Abstract
Vertebral artery dissection (VAD) is not uncommon in the young adult population. Owing to the various clinical manifestations; the diagnosis of VAD mainly depends on imaging examinations. Ultrasound has found an increasingly wide utilization in the diagnosis of VAD because of the continuous improvement in the resolution of ultrasonic instruments and accessibility.
We retrospectively collected the data of patients with a US-proven extracranial vertebral artery dissection. In accordance with the sonographic findings, all patients were classified as having intramural hematoma, double-lumen dissection, and occlusion dissection. The patients’ age, sex, risk factors for cerebrovascular diseases, and sonographic characteristics were analyzed.
A total of 37 cases of US-proven extracranial vertebral artery dissections were included in this study. Thirty patients presented with intramural hematoma dissection, 1 had double-lumen dissection and 6 had occlusion dissection. No dissecting aneurysm was found in any of the patients. Concerning a subsequent angiographic examination, 13 patients failed to undergo the examination for various reasons. The remaining 24 patients underwent digital subtraction angiography (DSA), magnetic resonance angiography (MRA), and computerized tomographic angiography (CTA), among whom 1 patient with intramural hematoma was underdiagnosed because the DSA result was interpreted as normal. One patient who underwent CTA had a contrast allergy. In the remaining patients, the results of other imaging examinations were consistent with the US results.
Intramural hematoma dissection is the most common type of extracranial vertebral artery dissection. Over other angiographic examinations US has a big diagnostic advantage for its direct view, accuracy, and low cost.
Keywords: dissection, ultrasound, vertebral artery
1. Introduction
Vertebral artery dissection (VAD) is not rare in the young adult population. It is the most prevalent among the different types of cervicocerebral artery dissection.[1,2] Owing to the lack of typical clinical manifestations, VAD is often misdiagnosed or underdiagnosed. With the rapid technological advancements in vascular imaging, arterial dissection is gradually increasingly being detected. Currently, the clinical diagnosis of arterial dissection mainly depends on imaging examinations. A suspected arterial dissection can be diagnosed using magnetic resonance imaging (MRI), magnetic resonance angiography (MRA), computerized tomographic angiography (CTA), ultrasonography (US), or digital subtraction angiography (DSA). The accuracy of each method varies according to the location and pathotype of the dissection. The guideline on the management of patients with extracranial carotid and vertebral artery disease recommends that comprehensive imaging modalities should be used to diagnose dissections.[3] In this study, we aimed to present the sonographic findings of extracranial VADs (eVADs) and to analyze the diagnostic reliability of US in comparison to various other imaging modalities.
2. Subjects and methods
This study received approval from our institutional review board. We retrospectively collected data from all patients with a US-proven eVAD at the central hospital in Baotou between January 2013 and May 2017. Clinical and demographic details were recorded. Carotid US was performed by experienced neurosonographers.
