Summary
Background & aims
Ageing increases risk of respiratory infections and impairs the response to influenza vaccination. Pre- and pro-biotics offer an opportunity to modulate anti-viral defenses and the response to vaccination via alteration of the gut microbiota. This study investigated the effect of a novel probiotic, Bifidobacterium longum bv. infantis CCUG 52486, combined with a prebiotic, gluco-oligosaccharide, on the B and T cell response to seasonal influenza vaccination in young and older subjects .
Methods
In a double-blind, randomized controlled trial, 58 young (18–35 y) and 54 older (60–85 y) subjects were supplemented with the synbiotic for 8 weeks. At 4 weeks they were administered with a seasonal influenza vaccine. B and T cell phenotype and responsiveness to in vitro re-stimulation with the vaccine were assessed at baseline, 4, 6 and 8 weeks.
Results
B and T cell profiles differed markedly between young and older subjects. Vaccination increased numbers of memory, IgA+ memory, IgG+ memory and total IgG+ B cells in young subjects, but failed to do so in older subjects and did not significantly alter T cell subsets. Seroconversion to the H1N1 subunit in the older subjects was associated with higher post-vaccination numbers of plasma B cells, but seroconversion was less consistently associated with T cell phenotype. B and T cell subsets from both young and older subjects demonstrated a strong antigen-specific recall challenge, and although not influenced by age, responsiveness to the recall challenge was associated with seroconversion. In older subjects, CMV seropositivity was associated with a significantly lower recall response to the vaccine, but the synbiotic did not affect the responsiveness of B or T cells to re-stimulation with influenza vaccine.
Conclusions
Antigen-specific B and T cell activation following an in vitro recall challenge with the influenza vaccine was influenced by CMV seropositivity, but not by a synbiotic.
Registered under ClinicalTrials.gov Identifier no. NCT01066377.
Keywords: Ageing, Influenza, Probiotic, Lymphocyte, Vaccination
Abbreviations: CIRS, cumulative illness rating scale; CMV, cytomegalovirus; Gl-OS, gluco-oligosaccharide; Treg, regulatory T cells; URTI, upper respiratory tract infection
1. Introduction
Immunosenescence reduces protection against infections and leads to poor responses to vaccination in older individuals [1]; as a result, influenza is a major cause of mortality in older adults [2], [3]. Poor vaccine efficacy against influenza in older individuals is not just a result of impaired antibody production, although this may be a contributing factor. Helper T cells play a vital role in the generation of vaccine-specific antibody production and viral clearance depends on cytotoxic T cells [4]. In fact, cellular immune function may even be better correlated with vaccine protection than the antibody response to influenza vaccination [5]. Repeated antigenic stimulation, activation and differentiation of T cells during ageing causes progressive loss of CD28 and shrinkage of the naïve and early memory cytotoxic T cell compartments [6], [7], altering both the quantity and quality of antibodies indirectly [8], [9]. Therefore, understanding the changes that occur in humoral and cell-mediated immunity with ageing is critical for developing strategies to protect against infection and maintain or enhance the response to vaccination.
Previous studies investigating the effects of probiotics on the response to vaccination have mainly focused on antibody production. While some studies have reported a modest effect of probiotics on the antibody response to vaccination in adults, trials in older subjects are largely inconsistent and data are limited [10]. The strain Bifidobacterium longum bv. infantis CCUG 52486 was originally isolated from a cohort of very healthy elderly subjects (independent life-style, free of chronic disease, and aged 90 years or over) in Italy as part of the CROWNALIFE EU FP5 project [11]. It has been demonstrated to have particular ecological fitness and anti-pathogenic effects in vitro, and it has immunomodulatory effects which are strongly influenced by the age of the host [12]. Furthermore, this strain has been fully genome sequenced so that genetic traits can potentially be related to biological effects. We recently reported that although a pre- and probiotic combination failed to reverse a marked impairment of the antibody response to influenza vaccination in older subjects, it did tend to improve production of vaccine-specific IgM and IgG in young subjects, but not older subjects, suggesting an age-dependent response to the intervention [13]. However, immunological characterization revealed that the older subjects randomized to the synbiotic had a significantly higher number of senescent (CD28−CD57+) helper T cells at baseline compared with those randomized to the placebo. They also had significantly greater tendency for seropositivity to cytomegalovirus (CMV) and higher plasma levels of anti-CMV IgG, which are associated with replicative senescence of T cells [13]. Moreover, higher numbers of CD28−CD57+ helper T cells were associated with failure to seroconvert to the Brisbane subunit of the vaccine, strongly suggesting that the subjects randomized to the synbiotic were already at a significant disadvantage in terms of likely ability to respond to the vaccine compared with those randomized to the placebo [13].
In this study, we examine the effects of the synbiotic on antigen-specific B and T cell activation following an in vitro vaccine recall challenge. This is important because previous studies have focussed almost entirely on antibody responses to vaccination and there is no information on the effects of pre- or pro-biotics on B and T cell recall responses to vaccination.
2. Methods
2.1. Ethics and trial registration
The study protocol was reviewed and approved by the University of Reading Research Ethics Committee (project number: 10/09) and the National Health Service (NHS) Research Ethics Committee for Wales (10/MRE09/5). The trial was registered with ClinicalTrials.gov (Identifier: NCT01066377) and conducted according to the guidelines laid down in the Declaration of Helsinki.
2.2. Participants
Prior to the influenza season of 2010–2011, young (18–35 y) and older (60–85 y) healthy adults were recruited from the population in and around Reading (UK) through newspaper and poster advertisements, email and radio from June 2010 to March 2011. Inclusion criteria were: a signed consent form, age 18–35 y or 60–85 y, body mass index (BMI) 18.5–30 kg/m2, good general health, as determined by medical questionnaires and laboratory data from screening blood and urine sample (fasting glucose, erythrocyte sedimentation rate, full blood count, liver function tests, renal profile, dipstick urinalysis), not pregnant, lactating or planning a pregnancy. Exclusion criteria included: allergy to the influenza vaccine, HIV infection, diabetes requiring any medication, asplenia and other acquired or congenital immunodeficiencies, any autoimmune disease, including connective tissue diseases, malignancy, cirrhosis, connective tissue diseases, current use of immunomodulating medication (including oral and inhaled steroids), self-reported symptoms of acute or recent infection (including use of antibiotics within last 3 months), taking lactulose or any other treatment for constipation, alcoholism and drug misuse. Additional exclusion criteria for older volunteers included: laboratory data which were outside the normal range for this age group and outside the ranges specified in the SENIEUR protocol [14], Barthel Index score of <16/100, cumulative illness rating scale (CIRS) score of >15 [15]. Additional exclusion criteria for the young subjects included laboratory data which were outside the normal range and influenza vaccination in the previous 12 months.
