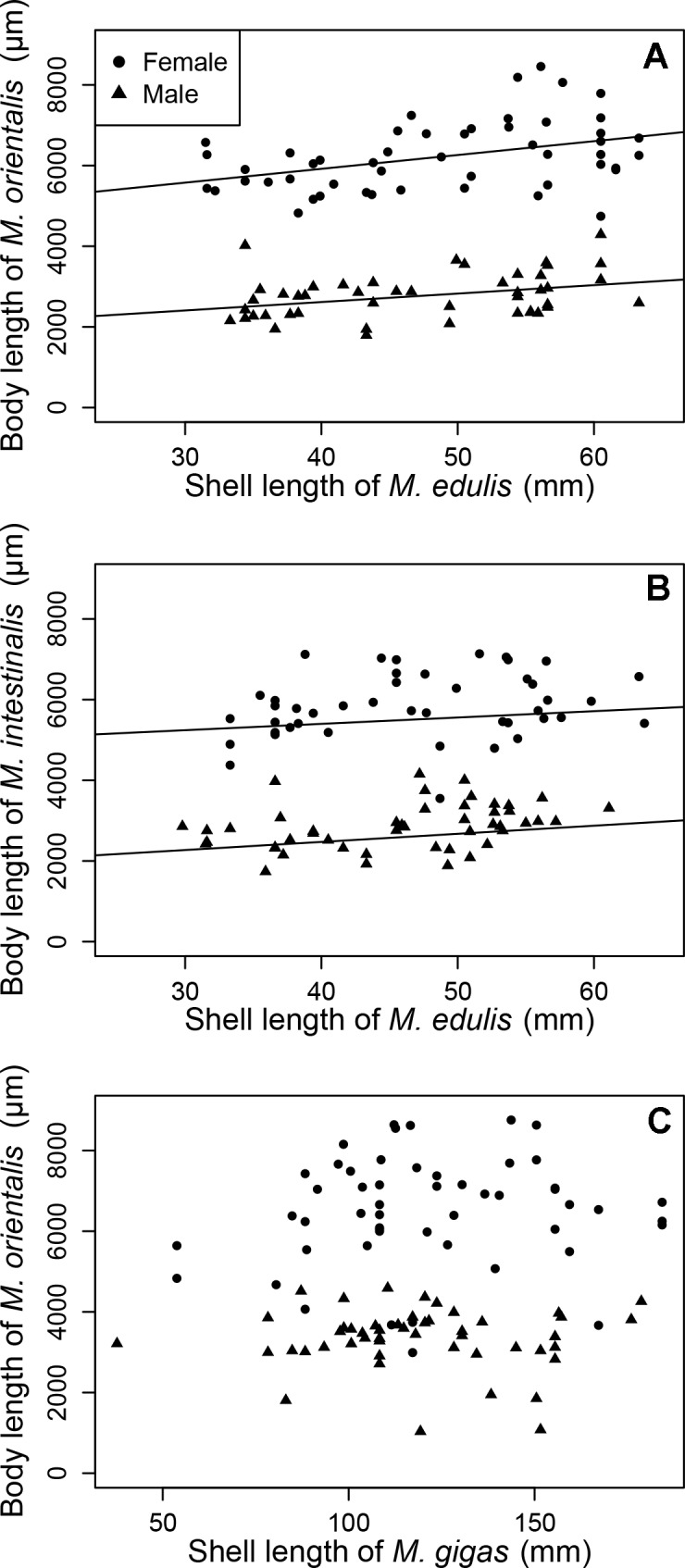
(A) M. orientalis in blue mussels (Mytilus edulis). (B) M. intestinalis in blue mussels. (C) M. orientalis in Pacific oysters (Magallana gigas). Fitted lines are significant regressions.