Excranial VADs are categorized as spontaneous dissections when they occur spontaneously or in association with a minor trauma.[4,5] The exclusion criteria are a history of obvious trauma and arterial dissection resulting from aortic arch dissections. The reference standards of US for diagnosing eVADs are as follows: multiple segmental intramural hematoma, membranous echo in the lumen, double-lumen structure, and irregular artery stenosis. The presence of any one of these standard findings can be used to make a definitive diagnosis.[6] The location of dissection is defined on the basis of the standard anatomical categories.[7] The V1 segment runs from the origin to C6; the V2 segment, from C6 to C2; the V3 segment, from C2 to the dura; and the V4 segment, from the dura to the basilar artery. In this study, eVAD involved V1 and V2. According to the pathophysiology of the arterial dissection, the sonographic findings were classified into 4 subtypes. Subtype 1 was defined as intramural hematoma dissection (Fig. 1), which is characterized by multi-intramural hematoma and wall thickening with or without stenosis of the lumen on B-mode US. In this subtype, the thickening wall shows hypoechoicity, isoechoicity, or hyperechoicity that correlates with the course of the dissection. Subtype 2 was defined as a double-lumen dissection characterized by a double lumen (Fig. 2). In this subtype, a membranous echo divides the lumen into a false and a true lumen, and it is mostly accompanied by local or whole-vasculature dilatation. Color Doppler US images show 2 beams of Doppler blood flow signals with the same or different color, depending on the mode of dissection (a broken mouth of the vessel wall or two). Subtype 3 was defined as an occlusion dissection characterized by an occlusive lumen (Fig. 3). It can be a local or whole occlusion. In this subtype, B-mode US reveals a membranous echo in the echo-filled (either homogeneous or heterogeneous) lumen. Color Doppler US reveals no Doppler blood flow signals in the lumen. Subtype 4 was defined as a dissecting aneurysm characterized by vascular dilatation. In this subtype, B-mode US reveals an obvious vascular dilatation to the extent of at least 1.5 times of the adjacent lumen, a membranous echo in the lumen, and an incomplete wall structure. The lumen of the dissecting aneurysm is either filled with complete thrombus, or partly filled with thrombus, or has no thrombus. The incomplete wall structure is the main difference between a dissecting aneurysm and an arterial aneurysm or a pseudoaneurysm. In addition, we analyzed the cornerstone of various imaging diagnosis, and reviewed the optimal imaging methods according to the various subtypes of dissection.
Figure 1.
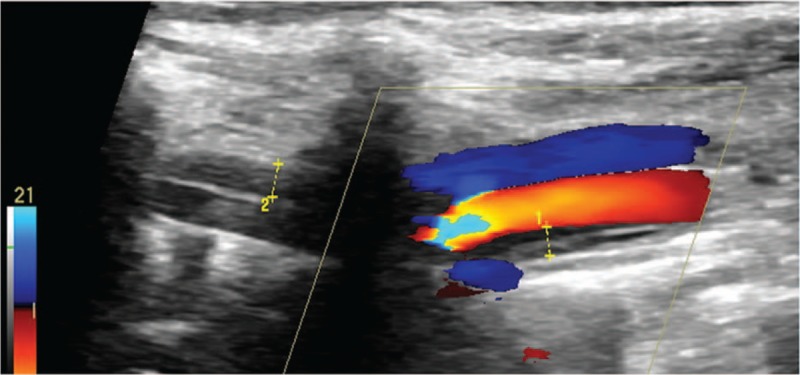
Intramural hematoma dissection of vertebral artery.
Figure 2.
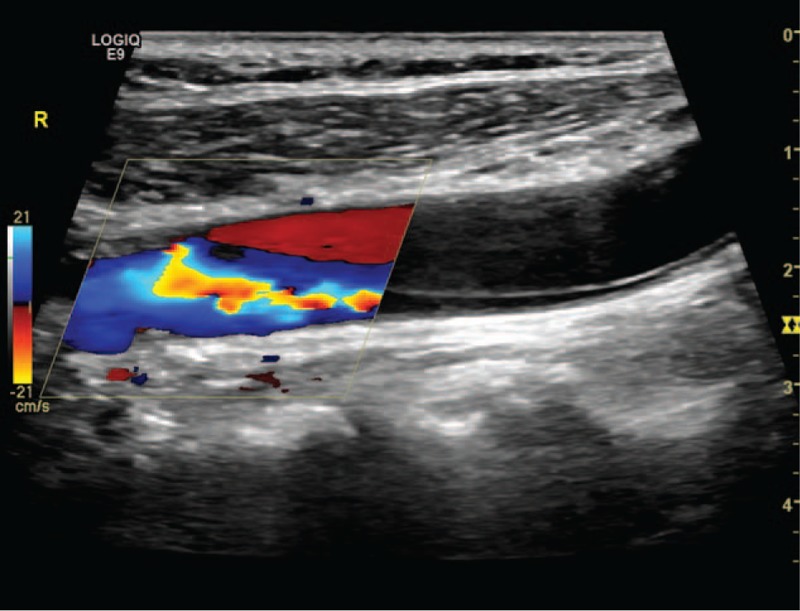
Double-lumen dissection of common carotid artery.
Figure 3.