2.3. Sample size
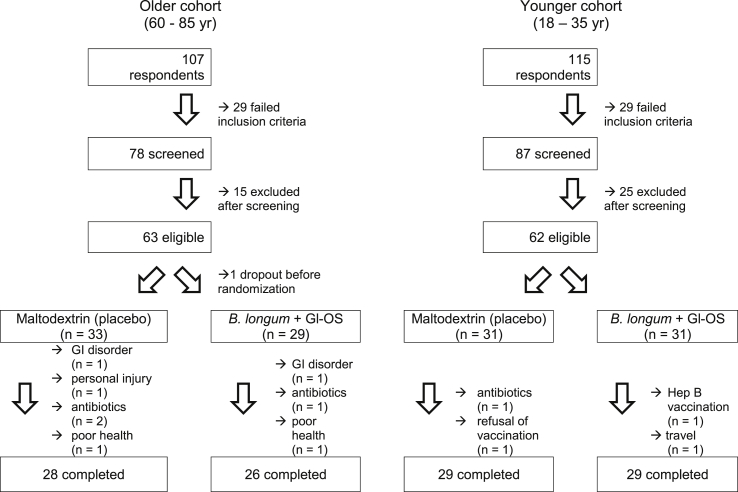
The primary outcome of the trial was the antibody response to vaccination, incorporating mean antibody titres, vaccine-specific Ig subclasses and seroprotection and seroconversion. Power calculations were based on mean antibody titres. Since the influenza vaccine is trivalent, it is unlikely that an intervention will alter the response to all three subunits in the same way. For example, in the study of Davidson et al. [16], there was no effect of probiotic on mean antibody titres in response to the H1N1 subunit, whereas the responses to both H3N2 and the B subunit were improved (72 vs 51 [SD 16.5] for H3N2 and 31 vs 25 [SD 7.1] for B subunit). Based on the smaller effect size for the B subunit, a sample size of 26 subjects per group within each cohort was determined to be sufficient for a two-tailed significance level of 5% and a power of 80%; this was adjusted to 30 subjects per group to allow for dropouts. Data on the co-primary endpoints, immunoglobulin subclasses, seroprotection and seroconversion, is very sparse, but a sample size of 26 subjects per group within each cohort was determined to be sufficient for a 376 mg/dL difference in circulating IgG levels in response to influenza vaccination, with an SD of 438 mg/dL, a two-tailed significance level of 5% and a power of 80% [17]. A total of 62 young subjects and 63 older subjects entered the study and 58 young and 54 older subjects completed the study (Fig. 1). Two subjects experienced adverse effects (gastrointestinal bloating) during the study, one on the placebo group and one in the synbiotic group; both withdrew from the study.
Fig. 1.
Recruitment flow diagram.
Reproduced from [13], published by Biomed Central.
2.4. Study design
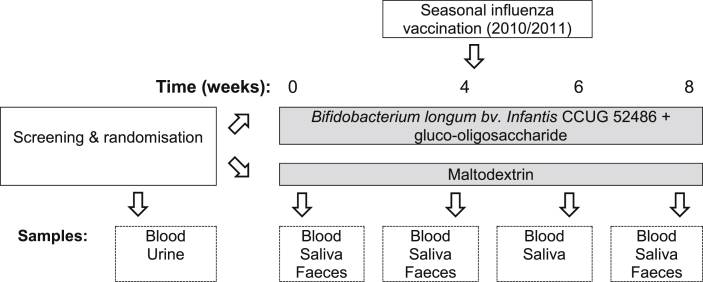
Subjects consumed B. longum bv. infantis CCUG 52486 (B. longum, 109 CFU in 1 g skim milk powder/day) combined with gluco-oligosaccharide (Gl-OS (BioEcolians, Solabia); 8 g/day)in a double-blind, placebo controlled randomised parallel group study design for 8 weeks. The synbiotic approach was selected because in vitro data examining the growth and survival of this strain indicated that it was very vulnerable compared with other strains, but survived much better in the presence of an oligosaccharide substrate (data not shown). When comparing a number of possible substrates, the low water activity of Gl-OS, combined with its ability to support the growth of the probiotic strain, made it a clear choice for a powdered product. This prebiotic also has bifidogenic effects in batch culture models [18]. The placebo used was maltodextrin (9 g/day); both the placebo and the pre- and pro-biotic were sourced, packaged and blinded by BioAgro S.A. (Italy). The powders were consumed sprinkled into water or milk or with breakfast cereal. Microbiological safety of the product was independently verified by Leatherhead Food Research associates (UK) prior to commencement of the study and viability of the probiotic strain was confirmed on a weekly basis during the study. During the three weeks prior to the study and during the intervention itself, subjects were requested not to consume fermented products such as yogurts, kefir etc. Subjects were randomized by a research nurse not involved in the analysis according to gender, age and BMI to receive the probiotic or placebo by covariate adaptive randomization. All investigators were blinded to the treatments, which were identical in appearance and labelled ‘A’ and ‘B’. A research assistant not involved in the analysis generated the random allocation sequence and a research nurse not involved in the analysis enrolled participants and assigned the interventions. After 4 weeks, subjects were administered with a single dose of the influenza vaccine (Influvac®sub-unit2010/2011 season, Abbott Biologicals B.V., lot number 1070166) containing A/California/7/2009 (H1N1), A/Perth/16/2009 (H3N2) and the B/Brisbane/60/2008-like strain by intra-muscular injection in the deltoid. Vaccination was carried out by a research nurse in the presence of a qualified clinician (MG). Details of the study schedule and samples collected are detailed in Fig. 2. Compliance was assessed by counting returned sachets and by copy numbers of B. longum, assessed by qPCR. None of the subjects in the young cohort had previously received seasonal influenza vaccination or swine flu vaccination. Three subjects in the older cohort had received swine flu vaccination, and forty subjects had previously been vaccinated for seasonal influenza, of whom thirty-seven had been vaccinated in the 2009/2010 period.
Fig. 2.
Study protocol.
Reproduced from [13], published by Biomed Central.
2.5. Blood sample processing
For serum, blood was collected into serum separator tubes and left at room temperature for 30 min to allow coagulation. Samples were centrifuged at 1300 × g for 10 min and aliquots of serum were collected and stored at −80 °C prior to analysis.
2.6. B cell phenotyping
B cell phenotyping was conducted by multi-parameter flow cytometry, using (FITC)-labelled anti-CD10, Pe-Cy7-labelled anti-IgD, Apc-Cy7-labelled anti-CD19, AmCyan-labelled anti-CD27, phycoerythrin (PE)-labelled anti-CD38, APC-labelled anti-IgA, PerCP-labelled anti-IgM, and Pacific blue-labelled anti-IgG (BD Biosciences, Oxon, UK). The lymphocyte population was gated using forward scatter/side scatter and fluorescence data for 10,000 events within the CD19+ population was collected and analysed using FlowJo software ©Tree star. Results expressed as absolute numbers in 1 ml of blood refer to data from flow cytometric analysis of samples of whole blood stained in TruCOUNT tubes. Non-specific staining was determined using mouse IgG1 as an isotype negative control for PE, APC-Cy7, AmCyan, PerCP, Pacific blue and APC-labelled antibodies and IgG2α as an isotype control for FITC and PE-Cy7-labelled antibodies. Immature B cells were identified by the presence of both CD19-APC-Cy7 and CD10-FITC within the lymphocyte population. Naïve B cells were identified by the presence of CD19-APC-CY7 and IgD and the absence of both CD10-FITC and CD27-AmCyan within the lymphocyte population. Memory B cells were identified by the presence of both CD19-APC-CY7 and CD27-AmCyan, the absence of CD10-FITC and the absence or low expression of CD38-PE within the lymphocyte population. Plasma B cells were identified by the presence of CD19-APC-CY7 and CD27-AmCyan, the absence of CD10-FITC and high expression of CD38-PE within the lymphocyte population. Memory B cells were further classified to subsets depending on their antibody expression. An IgD-PE-Cy7 vs IgM-PerCP plot was used to identify IgM+IgD+ (non class switched; NCS) memory B cells and an IgG-Pacific Blue vs. IgA-APC plot was used to identify IgA+ and IgG+ memory B cells. Total IgA+ and IgG+ B cells were identified using an IgA-APC vs. IgG-Pacific Blue plot.
2.7. T cell phenotyping
Peripheral blood mononuclear cells (PBMC: 1 × 106) were stained with the following fluorochrome-conjugated monoclonal antibodies: (PerCP) labelled anti-CD3, (AmCyan) labelled anti-CD4, (APC-Cy7) labelled anti-CD8, (PE-Cy7) labelled anti-CD25, (Pacific Blue) labelled anti-CD28, (APC) labelled anti-CD57, (FITC) labelled anti-CD26, (PE) labelled anti-CD127 (Becton Dickinson, UK) and analysed by multiparameter flow cytometry (FACS Canto II, BD Biosciences) using BD FACSDiva™ software. The lymphocyte population was gated using forward scatter/side scatter and fluorescence data collected for 10,000 events within the CD3+ population. The results are expressed as absolute numbers in 1 ml of blood, using data from flow cytometric analysis of samples of whole blood stained in TruCOUNT tubes. Non-specific staining was determined using mouse IgG1 as an isotype negative control for PerCP, AmCyan, APC-Cy7, PE-Cy7, Pacific Blue, FITC and PE-labelled antibodies and IgM as an isotype control for APC-labelled antibodies.
