Abstract
This study is to investigate the prevalence and risk factors of nonalcoholic fatty liver disease (NAFLD) and to analyze the effect of comprehensive community intervention on NAFLD in community residents in Urumqi, China.
Cluster sampling method with street community as a unit was adopted in this study. Questionnaire survey, body measurement, blood biochemistry (including liver function, fasting blood glucose [FPG], and uric acid [UA]) examination as well as liver B ultrasound were performed. Then, comprehensive intervention was conducted in NAFLD patients.
A total of 1000 people were enrolled, including 344 men and 656 women, with an average age of 51.79 ± 4.28 years. Of them, 660 were Han Chinese, 327 were Uygur, and 13 were Hui. The overall prevalence rate of NAFLD was 54.3%. The prevalence rate of NAFLD is higher in middle-aged population and is higher in ethnic minority than that in Han. NAFLD was associated with the past medical history of metabolic diseases. The factors of body mass index (BMI), systolic blood pressure (SBP), diastolic blood pressure (DBP), waist circumference, hip circumference, neck circumference, subcutaneous fat thickness, FPG, alanine aminotransferase, and aspartate aminotransferase were identified as risk factors for NFALD. Neck circumference predicts the occurrence of NAFLD in female better, whereas subcutaneous fat predicts the occurrence of NAFLD in male better. After 8 months of community intervention in NAFLD patients, the changes of BMI, SBP, DBP, waist circumference, neck circumference, subcutaneous fat thickness, and UA were statistically significant (P < .05).
The prevalence rate of NAFLD is high in Urumqi, China. Community intervention is effective in reducing the degree of NAFLD and promoting the overall health of NAFLD patients.
Keywords: community intervention, nonalcoholic fatty liver disease, prevalence rate, risk factors
1. Introduction
Nonalcoholic fatty liver disease (NAFLD) is a genetic, environmental, and metabolic and stress associated liver disease without history of excessive drinking and is characterized by steatosis and fat storage in liver parenchymal cells.[1] The disease spectrum of NAFLD includes nonalcoholic simple fatty liver, nonalcoholic steatohepatitis, liver fibrosis, liver cirrhosis, and hepatocellular carcinoma.[2] As a reversible disease, early detection, intervention, and treatment of NAFLD can cure the majority of patients.[3] NAFLD is a part of the metabolic syndrome, associated with many metabolic features, such as obesity, hypertension, diabetes, and hyperlipidemia.[4] In Europe, the overall prevalence rate of NAFLD among adults is 20% to 34%, among which the prevalence rate in Sweden, Germany, Spain, and Italy is 39.0%, 40.0%, 25.8%, and 25.0%, respectively.[5–7] The prevalence rate of NAFLD is 5% to 28%, 12%, 17%, and 5% in India, Philippines, Malaysia, and Singapore, respectively.[8] NAFLD affects 20% to 30% of the total population in North America, with prevalence rates of 30% in the United States and 25% in Canada.[9] In China, NAFLD affects about 5% to 24% of the population.[8] Thus, NAFLD is already a global public health issue.
Study has shown that the prevalence rate of NAFLD is associated with the age of patients.[10] China is a populous country and has become an aging population society.[11] However, the prevalence and the risk factors of NAFLD in middle-aged population are still unclear. In this study, the prevalence rate of NAFLD in the middle-aged population in Urumqi, China and its main risk factors were investigated. Comprehensive intervention was performed on the screened NAFLD patients.
2. Materials and methods
2.1. Subjects and data collection
This is a cross-sectional study. The communities of Fuxing and Beiyuanchun in Shayibake District and the communities of Heju and Qidaowan East Street in Shuimogou District in Urumqi, China were investigated. All subjects were asked by the investigators to fill in the questionnaire using a unified questionnaire. The questionnaire was designed due to the diet structure and living behavior of the local residents in Xinjiang and included general information, past history, eating habits, lifestyle habits, physical measurement indicators, and laboratory tests. Physical measurements including height, body mass, blood pressure, waist circumference, hip circumference, neck circumference, and triceps parts were performed, and the body mass index (BMI) was calculated. Then, 5 mL of the venous blood (at least fasting for more than 8 hours) was collected for blood biochemical tests. Finally, the abdominal B ultrasound was performed.
The inclusion criteria were as follows:
-
(i)
Subjects were in line with the diagnostic criteria of NAFLD.
-
(ii)
The age of subject was between 45 and 59 years.
-
(iii)
Subjects with clear awareness and able to answer questions correctly and move freely.
-
(iv)
Subjects were local residents living in the community for more than 2 years.
-
(v)
Informed consent was obtained from each subject who voluntarily participated in this study.
The exclusion criteria were as follows:
-
(i)
Patients with alcoholic fatty liver disease. For long-term drinkers, generally more than 5 years, those with alcohol consumption ≥40 g/day in men and ≥20 g/day in women were excluded. For drinkers with large alcohol consumption in 2 weeks, those with alcohol consumption >80 g/day[12] were excluded.
-
(ii)
Subjects were with hepatitis such as viral hepatitis (hepatitis C, hepatitis B, and others), drug-induced hepatitis, and cirrhosis.
-
(iii)
Subjects were patients with malignant tumors.
-
(iv)
Subjects were with woman acute gestational fatty liver or specific types of liver diseases such as Reye syndrome, Wilson disease, glycogen accumulation disease, and autoimmune liver disease.
-
(v)
Subjects were with industrial toxic contact history such as carbon tetrachloride, yellow phosphorus, dimethyl nitrosamines, and diethyl nitrosamines.
