Abstract
Ulcerative colitis (UC) is a chronic relapsing disease without satisfactory treatments, in which intestinal inflammation and disrupted intestinal epithelial barrier are two main pathogeneses triggering UC. Berberrubine (BB) is deemed as one of the major active metabolite of berberine (BBR), a naturally-occurring isoquinoline alkaloid with appreciable anti-UC effect. This study aimed to comparatively investigate the therapeutic effects of BB and BBR on dextran sodium sulfate (DSS)-induced mouse colitis model, and explore the potential underlying mechanism. Results revealed that BB (20 mg/kg) produced a comparable therapeutic effect as BBR (50 mg/kg) and positive control sulfasalazine (200 mg/kg) by significantly reducing the disease activity index (DAI) with prolonged colon length and increased bodyweight as compared with the DSS group. BB treatment was shown to significantly ameliorate the DSS-induced colonic pathological alternations and decreased histological scores. In addition, BB markedly attenuated colonic inflammation by alleviating inflammatory cell infiltration and inhibiting myeloperoxidase (MPO) and cytokines (TNF-α, IFN-γ, IL-1β, IL-6, IL-4 and IL-10) productions in DSS mice. Furthermore, BB treatment substantially upregulated the expression of tight junction (TJ) proteins (zonula occludens-1, zonula occludens-2, claudin-1, occludin) and mRNA expression of mucins (mucin-1 and mucin-2), and decreased the Bax/Bcl-2 ratio. In summary, BB exerted similar effect to its analogue BBR and positive control in attenuating DSS-induced UC with much lower dosage and similar mechanism. The protective effect observed may be intimately associated with maintaining the integrity of the intestinal mucosal barrier and mitigating intestinal inflammation, which were mediated at least partially, via favorable modulation of TJ proteins and mucins and inhibition of inflammatory mediators productions in the colonic tissue. This is the first report to demonstrate that BB possesses pronounced anti-UC effect similar to BBR and sulfasalazine with much smaller dosage. BB might have the potential to be further developed into a promising therapeutic option in the treatment of UC.
Introduction
Ulcerative colitis (UC), a subtype of inflammatory bowel disease (IBD), is clinically characterized by acute abdominal pain, weight loss, diarrhea, even hematochezia which severely lower the quality of life [1]. Nowadays, there are different drugs for UC treatment, including 5-aminosalicylic acid, steroid hormone, immunosuppressive agents and anti-tumor necrosis factor-α (anti-TNF-α) agent. However, the frequency and severity of side effects, inconvenient dosing regimen, and partially prohibitive price limit their clinical application [2]. Therefore, it is of great significance to seek effective alternative for the treatment of UC.
Inflammation responses are one of the most crucial factors causing UC [3]. The increased pro-inflammatory cytokines such as TNF-α, IFN-γ, and IL-1β, extend the inflammatory cascade and eventually lead to intestinal/colonic tissue damage in UC induced by DSS [4]. Moreover, the onset of UC is accompanied by obvious diffused intestinal inflammation which is closely associated with the increased permeability of intestinal epithelial barrier [5, 6].
The intestinal epithelial barrier, physically protecting the intestine from luminal bacteria and toxins, is composed of the mucous layer, epithelial cells and intercellular junctions. Components of the mucous layer are mucins, including mucin-1 and mucin-2, which are secreted by intestinal epithelium goblet cells and markedly decreased in colitis subjects [5, 7]. Tight junctions (TJs) are the most apical structure of the junction complex, which is intercellular structure in epithelial cells and play crucial roles in cell-cell recognition and paracellular motion of substances. TJs regulate the mucosal barrier capability [8], which comprised of various transmembrane proteins including occluding and claudins, coupling each other with zonaoccludens (ZO, cytoplasmic peripheral membrane proteins including ZO-1 and ZO-2) within cells [9, 10]. Once the barrier is impaired, bacteria and toxins may easily penetrate and aggravate the release of multiple cytokines, thus evoking the occurrence and development of UC. Therefore, maintaining the integrity of the gut barrier structure and function provide an invaluable contribution to the treatment of UC, and strategies based on this are now essential lines of treatment for UC [11].
Traditional Chinese medicines (TCMs) remain a fundamental therapeutic solution in the treatment of various diseases due to their long history of clinical practice and reliable therapeutic efficacy. Rhizoma Coptidis (the rhizoma of Coptischinensis Franch., Huanglian in Chinese), officially listed in the Chinese Pharmacopoeia, has been used in China for a long history to treat gastroenteritis including abdominal pain, diarrhea and IBD [12]. Berberine (BBR), the most abundant and major active isoquinoline alkaloid of Rhizoma Coptidis [13], has been reported to exert therapeutic effect on colitis and inhibits inflammatory responses in colonic macrophages and epithelial cells in DSS-treated mice [14]. Indeed, BBR is a safe and effective agent commonly used for the treatment of gastrointestinal disorders like diarrhea in China. However, there are some limitations of BBR due to its low bioavailability and poor intestinal absorption [15]. BBR undergoes extensive metabolism in vivo after oral administration which results in its extremely low plasma exposure [16]. Hence, increasing researchers have focused their attention on the metabolites of BBR, which were also believed to contribute a lot to its pharmacological effects [17].
Berberrubine (BB), one of the main metabolites of BBR in vivo [18] (Fig 1), is more lipophilic than BBR and has higher plasma concentration after BBR oral administration owing to its more efficient intestinal absorption. Previous study has suggested that it is potentially more pharmacologically active than BBR [19]. Indeed, BBR and BB are both found in the medicinal plant Berberis vulgaris, which has been shown to possess anti-UC effect [20, 21]. Increasing evidences have shown that BB exerted multiple activities, such as anti-inflammation [13], anti-microbial [22], anti-tumor [23], lipid-lowering [24], and anti-oxidation [25]. In this study, we made pioneering endeavor to assess the potential protective effects and underlying mechanism of BB in comparison with BBR in DSS-induced colitis by measuring macroscopic score, histological alternations, inflammation markers such as MPO and pro-inflammatory cytokines productions. In addition, we further detected the expression of colonic TJs and apoptosis-related proteins, and the mRNA expression of colonic mucins. Results indicated that BB effectively prevented DSS-induced colitis in mice, and the protective effect might be intimately associated with attenuating intestinal inflammation and maintaining the integrity of the intestinal mucosal barrier.
Fig 1. Chemical structures of berberine and berberrubine.
Materials and methods
Chemicals and reagents
Berberine hydrochloride (purity > 98% by HPLC) was provided by Xi’an Realin Biotechnology Co. Ltd. (Xi’an, China). Dextran sulfate sodium (DSS) was obtained from MP Biomedicals (LLC, France). Sulfasalazine (SASP) was supplied by Sunve Pharmaceutical Co. Ltd. (Shanghai, China). MPO detection kit was purchased from Nanjing Jiancheng Bioenginerring Institute (Nanjing, China). ELISA kits (TNF-α, IFN-γ, IL-6, IL-1β, IL-4 and IL-10) were purchased from eBioscience (MA, USA). BCA protein assay kit was purchased from Thermo Scientific Pierce (Rockford, USA). Sodium carboxymethyl cellulose (CMC-Na) was purchased from Guangzhou Chemical Reagent Factory (Guangzhou, China). All other reagents and chemicals used in the study were of analytical grade.