Total T cells were identified by the presence of CD3-PerCP and location within the lymphocyte population in the FSC/SSC plot. Helper and cytotoxic T cells were identified by the presence of CD4+AmCyan and CD8+APC-Cy7 respectively within the CD3+ T cell population. CD25, CD26, CD28, CD57 and CD127 were used to identify T cell subsets as shown in Supplementary Table 1.
2.8. Re-stimulation of PBMC with the influenza vaccine
PBMC (106) were incubated in the presence or absence of 20 μl influenza vaccine at 5 μg/ml for 6 days in medium containing RPMI, 10% bovine calf serum and 1% antibiotics in an air-CO2 (19:1) atmosphere. Cells were then stained with appropriate antibodies or isotype controls (as above) and activation of B and T cells assessed using (APC)-labelled anti-CD25. The lymphocyte population was gated using forward scatter/side scatter and fluorescence data for 10,000 events within the CD3+ population were collected and analysed using BD FACSDiva™ software.
2.9. Analysis of anti-CMV IgG antibodies
Concentrations of anti-CMV IgG antibodies were analysed by ELISA according to the manufacturer's instructions (ab108724 Anti-Cytomegalovirus (CMV) IgG Human Elisa Kit, Abcam, UK) and read in a microplate reader (GENios) at 450 nm, with 620 nm as a reference wavelength. CMV seropositivity was defined as antibody levels >11 AU/ml in accordance with the manufacturer's instructions.
2.10. Statistical analysis
Data were analysed using SPSS software (version 21). Differences between groups at baseline were identified using independent t-tests where appropriate. For the primary and continuous secondary endpoints, a Linear Mixed Model (LMM) was implemented. A first order autoregressive covariance structure was selected AR (1), with fixed factors of time (repeated measures for 3 timepoints; baseline, 6 weeks and 8 weeks), age and treatment and subject as a random effect. Since there were no effects of the synbiotic prior to vaccination (independent t tests comparing baseline with week 4), the decision was taken to use only one ‘baseline’ timepoint in the model, and the week 4 timepoint was consequently not included. Thus, the factor ‘time’ relates primarily to the effect of vaccination. Only main effects are reported as there were no two-way interactions between the variables. Following this main initial analysis, the data were split by cohort (young/older) and the analysis was repeated in the same manner to determine time and treatment effects within each cohort. The distribution of the data was checked using the Kolmogorov–Smirnov test. If data were not normally distributed, they were log transformed. Additional exploratory analyses examining differences between seroconverters and non-seroconverters (at week 6) and individuals who were CMV− vs CMV+ were conducted by independent t-tests. To account for multiple primary endpoints, two sided P values of 0.01 or less were considered statistically significant. All missing data were classed as missing at random and only available data were analysed.
3. Results
3.1. Subject characteristics
The characteristics of the subjects recruited to the study are described in Supplementary Table 2. Of the 125 volunteers who started the trial, 112 completed (Fig. 1).
3.2. Effect of ageing and vaccination on B and T cell phenotype
Older subjects had lower numbers of all classes of memory and plasma B cells than young subjects at baseline (Table 1). When young and older subjects were analysed separately, vaccination (time effect) increased numbers of memory, IgA+ memory, IgG+ memory, NCS memory and total IgG+ B cells in young subjects, but not in older subjects (LMM, effect of time in young subjects P < 0.001, P < 0.01, P < 0.001, P < 0.001 and P < 0.001 respectively; Table 1).
Table 1.
Effects of vaccination and treatment with synbiotic on the B cell profile in young and older subjects.
| Absolute number × 1000/ml blood |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Immature | Naïve t |
Memory at |
IgA+ memory at |
IgG+ memory at |
NCS memory at |
Plasma a |
Total IgA+ a |
Total IgG+ at |
|||
| Young (n = 58) | Placebo | Baseline | 7.4 ± 1.2 | 152.1 ± 14.3 | 76.0 ± 6.8 | 15.2 ± 1.9 | 12.4 ± 2.1 | 48.4 ± 4.2 | 3.4 ± 0.4 | 22.5 ± 2.4 | 18.9 ± 2.8 |
| 6 weeks | 7.4 ± 0.8 | 155.4 ± 11.9 | 95.9 ± 9.4 | 19.0 ± 2.3 | 16.6 ± 3.3 | 60.3 ± 5.9 | 4.6 ± 1.1 | 27.7 ± 2.9 | 24.9 ± 4.6 | ||
| 8 weeks | 7.5 ± 1.4 | 155.6 ± 15.0 | 85.7 ± 8.1 | 15.9 ± 1.8 | 14.3 ± 2.5 | 55.5 ± 5.5 | 2.9 ± 0.4 | 22.9 ± 2.2 | 21.4 ± 3.3 | ||
| Synbiotic | Baseline | 7.0 ± 0.7 | 131.3 ± 8.7 | 62.8 ± 5.3 | 13.6 ± 1.9 | 11.2 ± 2.2 | 38.0 ± 3.0 | 5.5 ± 1.0 | 22.4 ± 2.6 | 18.7 ± 3.2 | |
| 6 weeks | 6.7 ± 0.7 | 138.4 ± 10.0 | 73.9 ± 7.6 | 14.3 ± 2.0 | 13.0 ± 2.9 | 46.6 ± 5.1 | 5.4 ± 1.6 | 22.4 ± 3.1 | 20.6 ± 3.9 | ||
| 8 weeks | 6.5 ± 0.7 | 133.0 ± 10.7 | 67.8 ± 5.9 | 13.7 ± 2.0 | 11.0 ± 2.0 | 43.1 ± 3.6 | 4.8 ± 0.9 | 21.3 ± 2.8 | 17.7 ± 2.7 | ||
| Older (n = 54) | Placebo | Baseline | 7.8 ± 0.9 | 122.3 ± 10.3 | 53.5 ± 5.0 | 9.5 ± 1.0 | 5.7 ± 1.3* | 38.3 ± 3.7 | 2.6 ± 0.6 | 14.4 ± 1.4* | 9.6 ± 1.7* |
| 6 weeks | 7.8 ± 1.0 | 122.4 ± 9.8 | 54.3 ± 4.9** | 10.2 ± 1.2* | 4.9 ± 0.6* | 39.1 ± 3.8* | 2.2 ± 0.4 | 15.2 ± 1.8** | 8.7 ± 1.0* | ||
| 8 weeks | 6.6 ± 0.7 | 112.6 ± 11.0 | 48.6 ± 4.1** | 9.2 ± 0.8* | 4.3 ± 0.5** | 35.1 ± 3.3* | 1.9 ± 0.3 | 13.6 ± 1.2** | 8.1 ± 0.9** | ||
| Synbiotic | Baseline | 7.5 ± 1.3 | 132.5 ± 17.0 | 54.5 ± 5.5 | 10.5 ± 1.4 | 6.5 ± 0.9 | 37.5 ± 4.4 | 2.6 ± 0.5 | 15.5 ± 1.9 | 11.3 ± 1.4 | |
| 6 weeks | 7.9 ± 1.5 | 132.8 ± 13.4 | 57.9 ± 6.1 | 10.9 ± 1.5 | 7.4 ± 1.1 | 39.6 ± 4.7 | 2.3 ± 0.3 | 16.2 ± 2.1 | 12.1 ± 1.8 | ||
| 8 weeks | 8.5 ± 1.3 | 131.6 ± 16.8 | 56.9 ± 7.6 | 9.8 ± 1.5 | 7.7 ± 1.3 | 39.4 ± 6.5 | 2.1 ± 0.3* | 14.8 ± 2.4 | 12.9 ± 2.1 | ||
Data are mean ± SE for n = 58 young and n = 54 older subjects and were analysed using a Linear Mixed Model (LMM) with fixed factors of time (repeated measures), age and treatment. There was no significant effect of treatment for either cohort. a Denotes a significant main effect of age (P < 0.01 at least) and t denotes a significant main effect of time (P < 0.01 at least) for the combined cohorts. When the effect of time was examined separately in the young and older cohorts, there were significant effects of vaccination on numbers of memory, IgA+ memory, IgG+ memory, NCS memory and total IgG+ B cells in the young subjects only; there were no significant effects in the older subjects (LMM, effect of time in young subjects P < 0.001, P < 0.01, P < 0.001, P < 0.001 and P < 0.001 respectively). *Denotes significantly different from young subjects within the same timepoint and treatment group at P < 0.01 and ** denotes significantly different from young subjects within the same timepoint and treatment group at P < 0.001 (post-hoc t-tests with Bonferroni correction).
