Abstract
Up-regulation of the dystrophin-related gene utrophin represents a promising therapeutic strategy for the treatment of Duchenne Muscular Dystrophy (DMD). In order to re-program the utrophin expression level in muscle, we engineered artificial zinc finger transcription factors (ZF-ATFs) that target the utrophin ʻAʼ promoter. We have previously shown that the ZF-ATF “Jazz”, either by transgenic manipulation or by systemic adeno-associated viral delivery, induces significant rescue of muscle function in dystrophic “mdx” mice. We present the full characterization of an upgraded version of Jazz gene named “JZif1” designed to minimize any possible host immune response. JZif1 was engineered on the Zif268 gene-backbone using selective amino acid substitutions to address JZif1 to the utrophin ‘A’ promoter. Here, we show that JZif1 induces remarkable amelioration of the pathological phenotype in mdx mice. To investigate the molecular mechanisms underlying Jazz and JZif1 induced muscle functional rescue, we focused on utrophin related pathways. Coherently with utrophin subcellular localization and role in neuromuscular junction (NMJ) plasticity, we found that our ZF-ATFs positively impact the NMJ. We report on ZF-ATF effects on post-synaptic membranes in myogenic cell line, as well as in wild type and mdx mice. These results candidate our ZF-ATFs as novel therapeutic molecules for DMD treatment.
Keywords: ZF-ATF, Utrophin, DMD, NMJ, AAV, Gene therapy
Highlights
-
•
Up-regulation of Dystrophin-related gene Utrophin is a promising therapeutic strategy for Duchenne Muscular Dystrophy (DMD).
-
•
Zinc Finger Artificial Transcription Factors (ZF-ATFs) have been designed to target and to activate Utrophin ‘A’ promoter.
-
•
ZF-ATFs ‘Jazz’ and ‘JZif1’ were delivered by AAV to muscle tissue in dystrophic mdx mouse.
-
•
Jazz and JZif1 improve neuromuscular junction (NMJ) morphology and correct dystrophic pathology in mdx mice.
1. Introduction
Duchenne Muscular Dystrophy (DMD) is a severe X-linked muscle degenerative disease caused by the absence of the cytoskeletal protein dystrophin [1]. Despite the considerable progress in understanding the pathogenesis and advancement of therapeutic approaches, there is no effective cure for DMD [[2], [3], [4]]. A promising strategy to cure DMD aims to up-regulate utrophin gene expression, a ubiquitously expressed autosomal paralogue of dystrophin gene [5,6]. The strategy is based on the ability of utrophin protein to partially replace defective dystrophin. Utrophin displays about 80% homology with dystrophin and it is able to perform similar functions. Notably, utrophin up-regulation does not raise any immune response. Many research groups and companies are studying the possibility of modulating utrophin gene expression using pharmacological approaches [3,4,[7], [8], [9]]. Several small molecules, including heregulin, nabumetone, l-arginine and SMT C1100 are able to up-regulate the utrophin gene by activating the utrophin ‘A’ promoter [3]. In particular, the drug SMT C1100/Ezutromid is currently in clinical trials by Summit Therapeutics [4,10]. In addition, a post-transcriptional modulation of utrophin is achieved by a recombinant biglycan (rhBGN), which can recruit utrophin to the sarcolemma [11,12]. Our strategy is based on the use of zinc finger artificial transcription factors (ZF-ATFs) designed ad hoc to re-program utrophin gene expression. ZF-ATFs are small transcriptional regulators, active at very low concentration and mimic the body's natural regulatory mechanism, resulting in the production of all the different isoforms of the targeted protein [13]. Moreover, ZF-ATFs are well tolerated and do not affect global transcription when expressed in animal models, as demonstrated with the ZF-ATF prototype gene Jazz [13,14]. In addition, our last generation of ZF-ATFs has been designed to minimize immunogenicity. Using selective amino acid substitution, the natural Zif268/Egr1 transcription factor was specifically re-directed to the utrophin ‘A’ promoter, giving rise to the novel ZF-ATF named ‘JZif1’. Importantly JZif1 and Jazz genes share the same 9 base pair DNA target sequence. For delivery of ZF-ATFs, we chose recombinant Adeno-associated viral (AAV) vectors which have been described as powerful and safe “gene transfer” vehicles for the treatment of neuromuscular disorders [[15], [16], [17], [18]]. The AAV vector was engineered to express therapeutic genes under the control of the human alpha-actin muscle specific promoter: “muscle-AAV” (mAAV). The mAAV has been combined with the use of the muscle-targeting AAV-serotype “8”. Intraperitoneal injection of mAAV8-JZif1 or mAAV8-Jazz into dystrophin-deficient mdx mice (5 days after birth) resulted in therapeutic re-programming of the muscle utrophin gene, with a consequent amelioration of morphological as well as functional parameters of disease progression. Here we demonstrate the beneficial effects of our ZF-ATFs in mdx mice and describe the molecular mechanisms through which ZF-ATFs up-regulate utrophin, induce rescue of muscle function and positively impact the neuromuscular junction (NMJ). NMJ are structurally impaired in dystrophin-deficient mice, displaying an aberrant fragmented distribution of Acetylcholine receptors (AChRs), resulting in discontinuous and dispersed motor end-plate morphology [[19], [20], [21], [22]]. AChR spatial distribution at the post-synaptic membrane is crucial for synaptic function [23,24]. Recent studies indicate that an impaired NMJ morphology leads to NMJ dysfunction that can play a crucial role in DMD pathogenesis [25,26]. The beneficial effect of our ZF-ATFs on post-synaptic clustering of AChRs and on NMJ morphology/plasticity could therefore contribute to improve various aspects of muscle physiology and ameliorate DMD-like symptoms in the mdx mice.
2. Material and methods
2.1. Constructs
DNA fragments containing ZF-ATF zinc finger domains were synthetized by GenScript (New Jersey, USA). JZif1 gene was cloned into mAAV vector [15] and into the pAAV vector (Stratagene, La Jolla, CA, USA). The pXP luciferase reporter construct contains a portion of the utrophin ‘A’ promoter [27].
2.2. Cell cultures, transfections and reporter gene assay
Human HeLa and AAV-293 cell lines were grown in DMEM (Gibco-BRL, Grand Island, NY, USA) supplemented with 10% foetal bovine serum (Gibco-BRL), l-glutamine and penicillin/streptomycin. Mouse C2C12 myoblasts cell line [28] was grown in DMEM supplemented with 20% foetal bovine serum. Cell cultures were maintained at 37 °C in a humidified atmosphere of 5% CO2. Transient transfections were performed using Lipofectin® Transfection Reagent and PLUS™ Reagent (for HeLa cells) (Invitrogen, Carlsbad, CA, USA), or TransIT®-LT1 (for C2C12 cells) Transfection Reagent (Mirus Bio, Madison, WI, USA). Cell extracts were prepared and assayed for luciferase (LUC) activity as previously described [29].
2.3. Immunofluorescence
Cells were fixed in 4% formaldehyde for 10 min, permeabilized with 0.1% Triton X-100 for 5 min and incubated with mouse monoclonal anti-myc 9E10 antibody (9E10 clone; 1:20, DSHB, Iowa City, IO). Immunofluorescence on tissue was performed as previously described [13]. The following antibodies were used: anti-utrophin monoclonal antibody (610896, 1:100, BD Transduction Laboratories, Franklin Lakes, NJ, USA) and anti-laminin polyclonal antibody (L9393, 1:500, Sigma Aldrich Corporation, St. Louis, Missouri, USA). Samples were analyzed by conventional epifluorescence microscope (Olympus BX51; Tokyo, Japan). Images were captured using a digital camera and merged using the IAS2000 software.
2.4. Western blot analysis
Western blotting was performed as previously described [29]. Quadriceps muscle samples were homogenized in lysis buffer (1% SDS, 1% NP40, 5% glycerol, 5 mM EDTA) supplemented with proteinase inhibitors using a rotary-blade homogenizer (OMNI International GLH, Kennesaw, Georgia, USA). Anti-myc monoclonal antibody (9E10 clone; 1:100, DSHB, Iowa City, IO); anti β-actin monoclonal antibody (A5441, 1:10,000, Sigma-Aldrich Corporation, St. Louis, Missouri, USA); anti-utrophin monoclonal antibody (Mancho3, 8A4 clone; 1:100 DSHB); anti Egr-1 rabbit polyclonal antibody (C-19 clone, 1:200, Santa Cruz, Santa Cruz, CA, USA); anti-laminin polyclonal antibody (L9393, 1:2,000, Sigma-Aldrich Corporation) were used. ImageJ software (National Institutes of Health, USA) was used to quantify the densitometry of the immunoblot bands.