Synthesis of berberrubine
Berberrubine was synthesized according to the previous study with some modifications [26]. Briefly, berberine hydrochloride (3 g) was microwaved at 180 °C for 30 min under vacuum condition, then dissolved in deionized water (300 ml), extracted by chloroform extraction (300 ml once for 3 times) and dried using the rotary evaporation apparatus to get the crude product, which was purified and concentrated using silica gel column chromatography (CH2Cl2: MeOH = 10:1, v/v) to obtain the red compound (berberrubine). Its structural identity was confirmed by comparing its spectral data (MS, 1H- and 13C-NMR) with those published previously [27]. Its purity was determined to exceed 95.58% by HPLC analysis.
Experimental design and sample collection
Six-week-old male Balb/c mice were obtained from the Laboratory Animal Center of Guangzhou University of Chinese Medicine (Guangzhou, China). Mice were housed under standard conditions at controlled temperature (22 ± 2 °C), humidity (50 ± 10%), and light (12 h light/dark cycle) and fed with standard diets and tap water ad libitum. Animal experiments were approved by the Institutional Animal Care and Welfare Committee of Guangzhou University of Chinese Medicine and performed in accordance with institutional protocols (2016047).
After acclimatization for 7 days, mice were divided into 6 groups based on the average body weight (n = 12 per group), control group, DSS group, BBL (berberrubine, 10 mg/kg) group, BBH (berberrubine, 20 mg/kg) group, BBR (berberine, 50 mg/kg, control drug) and sulfasalazine (SASP, 200 mg/kg, positive drug). BB, BBR and SASP were also dissolved in 0.5% CMC-Na. Except the control group, colitis was induced by oral intake of 3% DSS (w/v, dissolved in drinking water) for 7 days in the other 5 groups. During the experimental period, normal control was given distilled water and DSS group was given 0.5% CMC-Na by gavage. Other groups were orally administered with the corresponding tested articles at the doses mentioned above (0.1 ml/10 g body weight) once daily for 7 consecutive days. The dosage of BBR was selected based on Lee’s study [28], and the dose of BB was adopted according to previous investigation [23] and our preliminary experiment.
Animals were euthanized by an overdose of inhalational diethyl ether on day 8 and colons were collected. The colon length, an indicator of colitis, was gauged from proximal rectum to the ileocecal junction [3]. Whereafter, colon was divided into 4 different pieces. One piece was placed in 4% paraformaldehyde for histological observation. The other piece was homogenized for MPO and cytokines measurements. The remaining two parts were stored at −80°C for further Western blotting and RT-PCR analyses. The colonic samples are all taken from the same position in the colons for every application.
Assessment of disease activity
During the experiment, body weight, stool character, and fecal occult blood were recorded daily. The disease activity index (DAI) was calculated by scoring weight loss, stool character and fecal occult blood based on the scoring system as shown in Table 1 as previously described [29].
Table 1. Scoring of disease activity index (DAI).
| Score | Bodyweight loss | Stool character | Fecal occult blood |
|---|---|---|---|
| 0 | 0 | Normal formed | Negative |
| 1 | = 1–5% | ||
| 2 | = 5–10% | Loose stool | Positive |
| 3 | = 10–20% | ||
| 4 | >20% | Diarrhea | Gross bleeding |
Histopathological examination
After 4% paraformaldehyde fixation for 24 h, colon tissues were then dehydrated, embedded in paraffin, sliced to 5 μm-thickness sections, and stained with H&E following routine protocol. Finally, slides were observed under a microscope. For each sample, six horizons were selected to assess the colonic inflammation and lesions degree, which were denoted as histological scores, according to the previous criterion [30].
Measurement of MPO activity and cytokines concentrations
The activity of MPO in the colonic tissue was measured using a MPO detection kit and the levels of cytokines (TNF-α, IFN-γ, IL-6, IL-1β, IL-4 and IL-10) were quantified by ELISA according to the instruction of manufacturers. To determine the MPO activity and the levels of these cytokines, tissues were homogenized with lysis buffer for 1 min and centrifuged at 14000 g, 4 °C for 15 min. Supernatants were collected for subsequent measurements. The amount of total protein was determined by BCA protein assay kit.
Mucin mRNA analysis by Real-time PCR
Total RNA was extracted from colonic samples using Trizol reagent (15596–026, Invitrogen, USA) according to the operating manual. Total RNA (2 μg) was retrotranscribed into cDNA using the GoScript™ reverse transcription system (Promega, Shanghai, China). The reaction condition was as follows: 70 °C incubation for 10 min, then at 42 °C for 60 min, and terminated at 75 °C for 15 min. And 5 μl cDNA was used in a Real-time PCR reaction which was performed with ABI 7500 Real-time PCR system (Life technology). Gene expression was detected using SYBR Green [FastStart Universal SYBR Green Master (Rox), Roche] based PCR with GAPDH as the internal reference. The primer sequences were designed by Invitrogen Biotechnology (Shanghai) Co. LTD. (Shanghai, China) and listed in Table 2. The cycle procedures were as follows: 95 °C initial denaturation for 2 min, then 45 cycles of 95 °C denaturation for 30 s, 60 °C annealing for 30 s and 72 °C extension for 30 s. A melting curve analysis, from 55 to 98 °C, was conducted after the finish of the amplification cycles. Finally, the relative expression levels were normalized to GAPDH and calculated using the 2-ΔΔCt method.
Table 2. Primers for Real-time PCR.
| Gene | Sequences (5'-3') | ProductSize (bp) | Genbank ID |
|---|---|---|---|
| GAPDH | F: ATTGTCAGCAATGCATCCTG | 102 | NM-001289726.1 |
| R: ATGGACTGTGGTCATGAGCC | |||
| Mucin-1 | F: CCAAGCGTAGCCCCTATGAG | 114 | NM-013605.2 |
| R: GTGGGGTGACTTGCTCCTAC | |||
| Mucin-2 | F: TTCCAACCCTCCTCCTACCAC | 189 | NM-023566.3 |
| R: CTCCACCATTCCACCAGACG |
The sequences are available through GenBank (http://www.ncbi.nlm.nih.gov/nuccore/) under the accession numbers listed above.