-
(vi)
Subjects refused to participate in this study.
Prior written and informed consent were obtained from every patient and the study was approved by the ethics review board of Xinjiang Medical University.
2.2. Diagnosis of NAFLD
NAFLD was diagnosed according to the previously described diagnostic criteria.[13] Patients were diagnosed as NAFLD if they met the 3 following diagnostic criteria: Subject had no history of drinking or alcohol consumption <140 g ethanol per week in men and <0 g ethanol per week in women; Subject did not have specific diseases that can cause fatty liver, such as viral hepatitis, drug-induced liver disease, total parenteral nutrition, hepatolenticular degeneration, and autoimmune liver disease; and Liver imaging of subjects was consistent with the diagnostic criteria for diffuse fatty liver. Ethanol amount conversion was calculated according to the equation as described previously: ethanol (g) = alcohol consumption (mL) × ethanol content (%) × 0.8 (ethanol specific gravity). The ethanol content was defined as 12% of the wine, 4% of the beer, 16% of rice wine, and 40% of liquor.
2.3. Intervention
The NAFLD patients were then randomly divided into intervention group and control group. Routine intervention was used in the control group. Routine and community intervention were used in the intervention group. The intervention time was 8 months. Briefly, routine intervention included setting up the NAFLD Prevention Bulletin, issuing NAFLD health education brochure, long-term placement of NAFLD promotional materials in the community, and conducting NAFLD health education seminar and exchange. Community intervention included establishing community health files for chronic diseases, targeted guidance to patients with NAFLD and other metabolic diseases (such as maintaining the BMI at 18.5–23.9 kg/m2 and monitoring blood pressure and blood glucose level), guiding the development of healthy lifestyles (such as encouraging aerobic exercise), dietary guidance for patients according to the severity of the disease (such as gradually reducing the intake of high fever, high fat, and high sugar food), monthly telephone follow-up, and home visits to the patients 1 time every 2 months.
2.4. Statistical analyses
Statistical analyses were performed using SPSS 17.0 (IBM-SPSS, Chicago, IL). The general demographic data of the subjects were described by the frequency and composition ratio. The results were expressed as the mean ± SD. The χ2 test was used to compare the prevalence rate of NAFLD in community residents with different demographic characteristics. The t test was used to compare the clinical observation indicators between the NAFLD patients and the normal population. The risk factors of NAFLD were analyzed by logistic regression analysis. The receiver operating characteristic curve was used to analyze the predictive value of obesity indexes in the incidence of NAFLD in female and male. When there was the homogeneity of variance and normality, 2 independent samples t test was used to compare the 2 sets of measurement data after intervention. When there was normality, but not the homogeneity of variance, t test was used. A P value less than 0.05 was considered statistically significant.
3. Results
3.1. Basic characteristics of included subjects and prevalence rate of NAFLD
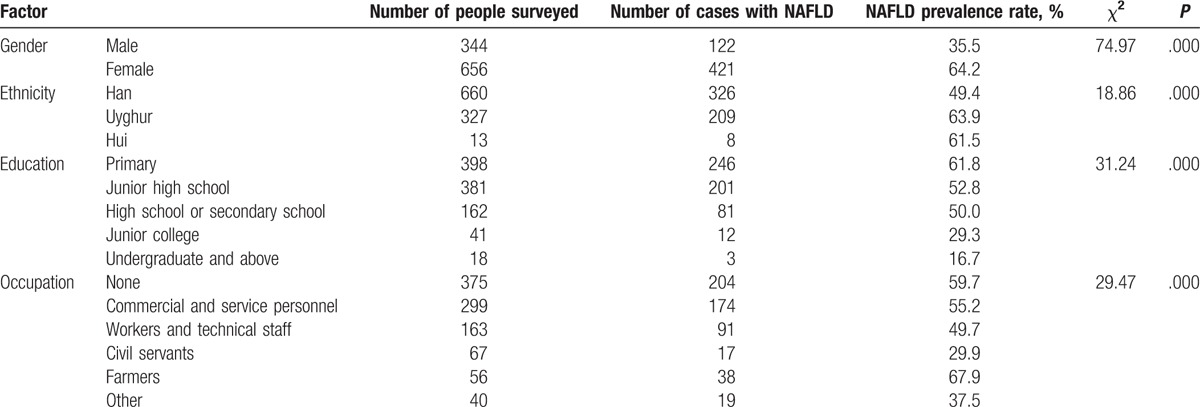
A total of 1000 people were selected, including 34.4% of men and 65.6% of women, with an average age of 51.79 ± 4.28 years, height of 1.60 ± 0.08 m, and weight of 67.38 ± 12.23 kg. Of them, 66% were Han, 32.7% were Uygur, and 1.3% were Hui (Table 1). Wherein, 543 cases of the patients were diagnosed with NAFLD, with the overall prevalence rate of 54.3% (Table 1). The prevalence rate of NAFLD in female patients (64.2%) was higher than that in male patients (35.5%), and the differences were statistically significant (Table 1). The prevalence rates of NAFLD in Uygur and Hui (63.9% and 61.5%, respectively) were higher than that in Han (49.4% of Han), and the differences were statistically significant (Table 1). These indicate that women are more likely to have NAFLD than men, and ethnic minorities are more likely to have NAFLD than Han in the middle-aged community.
Table 1.
Basic characteristics of included subjects.