Geographical region
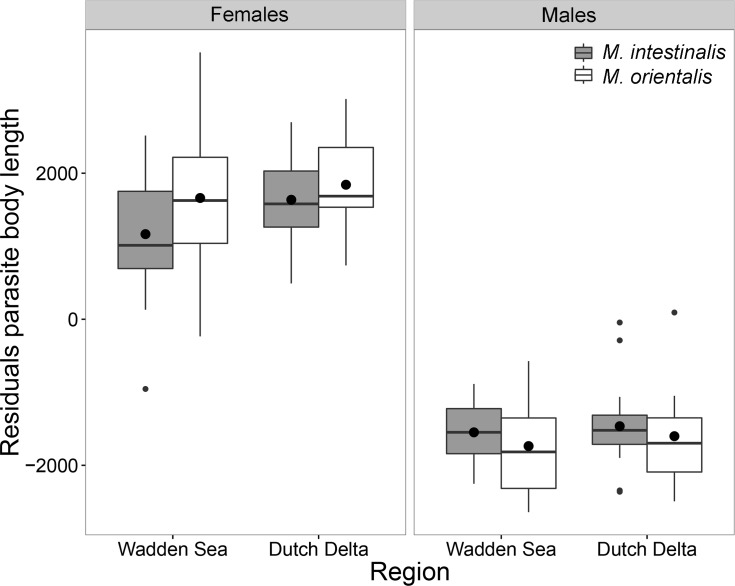
Body length of both parasite species (corrected for host size) in blue mussels did not differ between regions (p = 0.350; Fig 5). Furthermore, within mussel hosts, M. orientalis was overall larger than M. intestinalis (F1,179 = 15.568, p < 0.001), but a significant interaction term with sex showed that males of M. orientalis males were smaller than those of M. intestinalis males (F1,179 = 7.110, p < 0.01). Furthermore, for both parasite species, females were larger than males (F1,179 = 1048.029, p < 0.001).
Fig 5. Parasite body length (corrected for host size using linear regression) for both introduced Mytilicola species.
Females (left) and males (right) of M. intestinalis (grey) and M. orientalis (white) in each surveyed region. The boxes represent the interquartile range, the whiskers denote the lowest and highest values within the 1.5 interquartile range, the black line in each box denotes the median, the large black dots represent the mean and the smaller dots outside the boxes are outliers.
In Pacific oysters, M. orientalis were larger in the Wadden Sea than in the Dutch Delta (F1,97 = 9.656, p < 0.01) and females were larger than males (F1,97 = 240.112, p < 0.001). In addition, a significant interaction term between region and sex (F1,189 = 9.297, p < 0.01), showed that males in the Dutch Delta are slightly larger than males in the Wadden Sea, while for females this is the opposite.
Reliability of morphological identification
Principal component analysis
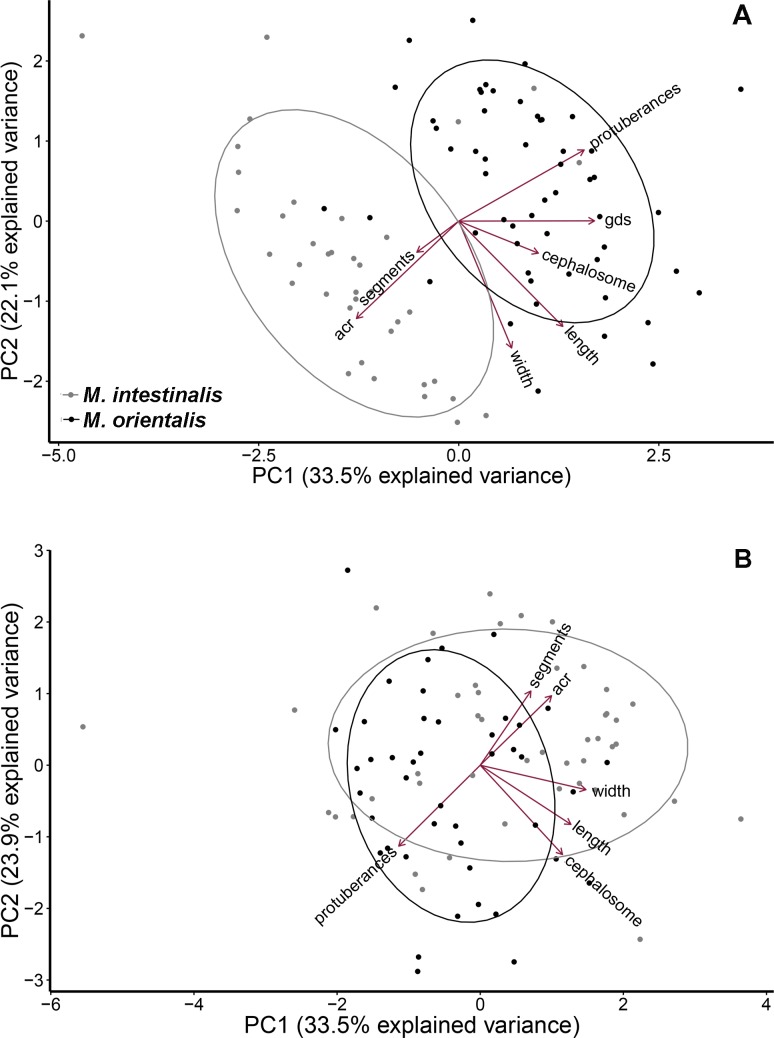
Morphological differences between parasite species, as visualized by Principal Component Analysis (PCA), showed limited overlap between Mytilicola species for females (n = 92, Fig 6A), but considerable overlap in males, as many individuals presented intermediate morphologies (n = 90, Fig 6B). This sex-related difference in species partitioning had consequences for the assessment of the reliability of morphological species identification for both sexes using Discriminant Function Analysis (DFA).