Western blot analysis
Total protein in the colon tissues was homogenized in ice-cold RIPA lysis buffer containing 50 mM Tris (pH 7.4), 150 mM NaCl, 1% sodium deoxycholate, 0.1% SDS, 10 mM NaF, 5 mM EDTA, 1 mM Na3VO4, and 1% protease inhibitor (Roche) for 1 min, incubated for 30 min on ice and centrifuged at 14000 g for 10 min. Protein concentration of liquid supernatant was determined using BCA assay kit (Thermo Scientific Pierce). The proteins were denatured, loaded on polyacrylamide gel (10 μg/well), separated by electrophoresis, and then transferred onto the PVDF membrane. This membrane was blocked with 5% defatted milk in TBST for 1 h and incubated at 4 °C overnight with primary antibodies against β-actin (internal control, Cell Signaling Technology), Bax, Bcl-2, ZO-1, ZO-2, claudin-1, or occluding (Santa Cruz). Subsequently, the membranes were incubated with HRP-conjugated secondary antibodies (Cell Signaling Technology), and blots were developed using the ECL detection reagents (Millipore) and analyzed by Image J software (Rawak Software, Inc. Germany).
Statistical analysis
Data were presented as means ± standard error (SEM) and imaged using Graphpad Prism5 software (GraphPad Prism, USA). Statistical significance between groups was evaluated by one-way ANOVA followed by the Dunnett’t test. Difference was considered significant at P < 0.05.
Results
BB mitigated the clinical symptoms in DSS-induced colitis mice
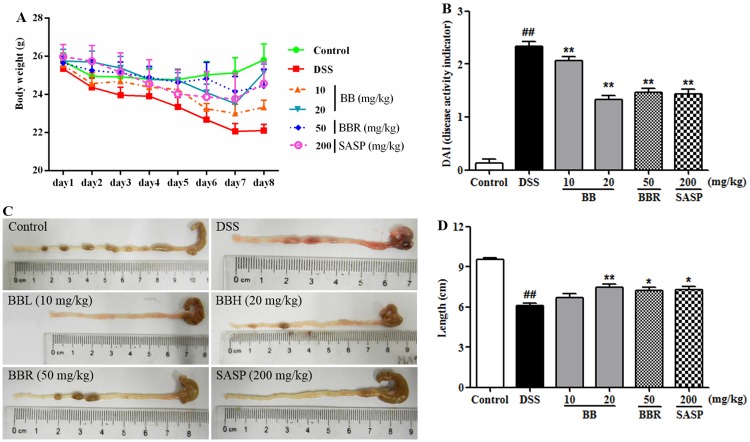
Consistent with previous study [31], challenging mice with DSS administration induced acute colitis characterized by bloody diarrhea, ulceration, colon shortening and loss of body weight, indicative of successful establishment of the UC murine model with typical symptoms. As shown in Fig 2A, the bodyweight of mice in control group was gradually increased, while the bodyweight of DSS group was sustainably and substantially reduced compared to the control group. In contrast, the bodyweight of mice in BB (20 mg/kg), BBR (50 mg/kg) or SASP (200 mg/kg) groups recovered greatly from day 5 to day 8.
Fig 2. BB remitted the clinical symptoms in mice with DSS-induced colitis.
(A) Bodyweight was shown as a mean value of daily weight from days 1 to 8. (B) DAI scores. (C) Gross morphology of colonic tissues. (D) Measuring result of gut length. Results are expressed as means ± SEM (n = 10). ##P < 0.01 vs. Control group,*P < 0.05 and**P < 0.01 vs. DSS group.
The DAI score, which is an indicator of the severity of colitis, is based on the results including weight loss, stool charter and fecal occult blood [32]. As shown in Fig 2B, DAI score for the DSS group was significantly higher as compared to the control group (P < 0.01). However, the DAI scores for the groups treated with BB (20 mg/kg), BBR or SASP were all significantly lower as compared to the DSS group (P < 0.05). As expected, colitis manifested severe intestinal inflammation, leading to colon shortening [5], a morphological indicator of colon inflammation. In accordance with the DAI, the colon of mice in DSS group was distinctly shorter than that of the control group on day 8, while mice treated with BB, BBR or SASP exhibited remarkably ameliorating colon shortening compared to the DSS-treated model mice (Fig 1C and 1D).
BB attenuated the colonic histopathological changes in mice colitis
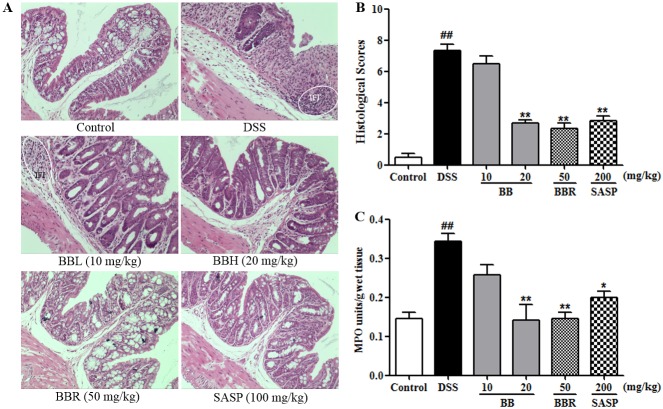
Histological characteristics of the colons were subsequently examined by H&E staining. Mice in control group maintained integrated normal colonic structures (Fig 2A), characteristic of clear outer membrane, muscular layer, submucosa and mucosa. However, DSS treatment elicited deteriorating pathological alternations, such as epithelium cell layer demolition, crypt lack, mucosa inflammation and cell infiltration, resulting in significantly higher histological scores as compared with the control group (Fig 3B). This colonic damage as determined pathologically was paralleled to that of macroscopically visible colonic injures. By contrast, mice treated with BB (20 mg/kg), BBR (50 mg/kg) or SASP (200 mg/kg) showed remarkably ameliorated intestinal injury, relieved mucosa inflammation and significantly decreased histological scores (Fig 3B) as compared to the DSS mice. Treatment with BB (10 mg/kg) was shown to only slightly ease the gut structural lesions, however, the histological score difference failed to reach statistical significance when compared with the DSS group.
Fig 3. BB mitigated the deteriorating colonic histopathological changes in mice with DSS-induced colitis.
(A) Representative H&E staining images of colonic sections from different treatments. (B) Histopathology scores. (C) MPO activity. The results shown are means ± SEM (n = 6). ##P < 0.01 vs. Control group, *P < 0.05 and **P < 0.01vs. DSS group.
MPO activity, an index to evaluate the neutrophil infiltration and inflammation, is believed to be proportional to the concentration of neutrophils in lesion tissue [33]. As shown in Fig 3C, DSS treatment significantly increased the MPO activity as compared with the control (P < 0.01). Whereas, BB (20 mg/kg), BBR (50 mg/kg) or SASP (200 mg/kg) treatment was found to appreciably decrease the elevated MPO activity induced by DSS, which was congruent with the histological findings.