3.2. Association of past medical history and NAFLD
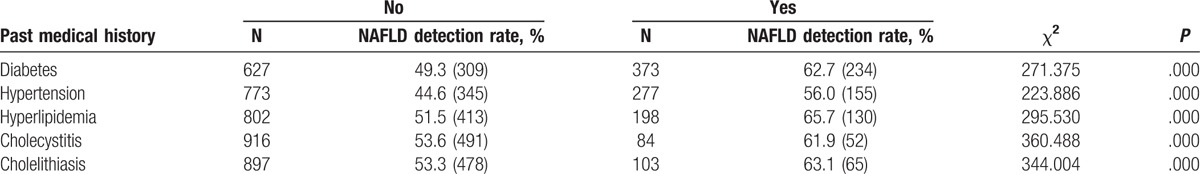
NAFLD is closely related to metabolic disorders.[14] Diabetes and hypertension are important risk factors for NAFLD.[15,16] The correlation between past medical history of metabolic disorders and NAFLD was investigated in this study. As shown in Table 2, the NAFLD prevalence rates in patients with diabetes, hypertension, hyperlipidemia, cholecystitis, and cholelithiasis were 62.7%, 56.0%, 65.7%, 61.9%, and 63.1%, respectively. The NAFLD prevalence rates of patients without diabetes, hypertension, hyperlipidemia, cholecystitis, and cholelithiasis were 49.3%, 44.6%, 51.5%, 53.6%, and 53.3%, respectively. The NAFLD prevalence rates of patients with metabolic disorders were significantly higher than those of patients without metabolic disorders (P < .05). These results indicate that the prevalence of NAFLD is closely related to diabetes, hypertension, hyperlipidemia, cholecystitis, and cholelithiasis in community residents in Urumqi, China.
Table 2.
Relationship between the past medical history of metabolic disorders and nonalcoholic fatty liver disease (NAFLD).
3.3. Analysis of risk factors for NAFLD
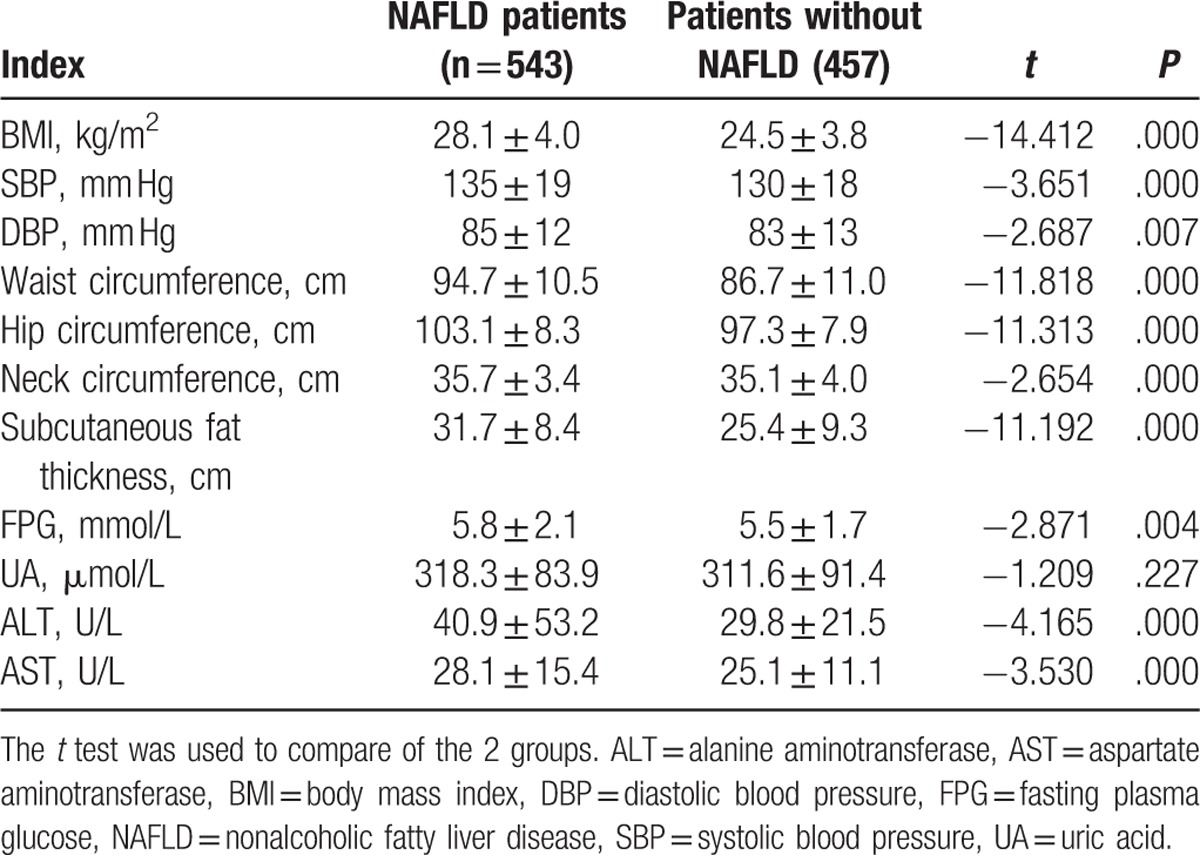
To analyze the risk factors for NAFLD, physical examination was performed. The clinical observations of patients with NAFLD were compared with those without NAFLD. As shown in Table 3, BMI, systolic blood pressure (SBP), diastolic blood pressure (DBP), waist circumference, hip circumference, neck circumference, subcutaneous fat thickness, fasting plasma glucose (FPG), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) levels in NAFLD patients were different from those without NAFLD. The differences were statistically significant (P < .05). This implies that these factors may have correlations with NAFLD.
