Abstract
Smoking is a risk factor for nonsmall cell lung carcinoma (NSCLC) and is associated with a lower response to epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKI). The purpose of this study is to examine the impact of the smoking status on the benefits from first-line EGFR-TKI in NSCLC patients with EGFR mutation.
This was a retrospective study of 159 patients with advanced NSCLC treated at the Beijing Hospital between January 2011 and December 2016. The follow-up was censored on December 2017. EGFR mutation status, smoking (nonsmoker vs <30 packs/year (light smoker) vs ≥30 packs/year (heavy smoker)), treatment, treatment response, and progression-free survival (PFS) were collected from the charts.
Median follow-up was 10.0 (1.0–36.6) months. Response rate was lower in heavy smokers compared with nonheavy smokers (19.0% vs 71.7%, P < .001). There was no difference in PFS between nonsmokers (median, 10.5 months) and light smoker (median, 11.0 months), and these 2 groups were pooled together. PFS was longer in nonheavy smokers compared with heavy-smokers (median, 10.7 vs 6.0 months, P < .001). Smoking ≥ 30 packs/year (HR = 2.48, 95% CI: 1.55–3.98, P < .001) was associated with PFS.
In patients with advanced NSCLC, the benefits and PFS of EGFR-TKI were better for nonheavy smokers than for heavy smokers.
Keywords: chemotherapy, epidermal growth factor receptor-tyrosine kinase inhibitors, nonsmall cell lung carcinoma, smoking, survival
1. Introduction
Lung cancer is one of the most frequent cancers worldwide, and is associated with high morbidity and mortality.[1] Nonsmall cell lung carcinoma (NSCLC) represents 85% to 90% of the cases.[2] NSCLC mostly affects adults aged ≥65 years[2] and have a predominance toward males.[3] Smoking is the most important cause of lung cancer.[2] Clinical studies found that the pathogenesis, clinical manifestation, and prognosis of smokers and nonsmokers are different.[4–6]
Genetic differences have been found in the tumors of nonsmokers versus smokers.[7–9] Indeed, nonsmokers with lung cancer are more likely to carry epidermal growth factor receptor (EGFR) mutations, mostly in exons 19 and 21,[7,10] but other mutations are also known (such as in exons 18 and 20).[11,12] About 10% of lung tumors in the United States harbor an EGFR mutations, compared with 35% in East Asians.[13,14] The presence of EGFR mutations is a key predictor of the efficacy of EGFR-tyrosine kinase inhibitors (TKI).[15,16] Nevertheless, EGFR-TKI drugs are not effective for all patients with EGRF gene sensitive mutation and NSCLC and a number of factors are associated with a better response to EGFR-TKI: East Asian ethnicity, female sex, never-smoking status, adenocarcinoma histology, EGFR mutations, and high EGFR protein expression.[17,18]
Smoking is related to lower rates of EGFR mutations and poor outcomes.[7,8] Igawa et al[19] showed that among patients harboring activating EGFR mutations, the response to gefitinib was higher among nonsmokers compared to smokers. Meta-analyses revealed better survival with erlotinib or gefitinib in never smokers compared to smokers.[20,21] Smoking status also affects the response to conventional chemotherapy, with nonsmokers achieving better response rates to pemetrexed than smokers.[22] Nevertheless, the predictors of response to EGFR-TKI remain controversial.[23] Therefore, improving the prediction of the efficacy of EGFR-TKI drugs is a current problem needing to be solved. In addition, previous studies suffer from heterogeneity in EGFR-TKI, disease stage, and line of treatment.
Therefore, the aim of the present study was to examine the impact of the smoking status on the benefits from first-line EGFR-TKI in NSCLC patients with EGFR mutation. The results could help selecting the patients who would benefit the most from treatments.
2. Methods
2.1. Study design and patients
This was a retrospective study of 159 patients with advanced NSCLC treated at the Beijing Hospital between January 2011 and December 2016. The inclusion criteria were: unresectable, locally advanced, and/or recurrence or metastasis of NSCLC; stage IIIb/IV NSCLC; known EGFR mutation status; first-line treatment (either erlotinib or gefitinib); complete baseline data; and complete follow-up data. NSCLC and EGFR mutation status were confirmed using specimens obtained by surgery, fiber-optic bronchoscopy, needle biopsy, pleural effusion analysis, or biopsy of metastatic lymph node or metastasis. The study was approved by the ethics committee of the Beijing Hospital. The need for individual consent was waived by the committee because of the retrospective nature of the study.
