Abstract
Background
Congenital cytomegalovirus (cCMV) infection is the most prevalent congenital infection acquired worldwide, with higher incidence in developing countries and among HIV-exposed children. Less is known regarding vertical transmission of parvovirus B19 (B19V) and enterovirus (EV). We aimed to assess the prevalence of CMV, B19V and EV vertical transmission and compare results of screening of congenital CMV obtained from two different specimens in a semirural Mozambican maternity.
Methods
A cross sectional study was conducted among pregnant mothers attending Manhiça District Hospital upon delivery. Information on maternal risk factors was ascertained. Dried umbilical cord (DUC) samples were collected in filter paper for CMV, B19V and EV detection by real-time polymerase chain reaction (RT-PCR), and nasopharyngeal aspirates (NPA) to test for CMV by RT-PCR. Maternal blood samples and placental biopsy samples were also obtained to investigate CMV maternal serology, HIV status and immunopathology.
Results
From September 2014 to January 2015, 118 mothers/newborn pairs were recruited. Prevalence of maternal HIV infection was 31.4% (37/118). CMV RT-PCR was positive in 3/115 (2.6%) of DUC samples and in 3/96 (6.3%) of NPA samples obtained from neonates. The concordance of the RT-PCR assay through DUC with their correspondent NPA sample was moderate (Kappa = 0.42 and p<0.001. No differences on cCMV prevalence were found among HIV-exposed and unexposed. All (100%) mothers were seropositive for CMV IgG. RT-PCR of EV and B19V in DUC were both negative in all screened cases. No histological specific findings were found in placental tissues. No risk factors associated to vertical transmission of these viral infections were found.
Conclusions
This study indicates the significant occurrence of vertical transmission of CMV in southern Mozambique. Larger studies are needed to evaluate the true burden, clinical relevance and consequences of congenital infections with such pathogens in resource-constrained settings.
Background
Despite the impressive reduction in child mortality in the last decades, neonatal mortality has declined more slowly and now accounts for nearly half (45%) of all under-5 child deaths[1]. Congenital and perinatal infections are well-known causes of neonatal morbidity and mortality and stillbirths in high-income countries (HIC)[2–4]. However, estimates of the global burden of congenital infections and attributable stillbirths, neonatal disease, disability or deaths due to mother-to-child transmission (MTCT) of these infections in low-income countries (LIC) are limited on account of a generalized scarcity of data[5]. Despite the positive impact in terms of health outcomes shown by the introduction of screening and treatment policies for several pregnancy-relevant infections such as human immunodeficiency virus (HIV), syphilis or malaria[6, 7]; pathogens that may also be vertically transmitted beyond these infections are rarely the focus of clinical practice and research[8, 9].
Several viruses, including among others cytomegalovirus (CMV), parvovirus B19V (B19V) and enterovirus (EV), may cause mild and self-limiting clinical manifestations among infected pregnant women, but more severe or even life-threatening disease in their offsprings. The Zika virus epidemic of 2016 in Latin America has contributed to highlight the emerging threat that maternal viral infections may carry for the health of the foetus and newborn[10].
Despite being the most prevalent congenital infection worldwide, congenital CMV infection (cCMV) remains largely neglected in the developed and developing world[11]. Although the global prevalence of cCMV has been reported to vary from approximately 0.2% to 2% (mean 0.65%), most of these studies have been conducted in high-income regions of Europe, USA or Japan were prevalence of cCMV ranges between 0.6–0.7%[12–14]. Data from LIC varies substantially, with some estimates peaking at 6–14%[15–17]. Higher overall rates of cCMV are found in countries with higher maternal CMV seroprevalence[12, 13, 18] and among infants exposed to HIV during pregnancy. Indeed, maternal HIV infection is thought to significantly increase (from 2.3% to 10.3%) the prevalence of cCMV in those HIV-exposed infants compared to those born to HIV-negative mothers, both in industrialized countries and also in LIC[19–23]. Furthermore, CMV infection seems to play a role as a cofactor for HIV disease progression in HIV/CMV co-infected newborns[24].
Different methods have been evaluated for cCMV screening based on saliva, urine and blood specimens, being saliva and urine the generally considered most appropriate samples[25]. Virus isolation from saliva or urine in rapid culture has been traditionally considered the standard method for identification of infants with cCMV but such methods appear unfeasible to perform for large screening efforts [26]. In contrast, real-time polymerase chain reaction (RT-PCR), also considered as gold standard [26–28], allows large numbers of specimens to be screened at a relatively low cost. However, RT-PCR based methods applied to dried-blood-spot (DBS), tested in countries where DBS are routinely collected for newborn metabolic screening, have shown a low sensitivity as a screening methodology[26]. Dried umbilical cord (DUC) sample-based PCR assays have demonstrated utility for diagnosis of cCMV, as part of retrospective investigations of the underlying aetiology of hearing impairment[29, 30]. Other samples such as nasopharyngeal aspirates (NPA) which are easily obtained and simple to store have been used to investigate respiratory pathogens of public health importance[31, 32]. However, to our knowledge, NPAs have not been evaluated as a screening methodology for cCMV diagnosis, although the Child Health and Mortality Prevention Surveillance Network (CHAMPS) aiming to know cause of death through innovative techniques such as the minimally invasive tissue sampling (MITS), proposes NPA as a standard specimen for diagnosis of cCMV[33, 34].
Knowledge gaps regarding the burden of other congenital infections associated to fatal foetal outcomes, such as B19V and EV, remain significant. B19V can cause a variety of foetal complications including spontaneous abortion, non-immune hydrops foetalis or intrauterine foetal death[35]. The epidemiology of B19V infection in pregnancy has been well studied in industrialized countries whereby prevalence has been estimated to vary from 1 to 5% in pregnant women with transmission rates to the foetus ranging between 17–33%[36]. However, the burden of this infection during pregnancy in LIC has been rarely documented and studies investigating in these settings active B19V infection in newborns have not been conducted.
Enteroviruses, which include coxsackieviruses and echoviruses, cause about one billion infections every year worldwide but their consequences during pregnancy have been seldom described[37]. Transplacental transmission of EV has been associated to stillbirths, non-immune hydrops foetalis and also severe neonatal infections, although the epidemiology and characterization of neonatal outcomes is not well documented[37].
Although data in LIC remain insufficient, higher burden of congenital infections may be assumed in regions like Southern Mozambique where HIV is highly prevalent and effective vaccines against pathogens such as rubella are partially or even not implemented[15, 38–41].
This is a pilot study exploring congenital acquisition of CMV, B19V and EV determined at birth. We additionally aimed to compare the results of two simple screening methodologies using RT-PCR for cCMV investigation, using DUC and NPA specimens obtained from the newborns.
Methods
Study site
The study was conducted in Manhiça, a semi-rural site in Southern Mozambique. The Manhiça Health Research Centre (CISM) runs a Demographic Surveillance System (DSS) in the area linked to a Hospital Morbidity Surveillance System (HMSS) ongoing at the Manhiça District Hospital (MDH) including all admitted children. A detailed description of MDH, CISM and the study area can be found elsewhere[42]. MDH is the referral hospital for the Manhiça district, covering a population of circa 183,000 inhabitants. The MDH includes adult and paediatric wards, together with a maternity, where an average of 3500–4000 deliveries takes place annually. Around 85% of all deliveries are institutionalized (A. Nhacolo, personal communication). It also includes an outpatient department and an antenatal care (ANC) clinic where pregnant women are routinely followed. As part of the National policy, all pregnant women are invited to attend ANC clinic during their pregnancy, where HIV testing and syphilis screening are routinely offered. Malaria transmission of moderate intensity is perennial with some seasonality and, intermittent preventive treatment during pregnancy (IPTp) for malaria prevention is recommended[42]. HIV prevalence in Manhiça district is amongst the highest in the world, with rates estimated at around 29% at the ANC clinic [40]. In 2013, MDH introduced WHO-recommended Option B+ for the prevention of mother-to-child HIV transmission[43], which is offered to mothers free of charge. No proactive strategies to screen for risk factors of neonatal sepsis or to prevent it are currently implemented in Mozambique.