Table 3.
Comparison of the indexes between the patients with NAFLD and those without.
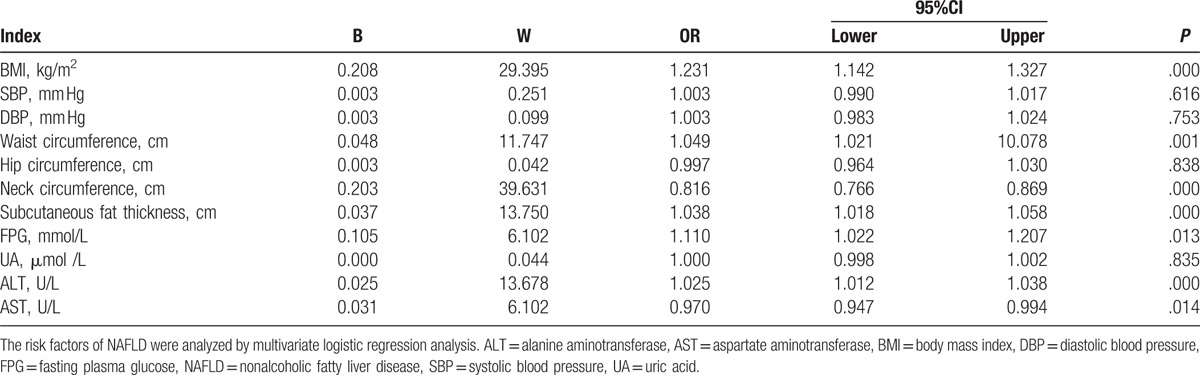
Next, multivariate logistic regression analysis was performed to further analyze the risk factors of NAFLD. Results showed that BMI, SBP, DBP, waist circumference, hip circumference, neck circumference, subcutaneous fat thickness, FPG, ALT, and AST levels were associated with NAFLD (Table 4). The correlation was statistically significant (P < .05), suggesting that these factors may be risk factors for NAFLD.
Table 4.
Multivariate logistic regression analysis of related factors of NAFLD.
Additionally, we plotted the receiver operating characteristic curve to analyze the predictive value of obesity indexes in the incidence of NAFLD in female and male. The results showed that the area under the curve of waist circumference, hip circumference, subcutaneous fat, and neck circumference were 0.711, 0.668, 0.652, and 0.649, respectively, in male (Fig. 1A) and 0.790, 0.760, 0.697, and 0.710, respectively, in female, respectively (Fig. 1B). This indicates that neck circumference predicts the occurrence of NAFLD in female population better, whereas subcutaneous fat predicts the occurrence of NAFLD in men better.
Figure 1.
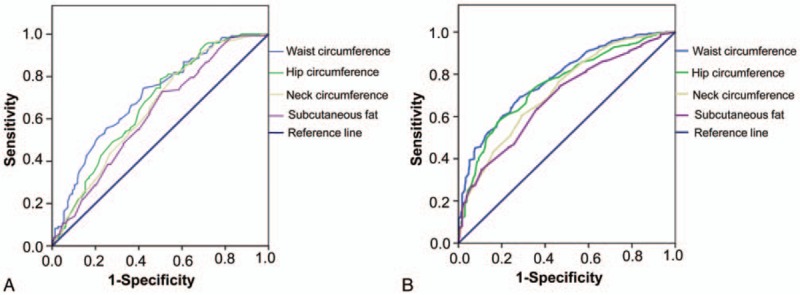
ROC curve of obesity indexes. ROC curve was used to analyze the predictive value of obesity indexes in the incidence of NAFLD in female and male. (A) ROC curve in male. Area under the curve of waist circumference, hip circumference, subcutaneous fat, and neck circumference in male were 0.711, 0.668, 0.652, and 0.649, respectively. (B) ROC curve in female. Area under the curve of waist circumference, hip circumference, subcutaneous fat, and neck circumference in female were 0.790, 0.760, 0.697, and 0.710, respectively. NAFLD = nonalcoholic fatty liver disease, ROC = receiver operating characteristic.
3.4. The effect of comprehensive intervention on patients with NAFLD
The 543 NAFLD patients were randomly divided into intervention and control group. After 8 months of intervention, a total of 462 people completed the follow-up. Totally 81 patients were censored. Of them, 3 patients were died, 30 patients dropped out of the study, 10 patients were hospitalized, and 38 patients were lost contact. The demographic data of the intervention (n = 220) and control group (n = 242) were compared. The results showed that there was no significant difference in age, sex, ethnicity, educational level, and occupational type between the 2 groups (P > .05, Table 5).
Table 5.
Comparison of demographic data between the intervention and control group.
