Abstract
Background
Tobacco dependence remains the leading preventable cause of death in the developed world. Smokers are disproportionately from lower socioeconomic groups, and may use the hospital emergency department (ED) as an important source of care. A recent clinical trial demonstrated the efficacy of a multicomponent intervention to help smokers quit, but the independent contributions of those components is unknown.
Methods
This is a full-factorial (16-arm) randomized trial in a busy hospital ED of 4 tobacco dependence interventions: brief motivational interviewing, nicotine replacement therapy, referral to a telephone quitline, and a texting program. The trial utilizes the Multiphase Optimization Strategy (MOST) and a novel mixed methods analytic design to assess clinical efficacy, cost effectiveness, and qualitative participant feedback. The primary endpoint is tobacco abstinence at 3 months, verified by participants’ exhaled carbon monoxide.
Results
Study enrollment began in February 2017. As of April 2017, 52 of 1056 planned participants (4.9%) were enrolled. Telephone-based semi-structured participant interviews and in-person biochemical verification of smoking abstinence are completed at the 3-month follow-up. Efficacy and cost effectiveness analyses will be conducted after follow-up is completed.
Discussion
The goal of this study is to identify a clinically efficacious, cost-effective intervention package for the initial treatment of tobacco dependence in ED patients. The efficacy of this combination can then be tested in a subsequent confirmatory trial. Our approach incorporates qualitative feedback from study participants in evaluating which intervention components will be tested in the future trial.
Keywords: Smoking cessation, Tobacco dependence treatment, Emergency department, Mixed methods
1. Introduction
Fifty years after the landmark 1964 Surgeon General’s report, smoking remains the leading cause of preventable death in the United States, with about 480,000 deaths per year [1]. In 2012, $289 billion in direct and indirect costs were associated with tobacco use. In 2015, 15.1% of Americans smoked [2], still far short of the Healthy People 2020 goal of 12% prevalence [3]. Certainly, significant progress has been made, with the prevalence of smoking among U.S. adults reduced from 42% in 1964. However, after half a century of research, regulation, policy advances, drug development, public service campaigns, and litigation, significant challenges in treating tobacco remain. Smoking is an addiction that has become increasingly disproportionate among the medically disadvantaged: those with low income or low education, the mentally ill, and individuals with other substance use disorders. These are among the groups identified in a 2006 NIH State-of-the-Science conference as those most in need of advances in treatment and treatment engagement [4].
These groups of smokers are commonly treated in hospital emergency departments (EDs). EDs are a frequent site of care for all Americans, with approximately 136 million visits across 4000 EDs in 2011 [5]. ED patients are disproportionately of low socioeconomic status, more likely to smoke compared with the general population, [6,7] and more likely to have limited or irregular access to primary care. ED smokers often present with illnesses caused or exacerbated by tobacco use or have injuries (e.g., lacerations, fractures) for which smoking impedes their healing [8–10]. Hence the ED visit represents an opportune time to discuss patients’ tobacco use, its relevance to their current visit, and to initiate tobacco treatment and aftercare [11]. This represents an evolving standard of treatment in the ED care of the patient who smokes [12].
Our group has recently demonstrated the efficacy of a multi-component intervention that includes behavioral and pharmacologic therapies in promoting tobacco abstinence among ED smokers [10]. In a randomized trial of 778 smokers, a combination of brief motivational interviewing, six weeks of nicotine patches and gum, referral to a smokers’ quitline, and a follow-up phone call resulted in a significant improvement in the three-month, biochemically confirmed abstinence rate: 12.2% vs. 4.9% [10]. Our model adapts the treatment paradigm known as Screening, Brief Intervention, and Referral to Treatment (SBIRT) [13]. The components of our intervention were: a brief adaptation of motivational interviewing (MI), called the Brief Negotiation Interview [14], initiation of nicotine replacement therapy (NRT) in the ED with provision of a 6-week supply of patches and gum, referral to the Connecticut State smokers’ telephone quitline (QL), provision of a smoking cessation brochure, and the provision of a booster phone call 3 days after enrollment [10]. Recent pilot studies conducted by our group showed the feasibility and potential efficacy of ED-initiated short-message-service (SMS) for tobacco dependence treatment [15,16].
One limitation of our work is that we cannot disentangle the contribution to abstinence of the individual components of the intervention. We assume that each is important, but we cannot model their contributions, or whether important interactions exist. It is therefore imperative to identify the most clinically effective and cost-effective components to create an efficacious intervention that can be delivered for the lowest cost possible in real-world ED settings.
1.1. Trial objectives
The goal of this study is to identify components of an ED-based intervention which are optimally effective for treating adult smokers.
2. Methods
2.1. Overview and study design
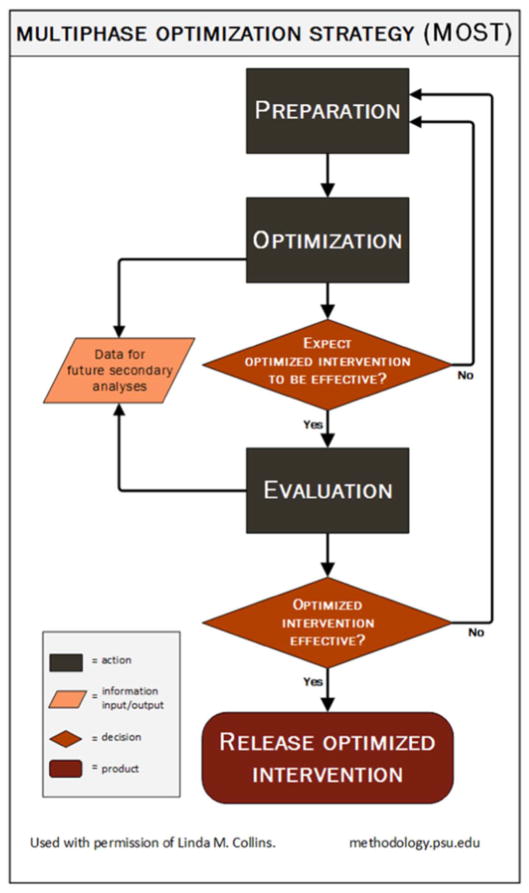
The MOST design, developed by Collins and outlined in Fig. 1, is an iterative process that often employs a factorial design (Optimization) followed by a traditional randomized clinical trial (Evaluation), that allows investigators to efficiently identify efficacious components of an intervention, subject to a cost constraint [17–21]. Intervention components may be studied in a full- or reduced-factorial design. MOST has been used to design interventions to treat tobacco dependence [21,22], but, to our knowledge, has not been previously used in the ED setting.
