Abstract
Early brain injury (EBI) plays a key role in determining the prognosis of patients suffering from subarachnoid hemorrhage (SAH). Resveratrol, a natural polyphenol, serves a neuroprotection function on EBI after SAH. However, the potential mechanism of resveratrol on EBI remains to be elucidated. Akt, also known as protein kinase B, and mammalian target of rapamycin (mTOR), the downstream protein of Akt, play key roles in cell survival and apoptosis, cell cycle regulation, and cellular protein homeostasis. In the present study, we examined the effect of resveratrol on EBI and their potential relationship with the Akt/mTOR pathway, autophagy, and apoptosis. Rats received intraperitoneal administration of resveratrol or vehicle immediately after establishing SAH model. We found that mortality and brain edema were significantly lower, whereas the neurological score was higher for resveratrol-treated rats. HE staining showed that resveratrol significantly reduced the neuronal pyknosis and swelling in the resveratrol-treated rats compared with SAH rats. The results were assessed by western blot, reverse transcription-PCR , and immunohistochemistry and immunofluorescence at 24 h after injury to determine changes in the expression of the Akt/mTOR signaling pathway, autophagy, and apoptosis proteins. Western blot analysis showed that the expression of beclin-1, LC3-II, LC3-II/LC3-I, and Bcl-2 was increased in resveratrol-treated rats, whereas the expression of p-Akt, p-mTOR, p62, cleaved caspase-3, caspase-9, and Bcl-2-associated X protein was decreased. Immunohistochemistry analysis of beclin-1, LC3-B treated with resveratrol alone or in combination with 3-methyladenine (autophagy inhibitor) suggested that resveratrol induced the autophagy process and the inhibitor blocked the occurrence of autophagy, and also increased the number of terminal deoxynucleotidyl transferase-mediated digoxigenin-DUTP-biotin nick-end labeling (+) cells. Taken together, these findings indicate that resveratrol exerts neuroprotective effects on EBI after SAH by regulating autophagy and apoptosis mediated by the Akt/mTOR pathway.
Keywords: Akt/mTOR pathway, apoptosis, autophagy, resveratrol, subarachnoid hemorrhage
Introduction
Subarachnoid hemorrhage (SAH) is a devastating disease that results from sudden cerebrovascular rupture and blood flow to the subarachnoid space, which can be divided into spontaneous SAH and traumatic SAH. Early brain injury (EBI) is considered to be the major factor for the poor prognosis associated with high morbidity and mortality after SAH, which occurs within 72 h after bleeding; thus, it is important to treat it as early as possible 1. Thus, the treatment of EBI is considered to be the principal goal for patients with SAH.
Autophagy is a phenomenon that exists widely in eukaryotic cells. Autophagy refers to the ability of cells to damage and degrade proteins and transport organelles to lysosomes for digestion and degradation to achieve the metabolic needs of the cells themselves and the renewal of associated organelles. Previous studies have suggested that autophagy was activated in ischemic stroke and cerebral hemorrhage animal models 2,3. In the SAH model, it was reported that autophagy-related pathways are activated, but the role of autophagy and the potential mechanism was not very clear.
Recently, studies have confirmed that autophagy is closely related to apoptosis, and apoptosis is a process of cell death caused by intracranial and extracorporeal death, also known as programmed cell death. Apoptosis is characterized by blebbing, cell shrinkage, nuclear fragmentation, chromatin condensation, and chromosomal DNA fragmentationglobal mRNA decay 4. Studies have been carried out in which animal experiments have confirmed that apoptosis exists in the early stage of spontaneous SAH, and the pathophysiological changes adversely affect the prognosis of patients 1. Autophagy and the morphological characteristics of apoptosis can develop at the same time in the same cell 5.
Resveratrol is a polyphenolic compound mainly present in plants such as grapes, veratrum, and polygonum cuspidatum. Some pharmacological studies found that resveratrol promotes antiplatelet aggregation, acts as an antioxidant, inhibits arachidonic acid and its metabolite formation, plays a role in tumor chemoprevention, and so on 6,7. Research on the neuroprotective effect of resveratrol has become a hotspot in the field of neuroscience. A previous study found that resveratrol induced autophagy by inhibiting the Akt/mammalian target of rapamycin (mTOR) signaling pathway 8. Some studies have indicated that resveratrol can serve a neuroprotective function by regulating apoptosis 9,10. Recent studies have found that autophagy and apoptosis have the same molecular mechanism; therefore, we hypothesize that autophagy and apoptosis are involved in the pathophysiological processes of SAH and there is an inverse relationship between them. Accumulating evidences have suggested that resveratrol has an effective therapeutic effect on SAH 11,12; however, to date, no study on resveratrol-mediated autophagy and apoptosis on SAH has been carried out and the potential mechanism has not been completely determined. The aim of our study was to evaluate the effect of resveratrol on mortality, neural functional recovery, and brain edema using a rat cerebral vascular puncture model and to identify a novel mechanism of action on SAH. We found that resveratrol reduced mortality, the amount of bleeding, and brain edema and improved neural functional recovery following SAH. These improvements in recovery are accompanied by enhancement of autophagy and inhibition of apoptosis regulated by the Akt/mTOR signaling pathway.
Materials and methods
Animal care and grouping
Male adult Sprague–Dawley rats (weighing 270–320 g, 8–10 weeks old) were purchased from the Experimental Animal Center of Xi’an Jiaotong University [license no. SCXK (Shaanxi) 2006-001]. Rats were housed in groups of six per cage and maintained at an ambient temperature of 22±1°C, under a 12-h light/dark cycle, with food/water ad libitum.