Older subjects had lower baseline numbers of CD26− helper, CD26high cytotoxic, CD26−CD28+ cytotoxic T cells and CD28−CD57− cytotoxic T cells, but higher numbers of CD26+ helper T cells (Th1) and senescent CD28−CD57+ helper and cytotoxic T cells than young subjects (Table 2), demonstrating clear evidence of immunosenescence in the older subjects. There was no significant effect of vaccination (time) on T cell subsets (Table 2).
Table 2.
Effects of vaccination and treatment with synbiotic on the T cell profile in young and older subjects.
| Absolute number × 1000/ml blood |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CD26+ helper a |
CD26− helper | CD26high cytotoxic a |
CD26int cytotoxic a |
CD26−CD28+ cytotoxic a |
CD26−CD28− cytotoxic | CD28−CD57+ cytotoxic a |
CD28−CD57− cytotoxic a |
|||
| Young (n = 58) | Placebo | Baseline | 308 ± 29 | 410 ± 20 | 28 ± 3 | 103 ± 10 | 225 ± 21 | 103 ± 11 | 65 ± 9 | 47 ± 5 |
| 6 weeks | 332 ± 30 | 454 ± 24 | 30 ± 4 | 124 ± 16 | 248 ± 23 | 112 ± 10 | 61 ± 8 | 54 ± 4 | ||
| 8 weeks | 322 ± 26 | 433 ± 25 | 27 ± 3 | 102 ± 9 | 240 ± 24 | 110 ± 12 | 69 ± 9 | 54 ± 6 | ||
| Synbiotic | Baseline | 341 ± 30 | 445 ± 36 | 30 ± 4 | 111 ± 10 | 233 ± 21 | 118 ± 11 | 73 ± 10 | 55 ± 6 | |
| 6 weeks | 372 ± 31 | 467 ± 32 | 30 ± 5 | 110 ± 11 | 224 ± 18 | 120 ± 14 | 79 ± 14 | 51 ± 5 | ||
| 8 weeks | 316 ± 30 | 394 ± 31 | 25 ± 4 | 104 ± 9 | 208 ± 17 | 121 ± 13 | 78 ± 12 | 53 ± 5 | ||
| Older (n = 54) | Placebo | Baseline | 435 ± 33* | 339 ± 24 | 15 ± 2** | 77 ± 7 | 108 ± 15** | 136 ± 26 | 120 ± 24 | 25 ± 3** |
| 6 weeks | 388 ± 33 | 315 ± 26** | 13 ± 2** | 75 ± 8 | 105 ± 15** | 131 ± 23 | 117 ± 22 | 23 ± 3** | ||
| 8 weeks | 415 ± 35 | 316 ± 26* | 14 ± 2* | 74 ± 8 | 94 ± 13** | 142 ± 28 | 127 ± 26 | 23 ± 4** | ||
| Synbiotic | Baseline | 408 ± 35 | 369 ± 35 | 14 ± 2* | 84 ± 12 | 112 ± 15** | 212 ± 37 | 181 ± 32* | 41 ± 9 | |
| 6 weeks | 446 ± 33 | 386 ± 29 | 14 ± 3* | 85 ± 15 | 99 ± 10** | 194 ± 46 | 169 ± 39 | 35 ± 10* | ||
| 8 weeks | 412 ± 34 | 361 ± 31 | 14 ± 4* | 89 ± 13 | 112 ± 17** | 219 ± 37 | 186 ± 30* | 45 ± 10 | ||
Data are mean ± SE for n = 58 young and n = 54 older subjects and were analysed using a Linear Mixed Model (LMM) with fixed factors of time (repeated measures), age and treatment. There were no significant effects of either time or treatment for either cohort. a Denotes a significant main effect of age (P < 0.01 at least). *Denotes significantly different from young subjects within the same timepoint and treatment group at P < 0.01 and ** denotes significantly different from young subjects within the same timepoint and treatment group at P < 0.001 (post-hoc t-tests with Bonferroni correction).
3.3. B and T cell phenotype influences seroconversion
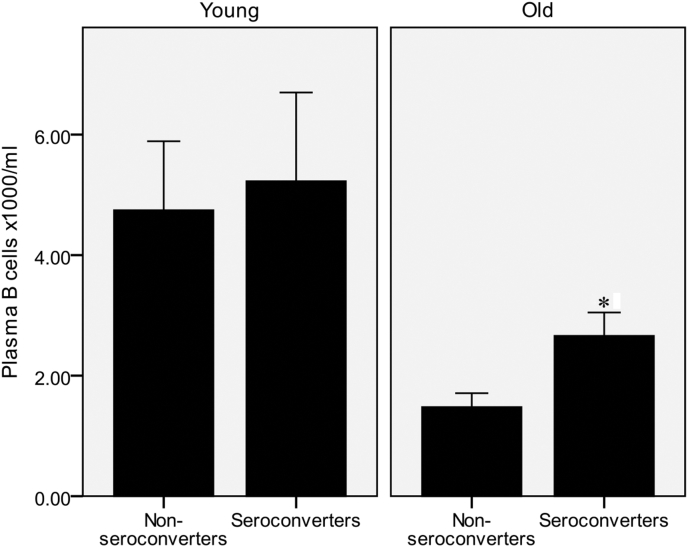
Seroconverters to the H1N1 subunit in the older cohort had significantly higher post vaccination numbers of plasma B cells (Fig. 3; independent t-test). For the H3N2 and Brisbane subunits, there were trends for associations with IgG+ memory and total B cells, but these were not statistically significant (data not shown).
Fig. 3.
Higher numbers of circulating plasma B cells are associated with seroconversion to H1N1 in the older cohort. Data are mean ± SE for n = 58 young and n = 54 older subjects. *Significantly different from non-seroconverters within the same age group (P < 0.01, independent t-test).