2.2. Data collection
Nonsmokers were defined as a lifetime smoking of <100 cigarettes. For smokers, 30 packs/year was used to discriminate between light and heavy smokers.[24–26] The EGFR mutation status had been tested routinely and data were obtained from the medical charts.
All patients received EGFR-TKIs (either erlotinib or gefitinib) as first-line treatment until disease progression or occurrence of intolerable toxicity. Treatment benefits were evaluated according to the Response Evaluation Criteria In Solid Tumor (RECIST).[27] The response rate (RR) represented the complete remission (CR) rate plus the partial remission (PR) rate. Progression-free survival (PFS) was determined as the time between start of treatment and any progression. The follow-up was censored on December 2017.
2.3. Statistical analysis
Continuous data were presented as mean ± standard deviation and analyzed using ANOVA and the Tukey post hoc test. Categorical data were presented as proportions and analyzed using the Chi-square or Fisher exact test, as appropriate. All statistical analyses were performed with SPSS 17.0 (IBM, Armonk, NY). The Kaplan–Meier method and the log-rank test were used to analyze the PFS. The Cox hazard model was used for the univariate analysis of risk factors for PFS. Two-sided P values <.05 were considered statistically significant.
3. Results
3.1. Characteristics of the patients
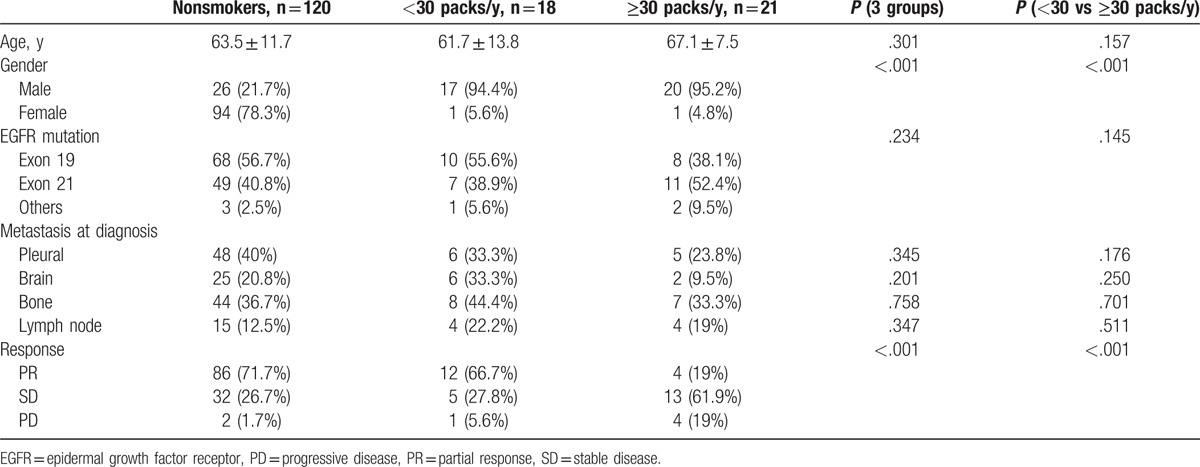
There were 120 nonsmokers, 18 light smokers, and 21 heavy smokers, all with an EGFR mutation. Table 1 presents the characteristics of the patients with an EGFR mutation. The age ranged from 29 to 89 years (median, 63 years). There were no differences in age, mutation, and metastases at NSCLC diagnosis among the 3 groups, but female gender was more frequent in the nonsmoker group (P < .001) and treatment response was better in the nonsmoker and <30 packs/year groups (P < .001). Smoking had no impact on the type of EGFR mutation (P = .145).
Table 1.
Characteristics of the patients.
3.2. Comparison of first-line EGFR-TKI therapy benefits for nonsmokers and smokers
Median follow-up was 10.0 (1–36.6) months. Response rate was lower in heavy smokers compared with nonheavy smokers (19.0% (4/21) vs 71.7% (86/120), P < .001). There was no difference in PFS between nonsmokers (median, 10.5 months) and light smoker (median, 11.0 months), and these 2 groups were pooled together. PFS was longer in nonheavy smokers compared with heavy smokers (median, 10.7 vs 6.0 months, P < .001) (Fig. 1).