Experimental design and drug delivery
One hundred and forty-one Sprague-Dawley rats were distributed randomly into five groups: the sham group (n=30), the SAH group (n=60), the SAH+resveratrol (n=30), and SAH+3-methyladenine (3-MA) (n=21). Animals in the sham group received 0.9% saline (intraperitoneal injection) after the sham operation. The SAH group received an equal amount of DMSO solution (0.1% dimethylsulfoxide in 0.9% saline). After SAH, the SAH+resveratrol group received 60 mg/kg resveratrol (intraperitoneal injection) 13 and the SAH+3-MA group received 60 mM 3-MA (intracerebroventricular administration) 14. Resveratrol and 3-MA were purchased from Abcam (ab120726; Cambridge, UK) and Cayman (13242; Ann Arbor, Michigan, USA), respectively.
Ethics statement
All protocols and procedures were performed in agreement with the Biomedical Ethics Committee of Animal Experiments of Shaanxi Province in China and complied with the principles and procedures of the Guidance Suggestions for the Care and Use of Laboratory Animals formulated by the Ministry of Science and Technology of PRC. All efforts were made to minimize animal suffering.
Rat subarachnoid hemorrhage model and sham operation
The SAH model was created by endovascular perforation as described previously 15. Briefly, animals were anesthetized by an intraperitoneal injection of 1% (w/v) pentobarbital sodium (35 mg/kg), the right common carotid artery bifurcation was exposed, and then the external carotid artery was separated and the blood flow was blocked with a vascular clamp. A blunted 3–0 single-stranded nylon suture was placed into the internal carotid from the common carotid artery bifurcation 18–20 mm to induce arterial rupture. The suture was retained for about 15 s and then pulled out; the wound was then closed. The sham group subjected to the same procedure as the SAH group, except that the vessel was not punctured.
Subarachnoid hemorrhage grading
To prevent differences in the severity of bleeding among the models of animals, our study selected a SAH grading system to examine the subarachnoid blood clots in the basal cisterns 16. The system divides the basis cistern into six regions, and scores the regions according to the number and distribution of blood clots in each region: 0, no SAH; 1, a small amount of SAH; 2, moderate number of blood clots; and 3, all the arteries in the region are covered by blood clots. The sum of scores represents the severity of SAH grading: 0–7, mild SAH; 8–12, moderate SAH; and 13–18, severe SAH. In this study, rats with a score of less than 3 and more than 15 were excluded.
Assessment of mortality and neurological function
The mortality of each group of rats was equal to the number of dead rats divided by the total number of rats. To quantify the neurological function of rats in each group, we used Garcia test neurological dysfunction scoring methods 17, which included the functional evaluation of autonomic exercise, exercise coordination, physical activity, and somatic sensation. The score ranged from 3 to 18; the lower the score, the more severe the neurological dysfunction.
Brain water content
Brain edema is a common pathophysiological change in bleeding that occurs after the creation of an intravascular puncture model. The brain water calculation was carried out using the method of wet and dry weight 18. Rats were routinely anesthetized and brains were rapidly separated into left hemisphere, right hemisphere and cerebellum. The puncture side of the cerebral hemisphere was considered the wet weight; this was then dried at 105°C for 24 h and the dry weight was obtained. The degree of brain edema was calculated as follows: brain water calculation (%)=[(wet weight−dry weight)/wet weight]×100%.
Hematoxylin and eosin staining
Sections were stained with hematoxylin and eosin (H&E) and mounted with neutral gum after dehydration with increasing concentrations of ethanol. Tissue sections were observed using a light microscope (BX-40; Olympus, Tokyo, Japan) at ×40 magnification.
RT-PCR
The total RNA was extracted from rat cortex using TRIzol Reagent (ThermoFisher Scientific, Pittsburgh, Pennsylvania, USA) according to the manufacturer’s instructions. The primers used for reverse transcription-PCR (RT-PCR) were as follows: LC3-B (134 bp) forward primer 5′-AGAGCGATACAAGGGTGAGAAG-3′ and reverse primer 5′-AGAAGGCTTGGTTAGCATTGAG-3′; beclin-1 (180 bp) forward primer 5′-GAATGGAGGGGTCTAAGGCG-3′ and reverse primer 5′-CTTCCTCCTGGCTCTCTCCT-3′; GAPDH (281 bp) forward primer 5′-TTCCTACCCCCAATGTATCCG-3′ and reverse primer 5′-CATGAGGTCCACCACCCTGTT-3′. The cycling conditions were as follows: 10 min at 95°C, followed by 40 cycles of 60 s at 95°C; final extension was performed at 60°C for 10 min.
Immumohistochemical staining
All sections were deparaffinized, rehydrated, and endogenous peroxidase was quenched with 3% (v/v) H2O2. Exposed antigen was repaired at high temperature. Sections were then blocked in 2% (v/v) normal goat serum and incubated in primary antibodies overnight with the following antibodies: rabbit monoclonal LC3-B (1 : 3200, catalog no. 3868; Cell Signaling Technology, Danvers, Massachusetts, USA) and rabbit polyclonal beclin-1 (1 : 200, catalog no. ab62557; Abcam). Sections were washed with PBS and incubated for 1 h with the secondary antibodies (1 : 5000). Then, we applied diaminobenzidine for chromogen. Results were obtained using a ×40 light microscope. The region selected for observation was located in the cortex. The densitometric analysis was carried out using an image analysis program (Image-Pro Plus 6.0; Media Cybernetics, Bethesda, Maryland, USA).