Fig 6. Principal component analysis of Mytilicola for each sex separately.
(A) Females (n = 92; seven morphological variables). (B) Males (n = 90, six variables). In grey, individual parasites that were molecularly identified as M. intestinalis; in black, similarly identified M. orientalis (gds = length of the genital double-somite; acr = angle between caudal rami and anteroposterior axis).
Discriminant function analysis for females
In total, 92 females of Mytilicola were entered into the DFA with the following prior probabilities per group: M. intestinalis 0.41 (n = 38) and M. orientalis 0.59 (n = 54). One discriminant function was extracted, and this was highly significant (Wilks’ λ = 0.227, F1,141 = 40.925, p = < 0.001), indicating a clear discrimination between groups. The first discriminant function with standardized discrimination coefficients for females is given by (gds = length of the genital double-somite; acr = angle between caudal rami and anteroposterior axis):
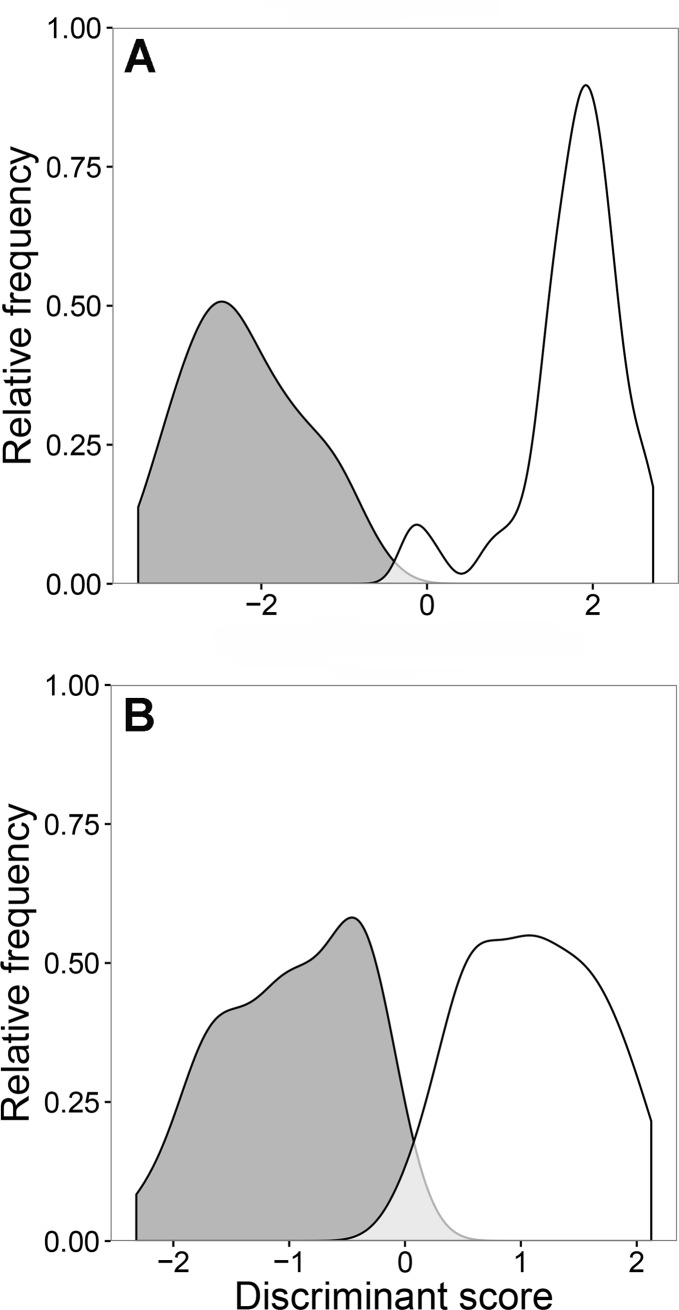
The discriminant distributions showed minimal overlap between the two groups, an indication that the discrimination was relatively accurate (Fig 7A). The classification of the groups was highly successful for females; 95% of molecularly identified M. intestinalis females and 91% of M. orientalis females were correctly assigned to the respective Mytilicola groups.