BB decreased colonic cytokines concentration in mice with colitis
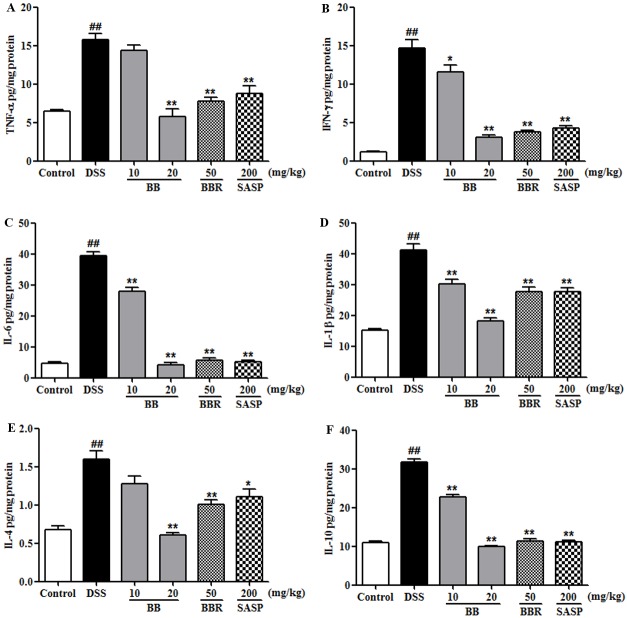
Development of colitis is often associated with chronic inflammation, characterized by inflammatory cellular infiltration and series of cytokines secretion, such as TNF-α, IFN-γ, IL-6, IL-1β, IL-4, and IL-10 [31, 34]. Cytokines perform essential roles in the intestinal immune system and are shown to have a central role in the pathophysiology of UC. Hence, we detected these above-mentioned indicators to estimate the potential anti-inflammatory effect of BB on DSS-induced colitis. Results indicated that DSS treatment induced remarkable augments of colonic levels of cytokines (TNF-α, IFN-γ, IL-6, IL-1β, IL-4 and IL-10) compared to the control group (Fig 4). However, treatment with BB (especially at the dose of 20 mg/kg), BBR (50 mg/kg) or SASP (200 mg/kg) obviously reversed these elevations induced by DSS (P < 0.05). These data indicated that treatment with BB effectively ameliorated the colonic inflammation caused by DSS.
Fig 4. BB suppressed colonic levels of inflammatory cytokines in mice with DSS-induced colitis.
(A) TNF-α, (B) IFN-γ, (C) IL-6, (D) IL-1β, (E) IL-4, and (F) IL-10 levels were examined by ELISA. The results shown are means ± SEM (n = 10). ##P < 0.01 vs. Control group, *P < 0.05 and **P < 0.01 vs. DSS group.
BB preserved intestinal barrier function in the colon of DSS-treated mice
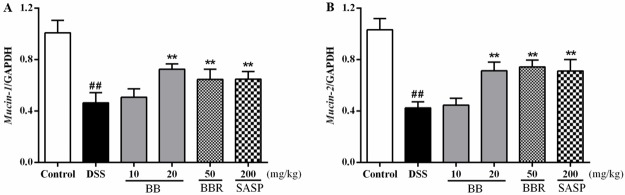
The colonic epithelium is composed of trefoil peptides such as mucin-1 and mucin-2, which play an important role in maintaining the barrier function of colon. To evaluate the potential effect of BB on colonic mucins, mRNA levels of the mucins (mucin-1 and mucin-2) in the colon of DSS-colitis mice were measured by RT-PCR. As shown in Fig 5, the gene expression of mucin-1 and mucin-2 was observably suppressed by DSS treatment relative to the control (P < 0.01). On the contrary, BB (20 mg/kg), BBR (50 mg/kg) and SASP (200 mg/kg) were shown to significantly upregulate the expression of these two genes (P < 0.01).
Fig 5. BB prevented disruption of intestinal integrity in mice with DSS-induced colitis.
Gene expression was normalized to GAPDH in each group. The results shown are means ± SEM (n = 10). ## P < 0.01 vs. Control group, *P < 0.05 and **P < 0.01 vs. DSS group.
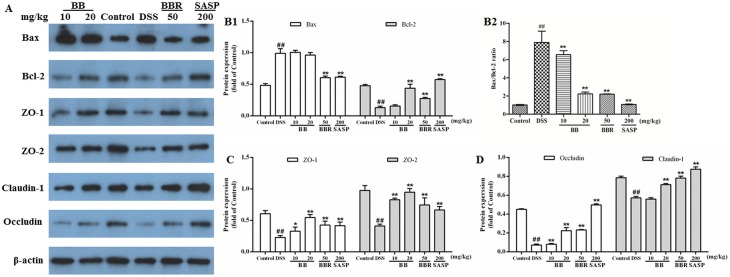
Intestinal epithelial tight junctions play a key role in protecting against inflammation, and disrupted tight junctions are a main cause of intestinal barrier dysfunction and inflammation. Apoptosis is also a well-known inducer of mucosal injury in many intestinal diseases, which can result in barrier dysfunction. Therefore, maintaining TJs and suppressing apoptosis to protect barrier function may provide benefits in the therapy of UC. To verify the involvement of apoptosis and epithelial TJ, the expression levels of apoptosis-associated proteins (Bax, Bcl-2) and TJ-associated proteins (ZO-1, ZO-2, claudin-1, occluding) were determined on colon tissues by Western blotting.
As shown in Fig 6A, the down-regulation of anti-apoptotic Bcl-2 and up-regulation of pre-apoptotic Bax were observed during DSS-induced colitis. By contrast, BB treatment markedly augmented the expression of Bcl-2 (P < 0.01) and slightly attenuated the expression of Bax, resulting in significantly decreased Bax/Bcl-2 ratio (P < 0.01) in colonic tissues. Furthermore, the protein expression of ZO-1, ZO-2, claudin-1 and occluding was all significantly down-regulated in mice treated with DSS alone, as compared with normal mice (P < 0.01). However, these proteins expression was prominently up-regulated by BB (especially at 20 mg/kg), BBR (50 mg/kg) and SASP (200 mg/kg) (Fig 6B and 6C). These results indicated that BB might prevent DSS-induced disruption of intestinal integrity by preserving tight junctional function and decreasing apoptosis.
Fig 6. BB alleviated the apoptosis and recovered the intestinal barrier function in mice with DSS-induced colitis.
Homogenates of colonic tissue were analyzed by Western blotting and representative images were shown (A). Quantitative results of expression levels of apoptosis-related proteins Bax and Bcl-2 (B1), Bax/Bcl-2 expression ratio (B2), tight junction-associated proteins (ZO-1 and ZO-2) (C), and occludin and claudin-1 (D). β-actin was used as a loading control. The results shown are means ± SEM (n = 6). ##P < 0.01 vs. Control group, *P < 0.05 and **P < 0.01 vs. DSS group.