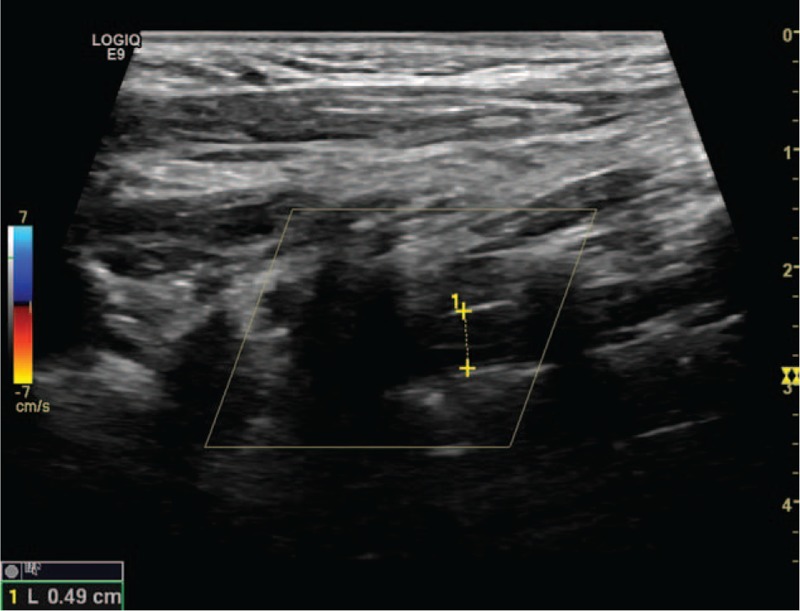
Occlusion dissection of vertebral artery.
3. Statistical analyses
One-way analysis of variance was performed to estimate the mean difference in age among the 3 subtypes. The results are expressed as mean ± standard deviation (range) in Table 1. The following variables were analyzed: sex, hypertension, diabetes mellitus, and hypercholesterolemia. The Fisher exact t test was performed for cross tabulation. All analyses were performed by using SPSS version 13.0.
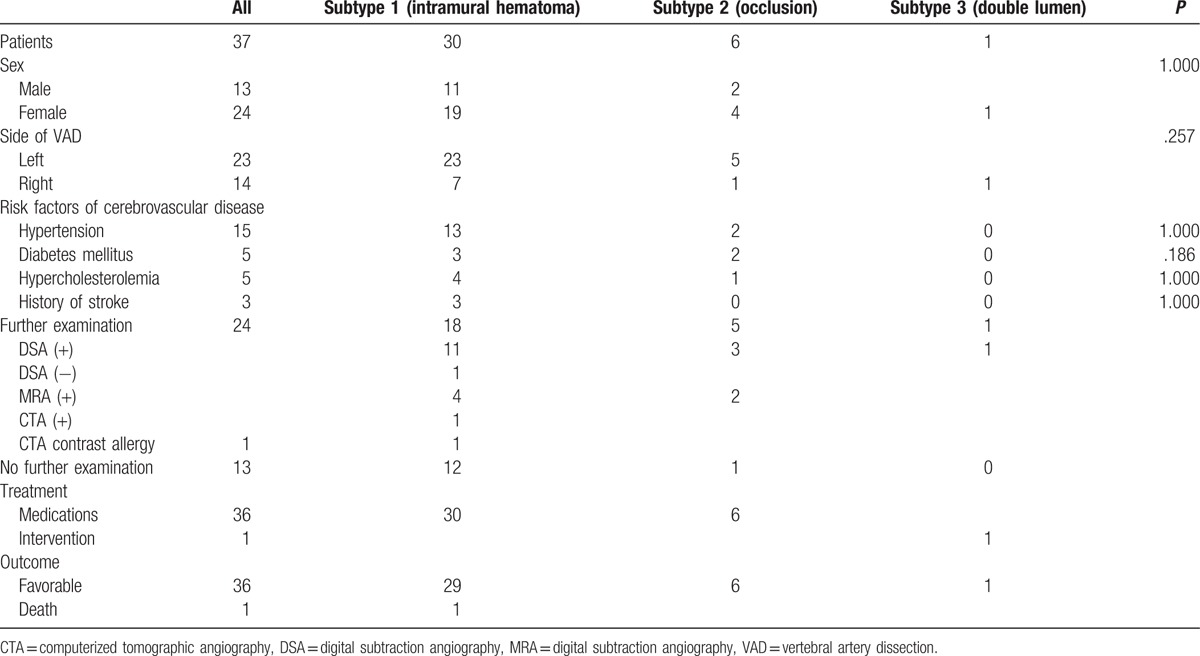
Table 1.