Fig 7. Discriminant distributions of Mytilicola for each sex separately.
(A) Females (n = 92) and (B) males (n = 90) of the parasitic copepods M. intestinalis (grey) and M. orientalis (white). The x-axis is the discriminant score of the first discriminant function and the y-axis is the relative frequency of the observations. The light grey area indicates “mistaken” assignments in comparison with molecular identifications.
The morphological features used for identification strongly differ in their importance for a correct assignment. One variable, the direction of the dorsolateral thoracic protuberances, had by far the highest coefficient in the discriminant function. Similarly, the backward selection procedure showed that the direction of the dorsolateral thoracic protuberances, the length of the genital double-somite and the angle between caudal rami and anteroposterior axis were the last variables to be dropped, indicating their importance in discriminating between the two parasite species.
When using only the two morphological features that were the easiest to assess (the direction of the dorsolateral thoracic protuberances and the angle between caudal rami and anteroposterior axis), a large proportion of individuals could still be correctly assigned to the genetically identified species. By using the former character alone, 97% of the M. intestinalis females and 87% of the M. orientalis females were correctly identified whereas use of the latter character alone resulted in correct identification of 81% of the M. orientalis females and 84% of the M. intestinalis females. An angle of 20° between the caudal rami and the anteroposterior axis was judged to be the cut-off point based on the group averages, < 20° being a “narrow” angle (M. intestinalis) and ≥ 20° being a “wide” angle (M. orientalis). From among all the genetically identified females, 94% of M. orientalis and 97% of M. intestinalis females were correctly identified by either using the direction of the dorsolateral thoracic protuberances or the angle between caudal rami and the anteroposterior axis.
Discriminant function analysis for males
In total, 90 males were entered into the DFA with the following prior probabilities per group: M. intestinalis 0.51 (n = 46) and M. orientalis 0.49 (n = 44). For males, one discriminant function was extracted and was highly significant (Wilks’ λ = 0.692, F1,88 = 6.152, p < 0.001). The first discriminant function with standardized discrimination coefficients for males is given by (acr = angle between caudal rami and anteroposterior axis):
For males, the discriminant distributions showed more overlap between the two groups than for females (Fig 7B). This also showed up in the procedure’s classification of the males, which was less reliable than that of the females described above; only 78% of genetically identified M. intestinalis males and 73% of M. orientalis males were correctly assigned to one of the two Mytilicola groups by the discriminant analysis.
As in females, one variable, the direction of the dorsolateral thoracic protuberances, had the highest coefficient in the discriminant function. Again, the backward selection procedure showed that the direction of the dorsolateral thoracic protuberances was the last variable to be dropped, indicating its importance in the discriminant function. While relying solely on this feature’s direction as morphological identification marker, some males could still be correctly assigned to the genetically identified species, but the success rate was not as high as that for females: 70% of the males of M. orientalis and 76% of those of M. intestinalis were correctly identified. The angle between the caudal rami and the anteroposterior axis of the body was less important in discriminating between groups for males than females, but if this feature alone was used as an identification criterion, 64% of the genetically identified M. orientalis and 63% of the M. intestinalis males were correctly identified. Among all the genetically identified males, 82% of those of M. orientalis and 78% of those of M. intestinalis males were correctly identified by either the direction of the dorsolateral thoracic protuberances or the angle between the caudal rami and anteroposterior axis.