Discussion
Berberine (BBR) is a commonly-used therapeutic agent for the treatment of gastroenteritis and infectious diarrhea in China. Previous study has suggested that BBR remitted DSS-induced colitis by inhibiting inflammatory responses in colonic macrophages and epithelial cells in DSS-treated mice [14]. Even though BBR possesses a low oral bioavailability, it has exhibited appreciable biological activities in vivo and the levels of its major metabolites like BB was relatively high [18]. These reports suggested that the metabolites might be active contributors to the biological activities of BBR in vivo. Indeed, various studies have revealed that the metabolites of BBR showed similar bioactivities. BB, the main primary metabolite of BBR, was more lipophilic and could be absorbed more efficiently in intestine than BBR [19]. Furthermore, BB was rapidly absorbed and widely distributed in various tissues [35]. Therefore, we hypothesized that BB might have similar beneficial or superior effect to BBR on UC. In the present work, pioneering endeavor was initiated to investigate the anti-UC effect of BB on DSS-induced colitis in comparison with BBR, and probe the possible underlying mechanism.
DSS-induced experimental colitis, one of the most widely used models of chemically induced colitis, has clinical symptoms similar to human UC [36–38]. In the present work, treatment with BB, as well as BBR, significantly ameliorated clinical symptoms of UC such as diarrhea, stool charter, bloody stool and bodyweight loss, thereby reducing the DAI score. Additionally, administration with BB (20 mg/kg) relieved signs of inflammation and mucosal injury as indicated by improved colon length and higher histological score, suggesting a protective effect of BB in the model of colitis. Noteworthy, BB was shown to exert similar effect to BBR and positive control SASP in attenuating DSS-induced UC with much smaller dosage.
Inflammatory responses, characteristic of gut barrier disruption in IBD, play a vital role in the pathogenesis of UC [39, 40]. Gut inflammation activates accumulated colonic macrophages, resulting in over-production of pro-inflammatory cytokines, such as TNF-α, IL-1β, IL-6 and IL-4, which further deteriorate UC [41] [42]. IFN-γ released by excessive function of Th1 cells is generally associated with intestinal inflammation especially in IBD [43, 44]. Furthermore, the level of MPO activity, proportional to the concentration of neutrophils in lesion tissue, was a common index to evaluate the neutrophil infiltration and inflammation [45]. Preceding investigations have reported BBR alleviated experimental colitis induced by DSS or trinitrobenzene sulfonic acid in rodent, which was closely associated with its anti-inflammatory activity. In this study, we found the induced productions of TNF-α, IFN-γ, IL-6, IL-4 and IL-1β by DSS treatment were dramatically suppressed by BB (especially at the dose of 20 mg/kg), BBR and SASP (Fig 4A–4E, P < 0.05). Besides, BB and BBR also decreased the levels of IL-10 in DSS-treated colitis mice (Fig 4F, P < 0.05). IL-10, which was mainly secreted by Treg cells, has been defined as anti-inflammatory cytokine. However, report has also suggested that T cells in UC patients expressed high level of IL-10 in intestine [46, 47], and high-level of IL-10 may result in the induction of pro-inflammatory cytokines in IBD [47]. The increased IL-10 in DSS mice in the present work was consistent with our preceding investigation [48] and other previous reports [49]. These results showed that suppression of inflammatory response in the colonic tissue of mice might contribute to the ameliorative effect of BB against DSS-induced colitis, which paralleled the histological evidence of protection.
The barrier dysfunction involved in the initiation and propagation of inflammation, and inflammatory cytokines could induce barrier dysfunction with vicious cycles. Most pro-inflammatory cytokines (e.g. IFN-γ, TNF-α, and IL-1β) could reduce key TJ-associated proteins such as intracytoplasmic proteins (ZO-1, ZO-2) and transmembrane proteins (occluding and claudin), resulting in disruption of intestinal TJ barrier and the development of intestinal inflammation [50]. Furthermore, the integrity of intestinal structure critically involves the function of the mucous layer, which comprised of mucin (primarily including mucin-1 and mucin-2) excreted by enterocyte, exerting lubricant and protective effect on intestinal tract [7]. Therefore, restoring intestinal barrier function may be a potentially beneficial strategy in the treatment of UC. Previous studies have indicated that BBR promoted barrier function in human retinal pigment epithelial cells, endothelial cells and Caco-2 human epithelial colorectal adenocarcinoma cells [51]. In this study, we observed the loss of colonic TJ (ZO-1, ZO-2, occludin and claudin-1) and colonic mucin (mucin-1 and mucin-2) expression in DSS-induced mice were dramatically suppressed by BB and BBR (Fig 6, P < 0.05). These results indicated that the ameliorative effect of BB against DSS-induced colitis might be tightly associated with its protection on intestinal mucosal barrier via enhancing the expression of TJ proteins and mucins.
Besides, increased apoptosis in intestinal epithelial cells also does serious damage to the mucosal barrier and induces gut inflammation. This has been reported in patients with UC, which suggested that the loss of epithelial cells in active UC was induced mainly by apoptosis in crypts of involved and adjacent uninvolved areas [52]. The same phenomenon also has been observed in CD patients [53] and murine models of colitis [54]. The key of keeping normal tissue homeostasis is to achieve balance of epithelial cell apoptosis and proliferation. The present study suggested that BB treatment significantly up-regulated the anti-apoptotic Bcl-2 protein expression and markedly decreased the Bax/Bcl-2 ratio, indicating the anti-apoptosis effect might contribute to the protection of BB against DSS-induced experimental UC.
In the current work, BB, the demethylation metabolite of BBR, was found to be more pharmacologically active than BBR in alleviating the DSS-induced colitis in mice, which was in concert with previous reports that BB possessed better hepatoprotective property [55] and OH scavenging activity [56] than BBR. These results suggested that the metabolite BB might contribute to the biological activities of BBR in vivo. The BB molecule is very similar to BBR except that the methoxy (OCH3) group at position 9 is replaced byhydroxyl group (OH) group, resulting in enhanced anti-UC activity. Indeed, the OH group at the C-9 position of BB was believed to be essential for its biological activities [56, 57]. Although the pharmacokinetic and pharmacodynamic relationships between BB and BBR in vivo merited further in-depth investigation, this observation provided important implication for the design of novel therapeutic entity with improved anti-UC efficacy. Furthermore, BB is also a naturally-occurring protoberberine alkaloid oriented from the medicinal plant Berberis vulgaris, which has been shown to possess anti-UC effect. Hence, it was deduced that BB might be one of the active principle obligatory for biological effect of Berberis vulgaris in the treatment of UC.
Since BB was a main metabolite of BBR, which serves as a safe and effective agent for the treatment of gastrointestinal diseases in clinic, BB was expected to provide a wide margin of safety. Furthermore, BB possessed similar anti-UC effect to BBR and the positive control SASP with much smaller dosage. Hence, BB might have the potential to be further developed into an effective candidate for the treatment of UC. Future studies for elucidating the detailed molecular mechanism underlying the anti-UC effect of BB, long-term safety and pharmacokinetics would likely pave an avenue for promising drug development process.