Fig. 1.
Multiphase Optimization Strategy (MOST).
Our group’s work in ED-initiated tobacco dependence treatment conducted to date would constitute the Preparation phase. MOST has been used in a number of smoking cessation trials [22–25], but not in the ED. MOST borrows 2 principles from engineering: (1) the resource management principle, which says that research resources should be managed strategically to maximize information gain in a timely fashion, and (2) continuous optimization, which says that a new cycle of research should begin soon after conclusion of the previous cycle, employing the information gained from that cycle [25].
Our study will use a full 2 [4] (i.e. 16-arm) factorial design to evaluate the effects of 4 intervention components at a fraction of the cost, using one-fourth of the participants it would take to conduct 4 individual experiments to evaluate each component separately. Table 1 outlines the 16 arms.
Table 1.
Arms of the trial.
| Arm | BNI | NRT | QL | Text |
|---|---|---|---|---|
| 1 | ||||
| 2 | ||||
| 3 | ||||
| 4 | ||||
| 5 | ||||
| 6 | ||||
| 7 | ||||
| 8 | ||||
| 9 | ||||
| 10 | ||||
| 11 | ||||
| 12 | ||||
| 13 | ||||
| 14 | ||||
| 15 | ||||
| 16 |
Green =component is offered.
Red = component is not.
BNI =Brief negotiated interview.
NRT = Nicotine replacement therapy.
QL = Quitline referral.
Text =Smoke-free text.
In addition, qualitative participant interviews will provide a nuanced understanding of how patients’ perceptions about using the various components and contextual factors may influence the feasibility of implementation. These perceptions and contextual factors may not otherwise be captured by traditional survey methods, and are therefore included in our mixed methods approach to improve the readiness of the final treatment package for real-world implementation. A subsequent traditional randomized trial is planned as a future study, which will require additional external support.
Our multicomponent intervention consists of the following: (1) a Brief Negotiation Interview (our brief adaptation of motivational interviewing [26]), delivered by a trained staff member; (2) provision of 6 weeks of nicotine patches and gum to the research participant, with application of the first patch in the ED; (3) active referral to the Connecticut Smokers’ Quitline; and (4) enrollment in the SmokefreeTXT short-messaging service (SMS) texting program for mobile phones. All patients will receive the smoking cessation brochure. Using MOST principles, the screening phase will use a 2× 2× 2× 2 full-factorial design to identify the components most likely to be efficacious in combination. Although the factorial design requires the allocation of participants to 16 different combinations of the 4 components (Table 1), evaluation of each individual component is performed comparing all of those receiving a component to all of those not receiving a component, making this an efficient design. For instance, evaluation of the BNI component will compare those randomized to conditions 1 through 8 to those in conditions 9 to 16. Based on the results of the screening phase in conjunction with findings from the qualitative analysis, we will design and propose a 2-arm randomized clinical trial comparing the efficacy of the multicomponent intervention package to usual care for the confirmatory phase.
2.2. Participants
2.2.1. Inclusion criteria
Patients who present to the adult ED at Yale-New Haven Hospital (YNHH) are eligible for the study if they are: (1) 18 years or older (2) have smoked at least 100 cigarettes in their lifetime (3) describe themselves as every-day or some-day smokers (4) smoke at least 5 cigarettes/day, on average (5) own a cellphone with texting capability, and (6) are able to give written informed consent.
2.2.2. Exclusion criteria
Patients are excluded for: (1) not being able to read or understand English; (2) currently receiving formal tobacco dependence treatment; (3) having a life-threatening or unstable medical, surgical, or psychiatric condition; (4) being unable to provide at least one collateral contact; (5) living out-of-state; (6) planning to leave the ED against medical advice (7) being pregnant (self-report or urine testing) or currently nursing or trying to conceive.
2.3. Setting
YNNH is part of an academic medical center that serves a moderately poor city in the northeastern United States; in 2015, 26.6% of New Haven’s 130,000 residents lived in poverty [27]. In 2013, women represented approximately 55% of the ED population; the mean age of ED adults was 41 years. The racial mix of our patients reflects that of New Haven: 65% White, not Hispanic; 23% African-American, not Hispanic, 10% Hispanic; 2% other. Payor status for ED smokers is approximately 55% Medicaid, 5% Medicare, 30% private insurance, and 10% self-pay. The Adult ED is a level one trauma center that treats 92,000 adult visits per year. The prevalence of smoking in the city is 18%, slightly above the national average [28].
2.4. Screening procedures and recruitment
Participants are being recruited during all days of the week from 8 am-10 pm. Potential participants will meet with a research assistant to be evaluated for eligibility. Patients are asked for verbal consent to complete the 2-item tobacco screener used by the Behavioral Risk Factor Surveillance System (Do you smoke cigarettes every day, some days, or not at all? Have you smoked at least 100 cigarettes in your entire life?). Patients who report smoking but are not eligible are given a handout recommending that they abstain from smoking, contact their primary care provider, and consider calling the quitline. Individuals who meet inclusion and exclusion criteria and consent to participate have baseline assessments performed and are then randomized to one of 16 combinations of components (Table 1).
2.5. Randomization
To assure equal intervention allocation and concealment of intervention allocation a random permuted block sequence was generated (via www.randomization.com) and intervention assignments distributed through the clinical trial data management system.