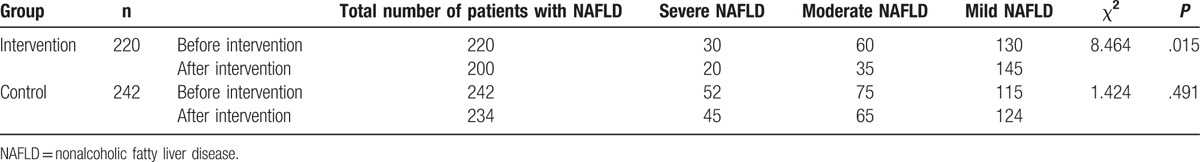
To study the effect of comprehensive intervention on patients with NAFLD, the imaging features of B ultrasound in the liver of the 2 groups were compared before and after the intervention. The prevalence rates of NAFLD patients of the 2 groups were firstly compared before the intervention. Result showed that there was no significant difference between the 2 groups (χ2 = 1.667, P = .434; data not shown). This suggests that the prevalence rate of the 2 groups were comparable. After intervention, 20 patients were cured in the intervention group and 8 patients were cured in the control group. In intervention group, there was statistically significant difference in the number of patients before and after intervention (Table 6). However, the difference was not obvious in control group (Table 6). These results show that comprehensive intervention has a good effect on the cure of NAFLD.
Table 6.
Comparison of abdominal ultrasonography before and after intervention.
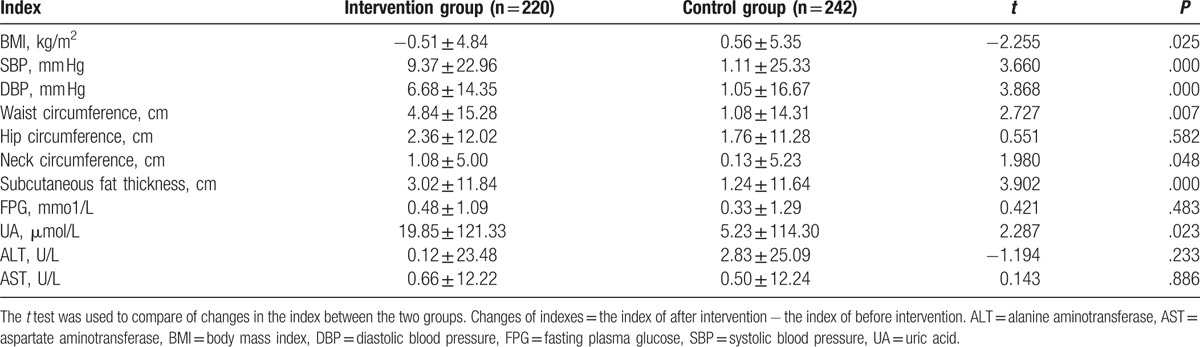
Next, the body measurements of the 2 groups were compared before and after the intervention. As shown in Table 7, BMI, SBP, DBP, waist circumference, hip circumference, neck circumference, subcutaneous fat thickness, FPG, uric acid (UA), ALT, and AST levels had no significant difference between the 2 groups before intervention. After intervention, BMI, SBP, DBP, waist circumference, hip circumference, neck circumference, subcutaneous fat thickness, FPG, UA, ALT, and AST levels were changed in the 2 groups (Table 7). The changes of these indexes in the intervention and control groups were compared. Results showed that there were significant differences in BMI, DBP, waist circumference, subcutaneous fat thickness, and FPG in the intervention group before and after intervention (P < .05) (Table 8). Although the differences in BMI, SBP, waist circumference, and subcutaneous fat in control group before and after intervention were significantly different (P < .05) (Table 8). These indicate that community intervention is effective in reducing the degree of NAFLD.
Table 7.
Comparison of the indexes between the intervention and control group before and after intervention.
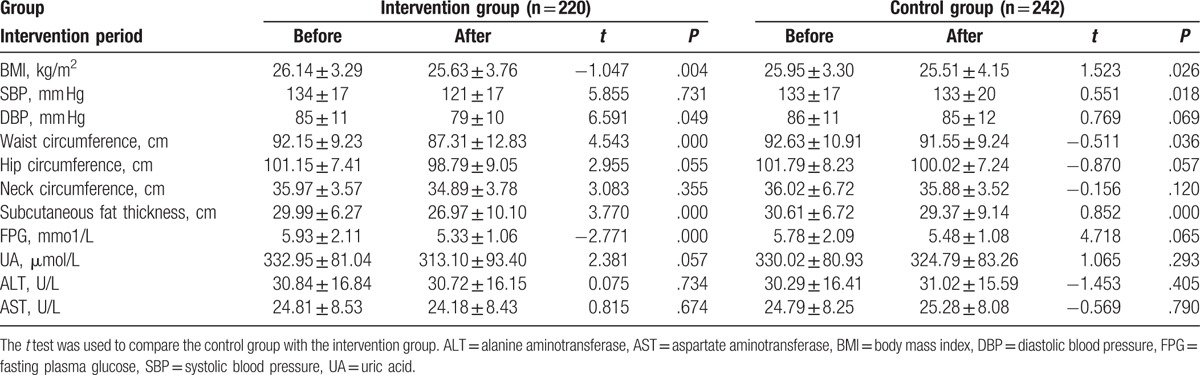
Table 8.
Comparison of the changes of indexes between the intervention and control group.
4. Discussion
NAFLD is a common clinical chronic liver disease, widely distributed in the world.[17] NAFLD affects 20% to 40% of the population in Western countries.[18] The prevalence rate of NAFLD is 5% to 24% in China,[8] and there are gender, age, and regional differences.[19–23] In this study, the total prevalence rate of NAFLD was 54.3% in Urumqi, significantly higher than other parts of China.[19–23] The prevalence rate of NAFLD of Uighur was very high. It is reported that the NAFLD prevalence rate of people with age ≥40 years was significantly higher than that of other age groups.[24] The age of the subjects were between 45 and 59 years in this study, and thus the NAFLD prevalence rate was relatively high. Cai [25] reported that the Uygur was more susceptible to NAFLD than other ethnic groups. This may be another reason leading to the high prevalence rate of NAFLD. Additionally, the diet of people in Xinjiang is mainly consisted of high fat and protein food[26] due to the long and cold winter, which may also result in the high prevalence rate of NAFLD.