2.5. Chromatin immunoprecipitation (ChIP) assay
Chromatin immunoprecipitation assays were performed as previously described [29]. Equal amounts of chromatin from each sample were immunoprecipitated overnight with anti-myc 9E10 monoclonal antibody (9E10 clone; 5 μg, DSHB, Iowa City, IO). The immunoprecipitated sonicated chromatin was amplified using human utrophin promoter-specific primers forward 5′-CGGCACGCACGGTTCACTCTGGAGCGC-3′ and reverse 5′-CAGCAACTTTGTTCCGGAAGATCAGCC-3′; human thymidine kinase (TK)-specific primers forward 5′-GCCCCTTTAAACTTGGTGGGCGG-3′ and reverse 5′-TTGCGCCTCCGGGAAGTTCACG-3′; human dystrophin promoter-specific primers forward 5′-GTGTTTTAAGAATTGGCACCAG-3′ and reverse 5′-AGTCTGAATAAGAGAAGCAGCA-3′; human TopBP1 promoter-specific primers forward 5′-TGCTCACCTCCACGTTTGACA-3′ and reverse 5′-GCCTCACTTACCTCGTTGGAG-3′ [30]; human chromosome 16 region–specific primers forward 5′-AGGACCACTCGCTGGGTAAGCA-3′ and reverse 5′-CGCGGAGGGTGACATGGGGT-3′; human chromosome 17 region–specific primers forward 5′-ACCTGTGTGTGGGTGGTGAGA-3′ and reverse 5′-CTGTAGGGCCCCAGGCACCAT-3′; human chromosome 19 region–specific primers forward 5′-AGTGCCTGGCATCAGCTT-3′ and reverse 5′-CGCCATTGCACTCCAGCAT-3′. The DNA sequence of the potential off target site present in chromosome 16 is: 5′-GCTGCTGgG-3′, with 8 out of 9 base pair matches with JZif1 target sequence. The DNA sequence of the potential off target site present in chromosome 17 is: 5′-GCTGCTGCG-3′, 100% match with JZif1 target sequence. The DNA sequence of the potential off target site present in chromosome 19 is: 5′-GCTGCTGCG-3′, with 100% match with JZif1 target sequence.
2.6. Quantitative real-time PCR (qPCR)
RNA was isolated using TRIzol® reagent (Gibco-BRL) and was then reverse transcribed using a High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA, USA). Quantitative real-time PCR assays were performed using the TaqMan Universal PCR Master Mix (Applied Biosystems) in an ABI Prism 7000 Sequence Detection System (Applied Biosystems) according to the manufacturer's protocol. β2M was used for the normalization of mRNA and relative expression was calculated using the comparative Ct methods (2−ΔΔCt). PCR primers and probes for the target gene (UTRN) and for housekeeping gene (β2M) were purchased as TaqMan Gene Expression Assays (Applied Biosystems) [15].
2.7. Production, purification and titration of recombinant mAAV8 stocks
Recombinant mAAV8 vector stocks were packaged by triple transfection of AAV-293 cells using the plasmid method “AAV Helper-Free System” (Stratagene). mAAV8 viral particles were purified from DMEM growth medium 72 h after transfection. The cell growth medium was extensively centrifuged, purified and concentrated 20:1. The viral titer was quantified by real-time PCR (v.p./mL) [15].
2.8. Animal care
Dystrophin-deficient C57BL/10ScSn-DMDmdx/J mice (mdx) and C57BL/6 wt mice were maintained under conventional housing conditions. All procedures on mice were carried out in accordance with the Directive 2010/63/EU of the European Community for the care and use of laboratory animals and our experimental protocol was approved by the Ethical Committee Italian Health Ministry (Permit numbers 498/2015 PR). Housing of the animals meets the behavioral needing of the species and was supervised by the responsible veterinarian.
2.9. Mice treatments
Mice were injected with viruses at 5 days of age. Systemic infection was achieved by intraperitoneal (i.p.) injection of 150 μl of viral suspension (5 × 1012 v.p./ml) (75 μl on each side of the lower quadrant of the abdomen) using a 0.3 ml Accu-Fine syringe (Roche Diagnostics). Control mice were injected with the same volume of saline solution. Mice were analyzed at two/three months of age.
2.10. Muscle tissue preparation
Mice were sacrificed 8–12 weeks after injections. Muscles were dissected and embedded in Tissue-Tek O.C.T. medium (Sakura Fine technical, NL) or directly snap frozen in liquid nitrogen for RNA and protein extraction. For histological and immunofluorescence analysis, transversal sections were obtained by cryostate CM1850UV at 2208C (Leica Microsystem GmbH, Wetzlar, Germany), placed onto polysine-coated microscope slides (Menzel Gmbh & Co, Iserlohn, Germany) and fixed with formaldehyde 4% in PBS for 10 min.
2.11. Histological analysis
Transversal sections (9 μm thick) of muscles were stained with hematoxylin and eosin (H&E; Roth, Germany) following the manufacturer's instructions. The entire cross-section, taken at mid-belly, was analyzed by microscope Olympus BX51 (Olympus Corporation) [13,15]. Images were captured using a digital camera at 10 x magnification and analyzed by ImageJ software. Five mice per group were analyzed and at least 200 myofibers were counted in each section.
2.12. Serum creatine kinase (CK) assay
The serum CK assay was performed as described by Radley-Crabb et al. [31]. CK activity was quantified in duplicate using the CK NAC Kit (Greiner Diagnostic GmbH, Bahlingen, Germany), and analyzed kinetically using the multilabel counter Victor 3 (PerkinElmer, Waltham, MA, USA). Data are expressed as units per liter (U/L).
2.13. Assays of mice performances by treadmill running
Exercise studies were performed as previously described and after the treadmill performance some mice were injected with Evans Blue dye [15].
2.14. Mechanical response of isolated muscles
As previously described [15], muscle excitability was examined ex-vivo by physiological assessment of the muscle force on isolated extensor digitorum longus (EDL) preparations of both hind limbs and of abdominal longitudinal strips (ABD), following direct electrical stimulation with rectilinear pulses of 0.5 ms duration at 0.05–0.2 Hz using variable voltage, until reaching the supramaximal voltage. At the end of the tension recordings, EDL and ABD muscles were subjected to a period of repetitive stimulations by trains of stimuli at a frequency of 40 Hz for 250 ms every second for 3 min. Following this procedure, able to obtain the muscle fatigue, tissues were removed from the chamber and placed in a Procion Orange solution (0.2%), in order to identify the percentage of damaged muscular fibers [13].
2.15. AChR clustering C2C12 myotubes
C2C12 myoblasts were seeded at a density of 1.7 × 104 cells/cm2 in Lab-Tek® Chamber Slides (Sigma-Aldrich Co.) previously coated with laminin‑1 (L2020; Sigma-Aldrich Co.) at 1 μg/cm2. 24 h after the transfection, the growth media was replaced with differentiation media containing 2% horse serum (Gibco-BRL). After 48 h Rat agrin (R&D Systems, Inc., Minneapolis, MN, USA) was included in the media at 10 ng/ml. Then, 18 h later, cultures were fixed in 4% formaldehyde for 30 min a room temperature, permeabilized for 10 min with 0.2% Nonidet P40, incubated with α‑bungarotoxin Alexa Fluor® 594 conjugate (B13423, 1:1000, Thermo Fisher Scientific, Waltham, MA, USA) at 1 μg/ml for 1 h at room temperature and mounted with ProLong Gold with Dapi (Thermo Fisher Scientific). Samples were examined by conventional epifluorescence microscope (Olympus BX51; Olympus Corporation). Images were captured using a digital camera at 20× magnification by a SPOT RT3 camera and elaborated by IAS2000 software. About twenty fields for each category were randomly captured and three independent experiments were averaged. Image analysis was performed with ImageJ software.
2.16. Morphological analysis of neuromuscular junctions (NMJ)
Mice were anesthetized with 400 mg/kg choral hydrate (Sigma Aldrich Co.) and transcardially perfused with phosphate buffered saline (PBS) followed by 4% paraformaldehyde (PFA) in PBS. The entire diaphragm was dissected and completely immersed in PBS. All subsequent incubation steps were performed on a rotating platform, at room temperature. Diaphragm was immersed in blocking buffer (3% BSA + 0.5% Triton-X in PBS) for at least 3 h, incubated in quenching solution (2 mg/ml sodium borohydride, in PBS) for three times, 10 min each time, and then washed three times with PBS. To visualize postsynaptic acetylcholine receptors (AChRs), diaphragms were incubated with Alexa Fluor 594-conjugated or Alexa Fluor 488 α-bungarotoxin (α-BTX) (B13423, B13422, 1:1,000, Thermo Fisher Scientific, Waltham, MA, USA), diluted in BSA 1% + Triton X-100 0.1%, for at least 2 h, washed 3 times with PBS, and then placed on a slide and mounted with ProLong Gold with Dapi (Termo Fisher Scientific). In order to identify each individual NMJ, the diaphragm was further flattened applying a pressure. Stained diaphragm was examined by confocal microscope (TCS-SP5, Leica Microsystem) acquiring a z-series of 20–25 optical sections at 4–4.5 μm intervals, by a ×20 magnification lens. Morphological analysis of neuromuscular junctions was carried out on two-dimensional projections at maximum intensity of each z-series generated with the LAF AF software platform (Leyca Microsystem) in the Tif format. For each hemi-diaphragm muscle, 3 separate regions were imaged following the trajectory of the post-synaptic receptor clusters. Approximately 200–250 junctions for the whole diaphragm muscle were analyzed in at least 3 animals per experimental group. AChR clusters were classified as continuous if they presented 3 or less continuous regions of receptor clustering and discontinuous if they presented >3 regions of receptor clustering.