2.6. Interventions
Our previous work employed a multicomponent approach to ED-initiated tobacco treatment, which used both pharmacologic and behavioral approaches. All components are evidence-based; most are cited in the 2008 Public Health Service clinical practice guideline [29]. The newest approach, text messaging on mobile phones, is supported by a Cochrane review [30]. Below we discuss these components, the rationale for each, and supportive evidence. Our intent is to use the MOST approach to identify the individual contributions of these components and to assemble them into an intervention that is effective, efficient, and scalable.
2.6.1. Brief Negotiation Interview (BNI)
Brief Negotiation Intervention (BNI) is a manual-guided brief intervention that is an adaptation of motivational interviewing and has been shown to be feasible and efficacious in the ED setting. The BNI manual for this study is based on one that we used in our previous trial. The purpose of the BNI is to assist patients in recognizing the problematic nature of and changing their tobacco use. The main goals of the interview are to elicit disincentives for smoking and compelling personal reasons/motives for quitting from the patient, thereby motivating them to change their smoking behavior by reducing the number of cigarettes smoked daily or abstaining completely. The 4 steps of the BNI are: 1) Raising the subject, 2) Providing feedback, 3) Enhancing motivation and 4) Negotiating a plan.
The BNI is delivered and audiotaped by trained RAs who have a bachelor’s-level education. Tapes are reviewed biweekly by the RAs and a clinical psychologist to assess fidelity to the scripted BNI protocol. In our prior work, the BNIs average 10–15 min in length. RAs received 10 h of training in our manualized BNI. The training included role-play of patient scenarios, followed by 1 week of shadowing an experienced RA performing BNIs on ED patients. These procedures were used successfully in our prior study [10].
If, during the BNI or other interactions, the participant discloses to the RA risky behaviors such as suicidality, intimate partner violence, or use of other substances, the RA notifies the treating ED physician.
2.6.2. Nicotine gum and patch, with initial dose in the ED
Participants randomized to NRT receive 6 weeks of patches (42 count) and gum (300 pieces of 2 mg). NRT dosing is tailored to the participant’s cigarette consumption (5–10 cigarettes/day: 14 mg patch and 2 mg gum;> 10 cigarettes/day: 21 mg patch and 2 mg gum). Patches come in 14 and 21 mg doses. The first patch is applied by an ED nurse at the index visit. The RA then conducts a brief educational session with the participant on the use of both the patch and the gum. This approach, which employs immediate cessation of tobacco, stands in contrast to the traditional model of setting a quit date several weeks after the initiation of tobacco dependence treatment. In our previous investigation, this approach was accepted enthusiastically by study participants. Combination NRT is provided because it is generally more efficacious than NRT monotherapy in promoting tobacco abstinence [29].
2.6.3. Text messaging
We are using the SmokefreeTXT program, developed by the National Cancer Institute of the NIH and available through Smokefree. gov. SmokefreeTXT is a text messaging service designed for U.S. adults who are trying to quit smoking. The program provides 24/7 encouragement, advice, and tips to help smokers quit, and stay quit. Messages are timed around the quit date, and the program lasts 6–8 weeks depending on chosen quit date. Users receive 1–5 messages per day and can receive additional quit support by texting one of SmokefreeTXT’s keywords.
The SmokefreeTXT library contains about 130 messages. In the previous pilot study, a modest number of modifications were made and primarily concerned time considerations (e.g., deletion of all messages concerning the 2-week lead-in to the actual quit date) and tailoring information to local resources for Connecticut residents. SmokeFreeTXT employs a number of behavior change strategies, including feedback and monitoring, social support, and shaping knowledge [31]. Based on the qualitative data we collected during our pilot study [16], we offer participants in the current trial a choice of how many messages they receive per day (up to 2 messages or up to 4 messages per day) and the time of day they receive the first message. We also added 18 messages for participants presenting to the ED with specific chief complaints (cardiac, respiratory, or wound care) during the index visit.
2.6.4. Active quitline referral
Participant information is faxed to the Connecticut QL’s service provider, Alere Wellbeing. QL staff then makes outbound calls to reach participants, provide information about services standardly offered by the Connecticut QL, and enroll interested participants in QL services. The Connecticut QL currently offers residents the option of enrolling in a one call, multiple call, or web-based tobacco cessation program. All enrollees in the multiple call or web-based programs who meet medical screening criteria are also eligible for two weeks of the nicotine patch, gum or lozenge mailed from the QL. The call programs combine individualized telephone counseling, text messaging, mailed or online materials, and an interactive online program to complement the phone-based program. After the initial assessment and planning call, participants in the multiple call program receive 4 additional proactive counseling calls. Calls are designed to help participants set a quit date, design a plan for cessation, develop problem-solving and coping skills, secure social support, and effectively use cessation medications, such as NRT. Quit coaches are required to have a bachelor’s degree, complete over 200 h of education and training in tobacco cessation treatment, and receive ongoing supervision. Calls are scheduled at convenient times and at relapse-sensitive intervals; calls in the multiple call program are typically completed within 2–3 months of program registration. Participants can also call for additional support between proactive calls. The Alere program is based on the 2008 Public Health Service clinical practice guideline [29] and grounded in Social Cognitive Theory [32]. Its effectiveness has been validated by 3 randomized trials [33–35], and several real-world evaluations [36–38]. In our prior study, 32% of participants in the intervention arm engaged in ≥1 call with the quitline [39].
Lastly, all participants are provided with a brochure that reviews the health hazards of smoking, and provides the phone number for the state quitline. In Connecticut, this brochure is produced by the State Department of Public Health, printed in English and Spanish, and is available in bulk at low cost. We believe that distributing this brochure to all study participants is reasonable and ethical. Because provision of the brochure is not manipulated in the experiment we will be unable to isolate any discrete effect of the brochure on tobacco abstinence.
2.7. Primary outcome measure
The primary efficacy endpoint is biochemically verified 7-day cessation at 3 months [40]. Tobacco cessation will be assessed by self-report and confirmatory biochemical testing with exhaled carbon monoxide. Patients who assert abstinence by phone interview will be asked to return to the hospital for biochemical assessment of exhaled carbon monoxide.