Immunofluorescence staining
Immunofluorescence was performed on sections of rat brain after SAH as described above (immunohistochemical staining). Briefly, the sections were deparaffinized, rehydrated, blocked with H2O2, followed by antigen retrieval using high temperature, and then blocked in 2% (v/v) normal goat serum and incubated in primary antibodies overnight with the following antibodies: rabbit monoclonal LC3-B (1 : 3200, catalog no. 3868, Cell Signaling Technology), rabbit polyclonal beclin-1 (1 : 200, catalog no. ab62557; Abcam), rabbit monoclonal SQSTM1/p62 (1 : 100, catalog no. ab109012; Abcam), and rabbit polyclonal active caspase-3 (1 : 1000, catalog no. ab49822; Abcam). Sections were incubated in secondary fluorescent antibodies Alexa 488 and Alexa 568 (1 : 400; Invitrogen, Carlsbad, California, USA) at room temperature, and the nuclei were marked with 4′,6-diamidino-2-phenylindole (1 μg/ml) for 10 min. The sections were viewed and images were acquired using a fluorescence microscope (Molecular Devices, Sunnyvale, California, USA).
Western blot
All the extraction operations were carried out on ice. Briefly, the tissues of the cortex were purified using RIPA. Equal aliquots of protein (40 μg) were subjected to 12% SDS-PAGE gels and transferred onto immobilon-P/PVDF membranes (Millipore Corp., Billerica, Massachusetts, USA). Then, membranes were blocked with TBST buffer (0.05 M Tris pH 7.4, 0.15 M NaCl, 0.05% Tween 20) containing 5% (w/v) skim milk powder for 1 h and incubated at 4°C overnight with specific antibodies: rabbit monoclonal anti-LC3-B (1 : 1000, catalog no. 3868, Cell Signaling Technology); rabbit polyclonal anti-beclin-1 (1 : 1000, catalog no. ab62557; Abcam); rabbit monoclonal anti-SQSTM1/p62 (1 : 10 000, catalog no. ab109012; Abcam); rabbit monoclonal anti-phospho-Akt (Ser473) (1 : 2000, catalog no. 4060; Cell Signaling Technology); rabbit monoclonal anti-Akt (1 : 1000, catalog no. 4685; Cell Signaling Technology); rabbit monoclonal anti-phospho-mTOR (Ser2448) (1 : 1000, catalog no. 5536; Cell Signaling Technology); rabbit monoclonal anti-mTOR (1 : 1000, catalog no. 2983; Cell Signaling Technology); rabbit polyclonal anti-Bcl-2-associated X protein (Bax) antibody (1 : 500, catalog no. 2772; Cell Signaling Technology); rabbit monoclonal anti-Bcl-2 antibody (1 : 1000, catalog no. 2870; Cell Signaling Technology); rabbit monoclonal anti-cleaved caspase-3 (1 : 1000, catalog no. 9664; Cell Signaling Technology); mouse monoclonal anti-caspase-9 (1 : 1000, catalog no. 9508; Cell Signaling Technology); and mouse monoclonal anti-β-actin (1 : 1000, catalog no. 3700; Cell Signaling Technology). After incubation, membranes were incubated with goat anti-rabbit or anti-mouse IgG-HRP secondary antibody (1 : 5000; Abcam) for 1 h at room temperature. The relative expression of protein bands was visualized using the ChemiDoc MP System (Bio-Rad protein assay; Bio-Rad, Segrate, Italy) with the ECL substrate (Millipore Corp.). In addition, to ensure equivalent amounts of protein, we used β-actin as an internal control. Densitometric quantification of the bands was performed using Image J software (version 1.29×; NIH, Bethesda, Maryland, USA).
TUNEL staining
The terminal deoxynucleotidyl transferase-mediated digoxigenin-DUTP-biotin nick-end labeling (TUNEL) assay was performed following the manufacturer’s instructions. Briefly, the sections were deparaffinized, rehydrated, and incubated in 0.2% (v/v) Triton X-100, and then we used a commercial cell death detection kit that was purchased from Roche Diagnostics (Indianapolis, Indiana, USA). Sections were washed with PBS and the nuclei were stained with 4′,6-diamidino-2-phenylindole for 10 min. Green fluorescence of apoptotic cells and blue fluorescence of nuclei were viewed and photographed by fluorescence microscopy (Promega, Madison, Wisconsin, USA).
Statistical analysis
Results are reported as mean±SD. We used SPSS 18.0 (SPSS Inc., Chicago, Illinois, USA) for the statistical analyses. One-way analysis of variance was used for all statistical analyses with multiple comparisons. For all P values, Student’s t-test was used for post-hoc analysis. A P value of less than 0.05 was considered statistically significant.
Results
Pathological changes in subarachnoid hemorrhage model rats
The brains of SAH rats showed varying degrees of edema and obvious blood clots near the Willis ring under macroscopic observation compared with the brains of sham rats. H&E staining showed neuronal pyknosis, swelling, torsion, cell body deformation, and extracellular space expansion in the cortex at 24 h after SAH. Resveratrol reduced these pathological changes on both macroscopic observation and H&E staining (Fig. 1).
Fig. 1.