Effects of preservation and storage method on morphology
We compared two groups of Mytilicola spp. that differed in preservation and storage methodology, using fresh specimens of similar size that were either preserved directly in ethanol or frozen prior to ethanol storage. Specimens became significantly smaller after being stored directly in ethanol (all p < 0.05), but the direction of the dorsolateral thoracic protuberances and the number of body segments carrying these protuberances remained the same (Table 2). Length measurements (body length, cephalosome length, body width, genital double-somite length) were, on average, 17.4% shorter after preservation in pure ethanol. In contrast, there was no significant difference in body length between fresh and frozen samples (all p > 0.05), but the latter shrunk significantly more after transfer to ethanol (p < 0.05 for each measurement, except p = 0.106 for genital double-somite length); however, the degree of shrinkage (9.0%) was less than in specimens directly preserved in ethanol (Table 2).
Table 2. Effects of preservation and storage methods on morphology of Mytilicola spp.
| Fresh → Ethanol | Frozen → Ethanol | |||||||
|---|---|---|---|---|---|---|---|---|
| % shrinkage | t | df | p | % shrinkage | t | df | p | |
| Body length | 17.2 | 2.348 | 31 | < 0.05 | 7.4 | 3.059 | 28 | < 0.05 |
| Cephalosome length | 22.2 | 4.112 | 32 | < 0.05 | 8.3 | 2.739 | 28 | < 0.05 |
| Body width | 14.1 | 2.624 | 31 | < 0.05 | 10.9 | 3.101 | 28 | < 0.05 |
| Genital double-somite length | 16.0 | 2.437 | 14 | < 0.05 | 9.5 | 1.709 | 17 | 0.106 |
| Mean | 17.4 | 9.0 | ||||||
Percentage of shrinking of various morphological measurements of Mytilicola spp. after storing fresh or frozen samples in ethanol, with significance assessed by Welch’s paired t-tests given in the adjacent columns.
Discussion
Our analyses show that in our study area, where both invasive species of parasitic copepods now co-occur, M. orientalis and M. intestinalis are morphologically differentiated to a significant degree, but with considerable overlap. No macroscopic diagnostic character, or combination of two or three characters, serves to distinguish them with full accuracy. The error rate of assignment to molecular species was more than three times higher in males (25%) than in females (7%). These error rates were obtained by a single observer (MAG) and may therefore represent a best-case scenario.
The present two species of Mytilicola were originally described from different parts of the globe (the Mediterranean Sea and Japan) and from different hosts (blue mussels and Pacific oysters). Now that the species' ranges and hosts have come to overlap in northwestern Europe, a sharp distinction between their morphologies is not seen. Whether the native populations of M. orientalis and M. intestinalis are more clearly differentiated morphologically would be interesting to find out but is unknown at present. A possible explanation for overlapping morphologies between species in sympatry is that they hybridise (co-occurrence within a host has been observed by Goedknegt et al. [26]); however, our nuclear DNA TaqMan assay detected no hybrids among 75 individuals from the present study (consisting of 35 individuals morphologically identified as M. intestinalis, and 40 as M. orientalis). Hybridisation thus appears not to be the cause for overlapping morphologies in the present instance.
For both males and females, the direction of the dorsolateral thoracic protuberances proved to be the most important discriminant factor in the multivariate analyses. Using only the direction of the dorsolateral thoracic protuberances for identification, the two species can still be correctly distinguished in many cases (females, 87–97%; males, 70–76%). The direction of the dorsolateral thoracic protuberances (folded inwards or extended outwards) is generally easier to observe in females as females of M. orientalis carry extended dorsolateral thoracic protuberances on almost every segment of the body whereas in males only the third and fourth body segments are extended [24] (unpublished observations by MAG). While this is a qualitative feature that is subject to personal observation bias, it has nonetheless been used by several authors up to now [15, 18, 19].
Another straightforward morphological feature, the angle between the caudal rami and the anteroposterior axis, has been mentioned previously as a useful distinguishing feature [18, 19], but in the present study this variable proved useful only for discriminating between females of the two species. Other morphological measurements such as body length and width and the lengths of the cephalosome and (females only) genital double-somite did not prove useful in discriminating between species in the discriminant analysis. Interestingly though, some of these features appeared to be related to the place of origin or the host of the parasites. Copepod body length was significantly related to location (but only for parasites originating from oysters), host size (only for parasites originating from mussels, although size is less easily measured in the irregularly shaped oysters, thus possibly obscuring any parasite size–host size relationship) and host species (only for M. orientalis). Because of collinearity, other body size measurements are likely related in the same way. If so, a series of more comprehensive morphological measurements will not increase the reliability of morphological identification. Besides measurements per se, the number of body segments carrying dorsolateral thoracic protuberances is not an important identification feature as it only discriminates between sexes (and life stages) [39]. In sum, among all the examined macroscopic morphological variables, the direction of the dorsolateral thoracic protuberances and, to a lesser extent, the angle between the caudal rami and the anteroposterior axis can be considered the most reliable and feasible identification features.