Baseline characteristics of patients with extracranial vertebral artery dissection.
4. Results
Thirty-seven patients (41% men) aged 30 to 85 years (mean, 52.81 ± 15.37 years) with an US-proven eVAD were included in this study. Of them, 23 patients (62%) presented with left eVAD and 14 patients presented with right vertebral dissection. According to the sonographic classification, 30 patients (81%) presented with intramural hematoma dissection, 1 (3%) had double-lumen dissection and 6 (16%) had occlusion dissection. No dissecting aneurysm was observed in this study. No differences in age and sex were observed among the different subtypes. No significant differences in risk factors for cerebrovascular disease were observed between subtypes 1 and 2. In subtype 1, 18 patients had further angiographic examination (12 with DSA, 4 with MRA, and 2 with CTA). The diagnosis was reconfirmed in 16 patients. Their imaging findings were all indirect signs including string-pearls signs on DSA or slender artery on MRA or CTA. One patient was underdiagnosed as having a normal-appearing lumen on DSA and another patient developed allergic reactions to the contrast agent in CTA. Twelve patients did not undergo further angiographic examinations, of whom 11 had favorable outcomes and 1 died of subarachnoid hemorrhage confirmed on head computed tomography. In subtype 2, 5 patients underwent further angiographic examination and 1 did not; however, no remarkable findings were obtained. Of the 5 patients, 3 were evaluated with DSA and 2 with MRA, where the occlusive vertebral arteries were all undeveloped. In subtype 3 dissection, 1 patient underwent DSA which showed the direct sign of double lumen and stent insertion therapy. The above-mentioned data are summarized in Table 1.
5. Discussion
The purpose of this study was to highlight the reliability and advantages of US in comparison with radiographic imaging examinations.
An arterial dissection arises from a tear in the vessel wall that allows the blood under arterial pressure to enter the wall of the artery, either subintimally or subadventitially. Therefore, the lumen is divided into a false and a true lumen. Signs most commonly appear in the subintimal lumen. If a thrombus is found in the subintimal lumen, namely in the false lumen, the dissection is defined as an intramural hematoma. If a thrombus is found both in the true and false lumens, the dissection is defined as an occlusion dissection. If no thrombus is observed either in the true or false lumen, the tearing membrane appears floating within the lumen, and the dissection is defined as a double-lumen dissection, which is typical but not as prevalent as the other types of dissection. Occurrence of signs in the subadventitia is relatively rare, which often results in an aneurysmal dilatation, namely a dissecting aneurysm. In this study, the typical arterial dissection, including a double lumen and an intimal flap, was observed in only 1 patient, whereas intramural hematoma dissections were more prevalent, similar to the reports in the literature.[7–9] Aneurysmal dissection, which relatively rarely occurs, if at all,[7–9] was also not found in this study.