2.8. Secondary outcome measures
Secondary outcomes include use of cessation medications and services, including use of the quitline, use of NRT, and use of other pharmacotherapies such as bupropion and varenicline, as well as changes in daily cigarette consumption. These will be assessed by self-report and fax reports from the CT quitline. A brief, structured interview called the Treatment Service Review (TSR) [41] will be administered to collect information on the type and amount of services received by participants, including ED visits, hospitalizations, primary medical care visits, quitline utilization, and self-help sources of support (e.g. web services). The TSR will be supplemented with questions on the use of smoking cessation medications.
2.9. Analytic strategy
2.9.1. Overview
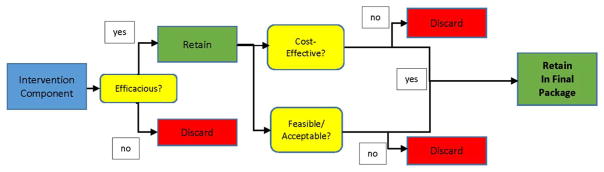
Intervention components will be analyzed over 3 domains, as outlined in Fig. 2. First, components will be assessed for clinical efficacy, using traditional measures such as biochemically verified tobacco abstinence. If clinically effective, components will then be analyzed for cost-effectiveness and feasibility/acceptability to participants.
Fig. 2.
Schematic view of analytic strategy after factorial trial.
2.10. Assessing clinical efficacy
Participants will be contacted by telephone at 1 and 3 months after enrollment. At 3 months, participants self-reporting tobacco abstinence will be asked to return to the hospital to measure exhaled carbon monoxide (CO). Participants whose breath CO levels are 9 ppm or less will be considered abstinent [40].
Multivariable logistic regression will be used to model the primary outcome, abstinence at 3 months. Per convention, in the primary analysis missing abstinence data, including absence of biochemical verification, will be coded as smoking. For this full-factorial design, the model will include main effects for each of the 4 components as well as all 2, 3 and 4-way interactions. The regression will also include baseline covariates: age, sex, race/ethnicity, and smoking characteristics, such as daily cigarette consumption. Main effects will be evaluated at the 0.05 significance level. Differences in proportions for the presence and absence of each component (i.e. main effects) will be estimated along with 95% confidence intervals using the bootstrap method [42]. A p-value of 0.10 will be used as a guide to flag potentially important interactions which will be explored graphically with a particular emphasis on identifying substantial synergistic effects or qualitative (i.e. where the impact of 1 component changes direction depending on the presence or absence of another component) effect modification. Similar analyses will be performed at 1-month follow-up. Additionally, a logistic regression with parameters estimated by weighted Generalized Estimating Equations (GEE) will incorporate both 1- and 3-month outcome data without imputing non-responders as relapse [43]. This analysis weights for the probability of response and is valid under the assumption that missing data are missing at random. Secondary outcomes at 1 and 3 months will also be evaluated by a generalized linear model with weighted GEE.
2.11. Justification of sample size
The goal of this proposal is to identify individual tobacco dependence treatment intervention components which show clinical efficacy, subject to constraints of cost effectiveness and acceptability. As each component is allocated to half the participants, the sample size to detect main effects in a full factorial MOST design depends not on the number of components evaluated, but rather the smallest clinically important difference between the presence and absence of a component. In our previous trial, we found a difference of 7.3% in biochemically confirmed abstinence between the control and intervention arms at 3 months. It is reasonable to expect that, in this factorial trial, the effect of individual components will be less than that seen in the multi-component trial [17,44]. Therefore, we have chosen an absolute difference of 5% for the main effects of each component. (We considered a narrower difference of 4%, but the sample size and resources needed become unfeasible.) With a two-sided 0.05 significance level, and an average abstinence proportion of 4.9% in the absence of a component, a total sample size of 860 participants will provide 80% power to detect a 5% increase in the abstinence rate. We will enroll 1056 participants to account for a 15% dropout rate by 3 months. We will investigate whether the effect of a component is dependent on the levels of other components (i.e. interactions), but our trial is not powered with the intent to detect these interactions.
2.12. Assessing cost effectiveness
2.12.1. Overview
We will perform a series of incremental cost effectiveness analyses (CEA) to determine whether the benefits of each component appear to be worth the added costs, partially accounting for the interactive effects of the components. Two outcomes will be considered: (1) number of abstinent smokers at 3 months (measured by biochemically verified 7-day tobacco abstinence) and (2) the cost per QALY saved. Following CEA recommendations [45,46], baseline analyses will adopt a societal perspective, considering all economic costs regardless of source. We also will calculate incremental cost effectiveness ratios (ICERs) from the payer’s perspective, excluding patient costs and, in sensitivity analysis, QL costs. We will use ICERs for other cessation interventions to select an acceptable maximum cost/quality-adjusted life year (QALY) saved for the tested program elements since we want to identify an ED cessation approach that is at least as cost-effective as approaches based in other settings [47–49].
2.12.2. Treatment costs
We will include costs of all smoking cessation treatment received by participants, broken down by component (Table 2). Resource costs include costs of administering the BNI and medication (a purchased item), administering the texting, and making the QL referral (i.e., IT costs, cost of clinician time to administer the intervention, medication delivered in hospital, incremental cost of longer follow-up booster phone call), costs related to QL use, outpatient treatment costs, other medical costs, and patient costs (e.g., time, transportation). In evaluating a prior trial, we measured RA time for an ED-based BNI session. Clinician time costs will include wages, fringe benefits, and overhead. Cost of patient time will be calculated using the average wage rate in the geographic area. Relevant medication use will be obtained from the medical record; cost of medication will be based on the average of the current formulary price for the top 5 health insurers in Connecticut. Quitline costs: We will get the number of counseling calls completed directly from QL records, then calculate costs of counselor and patient time related to the calls (both scheduled QL calls and participant-initiated calls). We will collect patient time on the website at 1 and 3 month assessments, as well as costs of any NRT shipped, and registration costs for quitline-managed web or texting services. We will exclude research costs because these would not be incurred if our intervention were standard care. Training and texting program setup costs will be excluded from the main analyses. Although these startup costs may be incurred, when distributed across many patients over many years, they would be negligible. We will collect information on training and setup costs and include in publications/sensitivity analysis, as these may be of interest to decision makers.