2.17. Magnetic resonance imaging
MRI studies were performed on a 7T preclinical magnetic resonance scanner (Bruker, BioSpec 70/30 USR; Karlsruhe, Germany), equipped with 450/675 mT/m gradients (slew-rate: 3,400-4,500 T/m/s; rise-time: 140 μs). A phased-array rat-heart coil with four internal preamplifiers was used as receiver, coupled with a 72 mm linear-volume coil as transmitter. Mice were analyzed under general anesthesia obtained by 1.5–2% isoflurane (Forane®; Abbott, North Chicago, Il, USA) vaporized in 100% oxygen, in prone position, with the right leg fixed in the center of the coil. Breathing and body temperature was monitored during MRI (SA Instruments Inc., Stony Brook, NY, USA). MRI protocol included T2-mapping (inflammation and necrosis detection), diffusion-mapping (fibers disruption and tissue reorganization), and Proton spectrometry (presence of fat and connective tissue) [32]. MRI post-processing and image analysis were performed with Paravision-5.0 software (Bruker).
2.18. Statistical analysis
Data are presented as either the mean ±S.D. or ±SEM. The differences between means were analyzed with a t-test. Statistical significance was defined at P < 0.05. Analysis of variance (ANOVA) for repeated measures was used to analyze the effect of treadmill running.
3. Results
3.1. The novel artificial transcription factor JZif1
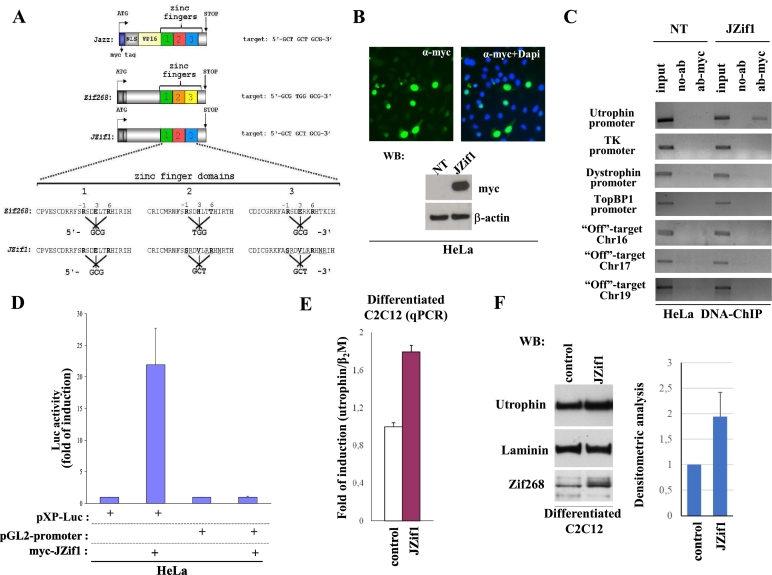
We previously demonstrated that the ZF-ATF artificial gene named ‘Jazz’ (Fig. 1A, top) (GenBank accession number AJ243577.1.), either by transgenic manipulation or by systemic adeno-associated viral vector delivery, induces significant rescue of muscle function in dystrophic mdx mice [[13], [14], [15]]. With the intent to move toward clinical application and to minimize potential deleterious immune responses, we developed a novel “almost natural” ZF-ATF gene using the backbone of the well-characterized Zif268/EGR1 human transcription factor (NCBI accession number NM_001964). As summarized in Fig. 1A, by substituting key amino acids in Zif268 alpha-helix zinc finger motifs, we re-directed the modified Zif268, re-named ‘JZif1’ (GenBank accession number MF377379) to utrophin ‘A’ promoter. The JZif1 DNA binding domain was designed following the same zinc finger signature previously used to engineer Jazz prototype gene [[33], [34], [35]]. JZif1 and Jazz bind the same nine base pair target sequence (5′-GCTCGTGCG-3′) presents in both the mouse and human utrophin gene ‘A’ promoters (Fig. 1A) (EMBL accession No. X95523). To verify whether JZif1 overcomes any impediments derived from its own primary structure and/or chromatin infrastructure in the utrophin promoter locus, we investigated its nuclear localization and performed chromatin immunoprecipitation experiments (ChIPs) (Fig. 1B and C). As shown in Fig. 1C, ChIP experiments in HeLa cells demonstrated that JZif1, as well as Jazz prototype gene, displayed binding specificity for the human genomic utrophin ‘A’ promoter. As expected, JZif1 did not bind the unrelated promoters belonging to thymidine kinase (TK) gene and dystrophin gene. Importantly, again by ChIP experiments, we showed that JZif1 did not bind the TopBP1 gene promoter that has been reported to be targeted and regulated by Zif268/Egr1 [30]. In addition, we verified that JZif1 did not bind the potential chromosomal “off-target” sites belonging respectively to Chromosome 16 (carrying eight base pair matches out of nine), to Chromosome 17 and to Chromosome 19 (both 100% nine base pair DNA target match).
Fig. 1.
The novel artificial transcription factor JZif1.
a. Schematic view of the novel artificial/natural transcription factor JZif1 and its prototype ‘Jazz’. Varied aminoacids in the Zif268 zinc finger region and transcription factors binding sites are indicated. b. Immunofluorescence images showing the nuclear localization of over-expressed myc-JZif1 in HeLa cells (top panel). The fluorescence intensity was normalized against the background signal. Western blot analysis of cell lysates from HeLa cells transiently transfected with myc-JZif1 (bottom panel). c. Chromatin immuno-precipitation (ChIP) was performed in HeLa cells (untransfected or transfected with the myc-JZif1 expression vector) using myc-tag monoclonal antibody or with no antibodies (no-Ab). Immuno-precipitates from each sample were analyzed by PCR performed with primers specific for the human utrophin promoter ‘A’. The PCR of unrelated promoters of thymidine kinase and dystrophin genes were included as controls. The PCR of TopBP1 gene promoter containing Zif268/Egr1 binding sites and the PCR of specific ‘off-target’ regions from chromosome 16, chromosome 17 and chromosome 19 were also included. A sample representing the linear amplification of the total input chromatin (input) was included (lane 1). d. Luciferase activity obtained upon co-transfection among myc-JZif1 and pXP-Luc utrophin promoter ‘A’ construct or the control reporter pGL2-Promoter (Promega) in HeLa cells. The data are presented as the means ± S.D. of three independent experiments that were performed in triplicate. e. Quantitative real time RT-PCR (qPCR) analysis of the utrophin gene mRNA expression in differentiated C2C12 cells transfected with the mAAV-JZif1 or mAAV control vectors. The gene expression ratio of utrophin, normalized as indicated, is shown as the mean ± S.D. from an experiment performed in triplicate. f. Representative Western blot analysis of utrophin protein levels in differentiated C2C12 cells transfected with the mAAV-JZif1 or mAAV control vectors. The antibodies used are indicated. Densitometric analysis represents the mean ± S.D. of four independent experiments (right panel).
The relative transcriptional activity of the JZif1artificial transcription factor was evaluated using luciferase activity assay; as shown Fig. 1D, expression of JZif1 revealed an approximately 20-fold induction of pXP-Luc vector [27], containing a portion of the utrophin ‘A’ promoter, while co-transfection of the control pGL2-promoter luciferase vector with JZif1 did not yield any luciferase activity. Next, we evaluated the capacity of JZif1 to up-regulate the endogenous utrophin gene expression by quantitative real-time PCR (qPCR) analysis of the utrophin mRNA levels in differentiated C2C12 myogenic cells. As shown in Fig. 1E, we observed an approximate 1.8-fold increase in utrophin transcript level in the presence of mAAV-JZif1 compared to the mAAV empty vector control. Western blot analysis using anti-utrophin antibody verified a direct correlation between utrophin mRNA expression and utrophin protein level (Fig. 1F).
3.2. Expression of JZif1 and utrophin up-regulation in mdx mice
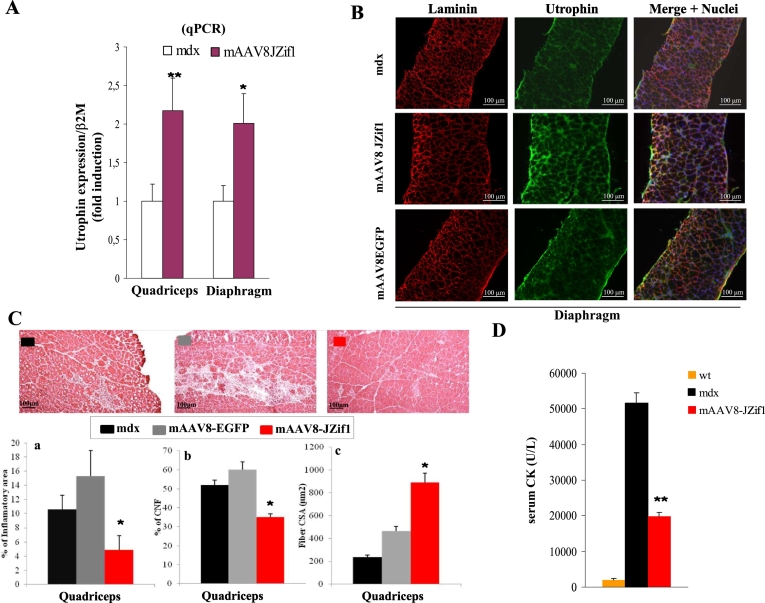
To characterize the biological activity of the JZif1 protein in vivo, we delivered JZif1 in mdx mice by infection with “muscle-AAV serotype 8” (mAAV8-JZif1) [15]. As shown in Fig. 2A, real-time PCR experiments revealed about 2-fold increase of utrophin mRNA expression in quadriceps and diaphragm isolated from mdx mice mAAV8-JZif1 intraperitoneally injected (i.p.) at 5 days and analyzed at two months of age. To determine whether increase of utrophin gene transcription, upon JZif1 treatment, was correlated with utrophin protein levels, Western blot analysis was performed (Fig. 1SA). Moreover, the histological analysis of the diaphragm stained with the utrophin antibody revealed a significant increase and consequent re-localization along the sarcolemma of utrophin protein in mAAV8-JZif1 mdx mice compared to control animals (Fig. 2B). These data demonstrate that the delivery of JZif1 by mAAV8 in mdx mice is able to re-program utrophin expression in muscle both at transcription and translation levels. To verify the possible therapeutic effects of utrophin up-regulation in mdx mice treated with mAAV8-JZif1, several DMD diagnostic parameters were evaluated. We quantified the extent of mononuclear inflammatory cell infiltration, the frequency of centrally nucleated myofibers (CNFs) and the fiber CSA (Cross section area), in muscle slices stained with hematoxylin and eosin (H&E) (Fig. 2C and Fig. 1SB). The results clearly showed a dramatic reduction in muscle pathology, as displayed by better tissue architecture as well as less pronounced degenerative/regenerative processes in mAAV8-JZif1 treated muscles. Moreover, in a subset of mice, we checked serum creatine kinase (CK) levels, an indicator of muscle damage in mdx mice. As shown in Fig. 2D, serum CK levels measured at 3 months of age, were elevated, as expected, in untreated mdx mice compared to wild type C57BL/6 mice and significantly reduced in JZif1-treated mdx mice.