Study design and population
This observational pilot study was conducted at the delivery wards of MDH, between September 15th 2014 and January 15th 2015, running continuously during working hours (8:00–16:00) and working days. We recruited pregnant women upon delivery (regardless of gestational age (GA) and their offspring. Participants were eligible for inclusion if they were >18 years old and able and willing to participate in the study and to provide informed consent after an explanation of the study. As this was a pilot study, in order to obtain a more representative sample of the study population, only the first three women seen every day were approached for recruitment.
Definitions
Gestational age was defined using fundal height, measured from the top of the mother's uterus to the top of the mother's pubic symphysis and assessed by a nurse specialist in maternal child health. A preterm baby was defined as that with a gestational age at birth of <37 weeks and a stillbirth case as an intrauterine death occurring after 28 weeks of GA. Low-birth weight was defined as weight at birth <2.500 grams[44]. Microcephaly was defined following the WHO growth standards[45]. Congenital CMV, B19V or EV infections were defined as detection of viral DNA/RNA by RT-PCR in dried cord umbilical samples obtained from neonates at birth[33]. Although cCMV diagnosis through NPA has not been validated, we also explored prevalence of CMV through positive RT-PCR in NPA specimens. Positive HIV status was defined according to national guidelines, which required for mothers two positive rapid testing (an initial discriminatory diagnostic test (Determine®) and a confirmatory test(Unigold®); and for children ≤ 18 months of age a positive rapid test in addition to a confirmatory positive PCR test.
Study procedures
A placental biopsy and two drops of umbilical cord blood collected in filter paper (Whatman 903®, Florham Park, NJ) were obtained immediately after delivery in order to determine DNA/RNA of CMV, B19V and EV and assess placental histopathology. In addition, a NPA was collected from neonates within the first two hours from birth, using a bulb aspiration kit conveniently pre-filled with sterile saline (M-PRO NPAK nasopharyngeal aspiration kit®). At least 1ml of NPA specimen mixed with sterile saline was collected and stored with the objective of screening for CMV DNA. A blood sample was collected from mothers for assessing anti-CMV antibodies. Maternal HIV status was determined and recorded if not previously registered in antenatal source documents. Other screening test results routinely performed at the ANC clinic, such as syphilis screening (using Rapid Plasma Reagin) or haemoglobin determination were also recorded.
DNA extraction and real time -PCR for viral amplification
CMV and B19V DNA and EV RNA were extracted from 1 drop of DUC (around 50 ul) and CMV from 400 ul of NPA specimen by using the NucliSENS® easyMag® (bioMérieux, Marcy l’Etoile, France) instrument according to the manufacturer’s procedure and eluted in 25 μl of elution buffer for DUC and 50 ul for NPA. Samples were tested for the presence of CMV DNA, B19V DNA and EV RNA with three different real-time PCR, CMV Q- PCR alert Kit®, Elitech Group Molecular Diagnostics, RealStar® Parvovirus B19V kit®, Altona Diagnostics and and a published in-house real-time RT-PCR assay for EV detection [46]. Positive results with cycle threshold values above 40 were classified as negative. Appropriate positive and negative controls were included in all the experiments. Samples were considered as valid when a clear positive or negative result was obtained.
Seroprevalence of CMV among pregnant women
Serum collected from the mothers was tested for the presence of anti-CMV immunoglobuline G (IgG) antibodies (AB) using the electrochemiluminescence immunoassay “ECLIA” intended for use on Elecsys and cobas immunoassay analyzers, according to the manufacturer’s instructions. Positive, negative, and cut-off controls were included in all runs, and positive samples were retested to confirm initial positive result.
Placental biopsy
A placental biopsy was obtained with a scalpel blade and immediately placed into 10% buffered formalin for transport to the laboratory. Each placental sample was embedded in paraffin wax, sectioned in 5μm tissue pieces and stained with standard haematoxylin–eosin for histopathologic examination. Different histopathological parameters were assessed including a macroscopic description (weight, oedema, haemorrhage, infarct and calcifications) and a microscopic description (inflammation and presence of microorganisms). Additional sections were saved for histochemical staining (PAS, Grocott and Gram) performed in an automated stainer (BenchMark Special Stains- Ventana Roche), following commercial recommendations. An immunohistochemical assay was performed on formaline-fixed paraffin-embedded tissue sections according to standard procedures, using the Ventana-Roche automated immunostainer system (BenchMark XT-Ventana Roche®). Antigen retrieval was performed by heat-induced epitope retrieval and B19V (Master Diagnostica®, Granada, SP) and CMV (Cell Marque®, Rocklin, CA, USA) antibodies were applied according to manufacturing instructions.
Infant follow-up
Clinical examination of all newborns, including the evaluation of head circumference and Apgar score at minute 1, 5 and 10, was performed at birth. Dubowitz score for postnatal GA determination was done in live neonates at least 12h after birth[47]. Maternal and child HIV status were registered. All participants were followed using the HMSS in order to check any hospital admissions to MDH during the first six months after birth. No specific imaging or hearing screening was performed in these children.
Statistical analysis
All data were prospectively collected using standardized questionnaires, which were double entered in specific study databases, created using Openclinica software. Discrepancies were solved after comparison with the original source documents by a senior data clerk, and in close collaboration with the study clinicians. Statistical analyses were performed using Stata 14.1 (Stata Corp., College Station, TX). Study variables were counted and summarized in frequency tables. Univariate and multivariate analyses were performed to identify risk factors for CMV, B19V and EV neonatal infection, separately. Firth logistic regression was used in order to address issues of separability, small sample sizes and bias of the parameter estimates. Variables that were found to be significantly associated with CMV, B19V and EV acquisition in the univariate analysis together with those related at a significance level of p<0.10 were entered into a multivariate model. Agreement between the results of DUC RT-PCR assays and those of NPA RT-PCR was assessed through Kappa statistic.
Ethical considerations
This protocol and all supporting documentation (Informed consent documents, Study questionnaires) were approved by the local bioethics committee of CISM (Comité Institucional de Bioética para Saúde) and by the National Bioethics Committee of Maputo in Mozambique, and by the Ethics Committee of the Hospital Clínic in Barcelona, Spain. Participants were asked to express their willingness to participate in the study by signing (or thumb-printing in case they were illiterate) a consent form. Participation in this study was voluntary, and study-related procedures did not interfere with the pregnant women’s or children’s standard clinical care.
Results
Maternal characteristics
During the study period, 118 pregnant women were recruited upon delivery at MDH (Fig 1). Table 1 summarizes the socio-demographic and clinical characteristics of participant mothers. Median age of recruited women was 22 years (Interquartile range, IQR 19–29), with >75% being younger than 30 years of age. Nearly a third of women (31.4%, 37/118) were confirmed HIV positive and only one of 118 had a positive syphilis test. Malaria test results were registered as negative in 5/118 women and no information about this disease was available for the rest of mothers. CMV IgG AB were detected in all (100%) women.