Table 2.
Components of economic analysis.
| Component | Health system cost | Participant cost | State costs |
|---|---|---|---|
| Screening | None. Screening information collected for all patients. | None. | None. |
| Motivational interview | Provider time performing motivational interview (wage × minutes). Average YNHH wage, conditional on provider training (i.e., MD, nurse practitioner, RA) will be used. | None. No additional time in hospital due to motivational interview. | None. |
| Medication (6 weeks) | Total price paid by hospital. In sensitivity analysis we will consider average wholesale price (AWP), since not all providers receive same medication discounts. | None. Assume all costs of medication borne by provider. | None. |
| Quitline | None. Provider bears no costs for quit line use. | Cost of time on QL (avg. wage rate ×min/call). | Marginal cost of QL user. |
| Texting | None. Texting will be automated. Once developed marginal cost of texting is small. | Cost of minutes to participant. | None. |
| Brochure | Printing costs of brochure. | None. | None. |
2.12.3. Measuring effectiveness
We will assess effectiveness (i.e., abstinence) biochemically at 3 months. With the MOST design, regression will estimate quit rates for the 4 potential intervention components, as well as the gains and losses that occur when combining them. Over time, abstinence reduces medical care utilization and improves quality of life. We will not track those savings in our patient cohort. Instead, we will update our adaptation of a popular model that simulates them [50].
2.12.4. Calculating cost effectiveness ratios
We will calculate incremental cost effectiveness ratios (ICERs) in 2 passes. A naive first pass will array cost and regression-adjusted quit rate for each of the 16 MOST cells, ignoring if differences are statistically significant. This naive look will let us drop clearly dominated cells where higher cost is associated with a lower quit rate, as well as any cells that proved infeasible in the clinical setting. In a refined pass, we will define incremental component cost effectiveness as ΔC/ΔE, where ΔC is the cost a component adds and ΔE is the effectiveness associated with the component. This refined look at the remaining candidates will use 2 effectiveness measures, the 3-month quit rate and the simulated quality-adjusted life year (QALY) gain. A QALY is a standard measure of health-related quality of life, defined so that a year in perfect health is valued at 1.0 and death is valued at 0.0 [46]. Even if 1 component costs more per quitter than another, it still may be worthwhile if it helps more smokers to quit at an acceptable cost per QALY gained. By estimating QALYs gained per quitter, we get a measure that lets us judge the cost of cessation gains relative to other smoking interventions, with a tentative ceiling of $5000 per QALY gained that we will refine in year 4 by updating our literature review on cessation interventions regularly used in medical settings.
The analysis will require estimates of the QALY losses and medical costs averted by smoking cessation. For QALY loss, the most recent estimates [51–53] are better than older estimates [54,55]. For medical costs, we expect to use the latest update to CDC’s SAMMEC model [1] which builds from the methods pioneered by V. Miller [56]. We will inflate all cost savings to the same year’s dollars as the program costs and compute present value of savings in future years at a 3% discount rate. To account for relapse, we will insert our chosen smoking costs and our observed quit rate pattern into the widely used BENESCO (benefits of smoking cessation on outcomes) Markov simulation model of cessation duration or a closely related model [47,48]. These models stem from a 2002 World Health Organization model. The resulting estimates will let us answer the question of which efficacious ED-based interventions maximize quits while staying below the acceptable cost per QALY gained.
To better inform our package choice, we will estimate 95% CIs around the ICERs. To estimate ratio variance, we will start with roughly estimated standard errors or distributional data for each number in the cost-effectiveness equation. We will use bootstrapping simulation methods that form an empirical probability distribution [57]. To handle estimates with unknown variance, notably the discount rate, we will conduct sensitivity analyses. The sensitivity analyses examine if different input assumptions would change package selection.
2.13. Assessing feasibility, acceptability
A novel feature of this study is the prespecified use of qualitative methods to assess the intervention. The goal of this analysis is to ensure that intervention components are considered feasible, practical, and acceptable by the patients who may use the package in real-world settings.
Similar to our approach in the pilot study, we will conduct phone-based, semi-structured interviews with a sub-sample of participants at the 3-month follow-up assessment. The interview will begin with a set of items that use a Likert format to obtain an overall rating of each component that participants received, both alone and in combination. We will use a purposive sampling strategy that will recruit equal numbers of participants from each component (i.e., either as a single component or in combination) to allow us to explore potential interactions of components. Consent for the qualitative component will have already been given at study enrollment. The semi-structured interviews will be audio-recorded and subsequently transcribed and analyzed thematically, Recruitment will continue until thematic saturation has been achieved.
Components scoring well in Likert assessments and found feasible and acceptable in interviews with participants (that are also clinically efficacious and cost-effective) will be retained to study in the future randomized trial.
The semi-structured interviews will be audio-recorded and subsequently transcribed, coded by study personnel with experience in coding, and analyzed thematically in an ongoing and iterative process [58,59] using qualitative analysis software ATLAS.ti (version 7.0; ATLAS. ti GmbH). Recruitment will continue until thematic saturation has been achieved. We anticipate interviewing 80–100 participants.
3. Results
As of 12 July 2017, 203 participants have enrolled in the trial. Their median age is 44 years (SD 12.6); 101 (50%) are male; their racial and ethnic composition is 97 (48%) white, 70 (34%) African American, and 36 (18%) other. Thirty-three (16%) participants identify as Hispanic. Their insurance coverage is 127 (63%) Medicaid, 6 (3%) Medicare, 20 (10%) Medicaid and Medicare, 47 (23%) private insurance, and 3 (1%) uninsured. Recruitment is proceeding on schedule. One-month and threemonth follow-ups have begun, including the qualitative interviews.