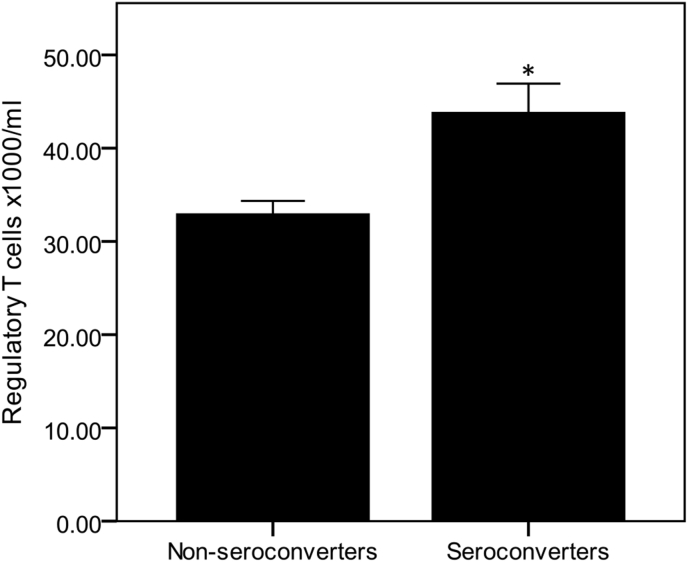
Seroconversion was less consistently associated with T cell phenotype. We previously reported that high numbers of senescent (CD28−CD57+) T cells were associated with failure to seroconvert to the influenza vaccine [13]. Further analysis of T cell phenotype demonstrated that seroconverters to all 3 subunits combined had significantly higher post vaccination numbers of Tregs (P < 0.001; combined cohorts, independent t-test; Fig. 4), and this was particularly significant for the Brisbane strain (P < 0.01, combined cohorts, independent t-test). Numbers of non-senescent CD26−CD28+ cytotoxic T cells 2 weeks post vaccination were also significantly higher in responders to Brisbane (P < 0.001, combined cohorts, independent t-test) (data not shown).
Fig. 4.
Higher numbers of regulatory T cells are associated with seroconversion to all subunits combined in the combined cohort. Data are mean ± SE for n = 58 young and n = 54 older subjects. *Denotes significantly different from non-seroconverters within the same age group (P < 0.01, independent t-test).
3.4. Effect of the synbiotic on B and T cell phenotype
Intervention with the synbiotic did not alter B or T cell phenotype in either young or older subjects prior to vaccination (data not shown), and for this reason, the Linear Mixed Model analysis was applied to data collected at baseline, 6 weeks and 8 weeks only. Following vaccination, numbers of IgG+ memory B cells tended to increase in the older subjects receiving the synbiotic, but not in those receiving the placebo (Fig. 5). This was not the case in the young subjects, where there was no effect of the symbiotic (data not shown). Numbers of CD25high total and helper T cells increased more in the older subjects who received the synbiotic than those receiving placebo (LMM, effect of treatment in the older cohort, P < 0.01; data not shown). As reported previously, older subjects who were randomized to the synbiotic had a significantly higher baseline number of senescent (CD28−CD57+) helper T cells and a trend towards higher baseline numbers of senescent (CD28−CD57+) cytotoxic T cells compared with age-matched subjects who were randomized to the placebo, and this was associated with failure to seroconvert to the Brisbane subunit of the vaccine [13]. However, there were no other phenotypic differences in the B or T cell populations in the randomized groups at baseline.
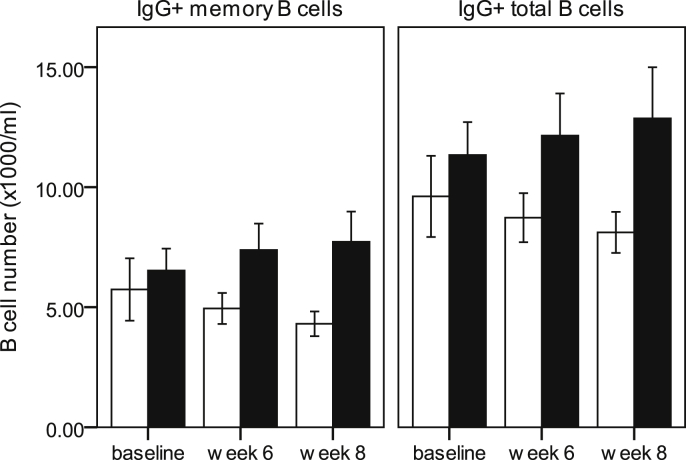
Fig. 5.
Effects of vaccination and synbiotic on numbers of IgG+ memory and IgG+ total B cells in older subjects. Data are mean ± SE for n = 54 older subjects. Numbers of IgG+ memory and IgG+ total B cells tended to increase in the older subjects receiving the synbiotic (■), but not in those receiving the placebo (□) (LMM, effect of treatment, older cohort, P = 0.068 and P = 0.09 respectively).
3.5. Responsiveness of B cells to in vitro re-stimulation with flu vaccine prior to vaccination is affected by ageing
As expected, vaccination increased B cell responsiveness to in vitro exposure to the vaccine. This was reflected in the higher proportion of activated (CD25+) cells within the naïve, memory and plasma B cell compartments, both in the combined and separate cohorts (Table 3). Activation of memory B cells (% CD25+) in response to in vitro re-stimulation with the vaccine was greater in young subjects than in older subjects (LMM, effect of age, p < 0.001; Table 3), and there was a similar trend for plasma B cells (P < 0.05).
Table 3.
Responsiveness of B cells to in vitro re-stimulation with flu vaccine.
| CD25 (%) Naïve t |
CD25 (%) Memory at |
CD25 (%) Plasma t |
CD25 MFI Naïve at |
CD25 MFI Memory t |
CD25 MFI Plasma t |
|||
|---|---|---|---|---|---|---|---|---|
| Young (n = 58) | Placebo | Baseline | 6.5 ± 0.7 | 15.5 ± 1.6 | 14.4 ± 2.2 | 171 ± 21 | 363 ± 83 | 1375 ± 347 |
| 6 weeks | 9.5 ± 1.0 | 22.5 ± 2.3 | 16.3 ± 2.2 | 219 ± 24 | 550 ± 111 | 1618 ± 499 | ||
| 8 weeks | 9.8 ± 1.2 | 23.6 ± 2.2 | 17.0 ± 1.8 | 231 ± 26 | 484 ± 90 | 1704 ± 490 | ||
| Synbiotic | Baseline | 8.0 ± 0.8 | 14.2 ± 1.6 | 12.5 ± 1.4 | 204 ± 21 | 160 ± 56 | 1554 ± 347 | |
| 6 weeks | 12.0 ± 1.0ˆ | 22.6 ± 1.9ˆ | 20.5 ± 2.4 | 299 ± 26 | 405 ± 70 | 2339 ± 678 | ||
| 8 weeks | 10.0 ± 0.9 | 18.2 ± 1.9 | 18.3 ± 2.9 | 212 ± 24 | 261 ± 71 | 1447 ± 748 | ||
| Older (n = 54) | Placebo | Baseline | 9.4 ± 1.8 | 8.9 ± 2.5 | 5.0 ± 1.9* | 502 ± 129 | 674 ± 338 | 637 ± 664 |
| 6 weeks | 12.7 ± 1.9 | 13.2 ± 2.5* | 12.7 ± 2.8 | 629 ± 137 | 1401 ± 672 | 3319 ± 965 | ||
| 8 weeks | 13.9 ± 2.2 | 14.0 ± 2.8* | 18.3 ± 3.3ˆ | 665 ± 152 | 1377 ± 560 | 2953 ± 1015 | ||
| Synbiotic | Baseline | 6.1 ± 0.9 | 6.8 ± 2.6 | 7.1 ± 4.5 | 235 ± 50 | 126 ± 280 | 907 ± 367 | |
| 6 weeks | 8.8 ± 1.2 | 11.6 ± 2.3* | 13.6 ± 3.5 | 315 ± 61 | 708 ± 228 | 2083 ± 760 | ||
| 8 weeks | 9.2 ± 1.2 | 13.5 ± 2.2 | 13.1 ± 4.0 | 275 ± 41 | 721 ± 157 | 1965 ± 483 | ||
Data are mean ± SE for n = 58 young and n = 54 older subjects and were analysed using a Linear Mixed Model (LMM) with fixed factors of time (repeated measures), age and treatment. There was no significant effect of treatment for either cohort. a Denotes a significant main effect of age (P < 0.01 at least) and t denotes a significant main effect of time (P < 0.01 at least) for the combined cohorts. When the effect of time was examined separately in the young and older cohorts, there were significant effects of vaccination in all B cell subsets (P < 0.01 at least). Activation of memory B cells (% CD25+) in response to in vitro re-stimulation with the vaccine was greater in young subjects than in older subjects (LMM, effect of age, P < 0.001). *Denotes significantly different from young subjects within the same timepoint and treatment group at P < 0.01 and ˆ denotes significantly different from baseline within the same age and treatment group at P < 0.01 (post-hoc t-tests with Bonferroni correction).