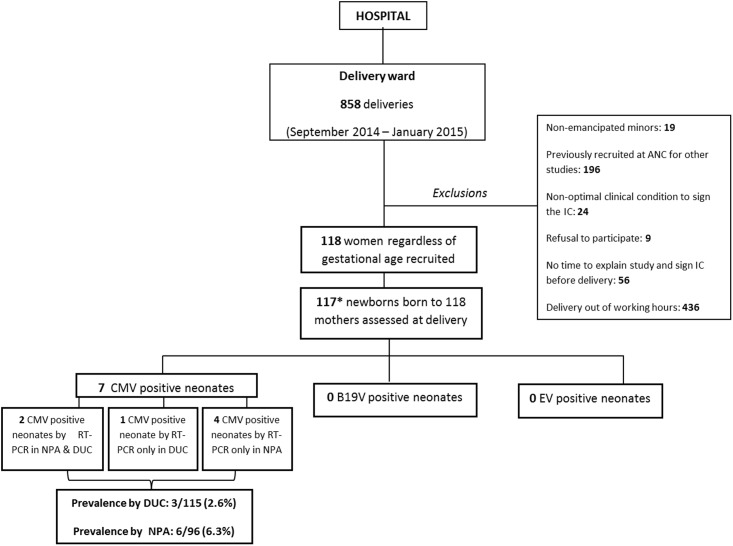
Fig 1. Study profile.
ANC: antenatal clinics; IC: informed consent; *One stillbirth whose mother refused to take samples. RT-PCR: real-time polymerase chain reaction, DUC: dried umbilical cord, NPA: nasopharyngeal aspirate.
Table 1. Socio-demographic and clinical characteristics of mothers participating in the study and univariate analysis of maternal risk factors associated to congenital CMV infection measured by different specimens.
| Total mothers recruited, n = 118 | Mothers of neonates CMV positive by DUC,n = 3ᶤ | Crude OR (95%CI)ᵟ | p-valueᵟ | Mothers of neonates CMV positive by NPA,n = 6ᶽ | Crude OR (95%CI)ᵟ | p-valueᵟ | |
|---|---|---|---|---|---|---|---|
| Socio-demographic characteristics | n (%) | n (%) | n (%) | ||||
| Age in years | 0.61 | 0.61 | |||||
| < 21 | 51 (43.2) | 2 (66.7) | 1.00 | 4 (66.7) | 1.00 | ||
| 22 to 29 | 40 (33.9) | 0 (0) | 0.23 (0.01–4.92) | 1 (16.7) | 0.23 (0.01–4.92) | ||
| ≥30 | 27 (22.9) | 1 (33.3) | 1.05 (0.13–8.42) | 1 (16.7) | 1.05 (0.13–8.42) | ||
| Seconday or tertiary education | 30 (25.4) | 0 (0.0) | 0.40 (0.02–8.06) | 0.55 | 3 (50.0) | 3.23 (0.68–15.36) | 0.14 |
| Employment | 6 (5.1) | 0 (0.0) | 2.34 (0.1150.28 | 0.59 | 0 (0.0) | 1.92 (0.89–41.36) | 0.68 |
| Obstetric History | n (%) | n (%) | n (%) | ||||
| Age of first pregnancy (median±IQR)) | 18.0 (17–20) | 18.0 (17–18) | 0.91 (0.27–3.11) | 0.89 | 18.0 (18–18) | 1.03 (0.76–1.41) | 0.84 |
| Gravidity (mean±SD) | 2.7 (±0.2) | 3.7 (±2.2) | 1.27 (0.81–2.01) | 0.30 | 1.5 (±0.2) | 0.63 (0.31–1.29) | 0.21 |
| Previous abortion n(%) | 11 (9.3) | 0 (0.0) | 1.26 (0.61–25.97) | 0.88 | 0 (0.0) | 0.75 (0.89–14.43) | 0.85 |
| History of current pregnancy | n (%) | n (%) | n (%) | ||||
| At least 3 antenatal visits during the pregnancy | 69 (58.5) | 2 (66.7) | 0.78 (0.98–6.15) | 0.81 | 5 (83.3) | 1.22 (0.19–8.01) | 0.83 |
| Gestational hypertension | 7 (6.1) | 0 (0.0) | 2.73 (0.12–62.25) | 0.53 | 1 (16.7) | 3.38 (0.47–24.33) | 0.23 |
| Vaginal discharge | 2 (1.7) | 0 (0.0) | 6.31 (0.25–157.63) | 0.26 | 0 (0.0) | 2.72 (0.12–62.86) | 0.53 |
| Investigations | n (%) | n (%) | n (%) | ||||
| Syphilis positive | 1 (0.5) | 0 (0.0) | 9.95 (0.34–290.3) | 0.32 | 0 (0.0) | 5.12 (0.19–140.92) | 0.33 |
| HIV positive | 37 (31.4) | 1 (2.7) | 1.26 (0.16–9.89) | 0.83 | 3 (50.0) | 2.19 (0.46–10.30) | 0.32 |
| HIV positive in HAART | 33 (89.2) | 1 (100.0) | 1.25 (0.11–4.43) | 0.86 | 3 (100.0) | 2.60 (0.48–14.04) | 0.25 |
| Anemia (<11g/dL) | 55 (72.4) | 0 (0.0) | 0.13 (0.01–3.26) | 0.21 | 2 (66.7) | 0.59 (0.07–4.88) | 0.63 |
| CMV IgG serum antibodies | 118 (100) | 3 (100) | 0.03 (0.00–1.81) | 0.09 | 6 (100) | 0.07 (0.06–3.92) | 0.20 |
IQR (interquartile range). SD: standard deviation. HAART: highly active antiretroviral therapy. CMV: cytomegalovirus. DUC: Dried umbilical cord.
ᶤResults based on 115 valid DUC samples. NPA: Nasopharingeal aspirate.
ᶽResults based on 96 valid NPA samples OR: odds ratio. CI; confindence intervals.
ᵟOR and P-value derived from Firth logistic regression.
Distal villous hypoplasia was observed in 27/117 (23.0%) of placental tissue samples evaluated. Neither microorganisms nor other significant findings were detected after evaluating tissue sections with standard tissue staining or in the immunohistochemical studies (Fig 2). No maternal risk factors associated to neonatal cCMV were found in the univariate analysis and thus, multivariate analysis was not performed (Table 1).
Fig 2. Placental histology from an infant with cytomegalovirus detected in dried umbilical cord blood.
Distal villous hypoplasia: many terminal villi are extremely small, with reduced stroma and capillaries number. No inmunohistochemical evidence of CMV could be found.
Neonatal outcomes and infant follow-up
One hundred and eighteen delivery outcomes from the 118 pregnant women were recorded (100%) at MDH. Characteristics of neonates born of mothers participating in the study are shown in Table 2. Neonatal outcomes included 110 live term babies, 7 preterm and 1 case of stillbirth born at 41 weeks of GA, whose mother refused permission to sample the foetus. Twenty-one (17.8%) newborns had low-birth-weight and three of them (2.5%) presented microcephaly at birth[48].
Table 2. Clinical characteristics of neonates born to mothers participating in the study.