4. Discussion
Interventions for addictive disorders commonly employ both behavioral approaches and pharmacotherapy. Evidence-based behavioral approaches may include motivational interviewing/enhancement, cognitive behavioral therapy, group approaches, or technology-facilitated approaches such as telephone quitlines, web-based programs, and text messaging. Pharmacotherapies for tobacco dependence include nicotine patch, gum, lozenge, nasal spray, inhaler, as well as varenicline and buproprion [29]. This study addresses the significant challenge of deciding how to best treat tobacco dependence in the ED where low income smokers typically get treated.
It is common to treat behavioral disorders and addictions with multi-component approaches. Given the complex behavioral, genetic, physiologic, and environmental factors that mediate disorders such as addiction, it is perhaps unsurprising that combinations of treatments often work better than monotherapy. Traditional approaches to assess efficacy typically involve randomized trials with two or more arms that test a package of interventions against control, or usual care. An important shortcoming of this approach is that it does not enable the investigator to disaggregate the effects of individual interventions or examine whether important interactions exist between interventions.
This study offers innovation on a number of fronts: (1) this is the first study to assess the efficacy of the individual components of EDinitiated tobacco dependence treatment; (2) this is the first ED-based study to use the MOST clinical trial methodology; (3) this is the first large study in the ED of mobile health technology for tobacco dependence treatment; (4) the treatment paradigm initiates NRT at the time of enrollment, without the traditional 2–3 week period prior to a formal “quit date;” (5) to our knowledge, this is the first MOST trial to add a qualitative component to the analytic strategy, incorporating qualitative and quantitative assessments of efficacy, cost effectiveness, and feasibility/acceptability, using a concurrent triangulation mixed methods approach [60].
5. Conclusion
This study is a full-factorial 16-arm trial using the MOST design to assess the effects of 4 evidence-based therapies in the treatment of tobacco dependence among adult smokers visiting a hospital ED. The therapies are nicotine patches and gum, brief motivational interviewing, telephone-based quitline counseling, and text messaging. The study uses a mixed methods approach to identify the most efficacious combination of treatments within a fixed cost constraint. Study endpoints include assessments of clinical efficacy (i.e. biochemically verified smoking cessation at 3 months), cost effectiveness analysis, and acceptability of the interventions to study participants. Recruitment of 1056 participants will conclude approximately in February 2019. Once the optimal combination of interventions is identified, a follow-on clinical trial will assess its efficacy.
Acknowledgments
Funding
The study is supported by Grant R01CA201873 from the National Cancer Institute of the National Institutes of Health. The funder played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
The authors thank Teresa O’Leary and Kimberly Beauchemin for their excellent research assistance.
Footnotes
Trial registration: Trial (NCT02896400) registered in ClinicalTrials.gov on September 6, 2016.
Ethics approval and consent to participate
This study was approved by the Human Investigation Committee of Yale University. All participants will provide written informed consent before participating.
Consent for publication
Not applicable.
Availability of data and material
Not applicable.
Competing interests
Dr. Toll received a grant from Pfizer for medicine only, and he testifies as an expert witness for plaintiffs who have filed litigation against the tobacco companies.
All other authors declare that they have no competing interests.
Authors’ contributions
SLB conceived of the study and wrote the first draft of the manuscript. BT, LC, LA, MP, TM, KV, and LG contributed to study design. JD directs the statistical analysis. JW manages the study. All authors read and approved the final manuscript.
References
- 1.U.S. Department of Health and Human Services. A report of the surgeon general. U.S. Department of Health and Human Services CfDCaP, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; Atlanta, GA: 2014. The health consequences of smoking— 50 years of progress. [Google Scholar]
- 2.Jamal A, King BA, Neff LJ, Whitmill J, Babb SD, Graffunder CM. Current cigarette smoking among adults — United States, 2005–2015. MMWR Morb Mortal Wkly Rep. 2016;65:1205–1211. doi: 10.15585/mmwr.mm6544a2. [DOI] [PubMed] [Google Scholar]
- 3.Office of Disease Prevention and Health Promotion. Tobacco Use: Objectives. U.S. Department of Health and Human Services; [Accessed 15 December 2014]. 2010. https://www.healthypeople.gov/2020/topicsobjectives/topic/tobacco-use/objectives. [Google Scholar]
- 4.NIH State-of-the-Science Panel. National Institutes of Health state-of-the-science conference statement: tobacco use: prevention, cessation, and control. Ann Intern Med. 2006;145:839–844. doi: 10.7326/0003-4819-145-11-200612050-00141. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention, National Hospital Ambulatory Medical Care Survey. [Accessed 15 December 2014];Emergency Department Summary Tables. 2011 at http://www.cdc.gov/nchs/data/ahcd/nhamcs_emergency/2011_ed_web_tables.pdf.