Fig. 2.
Expression of JZif1 and utrophin up-regulation in mdx mice.
a. Quantification by real-time PCR (qPCR) of utrophin transcripts from quadriceps and diaphragm muscles isolated from 2-month old mdx male mice either untreated or intraperitoneal injected at 5 days with mAAV8-JZif1. The gene expression ratio between utrophin and β2-microglobulin (β2M) is shown. Values are expressed as means ± S.D. of at least five different mice. (*P < 0.05, **P < 0.002). b. Immunohistochemistry of the diaphragm muscle isolated from 2-month old untreated, mAAV8-JZif1 or mAAV8-EGFP-treated mdx mice (four mice were analyzed in each group) and stained with the anti-utrophin antibody (green). The extracellular matrix is counterstained with the anti-laminin polyclonal antibody (red) and nuclei with Dapi (blue). The fluorescence intensity was normalized against the background signal. c. Representative images (H&E staining) and quantification graphs of inflammatory infiltrate (a), Centrally Nucleated Fibers (CNF) (b), Fiber Cross-sectional Area (CSA) (c) of quadriceps muscle from 2-month old mdx mice treated at 5 days of age with mAAV8-JZif1 as compared with mAAV8-EGFP treated or untreated mdx mice (five mice were analyzed in each group). Images quality: 640 × 512 pixels. All values are expressed as the mean ± SEM the statistically significant differences were calculated by Student t-test (*P < 0.05 JZif1 vs mdx). d. Quantification of serum CK levels in wt C57BL/6 mice (n = 5), untreated mdx (n = 5) and mAAV8-JZif1 treated mdx mice (n = 5) at 3 months of age. Data represent means (±S.D.) and statistically significant differences were calculated by Student t-test. (**P < 0.002 JZif1 vs mdx).
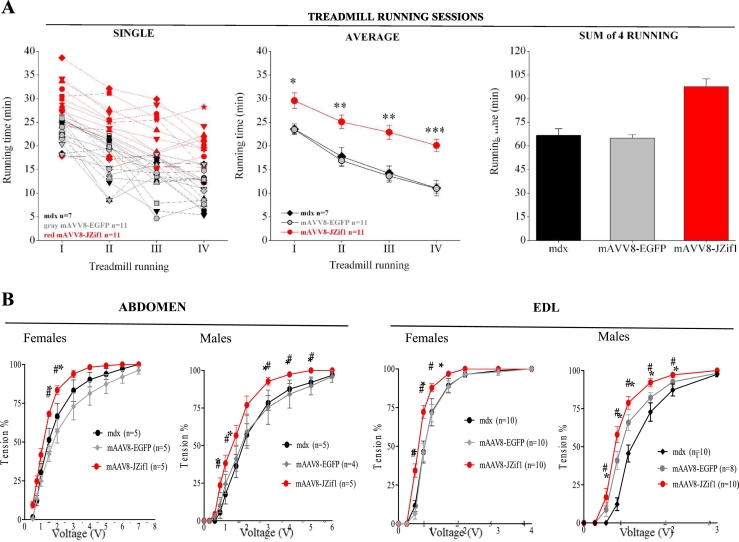
3.3. JZif1 delivery induces muscle functional recovery in mdx mice
Utrophin up-regulation achieved by JZif1 delivery effectively prevented muscle damage in mdx mice. In addition to histochemical analyses, we assessed the effects of JZif1 on dystrophic muscle function in vivo. Single session performance (first and second panel) and total running time performance (third panel) relative to four weekly treadmill trials with exhaustive exercise protocol were determined in untreated mdx mice, mAAV8-EGFP and mAAV8-JZif1 treated mdx mice (Fig. 3A). The mAAV8-JZif1 treated mdx mice showed significant improvement in exercise performance as compared to mAAV8-EGFP treated or untreated mdx mice. Notably, mAAV8-JZif1 treated mdx mice displayed running times close to those of wt mice (Fig. 1SC). JZif1 and Jazz prototype genes delivered in mdx mice induced a similar performance in treadmill trials (Fig. 1SC). Furthermore, JZif1 expression improved mdx sarcolemma integrity post exercise as shown by reduced in vivo Evan's blue dye uptake (Fig. 2SA). To verify whether JZif1 expression improved the mechanical response in dystrophic muscle, we also measured the contractile activity ex vivo. Isolated abdominal muscle strips and extensor digitorium longus (EDL) were examined by physiological assessment of muscle excitability using variable voltages until reaching the supramaximal value. As shown in Fig. 3B, both the abdomen and EDL muscles from mAAV8-JZif1 treated mdx mice showed a significant decrease in stimulation threshold for the muscle fibers, compared with control mice. Similarly, to the treadmill test, mAAV8-JZif1 and mAAV8-Jazz treated mdx mice displayed mechanical force response close to the wild type mice category (Fig. 1SD). At the end of the force test, we also assessed contraction-induced injury of the sarcolemma by staining each muscle with Procion Orange dye. Quantification of the dye-positive area in each section confirmed the increased ability of JZif1 to maintain the membrane integrity of stressed fibers (Fig. 2SB). Our results demonstrate the remarkable functional improvement of muscle performance in mdx mice expressing either JZif1or its prototype Jazz [15].
Fig. 3.
JZif1 delivery induces muscle functional recovery in mdx mice.
a. Single session performance (left) and total running time (right) relative to four weekly treadmill trials with exhaustive exercise protocol. Untreated mdx mice, mAAV8-EGFP (controls) or mAAV8-JZif1 mdx mice injected at day 5 after birth were tested at 3 months of age. For each group, lines indicate the mean duration of running time during each trial (left), and columns indicate the cumulative running time over the four consecutive trials (right). Number of animals is n = 11 for each treated group and n = 7 for untreated group. Data represent means (±SEM) and statistically significant differences were calculated by Student t-test. (*P < 0.05 vs mdx, **P < 0.002 vs mdx, *** P < 0.001 vs mdx). b. Mechanical response of isolated muscles of resting female and male mdx mice treated at 5 days and examined at 2 months. Effects of electrical stimulation on isotonic contractions in abdominal muscles and EDL from mAAV8-JZif1 and mAAV8-EGFP treated or untreated control mice. Muscles contractile force for each voltage was determined and considered as % of maximal contraction. Data represent means (±SEM) from n muscle samples and statistically significant differences were calculated by Student t-test. (*P < 0.05 JZif1vs mdx).
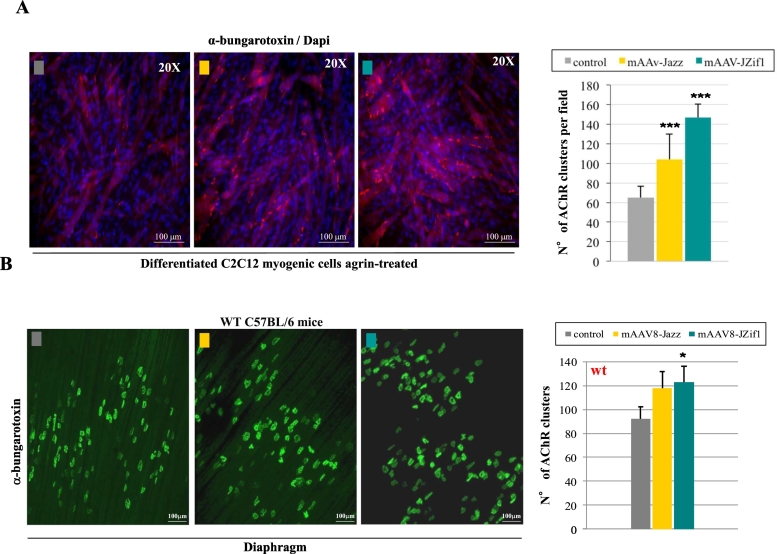
3.4. JZif1 and Jazz enhance the formation of post-synaptic acetylcholine receptor (AChR) clusters
To investigate the molecular mechanisms underlying JZif1 and Jazz rescue of muscular function, we focused on utrophin related pathways, at the neuromuscular junction (NMJ) where utrophin co-localizes with acetylcoline receptors (AChRs) at the crests of post-junctional folds. As first step, we investigated a possible effect of JZif1 and Jazz on post-synaptic AChR clustering in C2C12 myotubes expressing control vector, mAAV-JZif1 or mAAV-Jazz. Myotubes were subsequently treated with recombinant neural agrin (10 ng/ml for 18 h) to induce the basal formation of AChR post-synaptic clustering and visualized by alpha-bungarotoxin (BTX) staining. A 2–3 fold increase of AChR clusters was observed in the presence of either Jazz or JZif1 gene expression compared to control cells (Fig. 4A). Next, the effects of ZF-ATFs on maximum z-stack projections of whole diaphragm were evaluated in wild type mice treated with either Jazz or JZif1. With both ZF-ATFs, we observed an increase in the number of AChR clusters/motor endplates compared to control wt mice (Fig. 4B).