| Neonates at birth n = 118 | Neonates CMV positive by DUC n = 3ᶧ | Crude OR (95%CI)ᵟ | p-valueᵟ | Neonates CMV positive by NPA n = 6ᶽ | Crude OR (95%CI)ᵟ | p-valueᵟ | |
|---|---|---|---|---|---|---|---|
| Clinical characteristics of newbornsᶤ | n (%) | n (%) | n (%) | ||||
| Gestational age at birth (live birth) | 0.65 | 0.21 | |||||
| Term newborn | 110 (94.1) | 3 (100.0) | 1.00 | 5 (83.3) | 1.00 | ||
| Pre term newborn | 7 (5.9) | 0 (0) | 2.01 (0.95–42.62) | 1 (16.7) | 3.54 (0.49–25.53) | ||
| Stillbirth | 1 (0.9) | 0 (0) | _ | _ | 0 (0) | _ | _ |
| Low birth weight (<2500gr) | 21 (17.8) | 0 (0) | 0.61 (0.03–12.21) | 0.75 | 1 (16.7) | 1.23 (0.19–8.09) | 0.22 |
| Head circumference in cm (mean±SD) | 35.6 (0.4) | 39.7 (5.2) | 1.13 (0.99–1.29) | 0.08 | 36.2 (2.9) | 2.69 (0.12–62.15) | 0.54 |
| Microcephaly | 3 (2.5) | (0) | 4.43 (0.19–103.20) | 0.35 | 1 (16.7) | 9.65 (1.07–87.12) | 0.04 |
| Perinatal asphyxia | 2 (1.7) | 0 (0) | 10.62 (0.36–309.66) | 0.17 | 0 (0) | 2.69 (0.12–62.15) | 0.54 |
| Jaundice | 0 (0) | 0 (0) | _ | _ | 0 (0) | _ | _ |
| Purpura | 0 (0) | 0 (0) | _ | _ | 0 (0) | _ | _ |
| Dubowitz neurological score (mean±SD)ˠ | 30.8 (0.2) | 31.0 (0.8) | 0.88 (0.64–1.22) | 0.46 | 31.7 (1.02) | 1.14 (0.79–1.65) | 0.47 |
| Malformations at birth | 2 (1.7) | 0 (0) | 6.25 (0.25–156.21) | 0.26 | 0 (0) | 2.69 (0.12–62.15) | 0.54 |
| Sick at birth | 2 (1.7) | 0 (0) | 6.25 (0.25–156.21) | 0.26 | 0 (0) | 2.69 (0.12–62.15) | 0.54 |
| Outcome | |||||||
| Admitted first 6 months of life | 7 (5.9) | 0 (0) | 2.01 (0.95–42.62) | 0.65 | 1 (16.7) | 3.55 (0.49–25.52) | 0.21 |
| Death after birthᶬ | 2 (1.7) | 0 (0) | 6.25 (0.25–156.21) | 0.26 | 0 (0) | 2.69 (0.12–62.15) | 0.54 |
CMV: cytomegalovirus. DUC: Dried umbilical cord.
ᶧResults based on 115 valid DUC samples. OR: odds ratio. CI; confidence intervals.
ᵟOR and P-value derived from Firth logistic. NPA: Nasopharingeal aspirate.
ᶽResults based on 96 valid NPA samples.
ᶤResults based on 117 patients. Mother of a stillbirth refused to take sample of the baby.
ˠSuboptimal neurological score following Dubowitz: <30.5.
ᶬ Death (including stillbirth and any deaths in the first 6 months after birth)
At least one specimen was collected from 117 neonates. A hundred and fifteen of the 117 DUC samples obtained were valid for viral determination. All of them were negative for B19V and EV.
Three of the 115 (2.6%) valid DUC samples were positive for CMV. This virus was also detected in 6/96 (6.3%) valid NPA samples obtained. A total of 7/117 (6.0%) newborns tested had at least one positive sample for CMV. Prevalence of cCMV infection measured by DUC among HIV-exposed neonates was 2.7% (1/37) and 8.1% (3/37) when CMV was detected through NPA, although no significant difference was found compared to the prevalence among HIV-unexposed in both cases (Table 1). One of the NPA-CMV positive cases was born preterm and with low birth weight while all neonates DUC-CMV positive were healthy at birth. CMV infection was associated to a higher risk of microcephaly at birth when CMV was determined by NPA (OR 9.65, 95% CI 1.07–87.12, p = 0.04) in the univariate analysis (Table 2).
Through HMSS, 7/117 infants born to mothers participating in the study were detected as admissions at MDH at least once during their first 6 months of life (Table 2). Two of them admitted within the first 24h after birth died due to clinical sepsis. Other causes of admission included perinatal asphyxia, bronchiolitis, diarrhoea and malaria. Among neonates were CMV was detected, only the preterm baby with a positive NPA for CMV was admitted at birth. In all of these cases, children were discharged fully recovered. No additional follow-up investigations were conducted.
Comparison of dried umbilical cord and nasopharyngeal aspirate RT-PCR Assays for CMV determination
A total of 94 pairs of samples (DUC and NPA) from 94 neonates were available for testing CMV by RT-PCR assay and comparing results. Of these, 2/94 neonates (2.1%) were positive for CMV by any test. A newborn with a positive RT-PCR assay through DUC had a negative NPA result, and the NPA RT-PCR assay also identified four additional neonates as infected although their DUC RT-PCR were negative (Fig 1). The overall concordance of the RT-PCR assay through DUC with their correspondent through NPA was moderate (Kappa = 0.42 and p<0.001).
Discussion
This study is a first attempt at proactively investigating vertical transmission of CMV, B19V and EV in Mozambique. The study was an opportunistic attempt to explore congenital transmission of these viruses, and was not specifically designed to evaluate the performance of the RT-PCR method of identification of cCMV in neonates as compared with the “gold-standard” for the detection of CMV (isolation of the virus in rapid cultures or PCR assay) since that has already been demonstrated[49–51]. The study shows a prevalence of cCMV infection assessed through dried umbilical cord samples of 2.6% and through NPA of 6.3%. Although specimens utilized in this study have not been validated for their use in cCMV diagnosis, they highlight a high burden of vertical CMV transmission. Contrarily, B19V and EV congenital transmission in the newborns was not found in this cohort although only DUC samples were analyzed. The prevalence of cCMV in this study detected through DUC was likely underestimated, as it has been demonstrated that real-time dried-blood-spot PCR assay has a lower sensitivity compared with the standard saliva rapid culture[26]. The dried umbilical cord samples have previously been used for retrospective studies to diagnose cCMV[29, 30] and although this sample type has never been compared to other accepted specimens for cCMV diagnosis, it would however be reasonable to assume that a positive DUC sample is likely a true positive. On the other hand, NPA has never been previously assessed as a specimen valid for cCMV diagnosis. A significant limitation of this study includes the fact that up to 30% of CMV-seropositive women will secrete CMV in their vaginal fluid, something that could possibly contaminate with CMV the nasopharynx of neonates[52]. Thus, the prevalence of 6.3% found in this study through NPA may potentially overestimate the true prevalence.
If the cCMV prevalence found through DUC can be a proxy of the true prevalence, these findings suggest higher prevalence rates than those reported in newborns from industrialized countries (<1%)[14] and falls within the range reported in a systematic review for developing countries (0.6%–6.1%)[53]. However, that review excluded studies reporting data from high at-risk populations for CMV transmission, such as HIV infected mothers, and restricted inclusion to studies having used the recommended gold standard specimens (saliva and urine) and techniques (cultures and PCR)[54]. Although it is likely that our cCMV prevalence was underestimated for the aforementioned reasons, other studies conducted in HIV endemic areas have reported similar congenital CMV prevalence to those shown here. A study conducted in Nigeria found a rate of 3.8% among neonates born to mothers with a low prevalence of HIV (4.8%)[55]. High prevalence of cCMV in HIV-exposed infants has been previously reported in two settings (South Africa and Zambia) with a maternal HIV prevalence similar to the one documented in our study area[23, 56–58]. The South African study was conducted among 748 HIV-exposed infants found a cCMV prevalence of 2.9%. No comparison with HIV-unexposed was performed [23]. Overall prevalence of cCMV among high-risk newborns admitted to a referral neonatal unit in Zambia was 3.8%, and 11.4% in those infants exposed to maternal HIV (Adjusted OR 6.66, 95% CI 2.13–20.9)[58]. HIV prevalence in our maternal cohort was very high (31.4%) and almost 90% of mothers were under highly active antiretroviral therapy (HAART), possibly explaining why the prevalence of cCMV was not higher among HIV-exposed newborns and why differences between HIV-exposed and unexposed neonates (2.7% vs. 2.6%%, OR 1.26 95% CI 0.16–9.89, p = 0.83) were not found[59]. Reasons to explain the difference between Zambian study results and our findings could be that the Zambian study was performed on admitted and therefore sick neonates and our study was conducted at time of birth. Another reason may be a better immune status of our HIV-infected mothers although information about HAART in this Zambian study was not available[58]. Immunosuppression in HIV-infected pregnant women likely leads to increased incidence of reinfection or reactivation, or prolonged CMV viral shedding, lengthening the opportunity for congenital transmission[21, 60]. Moreover, an association of CMV transmission with advanced maternal immunosuppression has been previously described[23].