The fact that both parasite species were somewhat differentiated morphologically between locations and hosts indicates that at local scale genetic drift can lead to subtle difference in morphology even while stabilizing selection over larger time sales maintains the overall morphological similarity between species.
For practical applications, our results suggest that initial preservation and storage of Mytilicola specimens either in ethanol or by freezing can be recommended if species identification in this genus is to be based on the direction of the dorsolateral thoracic protuberances, because this feature is not affected by the mode of preservation or manner of storage. However, preservation and storage of fresh samples in ethanol is advisable for molecular identification. If morphological measurements are intended, shrinkage in body size (particularly with respect to the length and width of the body and the lengths of the cephalosome and genital double-somite) must be taken into account compared to fresh samples. Our study indicates that shrinkage can be reduced if samples are first frozen and then transferred to ethanol. Regarding species identification, the method of choice will depend on the level of accuracy required for the research question at hand. If 100% accuracy is needed, then identification with molecular methods will be required for both sexes. However, if lower accuracy is acceptable, identification can be based on the direction of the dorsolateral thoracic protuberances leading to 87–97% and 70–76% accuracy in females and males, respectively. Alternatively, as the error rate of especially females is quite low and individuals of both sexes co-occur simultaneously in one host [26], future monitoring surveys may also be based on the morphological examination of females alone. Nevertheless, as these data were collected by one experienced observer, the reliability of morphological identification is likely to vary among multiple and/or unexperienced observers.
Conclusions
No macroscopic diagnostic character that can be checked by relatively fast and simple morphological observations can be used to reliably discriminate between the two co-occurring invasive parasite species Mytilicola orientalis and M. intestinalis in their invaded range in northwest Europe. This is a good example of how the identification of morphologically similar invaders can be compromised. Even when one invader is known to occur in an ecosystem, the presence of morphologically similar relatives can be missed, making the latter “cryptic invaders”. More attention should be paid to the potential existence of cryptic invasions of parasites. When site of collection and/or host species is no longer operationally discriminative between similar species, a closer examination of potential morphological and genetic means of differentiating them may become necessary. In the present case, molecular identification proved more reliable than morphological species determination, but this need not always to be true.
Supporting information
The lower diagonal elements contain the (absolute) correlations. Collinearity was especially shown for body length and other morphological measurements.
(DOCX)
Lanes 1–8: M. intestinalis (diagnostic bands 366 and 217 bp, indicated by black arrows in lane 1); lanes 9–14, 16 and 17: M. orientalis (diagnostic bands 286, 222 and 75 bp; indicated by black arrows in lane 10; the 75 bp band is usually faint); lane 15: ambiguous pattern showing bands from both species; traces of incompletely digested DNA are often present; unlabelled lanes are intact PCR products before restriction; sizing ladders consist of bands from 100 to 1000 bp spaced by 100 bp intervals.