- 6.Lowenstein S, Tomlinson D, Koziol-McLain J, Prochazka A. Smoking habits of emergency department patients: an opportunity for disease prevention. Acad Emerg Med. 1995;2:165–171. doi: 10.1111/j.1553-2712.1995.tb03189.x. [DOI] [PubMed] [Google Scholar]
- 7.Lowenstein SR, Koziol-McLain J, Thompson M, et al. Behavioral risk factors in emergency department patients: a multisite study. Acad Emerg Med. 1998;5:781–787. doi: 10.1111/j.1553-2712.1998.tb02504.x. [DOI] [PubMed] [Google Scholar]
- 8.Bernstein SL, Bijur P, Cooperman N, et al. A randomized trial of a multicomponent cessation strategy for emergency department smokers. Acad Emerg Med. 2011;18:575–583. doi: 10.1111/j.1553-2712.2011.01097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernstein SL, Arnsten JH, Bijur PE, et al. Concurrent use of alcohol or illicit substances improves response to ED SBIRT for adult smokers. J Subst Abus Treat. 2013;44:139–142. [Google Scholar]
- 10.Bernstein SL, D’Onofrio G, Rosner J, et al. Successful tobacco dependence treatment achieved via pharmacotherapy and motivational interviewing in low-income emergency department patients. Ann Emerg Med. 2015;66:140–147. doi: 10.1016/j.annemergmed.2015.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boudreaux ED, Baumann BM, Camargo CA, Jr, O’Hea E, Ziedonis DM. Changes in smoking associated with an acute health event: theoretical and practical implications. Ann Behav Med. 2007;33:189–199. doi: 10.1007/BF02879900. [DOI] [PubMed] [Google Scholar]
- 12.Bernstein SL. Tobacco-related illnesses and management. In: Todd KH, Thomas CR Jr, editors. Oncologic Emergency Medicine: Principles and Practice. Springer; Switzerland: 2016. [Google Scholar]
- 13.Babor TF, McRee BG, Kassebaum PA, Grimaldi PL, Ahmed K, Bray J. Screening, brief intervention, and referral to treatment (SBIRT) Subst Abus. 2007;28:7–30. doi: 10.1300/J465v28n03_03. [DOI] [PubMed] [Google Scholar]
- 14.D’Onofrio G, Fiellin DA, Pantalon MV, et al. A brief intervention reduces hazardous and harmful drinking in emergency department patients. Ann Emerg Med. 2012;60:181–192. doi: 10.1016/j.annemergmed.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernstein SL, Rosner J, Toll B. A multicomponent intervention including texting to promote tobacco abstinence in emergency department smokers: a pilot study. Acad Emerg Med. 2016;23:803–808. doi: 10.1111/acem.12990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grau LE, Pham T, O’Leary T, Rosner J, Toll B, Bernstein SL. Smokers’ perspectives on texting for tobacco dependence treatment: a qualitative analysis. Nicotine Tob Res. 2017;19:307–313. doi: 10.1093/ntr/ntw184. [DOI] [PubMed] [Google Scholar]
- 17.Collins LM, Dziak JJ, Kugler KC, Trail JB. Factorial experiments: efficient tools for evaluation of intervention components. Am J Prev Med. 2014;47:498–504. doi: 10.1016/j.amepre.2014.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collins LM, Dziak JJ, Li R. Design of experiments with multiple independent variables: a resource management perspective on complete and reduced factorial designs. Psychol Methods. 2009;14:202–224. doi: 10.1037/a0015826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collins LM, MacKinnon DP, Reeve BB. Some methodological considerations in theory-based health behavior research. Health Psychol. 2013;32:586–591. doi: 10.1037/a0029543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collins LM, Murphy SA, Nair VN, Strecher VJ. A strategy for optimizing and evaluating behavioral interventions. Ann Behav Med. 2005;30:65–73. doi: 10.1207/s15324796abm3001_8. [DOI] [PubMed] [Google Scholar]
- 21.Collins LM, Murphy SA, Strecher V. The multiphase optimization strategy (MOST) and the sequential multiple assignment randomized trial (SMART): new methods for more potent eHealth interventions. Am J Prev Med. 2007;32:S112–118. doi: 10.1016/j.amepre.2007.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McClure JB, Derry H, Riggs KR, et al. Questions about quitting (Q2): design and methods of a Multiphase Optimization Strategy (MOST) randomized screening experiment for an online, motivational smoking cessation intervention. Contemp Clin Trials. 2012;33:1094–1102. doi: 10.1016/j.cct.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fraser D, Kobinsky K, Smith SS, Kramer J, Theobald WE, Baker TB. Five population-based interventions for smoking cessation: a MOST trial. Transl Behav Med. 2014;4:382–390. doi: 10.1007/s13142-014-0278-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baker TB, Mermelstein R, Collins LM, et al. New methods for tobacco dependence treatment research. Ann Behav Med. 2011;41:192–207. doi: 10.1007/s12160-010-9252-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collins LM, Baker TB, Mermelstein RJ, et al. The multiphase optimization strategy for engineering effective tobacco use interventions. Ann Behav Med. 2011;41:208–226. doi: 10.1007/s12160-010-9253-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller WR, Rollnick S. Motivational Interviewing: Preparing People for Change. 2. Guilford Press; New York: 2002. [Google Scholar]
- 27.United States Census Bureau. [Accessed 11 July 2017];American Fact Finder. 2017 https://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?src=CF2015.
- 28.Abraham M, Buchanan M. Greater New Haven Community Index 2016. New Haven, CT: DataHaven; [Accessed 2 January 2017]. 2016. at http://www.ctdatahaven.org/sites/ctdatahaven/files/DataHaven_GNH_Community_Index.pdf. [Google Scholar]
- 29.Fiore MC, Jaén CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Rockville, MD: US Department of Health and Human Services; 2008. [Google Scholar]
- 30.Whittaker R, McRobbie H, Bullen C, Borland R, Rodgers A, Gu Y. Mobile Phone-based Interventions for Smoking Cessation. Cochrane Database of Systematic Reviews. 2012 doi: 10.1002/14651858.CD006611.pub3. [DOI] [PubMed]
- 31.Stoyneva I, Coa K, Pugatch J, Sanders A, Schwarz M, Cole-Lewis H. SmokefreeTXT behaviour change technique analysis. J Smok Cessat. 2016:1–13. [Google Scholar]