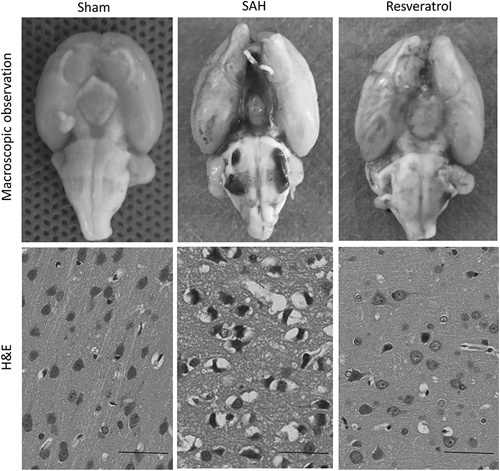
Pathological changes after SAH in rat cortex. Pathological changes after SAH were confirmed by macroscopic observations and H&E staining. Scale bar=50 µm. H&E, hematoxylin and eosin; SAH, subarachnoid hemorrhage.
Mortality, subarachnoid hemorrhage grade, neurological score, and water content
Compared with the sham groups, the SAH groups showed an obvious increase, but the resveratrol-treated groups showed a significant decrease in mortality (Fig. 2a), SAH grading (Fig. 2b), and water content (Fig. 2d). In contrast, the neurological score reduced in the SAH group compared with the sham group; the resveratrol-treated group showed a significant increase in the neurological score (Fig. 2c).
Fig. 2.
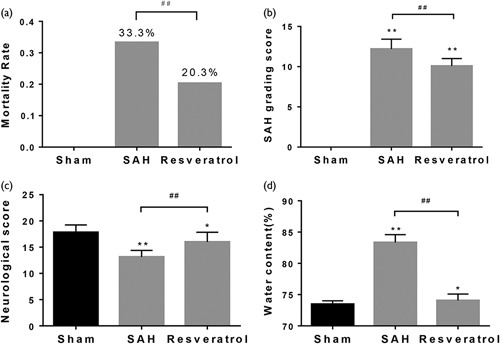
General evaluation of SAH models and the effect of resveratrol on grading score, neurological function, and brain water content at 24 h. (a) Evaluation of mortality. (b) Quantification of SAH severity. (c) Quantification of neurological function. (d) Evaluation of brain water content. Values are presented as the mean±SD (n=6/group). *P<0.05; **P<0.01; ##P<0.01, compared with the sham group. SAH, subarachnoid hemorrhage.
Resveratrol downregulates the Akt/mTOR signaling pathway and promotes the autophagy process in subarachnoid hemorrhage model rats
To address whether resveratrol reduced mortality and brain water content following SAH by activating autophagy, we examined the expression of autophagy in brain cortex tissue. We observed that the protein expression of beclin-1, LC3-II, and LC3-II/LC3-I was significantly higher at 24 h after SAH in resveratrol-treated rats than in rats that received the vehicle or sham rats, whereas the expression of p62, p-Akt, and p-mTOR was reduced in resveratrol-treated animals accordingly. There was no difference in the expression of Akt and mTOR among the sham, SAH, and resveratrol groups (Fig. 3). The results of mRNA expression showed that beclin-1 and LC3-B were significantly higher, whereas p62 was lower in resveratrol-treated rats than untreated rats as well (Fig. 4a–c). Furthermore, immunofluorescence analysis also found a marked increase in beclin-1 and LC3-B, whereas p62 decreased in the resveratrol-treated SAH rats (Fig. 4d–f). The expression of LC3-B and beclin-1 as assessed by immunohistochemistry analysis was consistent with the immunofluorescence analysis (Fig. 4g). Thus, the results suggest that resveratrol regulates the Akt/mTOR signaling pathway and promotes autophagy in SAH rats.
Fig. 3.
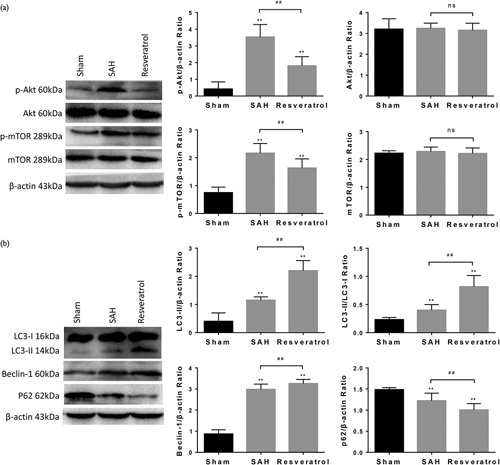
Resveratrol promotes biochemical markers of autophagy after SAH in rats. Western blot analysis was carried out to measure the expression of p-Akt, p-mTOR, LC3-B, beclin-1, and p62 in brain tissue from SAH rats treated with resveratrol. The expression of β-actin was used as an internal control. Values are presented as the mean±SD (n=6/group). **P<0.01; ##P<0.01, compared with the sham group. mTOR, mammalian target of rapamycin; SAH, subarachnoid hemorrhage.
Fig. 4.