Figure 1.
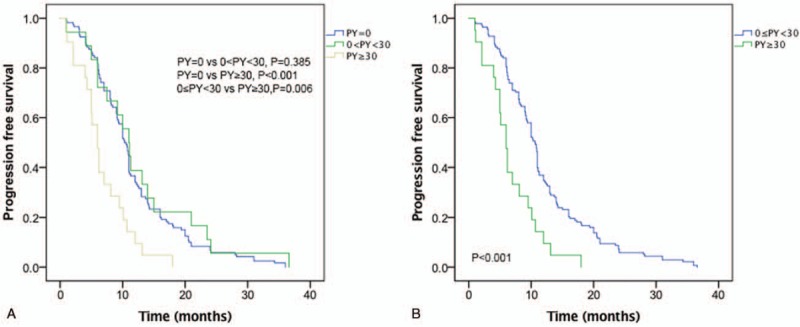
Progression-free survival. (A) Nonsmokers versus <30 packs/year versus ≥30 packs/year (P < .001). (B) <30 packs/year versus ≥30 packs/year (P < .001).
3.3. Cox hazard analysis
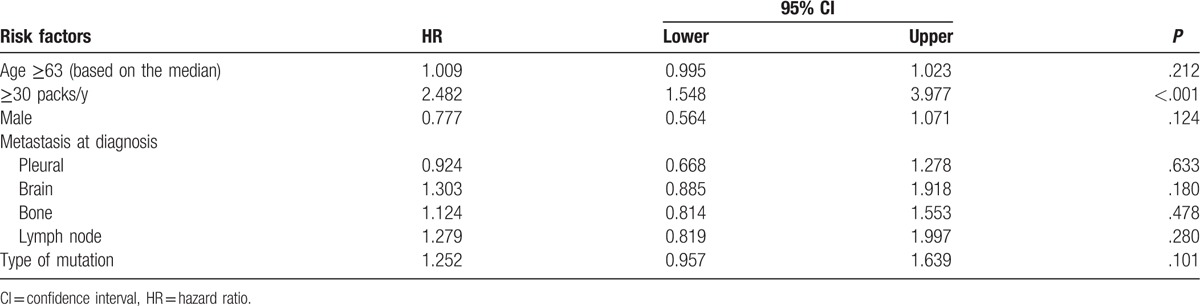
Table 2 presents the Cox hazard analysis. Smoking ≥30 packs/year (HR = 2.48, 95% CI: 1.55–3.98, P < .001) was associated with PFS. Since no other factor was associated with PFS on univariate analysis, no multivariate analysis could be performed.
Table 2.
Univariate analysis of PFS among patients with EGFR-mutated NSCLC treated with an EGFR-TKI.
4. Discussion
Smoking is a risk factor for NSCLC[2] and is associated to a lower response to EGFR-TKI.[20,21] Nevertheless, the predictors of response to EGFR-TKI remain controversial.[23] Therefore, improving the prediction of the benefits from EGFR-TKI drugs is a current problem needing to be solved. In addition, previous studies suffer from heterogeneity in the EGFR-TKI, disease stage, and line of treatment. Therefore, the present study aimed to examine the impact of the smoking status on the benefits from first-line EGFR-TKI in NSCLC patients with EGFR mutation. The results showed that in patients with advanced NSCLC, the benefits and PFS of EGFR-TKI were better for nonheavy smokers than for heavy smokers, which is supported by a Korean study.[26]
Smoking is the main risk factor for lung cancer.[2] The pathological type found among nonsmokers is mostly adenocarcinoma, while squamous cell carcinoma and small cell lung cancer are very rare.[2] Furthermore, there are significant differences in the gene mutation patterns between smoking and nonsmoking lung cancer patients.[7–9] The frequency of EGFR gene mutation is higher for nonsmokers with lung adenocarcinoma than for smokers.[7,10] In lung cancer, KRAS mutations are present in 15% to 25% of patients with lung cancer,[28] but they are rare in lung squamous carcinoma,[28] tumors harboring mutations in EGFR or ALK, and in East Asians.[29,30] In addition, KRAS mutations are even rarer in never-smoker lung cancer patients versus former/current smokers.[7,9,29–32] The exact prognostic impact of KRAS mutations is poorly known, but KRAS mutations are negative predictors of radiologic response to EGFR tyrosine kinase inhibitors.[33,34] In addition, some studies have reported that PD-L1 expression is different between smoking and nonsmoking lung cancer patients.[35] Lung cancer PD-L1 expression is significantly higher in smokers,[36,37] but this is controversial.[38,39] Nevertheless, a recent meta-analysis showed that PD-L1 expression was associated with poor survival of patients with lung cancer.[40] Taken together, KRAS mutations and PD-L1 expression could be involved in the impact of smoking on lung cancer survival, but additional studies are necessary to examine these factors in relation to heavy smoking.