The neuroimaging diagnosis of VAD can be performed with MRI, CTA, US, or DSA. DSA has long been considered the gold standard for the diagnosis of dissection; however, it lacks visualization of the arterial wall or mural hematoma. Hence, the diagnosis is sometimes inaccurate. In the present study, because of a subtle regular intramural hematoma with almost no stenosis in the true lumen, the dissection was underdiagnosed in the DSA evaluation because of a normal-appearing lumen. DSA is gradually being replaced by noninvasive imaging methods.[10] Concerning intramural hematoma, cross-sectional images of MRI have shown an advantage. Cristina et al[11] believe that MRI is the only reliable method for exploring the arterial wall. Provenzale[12] strongly suggested that MRI should be recommended as the first-line imaging screening tool. Blum and Yaghi[13] recommended that MRA along with T1 axial cervical MRI with the fat saturation technique should be prioritized for the diagnosis, owing to its lack of radiation, high sensitivity and specificity, and ability to visualize an intramural hematoma. However, the contraindications and high cost limit the wide application of MRI. As MRA and DSA were unable to display wall structures, including the presence of intramural hematomas, dissection was diagnosed on the basis of a series of indirect signs such as an irregular lumen and the string sign. By contrast, duplex US unusually but clearly and distinctly depicts intramural hematomas. Thus, US has been a useful reference imaging modality in the clinical assessment of patients suspected as having a cervical artery dissection.[14] A related study[15] highlighted the importance of identifying wall hematomas especially in dissections with a normal-appearing lumen, which is easily overlooked on MRA. Herein, multisection CTA showed a relative advantage. CTA has a higher resolution and is more readily available than MRI; however, it requires radiation exposure and the use of intravenous contrast, which may be contraindicated in patients with kidney failure and contrast allergy.[16] In the present study, a young man aged 30 years developed an allergy during CTA, and US became his examination of choice during hospitalization and after discharge. However, for viewing the lumen of the occlusive artery, the above-mentioned imaging methods except US are all ineffective. In 6 patients with occlusive dissection, US ascertained the cause of the occlusion on the basis of lumen echogenicity, which determine the follow-up treatment different from other common atherosclerosis occlusion. The benefits of treatment to patients according to a clear etiology are self-evident. But, for the moment, the etiological evaluation of the occlusive artery is uncommon because general examinations such as DSA, MRA or CTA cannot display occlusive artery except US. US can display membranous echogenicity, thrombosis, plaques and so on in the lumen of occlusive artery and show a specific advantage. In this study, as a first-line examination method, US was impressive because it allows an accurate and prompt diagnosis. In subsequent medical examinations, CTA, MRA, or DSA and MRI verified the results of US. US should be the first-choice diagnostic modality for eVADs in clinical practice. After identifying the definite cause, effective treatment should be initiated to prevent unnecessary examinations and to spare medical resources, in which US plays an immeasurable role. In some studies[7,17] that followed-up cervical artery dissection cases, confirmed recanalization and recurrence of cervical artery dissections were not rare. A related study[7] reported that the rate of complete recanalization was up to 64% at 12 months. Another study[17] of 105 cervical artery dissections revealed a complete recanalization rate of 51% and recurrence rate of 29%, and that a family history of arterial dissection was strongly associated with recurrence. The outcomes of eVAD were often favorable; however, caution should always be taken. In the study of Morris et al,[18] which included 2791 patients with dissections without ischemia, the risk of stroke after cervical artery dissection unaccompanied by ischemia at the time of diagnosis was observed in the first 2 weeks.
Our study shows that intramural hematoma is the most common ultrasonographic finding in eVADs, and US is suitable for the diagnosis of intramural hematoma. In terms of visualizing the lumen of the occlusive artery, US is uniquely capable. Therefore, US has an overwhelming advantage in the diagnosis of eVAD.
6. Limitations
This was a retrospective, observational study with a small sample size and operator dependency has always been a drawback of ultrasound examination. Additionally, the etiological evaluation of the occlusive artery encountered in US is not often, so the value of US in the occlusion of vascular disease has not been recognized widely.
Footnotes
Abbreviations: CTA = computerized tomographic angiography, DSA = digital subtraction angiography, eVADs = extracranial VADs, MRA = magnetic resonance angiography, US = ultrasonography, VAD = vertebral artery dissection.
Availability of data and materials: All data generated or analyzed during this study are included in this published article and its supplementary information files.
Competing interests: The authors declare that they have no competing interests.
Ethics approval and consent to participate: The Institutional Ethics Committee of Baotou Central Hospital approved this study.
The authors have no funding and no conflicts of interest to disclose.