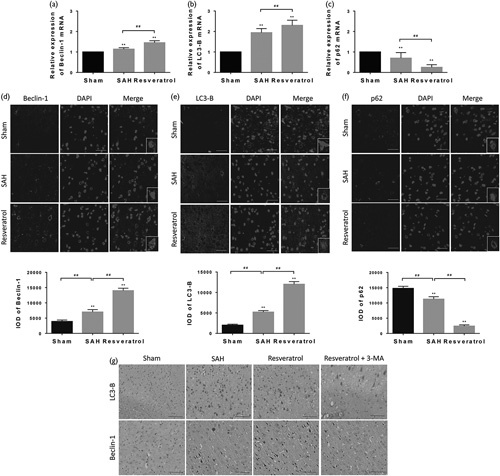
To confirm that resveratrol promoted autophagy in the SAH rats, beclin-1 (a, d and g), LC3-B (b, e and g), and p62 (c, f) were determined by RT-PCR, immunofluorescence, and immumohistochemistry, respectively (scale bar=50 µm; n=6). Positive cell levels were counted in 10 random fields (×40 magnification). The small figures located in the bottom right corner represent the magnification of particular sections. Values are presented as the mean±SD (n=6/group). **P<0.01; ##P<0.01, compared with the sham group. SAH, subarachnoid hemorrhage.
Resveratrol inhibits apoptosis in subarachnoid hemorrhage model rats
To determine whether resveratrol alleviated pathological changes in brain tissues and increased the neurological score by inhibiting apoptosis, we examined the expression of apoptosis proteins in the brain of treated and untreated SAH rats. As is known, cleaved caspase-3 is a proapoptosis protein and Bcl-2 is a prosurvival protein. Bax, another member of the Bcl-2 family, could combine with Bcl-2 to form a dimer, which usually has a proapoptotic property. In this study, the western blot and immunohistochemistry results showed that cleaved caspase-3 and Bax were upregulated in SAH rats compared with sham rats, whereas Bcl-2 was obviously downregulated. Nevertheless, the administration of resveratrol reduced the expression of cleaved caspase-3, Bax and increased the expression levels of Bcl-2 significantly following SAH (Fig. 5). Taken together, our results indicate that resveratrol markedly suppresses apoptosis in SAH rats.
Fig. 5.
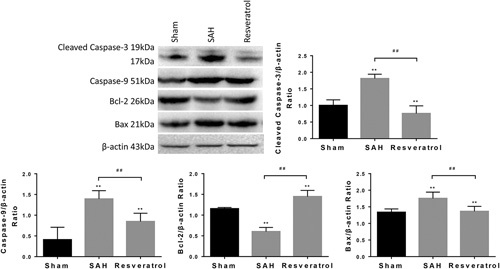
Resveratrol inhibits protein expression of apoptosis in SAH rats. The protein expressions of cleaved caspase-3, caspase-9, Bcl-2, and Bax protein were determined by western blot analysis. The expression of β-actin was used as an internal control. Values are presented as the mean±SD (n=6 per group). **P<0.01; ##P<0.01, compared with the sham group. Bax, Bcl-2-associated X protein; SAH, subarachnoid hemorrhage.
Resveratrol downregulates apoptosis but upregulates autophagy in subarachnoid hemorrhage model rats
The results thus far indicate that resveratrol is involved in the regulation of autophagy and apoptosis, but the relation between autophagy upregulation and blockage of apoptosis by resveratrol treatment after SAH remains unclear. To address this issue, we performed double-labeling immunofluorescence staining of p62 and cleaved caspase-3 (Fig. 6). We found that cleaved caspase-3 was upregulated in SAH rats compared with sham rats, whereas p62 was significantly downregulated. Nevertheless, administration of resveratrol significantly reduced the expression of cleaved caspase-3 and p62 following SAH. Thus, these findings indicate the relation between autophagy upregulation and blockage of apoptosis.
Fig. 6.
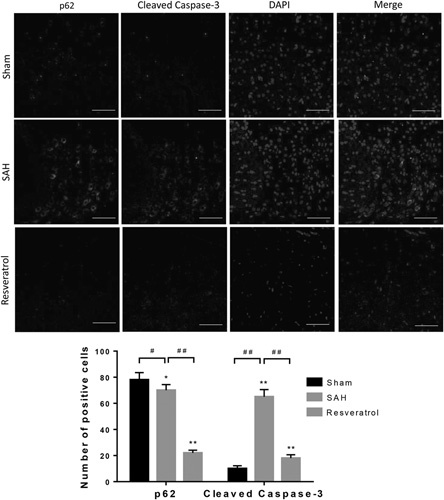
Resveratrol upregulates autophagy and inhibits apoptosis following SAH in rats. Double immunofluorescence staining was performed with the antibody to p62 and antibodies to cleaved caspase-3 (scale bar=50 µm; n=6). SAH, subarachnoid hemorrhage.
*P<0.05; **P<0.01; #P<0.05; ##P<0.01, compared with the sham group.
Inhibitor of the PI3K/Akt/mTOR signaling pathway reduces autophagy expression and increases apoptosis expression
We next examined the impact of 3-MA, a well-established inhibitor of autophagy. 3-MA inhibits autophagy by blocking autophagosome formation by the inhibition of type III phosphatidylinositol 3-kinases 19. Administration of 3-MA to rats showed that the proportion of beclin-1-positive and LC3-B-positive neurons decreased significantly by immunohistochemistry analysis (Fig. 4g). This result indicates that the upregulation effect of resveratrol is related to the activation of the Akt/mTOR pathway. Moreover, to determine the effect of resveratrol on apoptotic response, we performed TUNEL staining to evaluate the level of apoptosis in the cortex of rats. TUNEL-positive cells were rarely found in the sham group. The total number of apoptotic cells was consistently increased in the SAH group. Significantly fewer TUNEL-positive cells were found in the resveratrol-treated group than in the SAH group. Furthermore, rats in the resveratrol+3-MA group had significantly more TUNEL-positive cells than in the resveratrol group (Fig. 7a and b). In brief, our finding indicates that resveratrol enhances autophagy and inhibits apoptosis by inducing the Akt/mTOR signaling pathway, and that there may be an inverse relationship between autophagy and apoptosis.