Studies showed that nonsmoking Asian females have more benefits from EGFR-TKI compared with the other groups.[17,18] The IPASS study showed that smoking history and pathological types are independent factors affecting gene mutation,[41] as confirmed by the PIONEER study.[10] The present study examined the impact of smoking status and gender on EGFR mutations and found that there was no significant difference in the EGFR mutations within the same smoking status, regardless of gender. The results by Girard et al[42] also suggested that the most important predictor of EGFR mutation is the smoking index, while gender was not an independent predictor, as in the present study.
In the IPASS study,[41] the objective response rate of gefitinib in patients with EGFR mutation was up to 71.2%, while for EGFR patients without mutations, it was only 1.1%. EGFR mutation is a strong predictor of tumor response. Nevertheless, EGFR-TKIs are not equally effective for all NSCLC patients with EGFR gene sensitive mutation.[17,18] Nonsmoking lung cancer patients have higher EGFR mutation rate.[7,10] Although many EGFR mutations are found in nonsmokers with adenocarcinoma, a significant proportion of patients with smoking history have mutated EGFR. In the present study, the effective rate of EGFR-TKI for different smoking statuses was analyzed, and the results showed that the effective rates of nonheavy smokers and heavy smokers with EGFR sensitive mutation were 71.8% and 18.8% respectively, showing a significant difference. The median PFS for nonheavy smokers and heavy smokers were 10.8 and 6.2 months, respectively, and the difference was statistically significant. Therefore, EGFR-TKIs have different efficiency in nonheavy smokers and heavy smokers. Compared with heavy smokers, nonheavy smokers with EGFR mutation had a higher effective rate and longer PFS after first-line TKI therapy.[26] A previous study of 153 NSCLC patients with EGFR mutation showed that the overall response rate and tumor-free survival (TFS) for smokers and nonsmokers were 66.7% and 9 months, and 10.7% and 5.4 months, respectively (P = .0002),[19] but this is controversial. Indeed, Zeng et al[23] reported that the median PFS of patients receiving first-line EGFR-TKI was similar, regardless of smoking status (nonsmokers vs smokers: 9.9 vs 9.1 months, P = .570). The present study showed that smoking alone had no impact of PFS, but that the dose of cigarettes (>30 packs/year) is an independent factor associated with PFS. Therefore, it is possible that not only the smoking status is associated with EGFR-TKI response, but also the amount of cigarette smoke, as supported by Kim et al.[26] In addition, Jain et al[43] showed that the mutation patterns were different between light and heavy smokers. Nevertheless, these results will have to be confirmed in future studies.
The present study is not without limitations. The sample size was small and from a single center. In addition, the retrospective nature of the study limited the data that could be analyzed. Among others, data about smoke exposure in nonsmokers (from cooking, heating, work, etc.) were not available. Additional studies are necessary to understand the risk factors for lung cancer and the factors associated with a better response to EGFR-TKI.
In conclusion, in patients with advanced NSCLC, the benefits and PFS of EGFR-TKI were better for nonheavy smokers than for heavy smokers.
Footnotes
Abbreviations: CR = complete remission, EGFR = epidermal growth factor receptor, EGFR-TKI = epidermal growth factor receptor-tyrosine kinase inhibitors, NSCLC = nonsmall cell lung carcinoma, PFS = progression-free survival, PR = partial remission, RECIST = Response Evaluation Criteria In Solid Tumor, RR = response rate, TFS = tumor-free survival, TKI = tyrosine kinase inhibitor.