(DOCX)
(DOCX)
(TXT)
(TXT)
Acknowledgments
The authors wish to thank everyone who provided samples, including Tabea Stier, Anne-Karin Schuster and Carolin Wendling. We are grateful to Anneke Bol and Eike Petersen for help with the molecular analysis and to Felipe Ribas for the schematic representations of the parasites. Project partners Dr. Kees (C.J.) Camphuysen and Dr. Christian Buschbaum are thanked for their general support. Stichting Natuurmonumenten is kindly acknowledged for allowing us to sample in their natural reserves. We thank the Netherlands Organization for Scientific Research (NWO) and the German Bundesministerium für Bildung und Forschung (BMBF) for funding (NWO-ZKO project 839.11.002).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The field sampling and molecular analysis were financed by the Netherlands Organization for Scientific Research (NWO) and the German Bundesministerium für Bildung und Forschung (BMBF; NWO-ZKO project 839.11.002). Funding was awarded to project leader David W Thieltges. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Elton CS. The ecology of invasions. London: Methuen; 1958. [Google Scholar]
- 2.Vitousek P, D’Antonio C, Loope LL, Westbrooks R. Biological invasions as global environmental change. Am Sci. 1996;84:468–478. [Google Scholar]
- 3.Mack RN, Simberloff D, Lonsdale WM, Evans H, Clout M, Bazzaz FA. Biotic invasions: causes, epidemiology, global consequences, and control. Ecol Appl. 2000;10:689–710. [Google Scholar]
- 4.Grosholz E. Ecological and evolutionary consequences of coastal invasions. Trends Ecol Evol. 2002;17:22–27. [Google Scholar]
- 5.Carlton JT. Deep invasion ecology and the assembly of communities in historical time In: Rilov G, Crooks JA, editors. Biological invasions in marine ecosystems: Ecological, management, and geographic perspectives. Berlin: Springer-Verlag; 2009. p 13–56. [Google Scholar]
- 6.Geller JB, Walton ED, Grosholz ED, Ruiz GM. Cryptic invasions of the crab Carcinus detected by molecular phylogeography. Mol Ecol 1997;6:901–906. [DOI] [PubMed] [Google Scholar]
- 7.Geller JB. Decline of a native mussel masked by sibling species invasion. Conserv Biol 1999;13:661–664. [Google Scholar]
- 8.Saltonstall K. Cryptic invasion by a non-native genotype of the common reed, Phragmites australis, into North America. PNAS. 2002;19:2445–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zabin CJ, Zardus J, Pitombo FB, Fread V, Hadfield MG. A tale of three seas: consistency of natural history traits in a Caribbean-Atlantic barnacle introduced to Hawaii. Biol Invas. 2007;9:523–544. [Google Scholar]
- 10.Steuer A. Mytilicola intestinalis n.gen. n.sp. aus dem Darme von Mytilus galloprovincialis Lam. (Vorläufige Mitteillung). Arb Zool Inst Wien. 1902;15:1–46. [Google Scholar]
- 11.Caspers H. Über vorkommen und Metamorphose von Mytilicola intestinalis Steuer (Copepoda paras.) in der südlichen Nordsee. Zool Anz. 1939;126:161–171. [Google Scholar]
- 12.Hockley AR. On the biology of Mytilicola intestinalis (Steuer). J Mar Biol Assoc UK. 1951;30:223–232. [Google Scholar]
- 13.Theisen BF. Mytilicola intestinalis Steuer in Danish Waters 1964–65. Medd Dan Fisk Havunders NS. 1966;4:327–337. [Google Scholar]
- 14.Korringa P. On the ecology and the distribution of the parasitic copepod Mytilicola intestinalis Steuer. Bijdr Dierk. 1968;38:47–57. [Google Scholar]
- 15.Mori T. Mytilicola orientalis, a new species of parasitic Copepoda. Zool Soc Japan. 1935;47:687–693. [Google Scholar]
- 16.Carlton JT. Biogeographical ecology of the introduced marine and estuarine invertebrates of the Pacific coast of North America [dissertation]. Davis: University of California; 2002.
- 17.His E. Observations préliminaires sur la présence de Mytilicola orientalis Mori (1935) chez Crassostrea gigas Thunberg dans le basin d’Arcachon. Bull. Soc. Géol. et Amis du Museum du Havre 1979:2;7–8. [Google Scholar]
- 18.Stock JH. Copepoda (Crustacea) associated with commercial and non-commercial Bivalvia in the Eastern-Scheldt, The Netherlands. Bijdr Dierk. 1993;63:61–64. [Google Scholar]
- 19.Elsner NO, Jacobsen S, Thieltges DW, Reise K. Alien parasitic copepods in mussels and oysters in the Wadden Sea. Helgol Mar Res. 2011;65:299–307. [Google Scholar]