References
- [1].Kwon JY, Kim NY, Suh DC, et al. Intracranial and extracranial arterial dissection presenting with ischemic stroke: Lesion location and stroke mechanism. J Neurol Sci 2015;358:371–6. [DOI] [PubMed] [Google Scholar]
- [2].Jung SC, Kim HS, Choi CG, et al. Spontaneous and unruptured chronic intracranial artery dissection: high-resolution magnetic resonance imaging findings. Clin Neuroradiol 2016. [DOI] [PubMed] [Google Scholar]
- [3].Brott TG, Halperin JL, Abbara S, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American Stroke Association, American Association of Neuroscience Nurses, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Congress of Neurological Surgeons, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of NeuroInterventional Surgery, Society for Vascular Medicine, and Society for Vascular Surgery. Vasc Med (London, England) 2011;16:35–77. [DOI] [PubMed] [Google Scholar]
- [4].Fusco MR, Harrigan MR. Cerebrovascular dissections—a review part I: spontaneous dissections. Neurosurgery 2011;68:242–57. discussion 257. [DOI] [PubMed] [Google Scholar]
- [5].Fusco MR, Harrigan MR. Cerebrovascular dissections: a review. Part II: blunt cerebrovascular injury. Neurosurgery 2011;68:517–30. discussion 530. [DOI] [PubMed] [Google Scholar]
- [6].Yang L, Ran H. The advantage of ultrasonography in the diagnosis of extracranial vertebral artery dissection: two case reports. Medicine 2017;96:e6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Arauz A, Marquez JM, Artigas C, et al. Recanalization of vertebral artery dissection. Stroke 2010;41:717–21. [DOI] [PubMed] [Google Scholar]
- [8].Zhang G, Chen Z. Medical and interventional therapy for spontaneous vertebral artery dissection in the craniocervical segment. BioMed Res Int 2017;2017:7859719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gunther A, Witte OW, Freesmeyer M, et al. Clinical presentation, magnetic resonance angiography, ultrasound findings, and stroke patterns in patients with vertebral artery dissection. Eur Neurol 2016;76:284–94. [DOI] [PubMed] [Google Scholar]
- [10].Shakir HJ, Davies JM, Shallwani H, et al. Carotid and vertebral dissection imaging. Curr Pain Headache Rep 2016;20:68. [DOI] [PubMed] [Google Scholar]
- [11].Cristina T, Elena T, Nicolae G, et al. Vertebral artery dissection: a contemporary perspective. Maedica 2016;11:144–9. [PMC free article] [PubMed] [Google Scholar]
- [12].Provenzale JM. MRI and MRA for evaluation of dissection of craniocerebral arteries: lessons from the medical literature. Emerg Radiol 2009;16:185–93. [DOI] [PubMed] [Google Scholar]
- [13].Blum CA, Yaghi S. Cervical artery dissection: a review of the epidemiology, pathophysiology, treatment, and outcome. Arch Neurosci 2015;2:e26670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Siepmann T, Borchert M, Barlinn K. Vertebral artery dissection with compelling evidence on duplex ultrasound presenting only with neck pain. Neuropsychiatr Dis Treat 2016;12:2839–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lum C, Chakraborty S, Schlossmacher M, et al. Vertebral artery dissection with a normal-appearing lumen at multisection CT angiography: the importance of identifying wall hematoma. AJNR Am J Neuroradiol 2009;30:787–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Berntsen EM. Cervical artery dissection—choice of diagnostic imaging. Tidsskrift Nor laegeforen 2017;137:166. [DOI] [PubMed] [Google Scholar]
- [17].Baracchini C, Tonello S, Meneghetti G, et al. Neurosonographic monitoring of 105 spontaneous cervical artery dissections: a prospective study. Neurology 2010;75:1864–70. [DOI] [PubMed] [Google Scholar]
- [18].Morris NA, Merkler AE, Gialdini G, et al. Timing of incident stroke risk after cervical artery dissection presenting without ischemia. Stroke 2017;48:551–5. [DOI] [PMC free article] [PubMed] [Google Scholar]