Conclusion
This was the first report to indicate that BB had pronounced protective effect on DSS-induced colitis by significantly easing the clinical symptoms, suppressing the inflammatory response and repairing mucosal barrier function. These effects were mediated, at least in part, via amelioration of pro-inflammatory cytokines production and modulation of TJ proteins, mucins genes and apoptosis-related proteins in the colonic tissue. The anti-UC effect of BB was similar to that of BBR and positive control SASP with much lower dosage. Therefore, we speculated that BB might be one of the active metabolites of BBR responsible for its anti-UC effect, and BBR together with BB might form the material basis of BBR in vivo. BB was believed to have the potential to be further developed into a promising therapeutic agent in the treatment of UC.
Acknowledgments
At the end of this paper, we would like to express our sincere thanks to all those who helped us during the writing of this paper, and express our deep gratitude to the colleagues who offered valuable suggestions to us in the academic studies. Without their help, this paper could not have reached its present form.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by: 1. Hongkong, Macao and Taiwan Science & Technology Cooperation Program of China (2014DFH30010), http://www.most.gov.cn/, (Zi-Ren Su: study design); 2. Science and Technology Planning Project of Guangdong Province, China (2013B090600007), http://www.gdstc.gov.cn/, (Zi-Ren Su: study design); 3. Science and Technology Planning Project of Guangdong Province, China (2013B090600026), http://www.gdstc.gov.cn/, (Hui-Fang Zeng: study design); 4. Guangdong International Cooperation Project (2013508102016), http://www.gdstc.gov.cn/, (Zi-Ren Su: study design); 5. Shenzhen Science and Technology Plan Project (ZDSYS201606081515458), http://www.szsti.gov.cn/, (Jian-Ping Chen: study design); 6. Shenzhen Science and Technology Plan Project (JCYJ20140408153331810), http://www.szsti.gov.cn/, (Jian-Ping Chen: study design); 7. Natural Science Foundation of Guangdong Province (2015A030310247), http://www.gdstc.gov.cn/, (Jian-Ping Chen: study design).
References
- 1.Lombardi VRM, Etcheverria I, Carrera I, Cacabelos R, Chacon AR. Prevention of Chronic Experimental Colitis Induced by Dextran Sulphate Sodium (DSS) in Mice Treated with FR91. Journal of Biomedicine and Biotechnology. 2012. doi: 10.1155/2012/826178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel CA. Review article: explaining risks of inflammatory bowel disease therapy to patients. Alimentary Pharmacology & Therapeutics. 2011;33(1):23–32. doi: 10.1111/j.1365-2036.2010.04489.x [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez-Canales M, Jimenez-Rivas R, Canales-Martinez MM, Garcia-Lopez AJ, Rivera-Yanez N, Nieto-Yanez O, et al. Protective Effect of Amphipterygium adstringens Extract on Dextran Sulphate Sodium-Induced Ulcerative Colitis in Mice. Mediators of Inflammation. 2016. doi: 10.1155/2016/8543561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perrier C, Rutgeerts P. Cytokine blockade in inflammatory bowel diseases. Immunotherapy. 2011;3(11):1341–52. doi: 10.2217/imt.11.122 [DOI] [PubMed] [Google Scholar]
- 5.Han F, Zhang H, Xia X, Xiong H, Song D, Zong X, et al. Porcine beta-defensin 2 attenuates inflammation and mucosal lesions in dextran sodium sulfate-induced colitis. Journal of immunology (Baltimore, Md: 1950). 2015;194(4):1882–93. Epub 2015/01/21. doi: 10.4049/jimmunol.1402300 . [DOI] [PubMed] [Google Scholar]
- 6.Kim T II. The Role of Barrier Dysfunction and Change of Claudin Expression in Inflammatory Bowel Disease. Gut and Liver. 2015;9(6):699–700. doi: 10.5009/gnl15430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayashi T, Ishida T, Motoya S, Itoh F, Takahashi T, Hinoda Y, et al. Mucins and immune reactions to mucins in ulcerative colitis. Digestion. 2001;63:28–31. doi: 10.1159/000051907 [DOI] [PubMed] [Google Scholar]
- 8.Laukoetter MG, Nava P, Nusrat A. Role of the intestinal barrier in inflammatory bowel disease. World journal of gastroenterology. 2008;14(3):401–7. Epub 2008/01/18. doi: 10.3748/wjg.14.401 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edelblum KL, Turner JR. The tight junction in inflammatory disease: communication breakdown. Current Opinion in Pharmacology. 2009;9(6):715–20. doi: 10.1016/j.coph.2009.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitic LL, Anderson JM. Molecular architecture of tight junctions. Annual review of physiology. 1998;60:121–42. Epub 1998/04/29. doi: 10.1146/annurev.physiol.60.1.121 . [DOI] [PubMed] [Google Scholar]
- 11.Fillon S, Robinson ZD, Colgan SP, Furuta GT. Epithelial Function in Eosinophilic Gastrointestinal Diseases. Immunology and Allergy Clinics of North America. 2009;29(1):171–+. doi: 10.1016/j.iac.2008.09.003 [DOI] [PubMed] [Google Scholar]
- 12.He K, Hu YR, Ma H, Zou ZY, Xiao YB, Yang Y, et al. Rhizoma Coptidis alkaloids alleviate hyperlipidemia in B6 mice by modulating gut microbiota and bile acid pathways. Biochimica Et Biophysica Acta-Molecular Basis of Disease. 2016;1862(9):1696–709. doi: 10.1016/j.bbadis.2016.06.006 [DOI] [PubMed] [Google Scholar]
- 13.Cui HS, Hayasaka S, Zhang XY, Hayasaka Y, Chi ZL, Zheng LS. Effect of berberrubine on interleukin-8 and monocyte chemotactic protein-1 expression in human retinal pigment epithelial cell line. Life Sciences. 2006;79(10):949–56. doi: 10.1016/j.lfs.2006.05.004 [DOI] [PubMed] [Google Scholar]
- 14.Yan F, Wang LH, Shi Y, Cao HW, Liu LP, Washington MK, et al. Berberine promotes recovery of colitis and inhibits inflammatory responses in colonic macrophages and epithelial cells in DSS-treated mice. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2012;302(5):G504–G14. doi: 10.1152/ajpgi.00312.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan GY, Wang GJ, Liu XD, Fawcett JP, Xie YY. The involvement of P-glycoprotein in berberine absorption. Pharmacology & Toxicology. 2002;91(4):193–7. doi: 10.1034/j.1600-0773.2002.t01-1-910403.x [DOI] [PubMed] [Google Scholar]