Our result also showed that the NAFLD prevalence rate of women was higher than that of men. This may be because that the majority of minority women in Xinjiang were mainly housewives with less physical labor after marriage.[27] Middle-aged women were prone to abdominal obesity.[28] The NAFLD prevalence rates of Uygur and Hui were higher than that of Han, which may be related to the dietary structure of various ethnic groups. The minorities generally eat more meat and less vegetables and seafood, and lack outdoor sports.[29] The NAFLD prevalence of people with less education level was higher than that of people with higher education level. Thus, the popularization of NAFLD health control knowledge is particularly important.
Study has shown that NAFLD is closed related to obesity, diabetes, hypertension, hyperlipidemia, and other metabolic syndromes.[30] Patients with metabolic syndrome have a higher risk of NAFLD in 4 to 11 times than normal people.[7] Therefore, the American Society of Clinical Endocrinologists lists NAFLD as one of the components of metabolic syndrome in 2003.[31] This study showed that the detection rate of NAFLD in patients with diabetes, hypertension, hyperlipidemia, cholecystitis, and cholelithias was high and that patients with hyperlipidemia had the highest detection rate of NAFLD (65.7%). It is shown that the prevalence rate of fatty liver in patients with hyperlipidemia is higher than that of persons with normal blood lipids, and patients with high triglycerid and total cholesterol have the highest prevalence rate of fatty liver.[32] The prevalence rate of fatty liver in patients with severe hypertriglyceridemia and mixed hyperlipidemia is 5 to 6 times higher than that of normal persons.[33] Therefore, these results suggest that active treatment of hyperlipidemia and effective lowering of blood lipid levels, especially triglyceride levels of is important to the prevention and control of fatty liver.
Multivariate logistic regression analysis showed that BMI, waist circumference, subcutaneous fat, FPG, and ALT were risk factors for NAFLD. Obesity is one of the independent risk factors for NAFLD.[34] BMI is an indicator of the overall obesity.[35] Waist circumference is an indicator of abdominal fat accumulation.[36] Subcutaneous fat is an important indicator of upper body fat content.[37] Xiang et al[38] have shown that the risk of fatty liver in overweight, degree I obesity, and degree II obesity group increased by 1.7, 1.9, and 9.1 times, respectively, compared with the normal body mass group. Ma et al[39] have found that the risk of fatty liver is higher in people with large waist circumference (>85 cm in male and >80 cm in female). These findings indicate that BMI management is the key to prevent NAFLD. Serum ALT is used as a marker of liver disease. Elevated serum ALT in patients with NAFLD mainly reflects the severity of liver inflammation rather than steatosis or hepatic fibrosis.[40] These suggest that serum ALT alone is not enough for the evaluation of NAFLD, and other clinical metabolic markers are needed.
This study also found that neck circumference predicts the occurrence of NAFLD in female better, whereas subcutaneous fat predicts the occurrence of NAFLD in men better. This may be because that under similar abdominal subcutaneous fat and visceral fat, men have less neck fat than women.[41] Additionally, the male triceps subcutaneous fat is greater than female in the same age group. For males, the subcutaneous fat layer reaches peak at the age of 50. Although for females, body fat in all parts rapidly accumulates at the age of 20 to 30, and rapidly thinned at the age of 50 to 60.[42]
Studies have found that many diseases are closely related to the poor lifestyles.[43] To prevent and treat these diseases, lifestyle intervention is the basic measure of disease control, and health education is the basis for the implementation of lifestyle intervention.[44] Studies have shown that health education can improve the initiative and compliance of therapeutic lifestyle in NAFLD patients.[3,45,46] In this study, intervention results showed that the number of NAFLD patients in intervention group decreased after intervention. However, the difference was not obvious in control group. The changes of body measurements were statistically significant between the intervention and control group. These indicate that comprehensive intervention has a good effect on the cure of NAFLD. Stunkard et al proposed behavioral therapy, which requires patients to correct the wrong way of lifestyle.[47] Dietary intervention can make more reasonable diet for NAFLD patients. Choosing low-calorie, low-sugar, low-fat, high-protein, and high-fiber foods is conducive to lipoprotein synthesis, removing fat accumulation in the liver and promoting liver cell regeneration, thereby improving fatty liver.[48] Aerobic exercise has a certain role in the prevention and treatment of NAFLD, which can improve liver histopathology and liver function.[49]
However, this study is a cross-sectional study, which has some limitations. For example, the prevalence and distribution of the disease and the etiological assumptions can be obtained from cross-sectional study. However, the causal relationship between disease and etiology cannot be determined. Therefore, this study cannot determine the causal relationship between NAFLD and its related factors. Cohort studies are warranted to further investigate the causal relationship between the relevant factors and the disease.
In conclusion, the prevalence rate of NAFLD is high in Urumqi. The risk factors of NAFLD in middle-aged population of Urumqi are BMI, blood pressure, neck circumference, waist circumference, hip circumference, subcutaneous fat thickness, FPG, UA, ALT, and AST. Community intervention is effective in reducing the degree of NAFLD. For NAFLD patients, health education and the change in bad habits could reduce the risk of NAFLD.