Seroconverters to the H3N2 and Brisbane subunits demonstrated greater responsiveness of memory B cells (% CD25+) to in vitro re-stimulation with the influenza vaccine than non-converters (P < 0.01 and P < 0.01 respectively for combined cohorts, data not shown). Responsiveness of plasma B cells to in vitro re-stimulation tended to be greater in seroconverters to Brisbane compared with non-seroconverters (P < 0.02 for combined cohorts; data not shown). These differences were not maintained when the young and older subjects were analysed separately.
3.6. Responsiveness of T cells to in vitro re-stimulation with flu vaccine
As expected, vaccination increased T cell responsiveness to in vitro exposure to the vaccine, but there was no significant effect of age. This increased responsiveness was reflected in the higher proportion of activated (CD25+) cells and of mean fluorescence intensity within the CD4+ and CD8+ T cell compartments when young and older subjects were combined (LMM, effect of time, combined cohorts, P < 0.001), and within the young cohort (LMM, effect of time P < 0.001 at least, young cohort; Table 4), but not the older cohort. Although this suggests a greater responsiveness to the vaccine of T cells from young subjects, there was no significant effect of age according to the LMM. Furthermore, unlike B cells, there was no clear relationship between the responsiveness of T cells to re-stimulation with influenza vaccine and the antibody response to the vaccine or seroconversion (data not shown).
Table 4.
Responsiveness of T cells to in vitro re-stimulation with flu vaccine.
| CD25 (%) Total T cells t |
CD25 (%) CD4+ T cells t |
CD25 (%) CD8+ T cells t |
CD25 MFI Total T cells t |
CD25 MFI CD4+ T cells t |
CD25 MFI CD8+ T cells t |
|||
|---|---|---|---|---|---|---|---|---|
| Young (n = 58) | Placebo | Baseline | 9.3 ± 0.6 | 9.2 ± 0.6 | 6.9 ± 0.6 | 329 ± 35 | 389 ± 43 | 99 ± 19 |
| 6 weeks | 11.8 ± 1.0 | 11.2 ± 0.9 | 9.6 ± 1.0 | 392 ± 57 | 447 ± 63 | 171 ± 23 | ||
| 8 weeks | 12.2 ± 1.3 | 11.3 ± 1.2 | 10.6 ± 1.5 | 386 ± 58 | 420 ± 67 | 186 ± 27 | ||
| Synbiotic | Baseline | 10.1 ± 0.6 | 9.5 ± 0.5 | 8.6 ± 0.7 | 332 ± 44 | 353 ± 38 | 154 ± 30 | |
| 6 weeks | 14.0 ± 0.9 | 12.9 ± 0.9 | 12.6 ± 0.8 | 476 ± 58 | 533 ± 64 | 225 ± 28 | ||
| 8 weeks | 11.3 ± 0.8 | 11.1 ± 1.0 | 9.8 ± 0.7 | 379 ± 54 | 427 ± 55 | 159 ± 22 | ||
| Older (n = 54) | Placebo | Baseline | 8.6 ± 1.3 | 7.2 ± 1.6 | 11.7 ± 2.3 | 397 ± 77 | 307 ± 136 | 621 ± 210 |
| 6 weeks | 12.6 ± 1.8 | 11.0 ± 1.9 | 13.9 ± 2.2 | 725 ± 129 | 537 ± 130 | 852 ± 254 | ||
| 8 weeks | 13.0 ± 1.9 | 11.7 ± 2.1 | 14.1 ± 2.4 | 710 ± 156 | 577 ± 188 | 782 ± 244 | ||
| Synbiotic | Baseline | 7.9 ± 1.5 | 7.9 ± 1.6 | 7.2 ± 1.9 | 324 ± 104 | 427 ± 180 | 267 ± 113 | |
| 6 weeks | 10.4 ± 2.0 | 10.6 ± 2.2 | 8.5 ± 2.1 | 483 ± 98 | 621 ± 174 | 252 ± 91 | ||
| 8 weeks | 11.9 ± 2.4 | 12.0 ± 2.6 | 10.6 ± 2.6 | 527 ± 121 | 798 ± 286 | 301 ± 77 | ||
Data are mean ± SE for n = 58 young and n = 54 older subjects and were analysed using a Linear Mixed Model (LMM) with fixed factors of time (repeated measures), age and treatment. There was no significant effect of either age or treatment for either cohort. t Denotes a significant main effect of time (P < 0.01 at least) for the combined cohorts. When the effect of time was examined separately in the young cohort, there were significant effects of vaccination in all T cell subsets (P < 0.01 at least), but this was not the case in the older cohort.
3.7. Influence of CMV status on responsiveness of naïve B cells and cytotoxic T cells to in vitro re-stimulation with the influenza vaccine
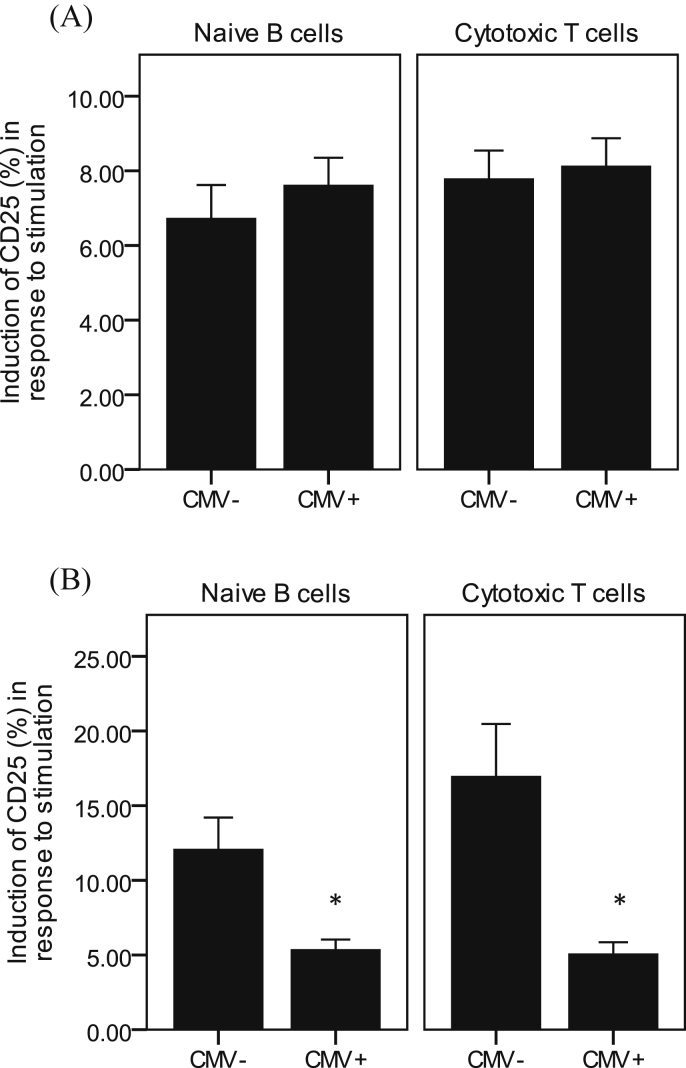
49% of young and 53% of older subjects were seropositive for CMV, with no significant difference between age groups. In young subjects, CMV seropositivity had no influence on responsiveness of either naïve B cells or cytotoxic T cells to in vitro re-stimulation with the vaccine (Fig. 6A). However, in older subjects, CMV seropositivity was associated with significantly lower responsiveness to the vaccine in these subsets (Fig. 6B). Other B and T cell subsets were not influenced by CMV seropositivity.
Fig. 6.