No risk factors independently associated with cCMV were found. Primiparity, acute placental malaria, HIV-exposure and jaundice have all been reported as independent risk factors for cCMV infection by other authors[19, 28, 58, 61]. Caution is needed when interpreting our findings, since sample size was small and likely insufficient to detect significant differences among infected and uninfected neonates. It has been estimated that 90% of infected newborns do not have obvious clinical signs of CMV congenital infection and of them, only 15% will develop long-term neurological sequelae, especially, neurosensory hearing loss[14]. However, no further examinations and follow-up were performed beyond birth and burden of hearing loss was not explored. cCMV infection is an important cause of hearing loss and better strategies to detect children at risk of this complication in LIC should be developed.
Maternal CMV IgG seroprevalence in this study was 100%. Immune status of mothers in our cohort prior to pregnancy was unknown. In these cases, isolated detection of CMV IgG or detection of specific IgM AB are inadequate single measures to diagnose maternal primary infection[28]. Estimates suggest that around 75% of all cCMV cases in industrialized countries occur in babies born to women with non-primary maternal infection (those women who are CMV seropositive before pregnancy[28, 62–64]) and the risk of intrauterine transmission has been estimated at 1% in CMV-seropositive mothers[14]. IgG CMV seroprevalence in developing countries is generally over 90% by adolescence and over 95% by early adulthood[53] and it has been demonstrated that the incidence of cCMV infection is parallel to maternal seroprevalence[28], suggesting that most of cases of vertical transmission of this virus result from non-primary maternal infection and may be due to reactivation of latent virus or reinfection with a new cytomegalovirus strain[28, 62–64]. This is the reason behind the current recommendations issued by The International Congenital Cytomegalovirus Recommendations Group of not conducting universal serological screening of pregnant women for primary CMV infection[28].
Maternal infections with B19V, CMV and EV have been associated with intrauterine foetal death[65]. This study did not focus in cases of abortion and only one stillbirth was registered during recruitment, which hinders our capacity to associate them to the aforementioned pathogens.
Our findings suggest a low prevalence of B19V and EV infections in Southern Mozambique. No B19V congenital infection in newborns was found in this study. To our knowledge, no prospective screening of congenital B19V and EV infections among neonates in developing countries has been conducted to date. A South African study exploring prevalence of B19V infection among pregnant women found 20 asymptomatic neonates born to IgM positive mothers, although no samples from newborns were obtained[66]. Different African studies on maternal B19 V seroprevalence found IgG AB between 24.9 and 80% and IgM between 3 and 19%[66–70]. Unfortunately, maternal seroprevalence was not performed in this study and further research should be done in order to know the real burden of congenital B19V.
Similarly, all babies were negative for EV and maternal seroprevalence studies were not performed. Data on incidence and consequences of EV during pregnancy and clinical outcomes are globally scarce. Case reports and small case series have suggested that EV infection may cause foetal loss, and maternal infections during the 2nd and 3rd trimester may also lead to in utero foetal anomalies and death, but also to severe neonatal infections[37]. However, no prospective studies investigating EV maternal prevalence and risk of transmission have been conducted.
The few and unspecific anatomopathological findings documented in this study, consisting on an accelerated placental maturation, could have resulted from a variety of causes, including maternal preeclampsia or other states of maternal vascular underperfusion, or, more likely in our setting, due to malnutrition or infectious such as HIV or malaria. However, the lack of ovular membranes and umbilical cord in placental samples did not allow ruling out possible infections of these tissues.
Our study has several limitations. First, diagnosis of cCMV through DUC specimen is a methodology not recommended for neonatal CMV screening, particularly as urine and saliva have been demonstrated to be the most reliable specimens [26, 28]. The use of NPA for cCMV has not been validated and this specimen could be contaminated by maternal secretions contained CMV, leading to an overestimation of the true cCMV prevalence. However, a similar issue occurs with saliva samples, since the risk of contamination of saliva samples with breast milk exists. We however chose to use RT-PCR methods, with known good performance, in those samples available from the study, both because the kind of samples and the molecular screening techniques could be a good approach to study several viruses simultaneously. Further studies to validate these particular specimens for cCMV diagnosis would help to know their specificity and positive predictive value, and shed a light on why an important number of samples provided invalid results. Second, further follow-up and additional investigations beyond six months of life were not performed and hearing impairment, which is frequently progressive and usually develops later during infancy, was not measured, leading to a potential underestimation of CMV- associated morbidity. Third, the study lacked sufficient statistical power to detect independent risk factors associated to higher risk of congenital CMV, B19V and EV infections given the small sample size, and the few positive results. Additionally, it is known that both, B19V and EV show seasonal or even epidemic patterns [71, 72]. Considering that the epidemiology of these viruses in unknown in Mozambique, we may have failed, during the short study period, to capture natural transmission of the pathogens. Finally, B19V and EV were only assessed in dried umbilical cord samples at birth and maternal seroprevalence of these viruses or their presence in other fluids such as amniotic fluid was not investigated. Understanding also that these viruses may not be detected in cord blood in the absence of a true viraemia, it appears difficult to properly correlate the burden of maternal infection and the associated vertical transmission of these viruses. Thus, the prevalence observed in this study could be importantly underestimated.
In conclusion, despite the small sample size and the use of non-standard specimens, this study demonstrates that the prevalence of vertical transmission of CMV may be high in southern Mozambique, although further research is needed to assess its clinical relevance in this area. Studies validating the sensitivity and specificity of NPA for CMV screening would be required before considering it for clinical use. Congenital B19V and EV infections seem to be less prevalent in this area. Further research to evaluate the consequences of vertical transmission of these viral infections in resource-constrained settings is needed.
Acknowledgments
We are indebted to the children and mothers participating in the study. The work of the clinical officers, data managers, laboratory workers and laboratory coordinator was important for the successful completion of the study.
Data Availability
Data are originally from the PIPAC study and are restricted since it contains sensitive patient information. To request access to the data, please contact the Internal Scientific Committee and Local Ethical Committee of Centro de Investigação de Manhiça (+258 823041570) and from National Ethical Committee of Mozambique (+258 214308114/21427131). The authorisation of these two ethics committees is required for data access. The authors of the PIPAC study can be contacted for questions regarding the data at: lola.madrid@isglobal.org.