Fig. 7.
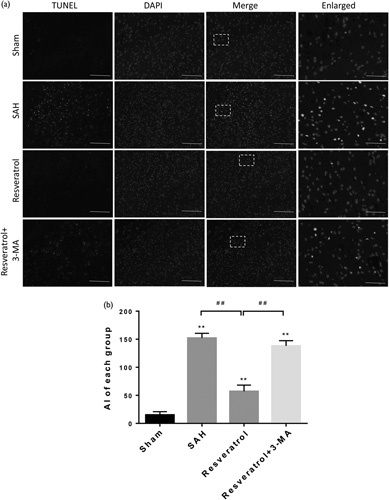
Resveratrol was related to cell apoptosis after SAH. The TUNEL assay was used to detect apoptotic cells. (a) Colocalization of TUNEL and nuclei (DAPI), were considered apoptotic cells (scale bar=100 µm; enlarged figures scale bar=50 µm). (b) The bar graphs showed the statistical results of apoptotic cells counts. The positive cell levels were counted in 10 random fields of the relevant regions (×40 magnification). Data were presented as mean±SD (n=6 per group). **P<0.01; ##P<0.01, compared with the sham group. Apoptotic index (AI), defined as the number of TUNEL-positive cells/mm2. SAH, subarachnoid hemorrhage; TUNEL, terminal deoxynucleotidyl transferase-mediated digoxigenin-DUTP-biotin nick-end labeling.
Discussion
The modes of cell death after SAH include apoptosis and necrosis. Recent studies have found that autophagy, apoptosis, and necrosis interacted with each other. In hypoxic–ischemic injury models, enhanced autophagy decreases the level of apoptosis and necrosis 20. Our study discovered that resveratrol reduced neuronal pyknosis, swelling, SAH severity and brain edema, and promoted neural functional recovery at 24 h following SAH. Resveratrol also increased autophagy activity as evidenced by the enhanced expression of beclin-1, LC3-B, and LC3-II/LC3-I, and inhibited the expression of p-Akt, p-mTOR, p62, and apoptosis proteins. The present study showed that resveratrol had a potentially protective mechanism to prevent or alleviate EBI.
EBI is a result of sudden intracranial vascular rupture; the area that is commonly affected is the cerebral cortex. In the present study, the SAH model was created by an endovascular puncture model. To identify the specific pathological changes associated with neurons, morphological observations were performed in the cortex. H&E staining showed the pathological features of acute neuron injury, such as neuronal pyknosis, swelling, torsion, and cell body deformation. Macroscopic observations also showed that the SAH model was established successfully.
Resveratrol is a polyphenolic compound that exerts neuroprotective effects because of its antioxidant, anti-inflammatory, and antiapoptotic properties in central nervous system diseases such as stroke, traumatic brain injury, and spinal cord injury 21–23. Our results are consistent with these studies. Resveratrol could decrease the mortality, SAH grading, and brain edema. In addition, the quantitative analysis of the neurological score, which is a direct index for the severity of EBI, also suggests the effectiveness of resveratrol in attenuating the early deterioration in neurological function induced by SAH.
Autophagy is a phenomenon that exists widely in eukaryotic cells and exerts a neuroprotective effect. Resveratrol promotes autophagy considerably and promotes the occurrence of autophagy. A previous study found that resveratrol alleviated ischemia/reperfusion injury in rats by autophagy 24. Our results showed that resveratrol altered the expression of autophagy in rats after SAH; the expression of beclin-1, LC3-II, and LC3-II/LC3-I increased, whereas the expression of p62 decreased as assessed by western blot, RT-PCR, immunofluorescence, and immunohistochemistry analyses. Then, we administered the autophagy inhibitor 3-MA to lateral ventricles of rats and found that it reversed the trend of autophagy expression. A previous study showed that the degree of cell death in the hippocampal neurons of brain tissue sections of patients who died because of SAH was significantly higher than that of patients who died of other causes 25. In a further study of SAH in rats, it was found that the apoptosis of EBI was significantly related to the prognosis of rats 26. In addition, Loos et al. 20 found that morphological features of autophagy and apoptosis can appear in the same cell. Thus, we next assessed whether resveratrol regulated apoptosis in rats with SAH. Our results showed that resveratrol inhibited apoptosis in SAH rats and the apoptosis-related proteins cleaved caspase-3, caspase-9, and Bax decreased in resveratrol-treated groups, whereas Bcl-2 increased as assessed by western blot.