Effect of CMV seropositivity on responsiveness of B and T cells to in vitro re-stimulation with the influenza vaccine. Data are mean ± SE for n = 45 young (A) and n = 44 older (B) subjects. *Denotes significantly different from CMV− subjects (P < 0.01, independent t-test).
3.8. Effect of the synbiotic on responsiveness of B and T cells to in vitro re-stimulation with influenza vaccine
There were no significant effects of the synbiotic on the responsiveness of either B cells or T cells to re-stimulation with influenza vaccine, which suggests that overall, there was no effect of treatment on antigen recall (data not shown).
4. Discussion
Influenza vaccination increased numbers of key B cell subsets in young subjects, but failed to do so in older subjects and this had a significant impact on seroconversion. B and T cell subsets demonstrated a strong response to the antigen-specific recall challenge for both young and older subjects, and although not influenced by ageing, responsiveness to the recall challenge was associated with seroconversion. In older subjects, CMV seropositivity was associated with a significantly lower recall response to the vaccine. Overall, there was little evidence of any effect of the synbiotic on the responsiveness of B or T cells to re-stimulation with influenza vaccine.
This study confirmed some of the well-documented age-related alterations in B and T cell phenotype, including restricted B cell diversity, reduced numbers of memory and plasma B cells and accumulation of terminally differentiated senescent CD28−CD57+ helper and cytotoxic T cells. In a previous paper, we demonstrated that these age-related alterations in the T cell profile were related to an impaired antibody response to the Brisbane subunit [13]. In the current study, we demonstrate that the number of circulating memory B cells following influenza vaccination increased to a significantly greater degree in the young subjects compared with the older subjects. This was correlated with the magnitude of the serological antibody response, which provides novel insight into the impact of ageing on the relationship between expanding B cell subsets and seroconversion following influenza vaccination [19]. Class-switching of memory and plasma B cells to IgA+ and IgG+ cells declines during ageing, resulting in a weaker humoral immune response and impaired protection against pathogens [20]. The current study demonstrated that numbers of isotype class-switched memory and total IgA+ and IgG+ B cells were significantly lower in the older subjects compared with the young subjects at baseline. Nevertheless, older subjects who seroconverted to H3N2 had greater numbers of IgA+ and IgG+ memory and total IgG+ B cells prior to vaccination. Similarly, seroconverters to the Brisbane subunit had greater numbers of total IgA+ B cells prior to vaccination. This is consistent with the suggestion of an association between the proportion of circulating class-switched B cells prior to influenza vaccination and the antibody response after vaccination [21].
In the current study, although seroconversion was less consistently associated with T cell phenotype, high levels of CD26+ Th1 memory cells prior to vaccination were related to an impaired antibody response to Brisbane, in addition to the CD28−CD57+ senescent T cells reported in our previous paper [13]. There was an increase in CD4+CD25high T cells and Tregs following vaccination in young subjects, which is consistent with a previous study [22]. Seroconverters to all 3 subunits combined had significantly higher post vaccination numbers of Tregs, and this was particularly significant for the Brisbane strain. The role of Tregs in humoral immunity and the antibody response to vaccination is unclear, although some studies report an inverse relationship between Tregs and the antibody response to vaccination [23]. It has been suggested that increases in CD4+CD25high T cells and Tregs after influenza vaccination increase levels of IL-10 and are negatively correlated with TGF-β, which results in suppression of the antibody response [22].
In vitro re-stimulation of B cells with the influenza vaccine results in induction of CD25 [24]. Morphologically, CD25+ B cells are larger in size and more granulated than CD25− B cells, they demonstrate greater expression of the IL-2 receptor and of the co-stimulatory molecules, CD80 and CD27, and have higher frequency and density of expression of IgA and IgG, but lower expression of MHC class II [25]. Functionally, CD25+ B cells have lower production of Ig than CD25- B cells, even though they have greater surface expression of Ig [25]. Despite lower expression of MHC class II, CD25+ B cells have greater antigen presentation activity than CD25− B cells, perhaps due to greater expression of CD80 and CD27. The greater antigen presentation activity contributes to greater in vitro stimulation of T helper cell proliferation compared to CD25− B cells. Antibody neutralization of CD25 removes this effect, demonstrating the importance of this surface molecule in B cell activation and function [25]. Vaccination increased the responsiveness of B cells to an antigen-specific recall challenge with the vaccine, evidenced by an increase in the percentage of CD25+ memory and plasma B cells, reflecting a strong secondary response of B cells to the vaccine. The responsiveness of B cells from young subjects to in vitro re-stimulation with the vaccine was significantly greater than that of older subjects. Memory and plasma B cells from seroconverters were more responsive to in vitro stimulation with the vaccine than non-converters, both before and after vaccination, for all three subunits combined and for the H3N2 and Brisbane subunits, but not for H1N1. Furthermore, impaired responsiveness in older subjects was associated with low antibody production in response to vaccination, which suggests that in vitro responsiveness of B cells to the influenza vaccine may be a useful functional marker of the immune response to vaccination. Vaccination also resulted in greater T cell responsiveness to an antigen-specific recall challenge, but unlike B cells, there was little or no influence of age. Furthermore, there was no clear relationship between the responsiveness of T cells to antigen recall and the antibody response to the vaccine or seroconversion. Interestingly, CMV seropositivity was associated with significantly lower responsiveness to the vaccine in older subjects only; this is relevant because latent infection with CMV has been demonstrated to result in a poor response to infection and vaccination [26].
We previously demonstrated that intervention with a novel synbiotic, B. longum + Gl-OS failed to reverse the impairment in the antibody response to influenza vaccination in older subjects. However, further immunological characterization revealed a greater degree of immunosenescence at baseline in older subjects randomized to the synbiotic, which could have explained the particularly poor response of these subjects to the vaccination. This highlighted the fact that interpretation of interventions examining the response to vaccination in older people may be highly dependent on their baseline immunological phenotype. In the current study, intervention with the synbiotic did not alter B or T cell phenotype in either young or older subjects prior to vaccination, but following vaccination, numbers of IgG+ memory B cells tended to increase more in the older subjects receiving the synbiotic than those receiving the placebo and numbers of CD25high total and helper T cells increased more in the older subjects who received the synbiotic than those receiving placebo. Thus, the greater degree of immunosenescence in the synbiotic group at baseline appears to have had little impact on numbers of memory B cells and helper T cells following vaccination. Overall, there were no other phenotypic difference in the B and T cell populations. There was also no effect of the synbiotic on the antigen-specific recall challenge, but this may well be due to the greater degree of immunosenescence in the older subjects randomized to the synbiotic masking any beneficial effects. Beneficial effects of probiotics on immune function have been reported in some, but not all, human studies [27] and some studies report decreased incidence of and/or duration of flu by probiotics after influenza vaccination [17], [28]. However, intervention studies evaluating the impact of probiotics on the immune response to vaccination are limited and report inconsistent results regarding vaccine-specific antibody production, with the majority being conducted in adults and only a few in elderly subjects [10]. Most of these studies simply report antibody titres, with no further immunological exploration [10]. This paper demonstrates that aspects of the humoral response to vaccination are markedly influenced by ageing, but resistant to manipulation by pre- and pro-biotics.
5. Conclusion
In conclusion, while vaccination altered the B and T cell profile differentially in young and older subjects, antigen-specific B and T cell activation following an in vitro recall challenge with the influenza vaccine was not altered by a synbiotic in either young or older subjects.
Conflict of interest
PY, CC, KT, ST and MG were involved in the conception and design of the study; SE, AP-K, CC, CM and LC were involved in the acquisition, analysis and interpretation of the data; PY led the preparation of the manuscript, with input from all authors. All authors have approved the final version of the manuscript. None of the authors has any conflict of interest.
Acknowledgements
This work was funded by a grant (BB/H00470X/1) from the Biotechnology and Biological Sciences Research Council Diet and Health Research Industry Club (BBSRC-DRINC).