The authors have no conflicts of interest to disclose.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Novello S, Barlesi F, Califano R, et al. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016;27:v1–27. [DOI] [PubMed] [Google Scholar]
- [3].Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–86. [DOI] [PubMed] [Google Scholar]
- [4].Ferketich AK, Niland JC, Mamet R, et al. Smoking status and survival in the national comprehensive cancer network non-small cell lung cancer cohort. Cancer 2013;119:847–53. [DOI] [PubMed] [Google Scholar]
- [5].Lee SJ, Lee J, Park YS, et al. Impact of smoking on mortality of patients with non-small cell lung cancer. Thorac Cancer 2014;5:43–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Muallaoglu S, Karadeniz C, Mertsoylu H, et al. The clinicopathological and survival differences between never and ever smokers with non-small cell lung cancer. J BUON 2014;19:453–8. [PubMed] [Google Scholar]
- [7].Chapman AM, Sun KY, Ruestow P, et al. Lung cancer mutation profile of EGFR, ALK, and KRAS: meta-analysis and comparison of never and ever smokers. Lung Cancer 2016;102:122–34. [DOI] [PubMed] [Google Scholar]
- [8].Gou LY, Niu FY, Wu YL, et al. Differences in driver genes between smoking-related and non-smoking-related lung cancer in the Chinese population. Cancer 2015;121(suppl 17):3069–79. [DOI] [PubMed] [Google Scholar]
- [9].Govindan R, Ding L, Griffith M, et al. Genomic landscape of non-small cell lung cancer in smokers and never-smokers. Cell 2012;150:1121–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Shi Y, Au JS, Thongprasert S, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol 2014;9:154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Yasuda H, Park E, Yun CH, et al. Structural, biochemical, and clinical characterization of epidermal growth factor receptor (EGFR) exon 20 insertion mutations in lung cancer. Sci Transl Med 2013;5:216ra177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Li AR, Chitale D, Riely GJ, et al. EGFR mutations in lung adenocarcinomas: clinical testing experience and relationship to EGFR gene copy number and immunohistochemical expression. J Mol Diagn 2008;10:242–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pao W, Miller V, Zakowski M, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci U S A 2004;101:13306–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Paez JG, Janne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004;304:1497–500. [DOI] [PubMed] [Google Scholar]
- [15].Tseng JS, Yang TY, Tsai CR, et al. Dynamic plasma EGFR mutation status as a predictor of EGFR-TKI efficacy in patients with EGFR-mutant lung adenocarcinoma. J Thorac Oncol 2015;10:603–10. [DOI] [PubMed] [Google Scholar]
- [16].Zhao D, Chen X, Qin N, et al. The prognostic role of EGFR-TKIs for patients with advanced non-small cell lung cancer. Sci Rep 2017;7:40374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Peled N, Yoshida K, Wynes MW, et al. Predictive and prognostic markers for epidermal growth factor receptor inhibitor therapy in non-small cell lung cancer. Ther Adv Med Oncol 2009;1:137–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Heist RS, Christiani D. EGFR-targeted therapies in lung cancer: predictors of response and toxicity. Pharmacogenomics 2009;10:59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Igawa S, Sasaki J, Otani S, et al. Impact of smoking history on the efficacy of gefitinib in patients with non-small cell lung cancer harboring activating epidermal growth factor receptor mutations. Oncology 2015;89:275–80. [DOI] [PubMed] [Google Scholar]
- [20].Sohn HS, Kwon JW, Shin S, et al. Effect of smoking status on progression-free and overall survival in non-small cell lung cancer patients receiving erlotinib or gefitinib: a meta-analysis. J Clin Pharm Ther 2015;40:661–71. [DOI] [PubMed] [Google Scholar]
- [21].Zhang Y, Kang S, Fang W, et al. Impact of smoking status on EGFR-TKI efficacy for advanced non-small-cell lung cancer in EGFR mutants: a meta-analysis. Clin Lung Cancer 2015;16:144.e1–51.e1. [DOI] [PubMed] [Google Scholar]
- [22].Igawa S, Sasaki J, Otani S, et al. Smoking history as a predictor of pemetrexed monotherapy in patients with non-squamous non-small cell lung cancer. Oncology 2016;91:41–7. [DOI] [PubMed] [Google Scholar]
