Abstract
The colorectal cancer (CRC) patients with microsatellite instability (MSI) have distinct clinicopathological characteristics consisting of factors predicting positive and negative outcomes, such as a high lymph node harvest and poor differentiation. In this study, we measured the value of MSI as a prognostic factor after controlling for these discrepant factors. A total of 603 patients who underwent curative surgery for stages I to III colorectal cancer were enrolled. The patients were divided into microsatellite instability high (MSI-H) and microsatellite stable/microsatellite instability low (MSS/MSI-L) groups. Propensity score matching was used to match clinicopathological factors between the 2 groups. MSI-H patients had a high lymph node harvest (median: 31.0 vs 23.0, P < .001), earlier-stage tumors (P < .001), advanced T stage (89.3% vs 74.0%, P = .018), and poor differentiation (19.6% vs 2.0%, P < .001). Survival analysis showed better survival in the MSI-H group, but the difference was not statistically significant (P = .126). Propensity score matching was performed for significant prognostic factors identified by Cox hazard regression. After the matching, the survival difference by MSI status was estimated to be larger than before, and reached statistical significance (P = .045). In conclusion, after controlling for pathological characteristics, MSI-H could be a potent prognostic factor regarding patient survival.
Keywords: colorectal neoplasms, microsatellite instability, propensity score, survival
1. Introduction
Microsatellite instability (MSI) is caused by mutations in DNA mismatch repair genes such as MLH1, MSH2, MSH6, and PMS2,[1] and it is found in 10% to 15% of sporadic colorectal cancers (CRCs).[2,3] The presence of MSI predicts a good outcome in colorectal cancer.[4] It was reported that the survival rate of CRC patients with MSI is up to 15% higher as compared with that of CRC patients with microsatellite stable (MSS) tumors.[5] MSI status has a pivotal role in treatment decisions for stage II CRC. The National Comprehensive Cancer Network (NCCN) guideline does not recommend chemotherapy for these patients because of the good prognosis of MSI high patients with stage II CRC. However, the reason for their good prognosis remains unclear. In addition, MSI has several problems that limit its use as a practical prognostic factor across all stages of CRC.
Several research groups have reported contradicting results that MSI was not statistically correlated with overall survival (OS) or disease-free survival (DFS).[6,7] This result may be explained by the fact that MSI CRCs have distinctive clinical features, with a mixture associated with both good and poor outcomes. MSI tends to show an increased total number of harvested lymph nodes (LNs) after surgery.[8–11] The increased harvesting of LNs presents a good prognosis by itself.[12] In addition, it has been reported that MSI has a positive correlation with not only an increase in harvesting LNs, but also in appropriate LNs harvest (LNs > 12), which is recommended by NCCN guidelines for accurate staging and a good prognosis.[11,13–15] On the other hand, there are studies showing that MSI has a strong association with poor differentiation pathologically, which is a robust factor predicting a poor outcome.[8,16,17]
Recent molecular biology studies have discovered some mechanisms for the correlation between MSI and prognosis, such as an immune reaction in cancers.[18,19] Even though a high LN harvest is an expression of the immune reaction in MSI-H patients, other external factors, such as surgical skill and the experience of the pathologist, can affect LN harvest. In order to establish a base for research about MSI as well as suitable practical usage, it is necessary to evaluate the exact value of MSI as a prognostic factor without the biases of the clinical features. Hence, the aim of this study was to determine the value of MSI as a prognostic factor after reducing the biases of clinicopathological features.
2. Methods
2.1. Patient selection
This research project was approved by the institutional review board of Korea University Guro Hospital (IRB No. KUGH14152-001). This included 603 consecutive patients who underwent curative surgery for colorectal cancer between September 2009 and December 2014 at the Korea University Guro Hospital in Seoul, South Korea. Patients were excluded if they had stage IV colon cancer or received preoperative concurrent chemoradiotherapy, which has been reported as a significant factor to decrease the number of harvested LNs after rectal cancer surgery.[20] Patients with suspected Lynch syndrome or patients with synchronous colon cancer were also excluded. The stage I patients received no chemotherapy and stage III patients received FOLFOX chemotherapy as first-line treatment. Almost all stage II patients received 5-FU chemotherapy, except for the stage II patients with high risk factors who underwent FOLOX chemotherapy. Patients with hereditary nonpolyposis CRC and familial adenomatous polyposis were excluded. The clinicopathological information of the patients was obtained from their electronic medical records through a retrospective review. Patient follow-up was conducted every 3 to 6 months after surgery in the outpatient care department of our hospital. The end-point of this study was cancer-specific death. To obtain accurate mortality information, the death-date and death-cause data of the patients were collected from the National Cancer Registration Program of Korea.
2.2. Operation and LN harvest
The standard treatment procedure for primary tumors was resection of the affected segment of the bowel, including the main tumor and en bloc resection of regional LNs. After surgery, the specimen was delivered to the Department of Pathology for staging evaluation. If the number of LNs found from the CRC specimen were not adequate (the number of LNs ≤ 12), another attempt was made to search for more LNs. However, additional techniques such as fat clearance were not used. The LN ratio (LNR) was calculated from the number of metastatic LNs divided by the total number of harvested LNs.
To analyze MSI status, genomic DNAs were extracted for PCR amplification from paraffin-embedded tumor tissues. The set of microsatellite markers consisted of 2 mononucleotides repeat markers (BAT25 and BAT26) and 3 dinucleotide repeat markers (D2S123, D5S346, and D17S250). MSI-H was defined as instability in 2 or more markers; MSI-L, as instability in a single marker; and MSS, as no evidence of instability in the markers. In previous studies, MSI-H showed a different clinicopathologic phenotype from MSI-L or MSS.[8] MSI-L also appears to originate from chromosomal instability, unlike MSI-H.[21] Therefore, we divided the patients into 2 groups: MSI-H and MSI-L/MSS. If the location of the tumor was proximal to the splenic flexure, we defined it as proximal colon cancer.