Fig. 4.
JZif1 and Jazz positively impact the formation of post-synaptic Acetylcholine Receptor clusters (AChR).
a. Myogenic cell line C2C12 was transiently transfected with either mAAV-Jazz and mAAV-JZif1 or the empty vector as control and the differentiated myotubes were treated with agrin (10 ng/ml for 18 h) to induce a basal formation of AChR clusters. The clusters were visualized with alpha-bungarotoxin (BTX) staining. The fields (about twenty for each category) were randomly captured in three independent experiments and data represent means (±S.D.). ***P < 0.001 indicate statistical significance by Student t-test (Jazz vs control, JZif1vs control). b. Maximum z-stack projections of whole diaphragm in wt C57BL/6mice, untreated and treated with mAAV8-Jazz or mAAV8-JZif1. Muscles were stained with BTX to visualize and count AChR clusters (right panel). Approximately, a total of 250 AChR clusters were examined for each diaphragm. Data represent the average ± SEM of four mice per group and statistically significant differences were calculated by Student t-test. (*P < 0.05 JZif1 vs wt).
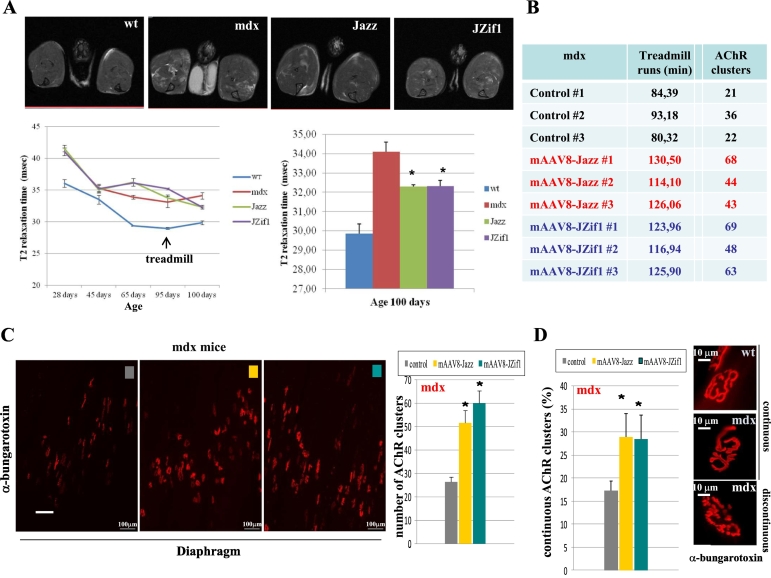
3.5. Correlation among MRI, treadmill performance and quantity/quality of AChR clusters in mdx mice
To further examine the range of responses to ZF-ATFs expression in vivo, the same sub-set of mdx mice were comparatively analyzed by magnetic resonance imaging (MRI), treadmill performance and AChR clusters analysis. MRI was used to monitor changes in the transverse relaxation time constant (T2) muscle. Because alterations in muscle T2 correlate with muscle damage, edema, inflammatory infiltration and fibrosis [36], T2 MRI was used to monitor the pathological changes in lower hindlimb muscles in untreated wt (C57BL/6) untreated or treated mdx mice at different times before and after exhaustive treadmill exercise (Fig. 5A). mAAV8-JZif1 and mAAV8-Jazz treated mdx mice demonstrated a better recovery from muscle damage five days after treadmill challenge, as compared with untreated littermates. Fig. 5A (top) shows representative MRI images of the fourth of 16 consecutive NMR sections of the lower hindlimbs taken for all experimental groups; the lighter areas indicate the presence of inflammatory infiltration or muscle damage. Part of the cohort of mice analyzed by MRI and treadmill test was also examined by NMJ evaluation. Maximum z-stack projections of the whole diaphragm in mdx mice showed a significant increase in the total number of the AChR clusters at post-synaptic membranes (Fig. 5B and C). Moreover, the AChR clusters analysis revealed that JZif1 and Jazz also improve the morphology of post-synaptic membrane, as indicated by an increase number of continuous (not fragmented) AChR clusters/motor endplates (Fig. 5D). Significantly, in the same groups of mdx mice, we found a positive correlation among: 1) profile of “T2 relaxation time parameter” (MRI); 2) muscle functionality (treadmill-running times); and 3) quantity/quality of NMJ post-synaptic membranes (BTX staining). Overall, these observations are consistent with an improvement in muscle function mediated by ectopic expression of ZF-ATFs.
Fig. 5.
Correlation among MRI, treadmill performance and quantity/quality of AChR clusters in mdx mice.
The same set of mdx mice were included in a cohort analyzed by: magnetic resonance imaging (MRI); treadmill performance and AChR clusters analysis. a. MRI was used to monitor changes in the transverse relaxation time constant (T2) muscle. On the top representative MRI images of the fourth of 16 consecutive NMR sections of the lower hind limbs for all experimental groups are shown. Alterations in muscle T2 are correlated to muscle damage, edema, inflammatory infiltration and fibrosis. T2 MRI changes in mdx mice lower hind limb muscles in untreated wt and untreated or treated mdx mice with mAAV8-Jazz or mAAV8-JZif1 at 5 days. All experimental groups were examined at the times indicated before and after an exhaustive treadmill exercise until the age of about 3 months. Statistical analysis with ‘unpaired t-test’ showed a significant difference in T2 relaxation time between untreated and treated mdx mice indicated by (*P < 0.05). The lighter areas indicate the presence of inflammatory infiltration or muscle damage. b. The table shows the correlation between treadmill running times and counts of AChR clusters. c. Maximum z-stack projections of whole diaphragm in mdx mice, untreated and treated with mAAV8-JZif1 ormAAV8-Jazz, analyzed post-treadmill. Muscles were stained with alpha-bungarotoxin to visualize and count AChR clusters at the postsynaptic membrane (right panel). Data represent the average ±SEM of 3 mice/group and statistically significant differences were calculated by Student t-test. (*P < 0.05 JZif1 vs mdx, Jazz vs mdx). d. The AChR clusters analysis, performed in mdx diaphragm reveals that JZif1 and Jazz improve also the morphology of postsynaptic membrane, as indicated by an increase number of continuous (not fragmented) AChR clusters. Approximately, a total of 200 AChR clusters were examined for each diaphragm. Data represent the average ±SEM of three mice per group and statistically significant differences were calculated by Student t-test. (*P < 0.05 JZif1 vs mdx, Jazz vs mdx).