Funding Statement
Quique Bassat had during the duration of the study a fellowship from the program Miguel Servet of the ISCIII (Plan Nacional de I+D+I 2008-2011, grant number: CP11/00269). Lola Madrid had a fellowship from the program Río Hortega of the ISCIII (CM13/00260). Rosauro Varo has a fellowship from the program Rio Hortega of the ISCIII (CD16/00024). CISM receives financial support from the Spanish Agency for International Cooperation. ISGlobal is a member of the CERCA Programme, Generalitat de Catalunya. SM, TN, CMA, EJC, CE, CC, MI, BV, VF, CLD and CM have nothing to declare. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J, et al. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet. 2016;388(10063):3027–35. Epub 2016/11/15. doi: 10.1016/S0140-6736(16)31593-8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams Waldorf KM, McAdams RM. Influence of infection during pregnancy on fetal development. Reproduction. 2013;146(5):R151–62. Epub 2013/07/26. doi: 10.1530/REP-13-0232 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Del Pizzo J. Focus on diagnosis: congenital infections (TORCH). Pediatr Rev. 2011;32(12):537–42. Epub 2011/12/03. doi: 10.1542/pir.32-12-537 . [DOI] [PubMed] [Google Scholar]
- 4.Neu N, Duchon J, Zachariah P. TORCH infections. Clin Perinatol. 2015;42(1):77–103, viii Epub 2015/02/14. doi: 10.1016/j.clp.2014.11.001 . [DOI] [PubMed] [Google Scholar]
- 5.Lawn JE, Blencowe H, Waiswa P, Amouzou A, Mathers C, Hogan D, et al. Stillbirths: rates, risk factors, and acceleration towards 2030. Lancet. 2016. Epub 2016/01/23. doi: 10.1016/S0140-6736(15)00837-5 . [DOI] [PubMed] [Google Scholar]
- 6.Ansbro EM, Gill MM, Reynolds J, Shelley KD, Strasser S, Sripipatana T, et al. Introduction of Syphilis Point-of-Care Tests, from Pilot Study to National Programme Implementation in Zambia: A Qualitative Study of Healthcare Workers' Perspectives on Testing, Training and Quality Assurance. PLoS One. 2015;10(6):e0127728 Epub 2015/06/02. doi: 10.1371/journal.pone.0127728 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gous NM, Scott LE, Potgieter J, Ntabeni L, Sanne I, Stevens WS. Implementation of multiple point-of-care testing in two HIV antiretroviral treatment clinics in South Africa. J Acquir Immune Defic Syndr. 2015. Epub 2015/10/21. doi: 10.1097/QAI.0000000000000872 . [DOI] [PubMed] [Google Scholar]
- 8.Nkhoma ET, Bowman NM, Kalilani-Phiri L, Mwapasa V, Rogerson SJ, Meshnick SR. The effect of HIV infection on the risk, frequency, and intensity of Plasmodium falciparum parasitemia in primigravid and multigravid women in Malawi. Am J Trop Med Hyg. 2012;87(6):1022–7. doi: 10.4269/ajtmh.2012.12-0392 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newman L, Kamb M, Hawkes S, Gomez G, Say L, Seuc A, et al. Global estimates of syphilis in pregnancy and associated adverse outcomes: analysis of multinational antenatal surveillance data. PLoS Med. 2013;10(2):e1001396 Epub 2013/03/08. doi: 10.1371/journal.pmed.1001396 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sikka V, Chattu VK, Popli RK, Galwankar SC, Kelkar D, Sawicki SG, et al. The Emergence of Zika Virus as a Global Health Security Threat: A Review and a Consensus Statement of the INDUSEM Joint working Group (JWG). J Glob Infect Dis. 2016;8(1):3–15. Epub 2016/03/26. doi: 10.4103/0974-777X.176140 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mussi-Pinhata MM, Yamamoto AY, Moura Brito RM, de Lima Isaac M, de Carvalho e Oliveira PF, Boppana S, et al. Birth prevalence and natural history of congenital cytomegalovirus infection in a highly seroimmune population. Clin Infect Dis. 2009;49(4):522–8. Epub 2009/07/09. doi: 10.1086/600882 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dollard SC, Grosse SD, Ross DS. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev Med Virol. 2007;17(5):355–63. Epub 2007/06/02. doi: 10.1002/rmv.544 . [DOI] [PubMed] [Google Scholar]
- 13.Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol. 2007;17(4):253–76. Epub 2007/06/21. doi: 10.1002/rmv.535 . [DOI] [PubMed] [Google Scholar]
- 14.Manicklal S, Emery VC, Lazzarotto T, Boppana SB, Gupta RK. The "silent" global burden of congenital cytomegalovirus. Clin Microbiol Rev. 2013;26(1):86–102. Epub 2013/01/09. doi: 10.1128/CMR.00062-12 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gumbo H, Chasekwa B, Church JA, Ntozini R, Mutasa K, Humphrey JH, et al. Congenital and postnatal CMV and EBV acquisition in HIV-infected Zimbabwean infants. PLoS One. 2014;9(12):e114870 Epub 2014/12/19. doi: 10.1371/journal.pone.0114870 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang XW, Li F, Yu XW, Shi XW, Shi J, Zhang JP. Physical and intellectual development in children with asymptomatic congenital cytomegalovirus infection: a longitudinal cohort study in Qinba mountain area, China. J Clin Virol. 2007;40(3):180–5. Epub 2007/10/09. doi: 10.1016/j.jcv.2007.08.018 . [DOI] [PubMed] [Google Scholar]
- 17.Bello C, Whittle H. Cytomegalovirus infection in Gambian mothers and their babies. J Clin Pathol. 1991;44(5):366–9. Epub 1991/05/01. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Waters A, Jennings K, Fitzpatrick E, Coughlan S, Molloy EJ, De Gascun CF, et al. Incidence of congenital cytomegalovirus infection in Ireland: implications for screening and diagnosis. J Clin Virol. 2014;59(3):156–60. Epub 2014/01/28. doi: 10.1016/j.jcv.2013.12.007 . [DOI] [PubMed] [Google Scholar]
- 19.Duryea EL, Sanchez PJ, Sheffield JS, Jackson GL, Wendel GD, McElwee BS, et al. Maternal human immunodeficiency virus infection and congenital transmission of cytomegalovirus. Pediatr Infect Dis J. 2010;29(10):915–8. Epub 2010/05/01. doi: 10.1097/INF.0b013e3181e0ce05 . [DOI] [PubMed] [Google Scholar]
- 20.Guibert G, Warszawski J, Le Chenadec J, Blanche S, Benmebarek Y, Mandelbrot L, et al. Decreased risk of congenital cytomegalovirus infection in children born to HIV-1-infected mothers in the era of highly active antiretroviral therapy. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2009;48(11):1516–25. Epub 2009/04/25. doi: 10.1086/598934 . [DOI] [PubMed] [Google Scholar]