Autophagy is a double-edged sword: on the one hand, autophagy is an important process of autologous cell repair and maintains the homeostasis of the cell environment to prevent further damage of cells; on the other, under special conditions, autophagy can induce cell damage or even death, and interact with apoptotic signals 27,28. Beclin-1, a homologue of the yeast autophagy gene in mammals, is an important positive regulator of autophagy. It can form complexes and further recruit the Atg5 complex and LC3 from the cytoplasm to promote the expansion of autophagosomes. Beclin-1 has a molecular weight of 60 kD and contains 450 amino acids and four specific domains: the Bcl-2 binding domain, the helix domain, the conserved domain, and the nuclear export signaling domain 29. The interaction between beclin-1 and antiapoptotic factor Bcl-2 may be involved in the initiation of autophagy and apoptosis, which is significant for cell survival and death 30.
mTOR is an evolutionarily conserved protein kinase belonging to the phosphatidylinositol kinase-dependent kinase superfamily member that functions as a serine/threonine kinase. In the cell growth, mTOR, as a central regulator, controls the basic biological processes, and regulates cell survival, division, migration, self-renewal, and cycle processes 31. The PI3K/Akt/mTOR signaling pathway mediates multiple cellular process, including cell survival, proliferation, migration, and autophagy. The PI3K/Akt/mTOR signaling pathway has been proven to be associated with autophagy and apoptosis in central nervous system diseases such as depression 32 and glioma 33. Consistent with these studies, in SAH rats, we observed that resveratrol treatment inhibited Akt/mTOR phosphorylation as assessed by western blot analysis and increased autophagy expression as well as decreased apoptosis expression. However, on administration of 3-MA, an inhibitor of PI3K, to the lateral ventricular, beclin-1 and LC3-B levels were suppressed significantly as indicated by immunochemistry analysis. Similarly, TUNEL-positive cells increased significantly. These results suggest that resveratrol induces autophagy and inhibits apoptosis by regulating Akt/mTOR pathways after SAH in rats. However, the potential mechanism is not very clear.
This is the first study to show that resveratrol suppresses neuronal apoptosis and promotes the autophagy process and then accelerates neurologic functional recovery by the activation of the Akt/mTOR autophagy signaling pathway in rats with SAH. Our results indicate a novel molecular mechanism for the neuroprotective effects of resveratrol and the potential clinical application of resveratrol in SAH therapy. However, this study has many limitations; the potential mechanism by which resveratrol acts by autophagy and apoptosis on SAH was not studied further and we only focused on the role of resveratrol in autophagy and apoptosis following SAH. As a natural polyphenolic compound, resveratrol has a variety of beneficial properties including antioxidant, anti-inflammatory, and antitumor effects. The other potential mechanisms of resveratrol on SAH should be investigated further.
Conclusion
These findings indicate that resveratrol exerts neuroprotective effects on SAH by regulating autophagy and apoptosis mediated by the Akt/mTOR signaling pathway.
Acknowledgements
D.G. designed and conceived the study and wrote the manuscript. D.G., J.Z., J.X. conducted the animal experiments. D.G. and T.H. analyzed the data. All authors discussed the conceptual and practical implications of the methods.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Cahill J, Calvert JW, Zhang JH. Mechanisms of early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab 2006; 26:1341–1353. [DOI] [PubMed] [Google Scholar]
- 2.He Y, Wan S, Hua Y, Keep RF, Xi G. Autophagy after experimental intracerebral hemorrhage. J Cereb Blood Flow Metab 2008; 28:897–905. [DOI] [PubMed] [Google Scholar]
- 3.Sheng R, Zhang LS, Han R, Liu XQ, Gao B, Qin ZH. Autophagy activation is associated with neuroprotection in a rat model of focal cerebral ischemic preconditioning. Autophagy 2010; 6:482–494. [DOI] [PubMed] [Google Scholar]
- 4.Yuan J, Yankner BA. Apoptosis in the nervous system. Nature 2000; 407:802–809. [DOI] [PubMed] [Google Scholar]
- 5.Lalaoui N, Lindqvist LM, Sandow JJ, Ekert PG. The molecular relationships between apoptosis, autophagy and necroptosis. Semin Cell Dev Biol 2015; 39:63–69. [DOI] [PubMed] [Google Scholar]
- 6.Xia EQ, Deng GF, Guo YJ, Li HB. Biological activities of polyphenols from grapes. Int J Mol Sci 2010; 11:622–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsieh TC, Wu JM. Resveratrol: Biological and pharmaceutical properties as anticancer molecule. Biofactors 2010; 36:360–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ge J, Liu Y, Li Q, Guo X, Gu L, Ma ZG, Zhu YP. Resveratrol induces apoptosis and autophagy in T-cell acute lymphoblastic leukemia cells by inhibiting Akt/mTOR and activating p38-MAPK. Biomed Environ Sci 2013; 26:902–911. [DOI] [PubMed] [Google Scholar]
- 9.Lin H, Tang H, Davis FB, Davis PJ. Resveratrol and apoptosis. Ann N Y Acad Sci 2011; 1215:79–88. [DOI] [PubMed] [Google Scholar]