4. Discussion
ZF-ATFs designed ad hoc to target disease-related genes offer therapeutic promise in gene therapy [[37], [38], [39], [40], [41],44]. We engineered several ZF-ATFs that up-regulated utrophin gene expression in muscle, and targeted both the human and mouse utrophin ‘A’ promoters [[13], [14], [15],33,35,45,46]. The prototype ZF-ATF named ‘Jazz’ was able to induce muscle functional recovery and to correct dystrophic pathology in mdx mice, making ZF-ATFs strong candidate for DMD treatment [[13], [14], [15]]. In this context, moving toward a possible clinical application, we focused on different crucial aspects: i) safe and tissue-restricted AAV delivery; ii) ZF-ATF tissue-restricted and persistent expression; iii) absence/reduction of immunogenicity induced by both ZF-ATFs and their delivery vehicles; and iv) characterization of molecular mechanisms underlying the therapeutic effects of ZF-ATFs. Our previous work faced some of these aspects, in particular our engineered AAV-vectors expressing therapeutic molecules are muscle restricted thanks to the use of both muscle specific promoter (mAAV) and AAV “serotype-8” [15]. Importantly, the muscle specific combination of AAV serotype ‘8’ with our mAAV vector, per se contributed to lowering host immune response [18]. Here, we report on the characterization of an upgraded version of the Jazz gene, that we named ‘JZif1’. Jazz prototype gene was engineered assembling the selected zinc finger domains, the viral Vp16 activation domain, the viral SV40 nuclear localization signal and the myc-tag [45]. JZif1 was designed on the natural human Zif268 gene backbone in order to minimize any possible host immune response. Notably, only thirteen amino acid substitutions were properly distributed in the alpha helix region of Zif268 three zinc finger domains to direct the novel ‘JZif1’ to both human and mouse utrophin ‘A’ promoters. It is important to underline that Zif268/Egr1 gene is highly conserved in mammalian, in particular between human and mouse and we have chosen to use human Zif268 as backbone for JZif1 gene in a vision of a possible gene therapy approach. We speculate that few amino acid changes in Zif268 alpha helix do not activate host immune response, since the alpha helix of thousands of natural zinc finger domains (>3,000 in the human genome) vary in these key positions in order to bind different DNA targets [38]. JZif1, as well as Jazz prototype gene, achieves muscle utrophin up-regulation inducing remarkable amelioration of the pathological phenotype in mdx mice. Morphological analysis of JZif1-treated mdx mice displayed better tissue architecture as well as less pronounced degenerative/regenerative processes. Consistently with data obtained with Jazz, JZif1 treated mdx mice displayed running times very close to wild type mice in treadmill test. The mdx muscle functional recovery was confirmed by ex vivo test on isolated muscles, where JZif1 expression clearly improves the mechanical response. To investigate the molecular mechanisms underlying JZif1 and Jazz mdx muscle rescue, we focused on the neuromuscular junction (NMJ), since utrophin localizes to and plays a role at the NMJ [23,24,[47], [48], [49], [50]]. Utrophin in partnership with the scaffold protein rapsyn may serve as backbone for consolidating post-synaptic domains, influencing different aspects, including AChR geometry/distribution, anchorage (to dystrophin glycoprotein complex DGC) and stability [26,51]. Importantly, several studies highlight that an impaired NMJ morphology/activity could contribute to DMD pathogenesis [25,26,52]. JZif1 and Jazz expression positively affects NMJ morphology/plasticity, improving the post-synaptic clustering of AChRs. In C2C12 myotubes, ZF-ATF utrophin up-regulation correlated with increased number of AChR clusters. ZF-ATF utrophin up-regulation, in both healthy wild type and mdx mice exerted a positive effect on NMJ at post-synaptic level, increasing the number of AChR clusters. These results are in good agreement with the improvement of muscular force previously observed in our wild type-Jazz transgenic mice [45]. Significantly, ZF-ATF expression also improved the morphology of post-synaptic membranes in mdx mice, as indicated by the percentage of continuous (not fragmented) AChR clusters versus discontinuous endplates. In ZF-ATF treated mdx mice, MRI analysis showed a better recovery after treadmill exercise. Significantly, in a sub-group of ZF-ATF treated and treadmill exercised mdx mice we were able to detect a positive correlation, highly consistent with a muscle functional rescue, among: 1) treadmill-running times, 2) quantity/quality of NMJ post-synaptic membranes, 3) profile of MRI-T2 relaxation time parameter. Remarkably, utrophin quality/quantity obtained by ZF-ATFs treatment in muscle is able to provide therapeutic effects. Significantly, our ZF-ATF therapeutic strategy is feasible independently from the type of deletion/mutation occurring in DMD patients and it can be combined and synergized with appropriate pharmacological treatments [53].
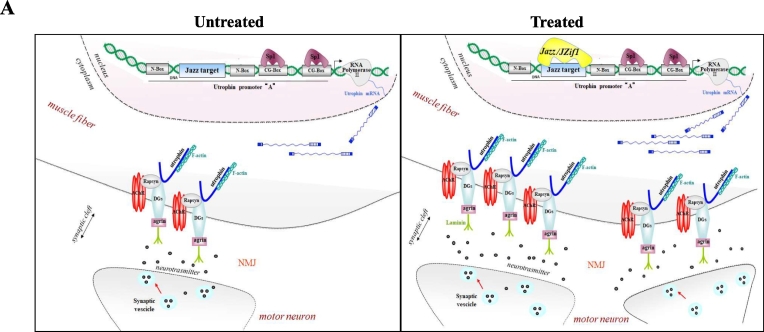
We can speculate that, inside the fiber syncytium, ZF-ATF expression increases the number of nuclei that maintain active utrophin gene transcription. This speculation could partially explain the results obtained on NMJ both in C2C12 myotubes and in wild type ZF-ATF treated mice. On the other hand, muscle up-regulation of all utrophin isoforms, obtained with ZF-ATF treatment in mdx mice, triggered functional and morphological muscle rescue differently from normally occurring utrophin up-regulation described in mdx untreated mice [9]. Here, we demonstrated that JZif1, as well as its prototype Jazz gene, delivered/expressed in muscle tissues by muscle AAV vector induce a remarkable amelioration of the pathological phenotype in mdx mice. We described a positive impact of our ZF-ATF expression on NMJ, both in physiological and pathological conditions. As depicted in the model (Fig. 6) we propose that the expression our ZF-ATFs improve the morphology/plasticity and augment the number of NMJ dictating a proper structural order in NMJ and sub-membrane domains. Moreover, ZF-ATF mediated utrophin up-regulation could exert a positive effect not only in receiving the signal-stimulus coming from the nerve, but also in promoting a retrograde stimulus toward the nerve [25,54,55]. These results candidate our ZF-ATFs for the treatment of DMD and potentially other diseases related to NMJ deficits. Our ongoing work points to further investigate utrophin related DMD pathways positively affected by our ZF-ATF treatments with the aim to deeply characterize the molecular mechanisms underlying JZif1 and Jazz induced functional rescue. The fine identification of ZF-ATF affected pathways related to DMD pathogenesis can suggest novel targets, as key factors within the NMJ network, for either gene therapy or novel pharmacological treatments.
Fig. 6.
The artificial transcription factors JZif1 and its prototype Jazz impact the neuromuscular junction (NMJ) by utrophin up-regulation.
The panels illustrate a schematic representation of the utrophin-associated protein complex at mammalian neuromuscular junction (NMJ). Dystroglycan (DG) associates with rapsyn and rapsyn links Acetylcholine Receptors (AChRs) to the utrophin associated protein complex. Utrophin links the entire complex to the F-actin cytoskeleton. The artificial transcription factors JZif1 and Jazz bind the utrophin promoter ‘A’ at the 9-base pair long target sequence, thereby enhancing transcription of utrophin gene. The expression of JZif1 (and Jazz) gene improves the morphology/plasticity of neuromuscular junction.
The following are the supplementary data related to this article.
Expression of JZif1 and utrophin up-regulation in mdx mice.
a. Western blot analysis of utrophin protein levels in quadriceps muscles isolated from 2-month old mdx mice uninfected or infected with mAAV8-JZif1 as reported on top. Representative individual mice are indicated with numbers. Detection of laminin was used to normalize the amount of proteins. Lower panel shows Western blot analysis of JZif1 (anti-Zif268 antibody) expression. Histogram shows densitometric analysis of expression levels of utrophin protein vs. laminin (Right). Values are expressed as means ± S.D. b. Quantification graphs of inflammatory infiltrate (a), Centrally Nucleated Fibers (CNF) (b), Fiber Cross-sectional Area (CSA) (c) of diaphragm and abdomen muscles from 2-month old mdx mice treated at 5 days of age with mAAV8-JZif1 as compared with mAAV8-EGFP treated or untreated mdx mice (8/10 mice were analyzed in each group). All values are expressed as the mean ± SEM and the statistically significant differences were calculated by Student t-test (*P < 0.05 JZif1 vs mdx). c. The wt, mdx-untreated mice and mdx mice i.p. injected at 5 days of age with either mAAV8-JZif1 or mAAV8-Jazz were subjected to treadmill trials. Columns indicate the cumulative mean time over three consecutive trials. Data represent means (±SEM) from 4/8 animals. Statistical analysis with “unpaired t-test” showed a significant effect of treatment on mice performance (**P < 0.002). d. Contractile activity of isolated abdomen and extensor digitorum longus (EDL) muscle were examined ex vivo by measure of muscle excitability, varying the voltage from 0.5 to 7 V, until the supramaximal voltage was reached. Data represent means (±SEM) from n = 4 animals in duplicate.
Rescue of muscle function by mAAV8-JZif1 treatment in mdx mice.
a. Evan's blue dye (EBD) uptake was used to compare skeletal muscle membrane integrity after treadmill exercise. Top: EBD uptake was also scored as a percentage of EBD positive myofibers (*P < 0.05). Bottom: Muscles from untreated and mAAV8-EGFP treated (controls) or mAAV8-JZif1 treated mdx mice were monitored for EBD uptake by fluorescence microscope. Scale Bar: 50 μm. b. Procion Orange dye uptake in sections of abdominal and EDL muscles after electrophysiological test. Representative images demonstrate the increased ability of mAAV8-JZif1 treated mdx mice to exclude dye from stretched fibers as compared with AAV8-EGFP treated or untreated control mice. Graphs show the mean (±SEM) area of dye-positive fibers, expressed as percentage of the total CSA of muscle sections. Statistical significance by Student t-test was indicated (*P < 0.05 JZif1 vs mdx).
Transparency document
Transparency document.
Acknowledgments
Acknowledgments
We thank Dr. John Hiscott and Dr. Libera Berghella for editing and critical reading of the manuscript. We thank Dr. R. G. Ruscitti for her precious constant assistance. This work was supported by Telethon-Italy (Grant no. GGP14073), Zingenix Ltd. Company, CNR-InterOmics Flagship Projects 2017 (Grant no. 19699) and FARMM-Onlus.
Author contributions
Conceived and designed the experiments: C.P., N.C., M.G.DC., E.M., Cl.P. Performed the experiments: C.P., G.S., F.G., I.C., M.G.DC., N.C., A.O., S.L., C.S. Analyzed the data: C.P., N.C., Cl.P., A.O., M.G.DC., G.S., E.M. Contributed reagents/materials/tools/services: A.E., T.C. Wrote the paper: C.P., N.C., Cl.P. All authors read and approved the final manuscript.