Footnotes
Abbreviations: ALT = alanine aminotransferase, AST = aspartate aminotransferase, BMI = body mass index, DBP = diastolic blood pressure, FPG = fasting plasma glucose, NAFLD = nonalcoholic fatty liver disease, SBP = systolic blood pressure, UA = uric acid.
SL and YX are the first coauthors.
Funding: This work was supported by Urumqi Science and Technology Project grant (No. Y14130051).
The authors have no conflicts of interest to disclose.
References
- [1].Mehta K, Van Thiel DH, Shah N, et al. Nonalcoholic fatty liver disease: pathogenesis and the role of antioxidants. Nutr Rev 2002;60:289–93. [DOI] [PubMed] [Google Scholar]
- [2].Machado MV, Cortez-Pinto H. Non-alcoholic fatty liver disease: what the clinician needs to know. World J Gastroenterol 2014;20:12956–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Arab A, Askari G, Golshiri P, et al. The effect of a lifestyle modification education on adiposity measures in overweight and obese nonalcoholic fatty liver disease patients. Int J Prev Med 2017;8:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Abenavoli L, Milic N, Di Renzo L, et al. Metabolic aspects of adult patients with nonalcoholic fatty liver disease. World J Gastroenterol 2016;22:7006–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Baclig MO, Lozano-Kuhne JP, Mapua CA, et al. Genetic variation I148 M in patatin-like phospholipase 3 gene and risk of non-alcoholic fatty liver disease among Filipinos. Int J Clin Exp Med 2014;7:2129–36. [PMC free article] [PubMed] [Google Scholar]
- [6].Park SH, Jeon WK, Kim SH, et al. Prevalence and risk factors of non-alcoholic fatty liver disease among Korean adults. J Gastroenterol Hepatol 2006;21(1 Pt 1):138–43. [DOI] [PubMed] [Google Scholar]
- [7].Hamaguchi M, Kojima T, Takeda N, et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med 2005;143:722–8. [DOI] [PubMed] [Google Scholar]
- [8].Amarapurkar DN, Hashimoto E, Lesmana LA, et al. How common is non-alcoholic fatty liver disease in the Asia-Pacific region and are there local differences? J Gastroenterol Hepatol 2007;22:788–93. [DOI] [PubMed] [Google Scholar]
- [9].Suzuki A, Angulo P, Lymp J, et al. Chronological development of elevated aminotransferases in a nonalcoholic population. Hepatology (Baltimore, MD) 2005;41:64–71. [DOI] [PubMed] [Google Scholar]
- [10].Liu CJ. Prevalence and risk factors for non-alcoholic fatty liver disease in Asian people who are not obese. J Gastroenterol Hepatol 2012;27:1555–60. [DOI] [PubMed] [Google Scholar]
- [11].Chen MH, Hao GC. Research on regional difference decomposition and influence factors of population aging in China. Zhongguo Renkou Ziyuan Yu Huan Jing 2014;24:136–41. [Google Scholar]
- [12].Association FLaALDSGOtCLD. Diagnosis and treatment guidelines for alcoholic liver disease (2010 revised edition). Zhong Hua Gan Zang Bing Za Zhi 2010;18:167–70. [Google Scholar]
- [13].Chinese Medicine Association of Liver Diseases FLaALD. Guidelines for the diagnosis and treatment of nonalcoholic fatty liver disease (Revised 2010). Chin J Hepatol 2010;18:163–6. [Google Scholar]
- [14].Qin L, Zhang W, Yang Z, et al. Impaired lung function is associated with non-alcoholic fatty liver disease independently of metabolic syndrome features in middle-aged and elderly Chinese. BMC Endocr Disord 2017;17:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Firneisz G. Non-alcoholic fatty liver disease and type 2 diabetes mellitus: the liver disease of our age? World J Gastroenterol 2014;20:9072–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Shi FY, Gao WF, Tao EX, et al. Metabolic syndrome is a risk factor for nonalcoholic fatty liver disease: evidence from a confirmatory factor analysis and structural equation modeling. Eur Rev Med Pharmacol Sci 2016;20:4313–21. [PubMed] [Google Scholar]
- [17].Bellentani S, Scaglioni F, Marino M, et al. Epidemiology of non-alcoholic fatty liver disease. Dig Dis 2010;28:155–61. [DOI] [PubMed] [Google Scholar]
- [18].Chitturi S, Farrell GC, Hashimoto E, et al. Non-alcoholic fatty liver disease in the Asia-Pacific region: definitions and overview of proposed guidelines. J Gastroenterol Hepatol 2007;22:778–87. [DOI] [PubMed] [Google Scholar]
- [19].Gao XC, Yang L. Epidemic status and related risk factors of nonalcoholic fatty liver disease in non-obese people. J Clin Intern Med 2015;297–9. [Google Scholar]
- [20].Wang CX. Prevalence of nonalcoholic fatty liver in Guangzhou physical examination population and analysis of related factors. Shantou University 2013. [Google Scholar]
- [21].Qiao LN, Dai GR, Zhang J. An epidemiological survey of prevalence and risk factors for fatty liver disease in adults residing in Yan’an, China. J Clin Hepatol 2015;82–7. [Google Scholar]
- [22].Cai YJ, Wang JJ, Deng LL. The elderly nonalcoholic fatty liver disease prevalence and related factors analysis in Xuanwu community. Chin J Primary Med Pharm 2011;18:3181–2. [Google Scholar]
- [23].Song QL. Prevalence of fatty liver in staff of plateau. J Prevent Med Chin People's Liberation Army 2001;19:269. [Google Scholar]