Footnotes
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.clnu.2017.01.011.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Weiskopf D., Weinberger B., Grubeck-Loebenstein B. The aging of the immune system. Transpl Int. 2009;22:1041–1050. doi: 10.1111/j.1432-2277.2009.00927.x. [DOI] [PubMed] [Google Scholar]
- 2.Thompson W.W., Shay D.K., Weintraub E., Brammer L., Cox N., Anderson L.J. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289:179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 3.Nordin J., Mullooly J., Poblete S., Strikas R., Petrucci R., Wei F. Influenza vaccine effectiveness in preventing hospitalizations and deaths in persons 65 years or older in Minnesota, New York, and Oregon: data from 3 health plans. J Infect Dis. 2001;184:665–670. doi: 10.1086/323085. [DOI] [PubMed] [Google Scholar]
- 4.Lambert N.D., Ovsyannikova I.G., Pankratz V.S., Jacobson R.M., Poland G.A. Understanding the immune response to seasonal influenza vaccination in older adults: a systems biology approach. Expert Rev Vaccines. 2012;11:985–994. doi: 10.1586/erv.12.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McElhaney J.E., Xie D., Hager W.D., Barry M.B., Wang Y., Kleppinger A. T cell responses are better correlates of vaccine protection in the elderly. J Immunol. 2006;176:6333–6339. doi: 10.4049/jimmunol.176.10.6333. [DOI] [PubMed] [Google Scholar]
- 6.Fagnoni F.F., Vescovini R., Passeri G., Bologna G., Pedrazzoni M., Lavagetto G. Shortage of circulating naive CD8(+) T cells provides new insights on immunodeficiency in aging. Blood. 2000;95:2860–2868. [PubMed] [Google Scholar]
- 7.Czesnikiewicz-Guzik M., Lee W.W., Cui D., Hiruma Y., Lamar D.L., Yang Z.Z. T cell subset-specific susceptibility to aging. Clin Immunol. 2008;127:107–118. doi: 10.1016/j.clim.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haynes L. Impaired CD4 T cell cognate function is responsible for age-related reductions in humoral responses. Exp Lung Res. 2005;31(Suppl. 1):78. [PubMed] [Google Scholar]
- 9.Haynes L. The effect of aging on cognate function and development of immune memory. Curr Opin Immunol. 2005;17:476–479. doi: 10.1016/j.coi.2005.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maidens C., Childs C., Przemska A., Dayel I.B., Yaqoob P. Modulation of vaccine response by concomitant probiotic administration. Br J Clin Pharmacol. 2013;75:663–670. doi: 10.1111/j.1365-2125.2012.04404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silvi S., Verdenelli M.C., Orpianesi C., Cresci A. EU project Crownalife: functional foods, gut microflora and healthy ageing – isolation and identification of Lactobacillus and Bifidobacterium strains from faecal samples of elderly subjects for a possible probiotic use in functional foods. J Food Eng. 2003;56:195–200. [Google Scholar]
- 12.You J.L., Yaqoob P. Evidence of immunomodulatory effects of a novel probiotic, Bifidobacterium longum bv. infantis CCUG 52486. FEMS Immunol Med Microbiol. 2012;66:353–362. doi: 10.1111/j.1574-695X.2012.01014.x. [DOI] [PubMed] [Google Scholar]
- 13.Przemska-Kosicka A., Childs C.E., Enani S., Maidens C., Dong H., Dayel I.B. Effect of a synbiotic on the response to seasonal influenza vaccination is strongly influenced by degree of immunosenescence. Immun Ageing. 2016;13:6. doi: 10.1186/s12979-016-0061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ligthart G.J., Corberand J.X., Fournier C., Galanaud P., Hijmans W., Kennes B. Admission criteria for immunogerontological studies in man – the Senieur Protocol. Mech Ageing Dev. 1984;28:47–55. doi: 10.1016/0047-6374(84)90152-0. [DOI] [PubMed] [Google Scholar]
- 15.Hudon C., Fortin A., Vanasse A. Cumulative illness rating scale was a reliable and valid index in a family practice context. J Clin Epidemiol. 2005;58:603–608. doi: 10.1016/j.jclinepi.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 16.Davidson L.E., Fiorino A.M., Snydman D.R., Hibberd P.L. Lactobacillus GG as an immune adjuvant for live-attenuated influenza vaccine in healthy adults: a randomized double-blind placebo-controlled trial. Eur J Clin Nutr. 2011;65:501–507. doi: 10.1038/ejcn.2010.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olivares M., Diaz-Ropero M.P., Sierra S., Lara-Villoslada F., Fonolla J., Navas M. Oral intake of Lactobacillus fermentum CECT5716 enhances the effects of influenza vaccination. Nutrition. 2007;23:254–260. doi: 10.1016/j.nut.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 18.Sarbini S.R., Kolida S., Gibson G.R., Rastall R.A. In vitro fermentation of commercial alpha-gluco-oligosaccharide by faecal microbiota from lean and obese human subjects. Br J Nutr. 2013;109:1980–1989. doi: 10.1017/S0007114512004205. [DOI] [PubMed] [Google Scholar]
- 19.Pinna D., Corti D., Jarrossay D., Sallusto F., Lanzavecchia A. Clonal dissection of the human memory B-cell repertoire following infection and vaccination. Eur J Immunol. 2009;39:1260–1270. doi: 10.1002/eji.200839129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scholz J.L., Diaz A., Riley R.L., Cancro M.P., Frasca D. A comparative review of aging and B cell function in mice and humans. Curr Opin Immunol. 2013;25:504–510. doi: 10.1016/j.coi.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frasca D., Diaz A., Romero M., Phillips M., Mendez N.V., Landin A.M. Unique biomarkers for B-cell function predict the serum response to pandemic H1N1 influenza vaccine. Int Immunol. 2012;24:175–182. doi: 10.1093/intimm/dxr123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang S.M., Tsai M.H., Lei H.Y., Wang J.R., Liu C.C. The regulatory T cells in anti-influenza antibody response post influenza vaccination. Hum Vacc Immunother. 2012;8:1243–1249. doi: 10.4161/hv.21117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X.F., Liu F., Zhou S., Xu Z.P., Hoellwarth J., Chen X.J. Partial regulatory T cell depletion prior to schistosomiasis vaccination does not enhance the protection. PLoS One. 2012:7. doi: 10.1371/journal.pone.0040359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waldmann T.A., Goldman C.K., Robb R.J., Depper J.M., Leonard W.J., Sharrow S.O. Expression of interleukin-2 receptors on activated human B-cells. J Exp Med. 1984;160:1450–1466. doi: 10.1084/jem.160.5.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brisslert M., Bokarewa M., Larsson P., Wing K., Collins L.V., Tarkowski A. Phenotypic and functional characterization of human CD25(+) B cells. Immunology. 2006;117:548–557. doi: 10.1111/j.1365-2567.2006.02331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Derhovanessian E., Maier A.B., Hahnel K., McElhaney J.E., Slagboom E.P., Pawelec G. Latent infection with cytomegalovirus is associated with poor memory CD4 responses to influenza a core proteins in the elderly. J Immunol. 2014;193:3624–3631. doi: 10.4049/jimmunol.1303361. [DOI] [PubMed] [Google Scholar]
- 27.Lomax A.R., Calder P.C. Probiotics, immune function, infection and inflammation: a review of the evidence from studies conducted in humans. Curr Pharm Des. 2009;15:1428–1518. doi: 10.2174/138161209788168155. [DOI] [PubMed] [Google Scholar]
- 28.Bunout D., Barrera G., Hirsch S., Gattas V., de la Maza M.P., Haschke F. Effects of a nutritional supplement on the immune response and cytokine production in free-living Chilean elderly. J Parenter Enteral Nutr. 2004;28:348–354. doi: 10.1177/0148607104028005348. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.