Conflicts of interest
C.P., G.S., M.G.DC., S.L., E.M., N.C., and Cl.P. are named inventors of the following National Research Council patent applications:
-
1)
U.S. Patent Application n. 15/328,833; European Patent Application n. 14002611.3
-
2)
U.S. Patent Application n. 14/909,854; European Patent Application n. 14746954.8
All other authors declare no competing interests.
Footnotes
The Transparency document associated with this article can be found in online version.
Contributor Information
Cinzia Pisani, Email: cinzia.pisani@uniroma1.it.
Claudio Passananti, Email: claudio.passananti@uniroma1.it.
References
- 1.McNally E.M., Pytel P. Muscle diseases: the muscular dystrophies. Annu. Rev. Pathol. 2007;2:87–109. doi: 10.1146/annurev.pathol.2.010506.091936. [DOI] [PubMed] [Google Scholar]
- 2.Konieczny P., Swiderski K., Chamberlain J.S. Gene and cell-mediated therapies for muscular dystrophy. Muscle Nerve. 2013;47:649–663. doi: 10.1002/mus.23738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guiraud S., Aartsma-Rus A., Vieira N.M., Davies K.E., Van Ommen G.J., Kunkel L.M. The pathogenesis and therapy of muscular dystrophies. Annu. Rev. Genomics Hum. Genet. 2015;16:281–308. doi: 10.1146/annurev-genom-090314-025003. [DOI] [PubMed] [Google Scholar]
- 4.Guiraud S., Davies K.E. Pharmacological advances for treatment in Duchenne muscular dystrophy. Curr. Opin. Pharmacol. 2017;34:36–48. doi: 10.1016/j.coph.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Miura P., Jasmin B.J. Utrophin upregulation for treating Duchenne or Becker muscular dystrophy: how close are we? Trends Mol. Med. 2006;12:122–129. doi: 10.1016/j.molmed.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Fairclough R.J., Wood M.J., Davies K.E. Therapy for Duchenne muscular dystrophy: renewed optimism from genetic approaches. Nat. Rev. Genet. 2013;14:373–378. doi: 10.1038/nrg3460. [DOI] [PubMed] [Google Scholar]
- 7.Guiraud S., Chen H., Burns D.T., Davies K.E. Advances in genetic therapeutic strategies for Duchenne muscular dystrophy. Exp. Physiol. 2015;100:1458–1467. doi: 10.1113/EP085308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malik V., Rodino-Klapac L.R., Mendell J.R. Emerging drugs for Duchenne muscular dystrophy. Expert Opin. Emerg. Drugs. 2012;17:261–277. doi: 10.1517/14728214.2012.691965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moorwood C., Khurana T.S. Duchenne muscular dystrophy drug discovery - the application of utrophin promoter activation screening. Expert Opin. Drug Discovery. 2013;8:569–581. doi: 10.1517/17460441.2013.777040. [DOI] [PubMed] [Google Scholar]
- 10.Ricotti V., Spinty S., Roper H., Hughes I., Tejura B., Robinson N., Layton G., Davies K., Muntoni F., Tinsley J. Safety, tolerability, and pharmacokinetics of SMT C1100, a 2‑arylbenzoxazole utrophin modulator, following single- and multiple-dose administration to pediatric patients with Duchenne muscular dystrophy. PLoS One. 2016;11 doi: 10.1371/journal.pone.0152840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amenta A.R., Yilmaz A., Bogdanovich S., McKechnie B.A., Abedi M., Khurana T.S., Fallon J.R. Biglycan recruits utrophin to the sarcolemma and counters dystrophic pathology in mdx mice. Proc. Natl. Acad. Sci. U. S. A. 2011;108:762–767. doi: 10.1073/pnas.1013067108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito M., Ehara Y., Li J., Inada K., Ohno K. Protein-anchoring therapy of biglycan for Mdx Mouse Model of Duchenne muscular dystrophy. Hum. Gene Ther. 2017;28:428–436. doi: 10.1089/hum.2015.088. [DOI] [PubMed] [Google Scholar]
- 13.Di Certo M.G., Corbi N., Strimpakos G., Onori A., Luvisetto S., Severini C., Guglielmotti A., Batassa E.M., Pisani C., Floridi A., Benassi B., Fanciulli M., Magrelli A., Mattei E., Passananti C. The artificial gene Jazz, a transcriptional regulator of utrophin, corrects the dystrophic pathology in mdx mice. Hum. Mol. Genet. 2010;19:752–760. doi: 10.1093/hmg/ddp539. [DOI] [PubMed] [Google Scholar]
- 14.Passananti C., Corbi N., Onori A., Di Certo M.G., Mattei E. Transgenic mice expressing an artificial zinc finger regulator targeting an endogenous gene. Methods Mol. Biol. 2010;649:183–206. doi: 10.1007/978-1-60761-753-2_11. [DOI] [PubMed] [Google Scholar]
- 15.Strimpakos G., Corbi N., Pisani C., Di Certo M.G., Onori A., Luvisetto S., Severini C., Gabanella F., Monaco L., Mattei E., Passananti C. Novel adeno-associated viral vector delivering the utrophin gene regulator jazz counteracts dystrophic pathology in mdx mice. J. Cell. Physiol. 2014;229:1283–1291. doi: 10.1002/jcp.24567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramos J., Chamberlain J.S. Gene therapy for Duchenne muscular dystrophy. Expert Opin. Orphan Drugs. 2015;3:1255–1266. doi: 10.1517/21678707.2015.1088780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hollinger K., Chamberlain J.S. Viral vector-mediated gene therapies. Curr. Opin. Neurol. 2015;28:522–527. doi: 10.1097/WCO.0000000000000241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chamberlain J.R., Chamberlain J.S. Progress toward gene therapy for Duchenne muscular dystrophy. Mol. Ther. 2017;25:1125–1131. doi: 10.1016/j.ymthe.2017.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lyons P.R., Slater C.R. Structure and function of the neuromuscular junction in young adult mdx mice. J. Neurocytol. 1991;20:969–981. doi: 10.1007/BF01187915. [DOI] [PubMed] [Google Scholar]
- 20.van Putten M., Kumar D., Hulsker M., Hoogaars W.M., Plomp J.J., van Opstal A., van Iterson M., Admiraal P., van Ommen G.J., 't Hoen P.A., Aartsma-Rus A. Comparison of skeletal muscle pathology and motor function of dystrophin and utrophin deficient mouse strains. Neuromuscul. Disord. 2012;22:406–411. doi: 10.1016/j.nmd.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 21.Pratt S.J., Shah S.B., Ward C.W., Inacio M.P., Stains J.P., Lovering R.M. Effects of in vivo injury on the neuromuscular junction in healthy and dystrophic muscles. J. Physiol. 2013;591:559–570. doi: 10.1113/jphysiol.2012.241679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pratt S.J., Shah S.B., Ward C.W., Kerr J.P., Stains J.P., Lovering R.M. Recovery of altered neuromuscular junction morphology and muscle function in mdx mice after injury. Cell. Mol. Life Sci. 2015;72:153–164. doi: 10.1007/s00018-014-1663-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sanes J.R., Lichtman J.W. Development of the vertebrate neuromuscular junction. Annu. Rev. Neurosci. 1999;22:389–442. doi: 10.1146/annurev.neuro.22.1.389. [DOI] [PubMed] [Google Scholar]
- 24.Ferraro E., Molinari F., Berghella L. Molecular control of neuromuscular junction development. J. Cachexia. Sarcopenia Muscle. 2012;3:13–23. doi: 10.1007/s13539-011-0041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pratt S.J., Valencia A.P., Le G.K., Shah S.B., Lovering R.M. Pre- and postsynaptic changes in the neuromuscular junction in dystrophic mice. Front. Physiol. 2015;9:252. doi: 10.3389/fphys.2015.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van der Pijl E.M., Van Putten M., Niks E.H., Verschuuren J.J., Aartsma-Rus A., Plomp J.J. Characterization of neuromuscular synapse function abnormalities in multiple Duchenne muscular dystrophy mouse models. Eur. J. Neurosci. 2016;43:1623–1635. doi: 10.1111/ejn.13249. [DOI] [PubMed] [Google Scholar]
- 27.Dennis C.L., Tinsley J.M., Deconinck A.E., Davies K.E. Molecular and functional analysis of the utrophin promoter. Nucleic Acids Res. 1996;24:1646–1652. doi: 10.1093/nar/24.9.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yaffe D., Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 1977;270:725–727. doi: 10.1038/270725a0. [DOI] [PubMed] [Google Scholar]
- 29.Onori A., Pisani C., Strimpakos G., Monaco L., Mattei E., Passananti C., Corbi N. UtroUp is a novel six zinc finger artificial transcription factor that recognises 18 base pairs of the utrophin promoter and efficiently drives utrophin upregulation. BMC Mol. Biol. 2013;14:3. doi: 10.1186/1471-2199-14-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Usskilat C., Skerka C., Saluz H.P., Hänel F. The transcription factor Egr-1 is a regulator of the human TopBP1 gene. Gene. 2006;380:144–1450. doi: 10.1016/j.gene.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 31.Radley-Crabb H.G., Fiorotto M.L., Grounds M.D. The different impact of a high fat diet on dystrophic mdx and control C57Bl/10 mice. PLoS Curr. 2011;3 doi: 10.1371/currents.RRN1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walter U., Krolikowski K., Tarnacka B., Benecke R., Czlonkowska A., Dressler D. Sonographic detection of basal ganglia lesions in asymptomatic and symptomatic Wilson disease. Neurology. 2005;64:1726–1732. doi: 10.1212/01.WNL.0000161847.46465.B9. [DOI] [PubMed] [Google Scholar]