- [24].Cui XW. Epidemiological investigation of nonalcoholic fatty liver in residents of Karamay city in Xinjiang. Xinjiang Medical University 2012. [Google Scholar]
- [25].Cai W. Study on the Relationship between Nonalcoholic Fatty Liver Disease and Lipid Metabolic Gene Polymorphism and Cytokines in Xinjiang Uygur and Han Nationalities. Xinjiang Medical University 2013. [Google Scholar]
- [26].Zhao L, Guo YJ, Najina W. Association between diet and venous thromboembolism in Uyghur patients. J Xinjiang Med Univ 2016;39:1377–81. [Google Scholar]
- [27].Wang X. The role of minority females in maintaining stability of Xinjiang Province. Soc Sci J Coll Shanxi 2016;28:25–9. 39. [Google Scholar]
- [28].He J, Guo H, Ding YS. Epidemiological study on overweight and obesity among rural adult residents in Hazakh, Uygur and Han populations in Xinjiang. Chin J Epidemiol 2013;34:1164–8. [PubMed] [Google Scholar]
- [29].Lin SL, Song JM, Xia HL. Unconditional logistic regression analysis of risk factors for nonalcoholic fatty liver disease in the Urumqi population. Chin J Hepatol 2014;22:953–4. [PubMed] [Google Scholar]
- [30].Saokaew S, Kanchanasuwan S, Apisarnthanarak P, et al. Clinical risk scoring for predicting non-alcoholic fatty liver disease in metabolic syndrome patients (NAFLD-MS score). Liver Int 2017;37:1535–43. [DOI] [PubMed] [Google Scholar]
- [31].Chinese Medical Association Diabetes Branch Metabolic Syndrome Research Collaborative Group. Suggestions on metabolic syndrome in the Chinese Medical Association Diabetes Branch. Chin J Diabetes 2004;12:156–60. [Google Scholar]
- [32].Lin XR, Lin GL, Feng XH. The relationship between fatty liver and hyperlipidemia. Modern Hospital 2006;6:64–5. [Google Scholar]
- [33].Yu L, Niu JQ. New advances in nonalcoholic fatty liver research. Chin J Gerontol. 2148–2151. [Google Scholar]
- [34].Zhang S, Du T, Li M, et al. Combined effect of obesity and uric acid on nonalcoholic fatty liver disease and hypertriglyceridemia. Medicine (Baltimore) 2017;96:e6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wu VJ, Pang D, Tang WW, et al. Obesity, age, ethnicity, and clinical features of prostate cancer patients. Am J Clin Exp Urol 2017;5:1–9. [PMC free article] [PubMed] [Google Scholar]
- [36].Rothberg AE, McEwen LN, Kraftson AT, et al. Impact of weight loss on waist circumference and the components of the metabolic syndrome. BMJ Open Diabetes Res Care 2017;5:e000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lee YH, Hsiao HF, Yang HT, et al. Reproducibility and repeatability of computer tomography-based measurement of abdominal subcutaneous and visceral adipose tissues. Sci Rep 2017;7:40389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Xiang GQ, Meng XY, Zhang H. Assessment of risk factors associated with fatty liver disease. World Chin J Digestol 2009;17:1038–41. [Google Scholar]
- [39].Ma JX, Chen PY, Zhou YJ. A1:1 matched case-control study on the risk factors of fatty liver in general people in guangdong province. Modern Prevent Med 2008;35:648–50. [Google Scholar]
- [40].Hwang ST, Cho YK, Yun JW, et al. Impact of non-alcoholic fatty liver disease on microalbuminuria in patients with prediabetes and diabetes. Intern Med J 2010;40:437–42. [DOI] [PubMed] [Google Scholar]
- [41].H L. Correlation between neck circumference and visceral fat and cardiovascular risk factors. Capital Medical University 2012. [Google Scholar]
- [42].Bai J, He Y, Hai X, et al. Skinfold thickness and body composition in Han nationality adults in Lanzhou. Chin J Anat 2014;37:527–32. [Google Scholar]
- [43].Heald A, Pendlebury J, Anderson S, et al. Lifestyle factors and the metabolic syndrome in Schizophrenia: a cross-sectional study. Ann Gen Psychiatry 2017;16:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Jarbol DE, Larsen PV, Gyrd-Hansen D, et al. Determinants of preferences for lifestyle changes versus medication and beliefs in ability to maintain lifestyle changes. A population-based survey. Prev Med Rep 2017;6:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Hong WJ. Influence of health education to the younger coronary heart disease patients. Chin Nurs Res 2008;22(12C):3323–4. [Google Scholar]
- [46].Dong F, Zhang Y, Huang Y, et al. Long-term lifestyle interventions in middle-aged and elderly men with nonalcoholic fatty liver disease: a randomized controlled trial. Sci Rep 2016;6:36783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Portincasa P, Grattagliano I, Palmieri VO, et al. Nonalcoholic steatohepatitis: recent advances from experimental models to clinical management. Clin Biochem 2005;38:203–17. [DOI] [PubMed] [Google Scholar]
- [48].Zhao HM, Xu L, Guo R. Motivational interviewing in the treatment of patients with non-alcoholic fatty liver disease. J Clin Hepatol 2012;15:93–6. [Google Scholar]
- [49].Oh S, So R, Shida T, et al. High-intensity aerobic exercise improves both hepatic fat content and stiffness in sedentary obese men with nonalcoholic fatty liver disease. Sci Rep 2017;7:43029. [DOI] [PMC free article] [PubMed] [Google Scholar]