- 20.Pogoda B, Jungblut S, Buck BH, Hagen W. Infestation of oysters and mussels by mytilicolid copepods: differences between natural coastal habitats and two offshore cultivation sites in the German Bight. J Appl Ichtyol. 2012;28:756–765. [Google Scholar]
- 21.Knowlton N, Weigt LA. New dates and new rates for divergence across the Isthmus of Panama. Proc R Soc Lond B. 1998;265:2257–2263. [Google Scholar]
- 22.Luttikhuizen PC, Campos J, van Bleijswijk J, Peijnenburg KTCA, van der Veer H. Phylogeography of the common shrimp, Crangon crangon (L.) across its distribution range. Mol Phyl Evol. 2008;46:1015–1030. [DOI] [PubMed] [Google Scholar]
- 23.Steuer A. Mytilicola intestinalis n. gen. n. sp. Zool Inst Univ Wien 1905;15:1–46. [Google Scholar]
- 24.Ho J-S, Kim I-H. Copepod parasites of Gastropoda from Korea. Kor J Zool. 1992;35: 240–255. [Google Scholar]
- 25.Aguirre-Macedo ML, Kennedy CR. Diversity of metazoan parasites of the introduced oyster species Crassostrea gigas in the Exe estuary. J Mar Biol Assoc UK. 1999;79:57–63. [Google Scholar]
- 26.Goedknegt MA, Schuster A-K, Buschbaum C, Gergs R, Jung AS, Luttikhuizen PC, van der Meer J, Troost K, Wegner KM, Thieltges DW. Spillover of the introduced parasitic copepod Mytilicola orientalis from the invasive Pacific oyster (Crassostrea gigas) to native hosts. Biol Invasions. 2016;19:365–379. [Google Scholar]
- 27.Lauckner G. Diseases of Mollusca: Bivalvia In: Kinne O, editor. Diseases of marine animals. Volume II: Introduction, Bivalvia to Scaphopoda. Hamburg: Biologische Anstalt Helgoland; 1983. p. 477–961. [Google Scholar]
- 28.Steele S, Mulcahy MF. Impact of the copepod Mytilicola orientalis on the Pacific oyster Crassostrea gigas in Ireland. Dis Aquat Org 2001;47:145–149. doi: 10.3354/dao047145 [DOI] [PubMed] [Google Scholar]
- 29.Korringa P. De aanval van de parasiet Mytilicola intestinalis op de Zeeuwse mosselcultuur. Visserij-Nieuws 1950;3 Suppl 1:S1–7. [Google Scholar]
- 30.Thieltges DW, Engelsma MY, Wendling CC, Wegner KM. Parasites in the Wadden Sea food web. J Sea Res 2012;82:122–133. [Google Scholar]
- 31.Dare PJ. The susceptibility of seed oysters of Ostrea edulis L. and Crassostrea gigas Thunberg to natural infestation by the copepod Mytilicola intestinalis Steuer. Aquaculture 1982;26:201–211. [Google Scholar]
- 32.Grizel H. Mytilicola orientalis MORI, parasitism In: Sindermann DJ, editor. Fiches d’identification des maladies et parasites des poissons, crustaces et mollusques. Copenhagen: Conseil International pour l’Exploration de la Mer; 1985. p 1–4. [Google Scholar]
- 33.Knowlton N. Sibling species in the sea. Annu Rev Ecol Syst. 1993;24:189–216. [Google Scholar]
- 34.Nygren A. Cryptic polychaete diversity: a review. Zool Scripta. 2014;43:172–183. [Google Scholar]
- 35.R Development Core Team. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2016. [Google Scholar]
- 36.Zuur AF, Ieno EN, Elphick CS. A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol. 2010;1:3–14. [Google Scholar]
- 37.Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Wagner HH. vegan: Community ecology package. R package version 2.0–9. 2016. [Google Scholar]
- 38.Venables WN, Ripley BD. Modern applied statistics with S. 4rd edn. New York: Springer, 2002. [Google Scholar]
- 39.Gee JM, Davey JT. Stages in the life history of Mytilicola intestinalis Steuer, a copepod parasite of Mytilus edulis (L.), and the effect of temperature on their rates of development. J Cons Int Explor Mer 1986;42:254–264. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The lower diagonal elements contain the (absolute) correlations. Collinearity was especially shown for body length and other morphological measurements.
(DOCX)
Lanes 1–8: M. intestinalis (diagnostic bands 366 and 217 bp, indicated by black arrows in lane 1); lanes 9–14, 16 and 17: M. orientalis (diagnostic bands 286, 222 and 75 bp; indicated by black arrows in lane 10; the 75 bp band is usually faint); lane 15: ambiguous pattern showing bands from both species; traces of incompletely digested DNA are often present; unlabelled lanes are intact PCR products before restriction; sizing ladders consist of bands from 100 to 1000 bp spaced by 100 bp intervals.
(DOCX)
(DOCX)
(TXT)
(TXT)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.