- 33.Corbi N., Libri V., Fanciulli M., Tinsley J.M., Davies K.E., Passananti C. The artificial zinc finger coding gene ‘Jazz’ binds the utrophin promoter and activates transcription. Gene Ther. 2000;7:1076–1083. doi: 10.1038/sj.gt.3301204. [DOI] [PubMed] [Google Scholar]
- 34.Corbi N., Libri V., Onori A., Passananti C. Synthetic zinc finger peptides: old and novel applications. Biochem. Cell Biol. 2004;82:428−436. doi: 10.1139/o04-047. [DOI] [PubMed] [Google Scholar]
- 35.Choo Y., Klug A. Physical basis of a protein-DNA recognition code. Curr. Opin. Struct. Biol. 1997;7:117–125. doi: 10.1016/s0959-440x(97)80015-2. [DOI] [PubMed] [Google Scholar]
- 36.Park J., Wicki J., Knoblaugh S.E., Chamberlain J.S., Lee D. Multi-parametric MRI at 14T for muscular dystrophy mice treated with AAV vector-mediated gene therapy. PLoS One. 2015;10 doi: 10.1371/journal.pone.0124914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klug A. The discovery of zinc fingers and their development for practical applications in gene regulation and genome manipulation. Q. Rev. Biophys. 2010;43:1–21. doi: 10.1017/S0033583510000089. [DOI] [PubMed] [Google Scholar]
- 38.Gersbach C.A., Gaj T., Barbas C.F. Synthetic zinc finger proteins: the advent of targeted gene regulation and genome modification technologies. Acc. Chem. Res. 2014;47:2309–2318. doi: 10.1021/ar500039w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eguchi A., Lee G.O., Wan F., Erwin G.S., Ansari A.Z. Controlling gene networks and cell fate with precision-targeted DNA-binding proteins and small-molecule-based genome readers. Biochem. J. 2014;462:397–413. doi: 10.1042/BJ20140400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gaj T., Sirk S.J., Shui S.L., Liu J. Genome-editing technologies: principles and applications. Cold Spring Harb. Perspect. Biol. 2016;8 doi: 10.1101/cshperspect.a023754. (pii: a023754) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sangamo Therapeutic Inc 2017. http://www.sangamo.com
- 44.Nelson C.E., Robinson-Hamm J.N., Gersbach C.A. Genome engineering: a new approach to gene therapy for neuromuscular disorders. Nat. Rev. Neurol. 2017;13:647–661. doi: 10.1038/nrneurol.2017.126. [DOI] [PubMed] [Google Scholar]
- 45.Mattei E., Corbi N., Di Certo M.G., Strimpakos G., Severini C., Onori A., Desantis A., Libri V., Buontempo S., Floridi A., Fanciulli M., Baban D., Davies K.E., Passananti C. Utrophin up-regulation by an artificial transcription factor in transgenic mice. PLoS One. 2007;2 doi: 10.1371/journal.pone.0000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Desantis A., Onori A., Di Certo M.G., Mattei E., Fanciulli M., Passananti C., Corbi N. Novel activation domain derived from Che−1 cofactor coupled with the artificial protein Jazz drives utrophin upregulation. Neuromuscul. Disord. 2009;19:158–162. doi: 10.1016/j.nmd.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 47.Deconinck A.E., Potter A.C., Tinsley J.M., Wood S.J., Vater R., Young C., Metzinger L., Vincent A., Slater C.R., Davies K.E. Postsynaptic abnormalities at the neuromuscular junctions of utrophin-deficient mice. J. Cell Biol. 1997;136:883–894. doi: 10.1083/jcb.136.4.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rafael J.A., Townsend E.R., Squire S.E., Potter A.C., Chamberlain J.S., Davies K.E. Dystrophin and utrophin influence fiber type composition and post-synaptic membrane structure. Hum. Mol. Genet. 2000;9:1357–1367. doi: 10.1093/hmg/9.9.1357. [DOI] [PubMed] [Google Scholar]
- 49.Banks G.B., Fuhrer C., Adams M.E., Froehner S.C. The postsynaptic submembrane machinery at the neuromuscular junction: requirement for rapsyn and the utrophin/dystrophin-associated complex. J. Neurocytol. 2003;32:709–726. doi: 10.1023/B:NEUR.0000020619.24681.2b. [DOI] [PubMed] [Google Scholar]
- 50.Grady R.M., Zhou H., Cunningham J.M., Henry M.D., Campbell K.P., Sanes J.R. Maturation and maintenance of the neuromuscular synapse: genetic evidence for roles of the dystrophin—glycoprotein complex. Neuron. 2000;25:279–293. doi: 10.1016/s0896-6273(00)80894-6. [DOI] [PubMed] [Google Scholar]
- 51.Aittaleb M., Martinez-Pena Y., Valenzuela I., Akaaboune M. Spatial distribution and molecular dynamics of dystrophin glycoprotein components at the neuromuscular junction in vivo. J. Cell Sci. 2017;130:1752–1759. doi: 10.1242/jcs.198358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rudolf R., Khan M.M., Labeit S., Deschenes M.R. Degeneration of neuromuscular junction in age and dystrophy. Front. Aging Neurosci. 2014;6:99. doi: 10.3389/fnagi.2014.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Arcangelis V., Strimpakos G., Gabanella F., Corbi N., Luvisetto S., Magrelli A., Onori A., Passananti C., Pisani C., Rome S., Severini C., Naro F., Mattei E., Di Certo M.G., Monaco L. Pathways implicated in Tadalafil amelioration of Duchenne muscular dystrophy. J. Cell. Physiol. 2016:224–233. doi: 10.1002/jcp.25075. [DOI] [PubMed] [Google Scholar]
- 54.Dobrowolny G., Aucello M., Rizzuto E., Beccafico S., Mammucari C., Boncompagni S., Belia S., Wannenes F., Nicoletti C., Del Prete Z., Rosenthal N., Molinaro M., Protasi F., Fanò G., Sandri M., Musarò A. Skeletal muscle is a primary target of SOD1G93A-mediated toxicity. Cell Metab. 2008;8:425–436. doi: 10.1016/j.cmet.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 55.Penney J., Tsurudome K., Liao E.H., Elazzouzi F., Livingstone M., Gonzalez M., Sonenberg N., Haghighi A.P. TOR is required for the retrograde regulation of synaptic homeostasis at the Drosophila neuromuscular junction. Neuron. 2012;12:166–178. doi: 10.1016/j.neuron.2012.01.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expression of JZif1 and utrophin up-regulation in mdx mice.
a. Western blot analysis of utrophin protein levels in quadriceps muscles isolated from 2-month old mdx mice uninfected or infected with mAAV8-JZif1 as reported on top. Representative individual mice are indicated with numbers. Detection of laminin was used to normalize the amount of proteins. Lower panel shows Western blot analysis of JZif1 (anti-Zif268 antibody) expression. Histogram shows densitometric analysis of expression levels of utrophin protein vs. laminin (Right). Values are expressed as means ± S.D. b. Quantification graphs of inflammatory infiltrate (a), Centrally Nucleated Fibers (CNF) (b), Fiber Cross-sectional Area (CSA) (c) of diaphragm and abdomen muscles from 2-month old mdx mice treated at 5 days of age with mAAV8-JZif1 as compared with mAAV8-EGFP treated or untreated mdx mice (8/10 mice were analyzed in each group). All values are expressed as the mean ± SEM and the statistically significant differences were calculated by Student t-test (*P < 0.05 JZif1 vs mdx). c. The wt, mdx-untreated mice and mdx mice i.p. injected at 5 days of age with either mAAV8-JZif1 or mAAV8-Jazz were subjected to treadmill trials. Columns indicate the cumulative mean time over three consecutive trials. Data represent means (±SEM) from 4/8 animals. Statistical analysis with “unpaired t-test” showed a significant effect of treatment on mice performance (**P < 0.002). d. Contractile activity of isolated abdomen and extensor digitorum longus (EDL) muscle were examined ex vivo by measure of muscle excitability, varying the voltage from 0.5 to 7 V, until the supramaximal voltage was reached. Data represent means (±SEM) from n = 4 animals in duplicate.
Rescue of muscle function by mAAV8-JZif1 treatment in mdx mice.
a. Evan's blue dye (EBD) uptake was used to compare skeletal muscle membrane integrity after treadmill exercise. Top: EBD uptake was also scored as a percentage of EBD positive myofibers (*P < 0.05). Bottom: Muscles from untreated and mAAV8-EGFP treated (controls) or mAAV8-JZif1 treated mdx mice were monitored for EBD uptake by fluorescence microscope. Scale Bar: 50 μm. b. Procion Orange dye uptake in sections of abdominal and EDL muscles after electrophysiological test. Representative images demonstrate the increased ability of mAAV8-JZif1 treated mdx mice to exclude dye from stretched fibers as compared with AAV8-EGFP treated or untreated control mice. Graphs show the mean (±SEM) area of dye-positive fibers, expressed as percentage of the total CSA of muscle sections. Statistical significance by Student t-test was indicated (*P < 0.05 JZif1 vs mdx).
Transparency document.