- 21.Kovacs A, Schluchter M, Easley K, Demmler G, Shearer W, La Russa P, et al. Cytomegalovirus infection and HIV-1 disease progression in infants born to HIV-1-infected women. Pediatric Pulmonary and Cardiovascular Complications of Vertically Transmitted HIV Infection Study Group. N Engl J Med. 1999;341(2):77–84. Epub 1999/07/08. doi: 10.1056/NEJM199907083410203 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mussi-Pinhata MM, Yamamoto AY, Figueiredo LT, Cervi MC, Duarte G. Congenital and perinatal cytomegalovirus infection in infants born to mothers infected with human immunodeficiency virus. J Pediatr. 1998;132(2):285–90. Epub 1998/03/20. . [DOI] [PubMed] [Google Scholar]
- 23.Manicklal S, van Niekerk AM, Kroon SM, Hutto C, Novak Z, Pati SK, et al. Birth prevalence of congenital cytomegalovirus among infants of HIV-infected women on prenatal antiretroviral prophylaxis in South Africa. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2014;58(10):1467–72. Epub 2014/02/26. doi: 10.1093/cid/ciu096 . [DOI] [PubMed] [Google Scholar]
- 24.Nigro G, Krzysztofiak A, Gattinara GC, Mango T, Mazzocco M, Porcaro MA, et al. Rapid progression of HIV disease in children with cytomegalovirus DNAemia. AIDS. 1996;10(10):1127–33. Epub 1996/09/01. . [PubMed] [Google Scholar]
- 25.Boppana SB, Ross SA, Shimamura M, Palmer AL, Ahmed A, Michaels MG, et al. Saliva polymerase-chain-reaction assay for cytomegalovirus screening in newborns. N Engl J Med. 2011;364(22):2111–8. Epub 2011/06/03. doi: 10.1056/NEJMoa1006561 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boppana SB, Ross SA, Novak Z, Shimamura M, Tolan RW Jr., Palmer AL, et al. Dried blood spot real-time polymerase chain reaction assays to screen newborns for congenital cytomegalovirus infection. JAMA. 2010;303(14):1375–82. Epub 2010/04/15. doi: 10.1001/jama.2010.423 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baquero-Artigao F, Grupo de estudio de la infeccion congenita por citomegalovirus de la Sociedad Espanola de Infectologia P. [Consensus document from the Spanish Society of Paediatric Infectious Diseases (SEIP) on the diagnosis and treatment of congenital cytomegalovirus infection]. An Pediatr (Barc). 2009;71(6):535–47. Epub 2009/10/10. doi: 10.1016/j.anpedi.2009.07.029 . [DOI] [PubMed] [Google Scholar]
- 28.Rawlinson WD, Boppana SB, Fowler KB, Kimberlin DW, Lazzarotto T, Alain S, et al. Congenital cytomegalovirus infection in pregnancy and the neonate: consensus recommendations for prevention, diagnosis, and therapy. Lancet Infect Dis. 2017;17(6):e177–e88. doi: 10.1016/S1473-3099(17)30143-3 . [DOI] [PubMed] [Google Scholar]
- 29.Tagawa M, Tanaka H, Moriuchi M, Moriuchi H. Retrospective diagnosis of congenital cytomegalovirus infection at a school for the deaf by using preserved dried umbilical cord. J Pediatr. 2009;155(5):749–51. Epub 2009/10/21. doi: 10.1016/j.jpeds.2009.04.033 . [DOI] [PubMed] [Google Scholar]
- 30.Koyano S, Araki A, Hirano Y, Fujieda K, Suzutani T, Yagyu K, et al. Retrospective diagnosis of congenital cytomegalovirus infection using dried umbilical cords. Pediatr Infect Dis J. 2004;23(5):481–2. Epub 2004/05/08. . [DOI] [PubMed] [Google Scholar]
- 31.Wejse C, Birkebaek NH, Nielsen LP, Andersen HM. Respiratory tract infections in cytomegalovirus-excreting and nonexcreting infants. Pediatr Infect Dis J. 2001;20(3):256–9. Epub 2001/04/17. . [DOI] [PubMed] [Google Scholar]
- 32.Meerhoff TJ, Houben ML, Coenjaerts FE, Kimpen JL, Hofland RW, Schellevis F, et al. Detection of multiple respiratory pathogens during primary respiratory infection: nasal swab versus nasopharyngeal aspirate using real-time polymerase chain reaction. Eur J Clin Microbiol Infect Dis. 2010;29(4):365–71. Epub 2010/01/30. doi: 10.1007/s10096-009-0865-7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.(CHAMPS) CHaMPS. Determination of Cause of Death (DeCoDe) Diagnosis Standards: guidance for standardized interpretation of CHAMPS data. [cited 2017 Nov 17]. https://champshealth.org/wp-content/uploads/2017/10/CHAMPS-Diagnosis-Standards-for-DeCoDe-1.pdf. 2017.
- 34.Farag TH, Koplan JP, Breiman RF, Madhi SA, Heaton PM, Mundel T, et al. Precisely Tracking Childhood Death. Am J Trop Med Hyg. 2017;97(1):3–5. Epub 2017/07/19. doi: 10.4269/ajtmh.16-0302 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Norbeck O, Papadogiannakis N, Petersson K, Hirbod T, Broliden K, Tolfvenstam T. Revised clinical presentation of parvovirus B19-associated intrauterine fetal death. Clin Infect Dis. 2002;35(9):1032–8. Epub 2002/10/18. doi: 10.1086/342575 . [DOI] [PubMed] [Google Scholar]
- 36.Ergaz Z, Ornoy A. Parvovirus B19 in pregnancy. Reprod Toxicol. 2006;21(4):421–35. doi: 10.1016/j.reprotox.2005.01.006 . [DOI] [PubMed] [Google Scholar]
- 37.Mereaux J, Picone O, Vauloup-Fellous C, Khediri Z, Benachi A, Mandelbrot L, et al. [Enterovirus infection during pregnancy: Underestimated cause of fetal and neonatal complications?]. Gynecol Obstet Fertil Senol. 2017;45(4):231–7. doi: 10.1016/j.gofs.2017.02.004 . [DOI] [PubMed] [Google Scholar]
- 38.Bollen LJ, Whitehead SJ, Mock PA, Leelawiwat W, Asavapiriyanont S, Chalermchockchareonkit A, et al. Maternal herpes simplex virus type 2 coinfection increases the risk of perinatal HIV transmission: possibility to further decrease transmission? AIDS. 2008;22(10):1169–76. Epub 2008/06/06. doi: 10.1097/QAD.0b013e3282fec42a . [DOI] [PubMed] [Google Scholar]
- 39.Hoffmann CJ, Thio CL. Clinical implications of HIV and hepatitis B co-infection in Asia and Africa. Lancet Infect Dis. 2007;7(6):402–9. Epub 2007/05/25. doi: 10.1016/S1473-3099(07)70135-4 . [DOI] [PubMed] [Google Scholar]
- 40.Gonzalez R, Munguambe K, Aponte J, Bavo C, Nhalungo D, Macete E, et al. High HIV prevalence in a southern semi-rural area of Mozambique: a community-based survey. HIV Med. 2012;13(10):581–8. Epub 2012/04/17. doi: 10.1111/j.1468-1293.2012.01018.x . [DOI] [PubMed] [Google Scholar]
- 41.Cutland CL, Schrag SJ, Zell ER, Kuwanda L, Buchmann E, Velaphi SC, et al. Maternal HIV infection and vertical transmission of pathogenic bacteria. Pediatrics. 2012;130(3):e581–90. doi: 10.1542/peds.2011-1548 . [DOI] [PubMed] [Google Scholar]
- 42.Sacoor C, Nhacolo A, Nhalungo D, Aponte JJ, Bassat Q, Augusto O, et al. Profile: Manhica Health Research Centre (Manhica HDSS). Int J Epidemiol. 2013;42(5):1309–18. Epub 2013/10/26. doi: 10.1093/ije/dyt148 . [DOI] [PubMed] [Google Scholar]
- 43.WHO. Use of Antiretroviral Drugs for Treating Pregnant Women and Preventing HIV Infection in Infants. Geneva, Switzerland: World Health Organization, 2012. [PubMed] [Google Scholar]
- 44.WHO. New born with low birth weight. [cited 2017 Nov 17]. http://www.who.int/whosis/whostat2006NewbornsLowBirthWeight.pdf. Geneva, Switzerland 2006.