- [23].Zeng Z, Chen HJ, Yan HH, et al. Sensitivity to epidermal growth factor receptor tyrosine kinase inhibitors in males, smokers, and non-adenocarcinoma lung cancer in patients with EGFR mutations. Int J Biol Markers 2013;28:249–58. [DOI] [PubMed] [Google Scholar]
- [24].Fujisawa T, Iizasa T, Saitoh Y, et al. Smoking before surgery predicts poor long-term survival in patients with stage I non-small-cell lung carcinomas. J Clin Oncol 1999;17:2086–91. [DOI] [PubMed] [Google Scholar]
- [25].Aberle DR, Adams AM, et al. National Lung Screening Trial Research Team. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kim MH, Kim HR, Cho BC, et al. Impact of cigarette smoking on response to epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors in lung adenocarcinoma with activating EGFR mutations. Lung Cancer 2014;84:196–202. [DOI] [PubMed] [Google Scholar]
- [27].Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 2009;15:7412–20. [DOI] [PubMed] [Google Scholar]
- [28].Brose MS, Volpe P, Feldman M, et al. BRAF and RAS mutations in human lung cancer and melanoma. Cancer Res 2002;62:6997–7000. [PubMed] [Google Scholar]
- [29].Riely GJ, Kris MG, Rosenbaum D, et al. Frequency and distinctive spectrum of KRAS mutations in never smokers with lung adenocarcinoma. Clin Cancer Res 2008;14:5731–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sun Y, Ren Y, Fang Z, et al. Lung adenocarcinoma from East Asian never-smokers is a disease largely defined by targetable oncogenic mutant kinases. J Clin Oncol 2010;28:4616–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kawaguchi T, Koh Y, Ando M, et al. Prospective analysis of oncogenic driver mutations and environmental factors: Japan Molecular Epidemiology for Lung Cancer Study. J Clin Oncol 2016;34:2247–57. [DOI] [PubMed] [Google Scholar]
- [32].Zheng S, Wang R, Zhang Y, et al. Former smokers with non-small-cell lung cancers: a comprehensive investigation of clinicopathologic characteristics, oncogenic drivers, and prognosis. Cancer Med 2016;5:2117–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Riely GJ, Ladanyi M. KRAS mutations: an old oncogene becomes a new predictive biomarker. J Mol Diagn 2008;10:493–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Riely GJ, Marks J, Pao W. KRAS mutations in non-small cell lung cancer. Proc Am Thorac Soc 2009;6:201–5. [DOI] [PubMed] [Google Scholar]
- [35].Calles A, Liao X, Sholl LM, et al. Expression of PD-1 and its ligands, PD-L1 and PD-L2, in smokers and never smokers with KRAS-mutant lung cancer. J Thorac Oncol 2015;10:1726–35. [DOI] [PubMed] [Google Scholar]
- [36].Inoue Y, Yoshimura K, Mori K, et al. Clinical significance of PD-L1 and PD-L2 copy number gains in non-small-cell lung cancer. Oncotarget 2016;7:32113–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Takada K, Okamoto T, Shoji F, et al. Clinical significance of PD-L1 protein expression in surgically resected primary lung adenocarcinoma. J Thorac Oncol 2016;11:1879–90. [DOI] [PubMed] [Google Scholar]
- [38].Cooper WA, Tran T, Vilain RE, et al. PD-L1 expression is a favorable prognostic factor in early stage non-small cell carcinoma. Lung Cancer 2015;89:181–8. [DOI] [PubMed] [Google Scholar]
- [39].D’Incecco A, Andreozzi M, Ludovini V, et al. PD-1 and PD-L1 expression in molecularly selected non-small-cell lung cancer patients. Br J Cancer 2015;112:95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zhang M, Li G, Wang Y, et al. PD-L1 expression in lung cancer and its correlation with driver mutations: a meta-analysis. Sci Rep 2017;7:10255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Goto K, Ichinose Y, Ohe Y, et al. Epidermal growth factor receptor mutation status in circulating free DNA in serum: from IPASS, a phase III study of gefitinib or carboplatin/paclitaxel in non-small cell lung cancer. J Thorac Oncol 2012;7:115–21. [DOI] [PubMed] [Google Scholar]
- [42].Girard N, Sima CS, Jackman DM, et al. Nomogram to predict the presence of EGFR activating mutation in lung adenocarcinoma. Eur Respir J 2012;39:366–72. [DOI] [PubMed] [Google Scholar]
- [43].Jain A, Lim C, Gan EM, et al. Impact of smoking and brain metastasis on outcomes of advanced EGFR mutation lung adenocarcinoma patients treated with first line epidermal growth factor receptor tyrosine kinase inhibitors. PLoS ONE 2015;10:e0123587. [DOI] [PMC free article] [PubMed] [Google Scholar]