- 16.Caliceti C, Franco P, Spinozzi S, Roda A, Cicero AFG. Berberine: New Insights from Pharmacological Aspects to Clinical Evidences in the Management of Metabolic Disorders. Current Medicinal Chemistry. 2016;23(14):1460–76. doi: 10.2174/0929867323666160411143314 [DOI] [PubMed] [Google Scholar]
- 17.Wang K, Feng X, Chai L, Cao S, Qiu F. The metabolism of berberine and its contribution to the pharmacological effects. Drug metabolism reviews. 2017;49(2):139–57. Epub 2017/03/16. doi: 10.1080/03602532.2017.1306544 . [DOI] [PubMed] [Google Scholar]
- 18.Zuo F, Nakamura N, Akao T, Hattori M. Pharmacokinetics of berberine and its main metabolites in conventional and pseudo germ-free rats determined by liquid chromatography/ion trap mass spectrometry. Drug Metabolism and Disposition. 2006;34(12):2064–72. doi: 10.1124/dmd.106.011361 [DOI] [PubMed] [Google Scholar]
- 19.Spinozzi S, Colliva C, Camborata C, Roberti M, Ianni C, Neri F, et al. Berberine and Its Metabolites: Relationship between Physicochemical Properties and Plasma Levels after Administration to Human Subjects. Journal of Natural Products. 2014;77(4):766–72. doi: 10.1021/np400607k [DOI] [PubMed] [Google Scholar]
- 20.Minaiyan M, Ghannadi A, Mahzouni P, Jaffari-Shirazi E. Comparative Study of Berberis vulgaris Fruit Extract and Berberine Chloride Effects on Acetic Acid-Induced Colitis in Rats. Iranian Journal of Pharmaceutical Research. 2011;10(1):97–104. [PMC free article] [PubMed] [Google Scholar]
- 21.Tanideh N, Afaridi E, Mehrabani D, Azarpira N, Hosseinzadeh M, Amini M, et al. The Healing Effect of Berberis vulgaris in Acetic Acid-Induced Ulcerative Colitis in Rat. Middle East Journal of Scientific Research. 2014;21(8):1288–94. [Google Scholar]
- 22.Kim SH, Lee SJ, Lee JH, Sun WS, Kim JH. Antimicrobial activity of 9-O-acyl- and 9-O-alkylberberrubine derivatives. Planta Medica. 2002;68(3):277–81. doi: 10.1055/s-2002-23128 [DOI] [PubMed] [Google Scholar]
- 23.I T, I Y. Antitumor activity of 13-methyl-berberrubine derivatives. D—7901854. (- 0386-846X (Print)):- 469–74. [DOI] [PubMed] [Google Scholar]
- 24.Li YH, Li Y, Yang P, Kong WJ, You XF, Ren G, et al. Design, synthesis, and cholesterol-lowering efficacy for prodrugs of berberrubine. Bioorganic & Medicinal Chemistry. 2010;18(17):6422–8. doi: 10.1016/j.bmc.2010.06.106 [DOI] [PubMed] [Google Scholar]
- 25.Pongkittiphan V, Chavasiri W, Supabphol R. Antioxidant Effect of Berberine and its Phenolic Derivatives Against Human Fibrosarcoma Cells. Asian Pacific journal of cancer prevention: APJCP. 2015;16(13):5371–6. Epub 2015/08/01. . [DOI] [PubMed] [Google Scholar]
- 26.Li RJ, Wu JB, He Y, Hai L, Wu Y. Synthesis and in vitro evaluation of 12-(substituted aminomethyl) berberrubine derivatives as anti-diabetics. Bioorganic & Medicinal Chemistry Letters. 2014;24(7):1762–5. doi: 10.1016/j.bmcl.2014.02.032 [DOI] [PubMed] [Google Scholar]
- 27.Zhong M, Ma YX, Liu JX, Di DL. A new quaternary protoberberine alkaloid isolated from Dicranostigma leptopodum (Maxim) Fedde. Natural Product Research. 2014;28(8):507–10. doi: 10.1080/14786419.2013.879586 [DOI] [PubMed] [Google Scholar]
- 28.Li CZ, Xi Y, Li S, Zhao Q, Cheng WJ, Wang ZT, et al. Berberine ameliorates TNBS induced colitis by inhibiting inflammatory responses and Th1/Th17 differentiation. Molecular Immunology. 2015;67(2):444–54. doi: 10.1016/j.molimm.2015.07.013 [DOI] [PubMed] [Google Scholar]
- 29.Murano M, Maemura K, Hirata I, Toshina K, Nishikawa T, Hamamoto N, et al. Therapeutic effect of intracolonically administered nuclear factor kappa B (p65) antisense oligonucleotide on mouse dextran sulphate sodium (DSS)-induced colitis. Clinical and Experimental Immunology. 2000;120(1):51–8. doi: 10.1046/j.1365-2249.2000.01183.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li J, Zhong W, Wang WW, Hu SP, Yuan JH, Zhang B, et al. Ginsenoside Metabolite Compound K Promotes Recovery of Dextran Sulfate Sodium- Induced Colitis and Inhibits Inflammatory Responses by Suppressing NF-kappa B Activation. Plos One. 2014;9(2). doi: 10.1371/journal.pone.0087810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu YS, Li XQ, Chen JQ, Chen TJ, Shi ZM, Lei MN, et al. The pentacyclic triterpene Lupeol switches M1 macrophages to M2 and ameliorates experimental inflammatory bowel disease. International Immunopharmacology. 2016;30:74–84. doi: 10.1016/j.intimp.2015.11.031 [DOI] [PubMed] [Google Scholar]
- 32.Pan T, Guo HY, Zhang H, Liu AP, Wang XX, Ren FZ. Oral administration of Lactobacillus paracasei alleviates clinical symptoms of colitis induced by dextran sulphate sodium salt in BALB/c mice. Beneficial Microbes. 2014;5(3):315–22. doi: 10.3920/BM2013.0041 [DOI] [PubMed] [Google Scholar]
- 33.Choudhary S, Keshavarzian A, Yong S, Wade M, Bocckino S, Day BJ, et al. Novel antioxidants Zolimid and AEOL11201 ameliorate colitis in rats. Digestive Diseases and Sciences. 2001;46(10):2222–30. doi: 10.1023/a:1011975218006 [DOI] [PubMed] [Google Scholar]
- 34.Atreya R, Mudter J, Finotto S, Mullberg J, Jostock T, Wirtz S, et al. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: Evidence in Crohn disease and experimental colitis in vivo. Nature Medicine. 2000;6(5):583–8. doi: 10.1038/75068 [DOI] [PubMed] [Google Scholar]
- 35.Zhao YQ, Yang N, Fei F, Sun RB, Feng SQ, He J, et al. Sensitive Analysis and Pharmacokinetic Study of Berberrubine Using LC-MS/MS. Chinese Herbal Medicines. 2017;9(3). [Google Scholar]