- 45.WHO. Partnership for Maternal, Newborn & Child Health, World Health Organization. Countdown to 2015: Building a Future for Women and Children, Mozambique Country Reports. Geneve: World Health Organization, 2012. [Google Scholar]
- 46.Selva L, Martinez-Planas A, Garcia-Garcia JJ, Casadevall R, Luaces C, Munoz-Almagro C. Comparison of an in-house real-time RT-PCR assay with a commercial assay for detection of enterovirus RNA in clinical samples. Eur J Clin Microbiol Infect Dis. 2012;31(5):715–9. doi: 10.1007/s10096-011-1364-1 . [DOI] [PubMed] [Google Scholar]
- 47.Dubowitz LM, Dubowitz V, Goldberg C. Clinical assessment of gestational age in the newborn infant. J Pediatr. 1970;77(1):1–10. Epub 1970/07/01. . [DOI] [PubMed] [Google Scholar]
- 48.Quinto L, Garcia-Basteiro AL, Bardaji A, Gonzalez R, Padilla N, Martinez-Espinosa FE, et al. The Challenge of Assessing Microcephaly in the Context of the Zika Virus Epidemic. J Trop Pediatr. 2017. Epub 2017/03/24. doi: 10.1093/tropej/fmx015 . [DOI] [PubMed] [Google Scholar]
- 49.Demmler GJ, Buffone GJ, Schimbor CM, May RA. Detection of cytomegalovirus in urine from newborns by using polymerase chain reaction DNA amplification. J Infect Dis. 1988;158(6):1177–84. Epub 1988/12/01. . [DOI] [PubMed] [Google Scholar]
- 50.Tsai CH, Tsai FJ, Shih YT, Wu SF, Liu SC, Tseng YH. Detection of congenital cytomegalovirus infection in Chinese newborn infants using polymerase chain reaction. Acta Paediatr. 1996;85(10):1241–3. Epub 1996/10/01. . [DOI] [PubMed] [Google Scholar]
- 51.Warren WP, Balcarek K, Smith R, Pass RF. Comparison of rapid methods of detection of cytomegalovirus in saliva with virus isolation in tissue culture. J Clin Microbiol. 1992;30(4):786–9. Epub 1992/04/01. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stagno S, Pass RF, Dworsky ME, Alford CA, Jr. Maternal cytomegalovirus infection and perinatal transmission. Clin Obstet Gynecol. 1982;25(3):563–76. Epub 1982/09/01. . [DOI] [PubMed] [Google Scholar]
- 53.Lanzieri TM, Dollard SC, Bialek SR, Grosse SD. Systematic review of the birth prevalence of congenital cytomegalovirus infection in developing countries. Int J Infect Dis. 2014;22:44–8. Epub 2014/03/19. doi: 10.1016/j.ijid.2013.12.010 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McGovern LM, Boyce TG, Fischer PR. Congenital infections associated with international travel during pregnancy. J Travel Med. 2007;14(2):117–28. Epub 2007/03/21. doi: 10.1111/j.1708-8305.2006.00093.x . [DOI] [PubMed] [Google Scholar]
- 55.Olusanya BO, Slusher TM, Boppana SB. Prevalence of congenital cytomegalovirus infection in Nigeria: a pilot study. Pediatr Infect Dis J. 2015;34(3):322–4. doi: 10.1097/INF.0000000000000555 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Phillips T, Brittain K, Mellins CA, Zerbe A, Remien RH, Abrams EJ, et al. A Self-Reported Adherence Measure to Screen for Elevated HIV Viral Load in Pregnant and Postpartum Women on Antiretroviral Therapy. AIDS Behav. 2017;21(2):450–61. Epub 2016/06/10. doi: 10.1007/s10461-016-1448-0 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stringer EM, Chintu NT, Levy JW, Sinkala M, Chi BH, Muyanga J, et al. Declining HIV prevalence among young pregnant women in Lusaka, Zambia. Bull World Health Organ. 2008;86(9):697–702. Epub 2008/09/18. doi: 10.2471/BLT.07.045260 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mwaanza N, Chilukutu L, Tembo J, Kabwe M, Musonda K, Kapasa M, et al. High rates of congenital cytomegalovirus infection linked with maternal HIV infection among neonatal admissions at a large referral center in sub-Saharan Africa. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2014;58(5):728–35. Epub 2013/11/23. doi: 10.1093/cid/cit766 . [DOI] [PubMed] [Google Scholar]
- 59.Frederick T, Homans J, Spencer L, Kramer F, Stek A, Operskalski E, et al. The effect of prenatal highly active antiretroviral therapy on the transmission of congenital and perinatal/early postnatal cytomegalovirus among HIV-infected and HIV-exposed infants. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2012;55(6):877–84. Epub 2012/06/08. doi: 10.1093/cid/cis535 . [DOI] [PubMed] [Google Scholar]
- 60.Mostad SB, Kreiss JK, Ryncarz AJ, Overbaugh J, Mandaliya K, Chohan B, et al. Cervical shedding of cytomegalovirus in human immunodeficiency virus type 1-infected women. J Med Virol. 1999;59(4):469–73. Epub 1999/10/27. . [PubMed] [Google Scholar]
- 61.van der Sande MA, Kaye S, Miles DJ, Waight P, Jeffries DJ, Ojuola OO, et al. Risk factors for and clinical outcome of congenital cytomegalovirus infection in a peri-urban West-African birth cohort. PLoS One. 2007;2(6):e492 Epub 2007/06/07. doi: 10.1371/journal.pone.0000492 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ornoy A, Diav-Citrin O. Fetal effects of primary and secondary cytomegalovirus infection in pregnancy. Reprod Toxicol. 2006;21(4):399–409. doi: 10.1016/j.reprotox.2005.02.002 . [DOI] [PubMed] [Google Scholar]
- 63.Stagno S, Pass RF, Dworsky ME, Henderson RE, Moore EG, Walton PD, et al. Congenital cytomegalovirus infection: The relative importance of primary and recurrent maternal infection. N Engl J Med. 1982;306(16):945–9. doi: 10.1056/NEJM198204223061601 . [DOI] [PubMed] [Google Scholar]
- 64.Wang C, Zhang X, Bialek S, Cannon MJ. Attribution of congenital cytomegalovirus infection to primary versus non-primary maternal infection. Clin Infect Dis. 2011;52(2):e11–3. Epub 2011/02/04. doi: 10.1093/cid/ciq085 . [DOI] [PubMed] [Google Scholar]
- 65.Petersson K, Norbeck O, Westgren M, Broliden K. Detection of parvovirus B19, cytomegalovirus and enterovirus infections in cases of intrauterine fetal death. J Perinat Med. 2004;32(6):516–21. doi: 10.1515/JPM.2004.128 . [DOI] [PubMed] [Google Scholar]
- 66.Schoub BD, Blackburn NK, Johnson S, McAnerney JM. Primary and secondary infection with human parvovirus B19 in pregnant women in South Africa. S Afr Med J. 1993;83(7):505–6. . [PubMed] [Google Scholar]
- 67.Emiasegen SE, Nimzing L, Adoga MP, Ohagenyi AY, Lekan R. Parvovirus B19 antibodies and correlates of infection in pregnant women attending an antenatal clinic in central Nigeria. Mem Inst Oswaldo Cruz. 2011;106(2):227–31. . [DOI] [PubMed] [Google Scholar]
- 68.Mirambo M MF, Majigo M, Mushi MF, Moremi N, Seni J, Matovelo D, Mshana SE. The magnitude and correlates of Parvovirus B19 infection among pregnant women attending antenatal clinics in Mwanza, Tanzania. BMC Pregnancy and Childbirth. 2017;17(176). doi: 10.1186/s12884-017-1364-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schwarz TF, Gurtler LG, Zoulek G, Deinhardt F, Roggendorf M. Seroprevalence of human parvovirus B19 infection in Sao Tome and Principe, Malawi and Mascarene Islands. Zentralbl Bakteriol. 1989;271(2):231–6. . [DOI] [PubMed] [Google Scholar]
- 70.Volker F, Cooper P, Bader O, Uy A, Zimmermann O, Lugert R, et al. Prevalence of pregnancy-relevant infections in a rural setting of Ghana. BMC Pregnancy Childbirth. 2017;17(1):172 doi: 10.1186/s12884-017-1351-3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shapiro D, Bodinayake CK, Nagahawatte A, Devasiri V, Kurukulasooriya R, Hsiang J, et al. Burden and Seasonality of Viral Acute Respiratory Tract Infections among Outpatients in Southern Sri Lanka. Am J Trop Med Hyg. 2017;97(1):88–96. doi: 10.4269/ajtmh.17-0032 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Young NS, Brown KE. Parvovirus B19. N Engl J Med. 2004;350(6):586–97. doi: 10.1056/NEJMra030840 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are originally from the PIPAC study and are restricted since it contains sensitive patient information. To request access to the data, please contact the Internal Scientific Committee and Local Ethical Committee of Centro de Investigação de Manhiça (+258 823041570) and from National Ethical Committee of Mozambique (+258 214308114/21427131). The authorisation of these two ethics committees is required for data access. The authors of the PIPAC study can be contacted for questions regarding the data at: lola.madrid@isglobal.org.