- 10.Bastianetto S, Menard C, Quirion R. Neuroprotective action of resveratrol. Biochim Biophys Acta 2014; 1852:1195–1201. [DOI] [PubMed] [Google Scholar]
- 11.Qian C, Jin J, Chen J, Li J, Yu X, Mo H, Chen G. SIRT1 activation by resveratrol reduces brain edema and neuronal apoptosis in an experimental rat subarachnoid hemorrhage model. Mol Med Rep 2017; 16:9627–9635. [DOI] [PubMed] [Google Scholar]
- 12.Zhang XS, Li W, Wu Q, Wu LY, Ye ZN, Liu JP, et al. Resveratrol Attenuates Acute Inflammatory Injury in Experimental Subarachnoid Hemorrhage in Rats via Inhibition of TLR4 Pathway. Int J Mol Sci 2016; 17:1331–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou XM, Zhou ML, Zhang XS, Zhuang Z, Li T, Shi JX, et al. Resveratrol prevents neuronal apoptosis in an early brain injury model. J Surg Res 2014; 189:159–165. [DOI] [PubMed] [Google Scholar]
- 14.Backer JM. The regulation and function of class III PI3Ks: novel roles for Vps34. Biochem J 2008; 410:1–17. [DOI] [PubMed] [Google Scholar]
- 15.Prunell GF, Mathiesen T, Diemer NH, Svendqaard NA. Experimental subarachnoid hemorrhage: subarachnoid blood volume, mortality rate, neuronal death, cerebral blood flow, and perfusion pressure in three different rat models. Neurosurgery 2003; 52:165–176. [DOI] [PubMed] [Google Scholar]
- 16.Sugawara T, Ayer R, Jadhav V, Zhang JH. A new grading system evaluating bleeding scale in filament perforation subarachnoid hemorrhage rat model. J Neurosci Methods 2008; 167:327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral-artery occlusion in rats – statistical validation. Stroke 1995; 26:627–634. [DOI] [PubMed] [Google Scholar]
- 18.Li DD, Song JN, Huang H, Guo XY, An JY, Zhang M, et al. The roles of MMP-9/TIMP-1 in cerebral edema following experimental acute cerebral infarction in rats. Neurosci Lett 2013; 550:168–172. [DOI] [PubMed] [Google Scholar]
- 19.Takatsuka C, Inoue Y, Matsuoka K, Moriyasu Y. 3-Methyladenine inhibits autophagy in tobacco culture cells under sucrose starvation conditions. Plant Cell Physiol 2004; 45:265–274. [DOI] [PubMed] [Google Scholar]
- 20.Loos B, Genade S, Ellis B, Lochner A, Engelbrecht AM. At the core of survival: autophagy delays the onset of both apoptosis and necrotic cell death in a model of ischemic cell injury. Exp Cell Res 2011; 317:1437–1453. [DOI] [PubMed] [Google Scholar]
- 21.Sakata Y, Zhuang H, Kwansa H, Koehler RC, Dore S. Resveratrol protects against experimental stroke: putative neuroprotective role of heme oxygenase 1. Exp Neurol 2010; 224:325–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ates O, Cayli S, Altinoz E, Gurses I, Yucel N, Sener M, et al. Neuroprotection by resveratrol against traumatic brain injury in rats. Mol Cell Biochem 2007; 294:137–144. [DOI] [PubMed] [Google Scholar]
- 23.Zhao HS, Chen SR, Gao K, Zhou ZP, Wang C, Shen Z, et al. Resveratrol protects against spinal cord injury by activating autophagy and inhibiting apoptosis mediated by the SIRT1/AMPK signaling pathway. Neuroscience 2017; 348:241–251. [DOI] [PubMed] [Google Scholar]
- 24.He Q, Li ZY, Wang YT, Hou YH, Li LY, Zhao J. Resveratrol alleviates cerebral ischemia/reperfusion injury in rats by inhibiting NLRP3 inflammasome activation through Sirt1-dependent autophagy induction. Int Immunopharmacol 2017; 50:208–215. [DOI] [PubMed] [Google Scholar]
- 25.Nau R, Haase S, Bunkowski S, Bruck W. Neuronal apoptosis in the dentate gyrus in humans with subarachnoid hemorrhage and cerebral hypoxia. Brain Pathol 2002; 12:329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sabri M, Kawashima A, Ai J, Macdonald RL. Neuronal and astrocytic apoptosis after subarachnoid hemorrhage: a possible cause for poor prognosis. Brain Res 2008; 1238:163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wen YD, Sheng R, Zhang LS, Han R, Zhang X, Zhang XD, et al. Neuronal injury in rat model of permanent focal cerebral ischemia is associated with activation of autophagic and lysosomal pathways. Autophagy 2008; 4:762–769. [DOI] [PubMed] [Google Scholar]
- 28.Koike M, Shibata M, Tadakoshi M, Gotoh K, Komatsu M, Waguri S, et al. Inhibition of autophagy prevents hippocampal pyramidal neuron death after hypoxic-ischemic injury. Am J Pathol 2008; 172:454–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pattingre S, Espert L, Biard-Piechaczyk M, Codogno P. Regulation of macroautophagy by mTOR and beclin 1 complexes. Biochimie 2008; 90:313–323. [DOI] [PubMed] [Google Scholar]
- 30.Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, et al. Bcl-2 antiapoptotic proteins inhibit beclin 1-dependent autophagy. Cell 2005; 122:927–939. [DOI] [PubMed] [Google Scholar]
- 31.Proud CG. Cell signaling. mTOR, unleashed. Science 2007; 318:926–927. [DOI] [PubMed] [Google Scholar]
- 32.Liu S, Li T, Liu H, Wang X, Bo S, Xie Y, et al. Resveratrol exerts antidepressant properties in the chronic unpredictable mild stress model through the regulation of oxidative stress and mTOR pathway in the rat hippocampus and prefrontal cortex. Behav Brain Res 2016; 302:191–199. [DOI] [PubMed] [Google Scholar]
- 33.Wang G, Dai F, Yu K, Jia Z, Zhang A, Huang Q, et al. Resveratrol inhibits glioma cell growth via targeting oncogenic microRNAs and multiple signaling pathways. Int J Oncol 2015; 46:1739–1747. [DOI] [PubMed] [Google Scholar]