- 36.Mennigen R, Nolte K, Rijcken E, Utech M, Loeffler B, Senninger N, et al. Probiotic mixture VSL#3 protects the epithelial barrier by maintaining tight junction protein expression and preventing apoptosis in a murine model of colitis. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2009;296(5):G1140–G9. doi: 10.1152/ajpgi.90534.2008 [DOI] [PubMed] [Google Scholar]
- 37.Seril DN, Liao J, Yang GY, Yang CS. Oxidative stress and ulcerative colitis-associated carcinogenesis: studies in humans and animal models. Carcinogenesis. 2003;24(3):353–62. Epub 2003/03/29. . [DOI] [PubMed] [Google Scholar]
- 38.Tanaka T. Animal models of carcinogenesis in inflamed colorectum: potential use in chemoprevention study. Current drug targets. 2012;13(14):1689–97. Epub 2012/11/13. . [DOI] [PubMed] [Google Scholar]
- 39.Salim SY, Soderholm JD. Importance of Disrupted Intestinal Barrier in Inflammatory Bowel Diseases. Inflammatory Bowel Diseases. 2011;17(1):362–81. doi: 10.1002/ibd.21403 [DOI] [PubMed] [Google Scholar]
- 40.Pastorelli L, De Salvo C, Mercado JR, Vecchi M, Pizarro TT. Central role of the gut epithelial barrier in the pathogenes of chronic intestinal inflammation: lessons learned from animal models and human genetics. Frontiers in Immunology. 2013;4 doi: 10.3389/fimmu.2013.00280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bain CC, Scott CL, Uronen-Hansson H, Gudjonsson S, Jansson O, Grip O, et al. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal immunology. 2013;6(3):498–510. Epub 2012/09/20. doi: 10.1038/mi.2012.89 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dohi T, Fujihashi K, Koga T, Shirai Y, Kawamura YI, Ejima C, et al. T helper type-2 cells induce ileal villus atrophy, goblet cell metaplasia, and wasting disease in T cell-deficient mice. Gastroenterology. 2003;124(3):672–82. doi: 10.1053/gast.2003.50092 [DOI] [PubMed] [Google Scholar]
- 43.Powrie F, Leach MW, Mauze S, Menon S, Caddle LB, Coffman RL. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;1(7):553–62. Epub 1994/10/01. . [DOI] [PubMed] [Google Scholar]
- 44.McGovern D, Powrie F. The IL23 axis plays a key role in the pathogenesis of IBD. Gut. 2007;56(10):1333–6. Epub 2007/09/18. doi: 10.1136/gut.2006.115402 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.El Kebir D, Jozsef L, Pan WL, Filep JG. Myeloperoxidase delays neutrophil apoptosis through CD11b/CD18 integrins and prolongs inflammation. Circulation Research. 2008;103(4):352–9. doi: 10.1161/01.RES.0000326772.76822.7a [DOI] [PubMed] [Google Scholar]
- 46.Melgar S, Yeung MMW, Bas A, Forsberg G, Suhr O, Oberg A, et al. Over-expression of interleukin 10 in mucosal T cells of patients with active ulcerative colitis. Clinical and Experimental Immunology. 2003;134(1):127–37. doi: 10.1046/j.1365-2249.2003.02268.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsuchiya K, Ikeda T, Batmunkh B, Choijookhuu N, Ishizaki H, Hotokezaka M, et al. Frequency of CD4+CD161+T Cell and Interleukin-10 Expression in Inflammatory Bowel Diseases. Acta Histochemica Et Cytochemica. 2017;50(1):21–8. doi: 10.1267/ahc.16035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qu C, Yuan ZW, Yu XT, Huang YF, Yang GH, Chen JN, et al. Patchouli alcohol ameliorates dextran sodium sulfate-induced experimental colitis and suppresses tryptophan catabolism. Pharmacological research. 2017;121:70–82. Epub 2017/05/01. doi: 10.1016/j.phrs.2017.04.017 . [DOI] [PubMed] [Google Scholar]
- 49.Mu HX, Liu J, Fatima S, Lin CY, Shi XK, Du B, et al. Anti-inflammatory Actions of (+)-3'alpha-Angeloxy-4'-keto-3',4'-dihydroseselin (Pd-Ib) against Dextran Sulfate Sodium-Induced Colitis in C57BL/6 Mice. J Nat Prod. 2016;79(4):1056–62. Epub 2016/02/26. doi: 10.1021/acs.jnatprod.5b01071 . [DOI] [PubMed] [Google Scholar]
- 50.Al-Sadi R, Boivin M, Ma T. Mechanism of cytokine modulation of epithelial tight junction barrier. Frontiers in bioscience (Landmark edition). 2009;14:2765–78. Epub 2009/03/11. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Valenzano MC, DiGuilio K, Mercado J, Teter M, To J, Ferraro B, et al. Remodeling of Tight Junctions and Enhancement of Barrier Integrity of the CACO-2 Intestinal Epithelial Cell Layer by Micronutrients. Plos One. 2015;10(7). doi: 10.1371/journal.pone.0133926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Iwamoto M, Koji T, Makiyama K, Kobayashi N, Nakane PK. Apoptosis of crypt epithelial cells in ulcerative colitis. The Journal of pathology. 1996;180(2):152–9. Epub 1996/10/01. doi: 10.1002/(SICI)1096-9896(199610)180:2<152::AID-PATH649>3.0.CO;2-Y . [DOI] [PubMed] [Google Scholar]
- 53.Di Sabatino A, Ciccocioppo R, Luinetti O, Ricevuti L, Morera R, Cifone AG, et al. Increased enterocyte apoptosis in inflamed areas of Crohn’s disease. Diseases of the Colon & Rectum. 2003;46(11):1498–507. [DOI] [PubMed] [Google Scholar]
- 54.Qin L, Yao ZQ, Chang Q, Zhao YL, Liu NN, Zhu XS, et al. Swimming attenuates inflammation, oxidative stress, and apoptosis in a rat model of dextran sulfate sodium-induced chronic colitis. Oncotarget. 2017;8(5):7391–404. doi: 10.18632/oncotarget.14080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.El-Salam MA, Mekky H, El-Naggar EMB, Ghareeb D, El-Demellawy M, El-Fiky F. Hepatoprotective properties and biotransformation of berberine and berberrubine by cell suspension cultures of Dodonaea viscosa and Ocimum basilicum. South African Journal of Botany. 2015;97:191–5. doi: 10.1016/j.sajb.2015.01.005 [Google Scholar]
- 56.Jang MH, Kim HY, Kang KS, Yokozawa T, Park JH. Hydroxyl radical scavenging activities of isoquinoline alkaloids isolated from Coptis chinensis. Archives of Pharmacal Research. 2009;32(3):341–5. doi: 10.1007/s12272-009-1305-z [DOI] [PubMed] [Google Scholar]
- 57.Kim SA, Kwon Y, Kim JH, Muller MT, Chung IK. Induction of topoisomerase II-mediated DNA cleavage by a protoberberine alkaloid, berberrubine. Biochemistry. 1998;37(46):16316–24. doi: 10.1021/bi9810961 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.