- 32.Bandura A. Human agency in social cognitive theory. Am Psychol. 1989;44:1175–1184. doi: 10.1037/0003-066x.44.9.1175. [DOI] [PubMed] [Google Scholar]
- 33.Orleans TC, Schoenbach VJ, Wagner EH, et al. Self-help quit smoking interventions: effects of self-help materials, social support instructions, and telephone counseling. J Consult Clin Psychol. 1991;59:439–448. doi: 10.1037//0022-006x.59.3.439. [DOI] [PubMed] [Google Scholar]
- 34.Swan GE, McAfee T, Curry SJ, et al. Effectiveness of bupropion sustained release for smoking cessation in a health care setting: a randomized trial. Arch Intern Med. 2003;163:2337–2344. doi: 10.1001/archinte.163.19.2337. [DOI] [PubMed] [Google Scholar]
- 35.Hollis JF, McAfee TA, Fellows JL, Zbikowski SM, Stark M, Riedlinger K. The effectiveness and cost effectiveness of telephone counselling and the nicotine patch in a state tobacco quitline. Tob Control. 2007;16:i53–i59. doi: 10.1136/tc.2006.019794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Curry SJ, Grothaus LC, McAfee T, Pabinak C. Use and cost effectiveness of smoking-cessation services under four insurance plans in a health maintenance organization. N Engl J Med. 1998;339:673–679. doi: 10.1056/NEJM199809033391006. [DOI] [PubMed] [Google Scholar]
- 37.Ringen K, Anderson N, McAfee T, Zbikowski SM, Fales D. Smoking cessation in a blue-collar population: results from an evidence-based pilot program. Am J Ind Med. 2002;42:367–377. doi: 10.1002/ajim.10129. [DOI] [PubMed] [Google Scholar]
- 38.El-Bastawissi A, McAfee T, Zbikowski SM, et al. The uninsured and Medicaid Oregon tobacco user experience in a real world, phone based cessation programme. Tob Control. 2003;12:45–51. doi: 10.1136/tc.12.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bernstein SL, Weiss JR, Toll BA, Zbikowski SM. Association between utilization of quitline services and probability of tobacco abstinence in low-income smokers. J Subst Abuse Treat. 2016;71:58–62. doi: 10.1016/j.jsat.2016.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob Res. 2003;5:13–25. [PubMed] [Google Scholar]
- 41.McLellan AT, Alterman AI, Cacciloa J, Metzger D, O’Brien CP. A new measure of substance abuse treatment: initial studies of the treatment service review. J Nerv Ment Dis. 1992;180:101–110. doi: 10.1097/00005053-199202000-00007. [DOI] [PubMed] [Google Scholar]
- 42.Efron B, Tibshirani R. An Introduction to the Bootstrap. Chapman & Hall/CRC; Boca Raton, FL: 1993. [Google Scholar]
- 43.Dmitrienko A, Molenberghs G, Chuang-Stein C, Offen W. Analysis of Clinical Trials Using SAS: A Practical Guide. SAS Institute, Inc; Cary, NC: 2005. [Google Scholar]
- 44.Wolbers M, Heemskerk D, Chau T, et al. Sample size requirements for separating out the effects of combination treatments: randomised controlled trials of combination therapy vs. standard treatment compared to factorial designs for patients with tuberculous meningitis. Trials. 2011;12(26) doi: 10.1186/1745-6215-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Drummond MF, Schulpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. 3. Oxford University Press; New York: 2007. [Google Scholar]
- 46.Gold MR, Siegel JE, Russell LB, Weinstein MC. Cost-Effectiveness in Health and Medicine. Oxford University Press; New York: 1996. [Google Scholar]
- 47.Howard P, Knight C, Boler A, Baker C. Cost-utility analysis of varenicline versus existing smoking cessation strategies using the BENESCO simulation model: application to a population of US adult smokers. PharmacoEconomics. 2008;26:497–511. doi: 10.2165/00019053-200826060-00004. [DOI] [PubMed] [Google Scholar]
- 48.Flack S, Taylor M, Trueman P. Cost-effectiveness of Interventions for Smoking Cessation: Final Report. National Institute for Health and Clinical Excellence; London: 2007. [Google Scholar]
- 49.Cromwell J, Bartosch WJ, Fiore MC, Hasselblad V, Baker T. Cost-effectiveness of the clinical practice recommendations in the AHCPR guideline for smoking cessation. Agency for Health Care Policy and Research, see comment. JAMA. 1997;278:1759–1766. [PubMed] [Google Scholar]
- 50.Levy DT, Graham AL, Mabry PL, Abrams DB, Orleans CT. Modeling the impact of smoking-cessation treatment policies on quit rates. Am J Prev Med. 2010;38:S364–S372. doi: 10.1016/j.amepre.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jia H, Lubetkin EI. Trends in quality-adjusted life-years lost contributed by smoking and obesity. Am J Prev Med. 2010;38:138–144. doi: 10.1016/j.amepre.2009.09.043. [DOI] [PubMed] [Google Scholar]
- 52.Strine TW, Okoro CA, Chapman DP, et al. Health-related quality of life and health risk behaviors among smokers. Am J Prev Med. 2005;28:182–187. doi: 10.1016/j.amepre.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 53.Vogl M, Wenig CM, Leidl R, Pokhrel S. Smoking and health-related quality of life in English general population: implications for economic evaluations. BMC Public Health. 2012;19(203) doi: 10.1186/1471-2458-12-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ahmad S, Billimek J. Estimating the health impacts of tobacco harm reduction policies: a simulation modeling approach. Risk Anal. 2005;25:801–812. doi: 10.1111/j.1539-6924.2005.00647.x. [DOI] [PubMed] [Google Scholar]
- 55.Glick HA, Doshi JA, Sonnad SS, Polsky D. Economic Evaluation in Clinical Trials. Oxford University Press; Oxford: 2007. [Google Scholar]
- 56.Miller VP, Ernst C, Collin F. Smoking-attributable medical care costs in the USA. Soc Sci Med. 1999;48:375–391. doi: 10.1016/s0277-9536(98)00344-x. [DOI] [PubMed] [Google Scholar]
- 57.Willan AR, Briggs AH. Statistical Analysis of Cost-effectiveness Data. John Wiley & Sons Ltd; West Sussex, UK: 2006. [Google Scholar]
- 58.Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3:77–101. [Google Scholar]
- 59.Braun V, Clarke V. Successful Qualitative Research: A Practical Guide for Beginners. SAGE Publications Ltd; Thousand Oaks, CA: 2013. [Google Scholar]
- 60.Cresswell JW, Clark VLP, Gutmann ML, Hanson WE. Advanced mixed methods research designs. In: Tashakkori A, Teddlie C, editors. Handbook of Mixed Methods in Social & Behavioral Research. SAGE Publications, Inc; Thousand Oaks, CA: 2003. [Google Scholar]