2.3. Statistical analyses
Statistical analyses were conducted by using R version 3.3.2 (R Foundation for Statistical Computing, Vienna, Austria). The Mann–Whitney test was used to analyze statistical differences in clinicopathological features of MSI status. For categorical variables, a chi-square test or Fisher exact test was used. The survival rate in each group was analyzed by using the Kaplan–Meier method with the log-rank test to estimate the statistical significance. The factors that affected survival were identified by using univariate analysis with the Cox hazard regression model. Akaike information criterion (AIC) and Bayesian information criterion (BIC) were used for selection of the better-fitted model in Cox analysis. In the multivariate analysis, the fitted model was decided by the lowest value of AIC. P value less than .05 was considered to be significant.
Propensity score matching[22] was performed using the R package, MatchIt, for reducing the bias of the pathologic characteristics of patients.[23] The significant prognostic factors revealed by univariate Cox analysis were selected as covariates and matched with the optimal method. The selected covariates are as follows: differentiation, T stage, N stage, and LNR. The propensity score was generated by a generalized linear model. With one to one matching, 56 people were assigned to each group.
3. Results
3.1. Comparison of clinicopathological features based on MSI status in all stages
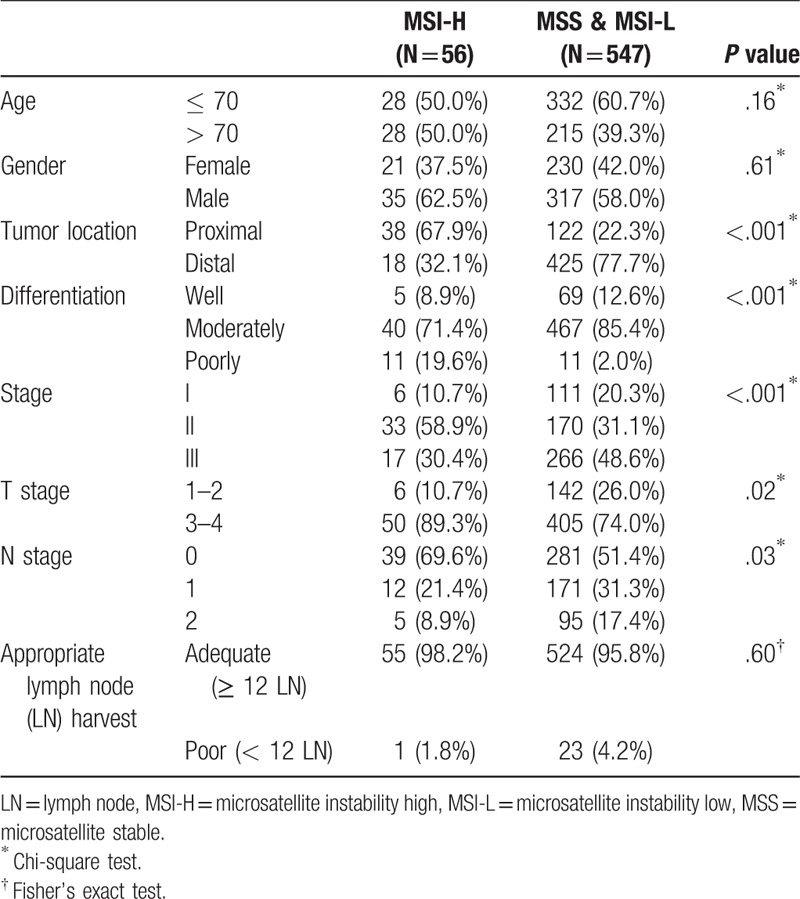
Among the 603 patients, 56 (9.3%) had MSI-H CRC (Table 1). MSI-H CRC presented with a proximal location (67.9% vs 22.3%, P < .001), advanced T stage (89.3% vs 74.0%, P = .02), and poor differentiation (19.6% vs 2.0%, P < .001). Most patients with MSI-H were stage II, whereas the patients with MSS/MSI-L accounted for the largest proportion of patients with stage III (P < .001). In the nodal stage, MSS/MSI-L patients showed more aggressive node metastasis (P = .03). Generally, MSI-H was more strongly correlated with early stage CRCs than MSI-L/MSS. There was no relationship between MSI status and appropriate LN harvest (> 12; P = .35).
Table 1.
Clinical and pathological features of 603 colorectal cancer patients who underwent surgical resection with a comparison between MSI-H patients and MSS patients.
3.2. Comparison of the total number of harvested LNs, metastatic LN, and LNR based on MSI status
Across all stages, the total harvest of LNs (THN) was statistically significantly higher in MSI-H patients (median: 31.0 vs 23.0, P < .001) (Table 2). The number of metastatic LNs was significantly lower in MSI-H patients, and LNR was also lower in MSI-H patients (P = .01). For further evaluation, THN, metastatic LNs, and LNR were examined in stage III. THN was also significantly higher in MSI-H of stage III (median: 32.0 vs 24.0, P = .01). Unlike in all stages, the number of metastatic LNs did not differ according to MSI status in stage III. However, LNR was still lower in MSI-H patients than in MSS/MSI-L patients (median: .06 vs.105, P = .01). The comparative distribution of lymph node features according to MSI status is shown in Figure 1.
Table 2.
Comparison of lymph node features according to MSI status.
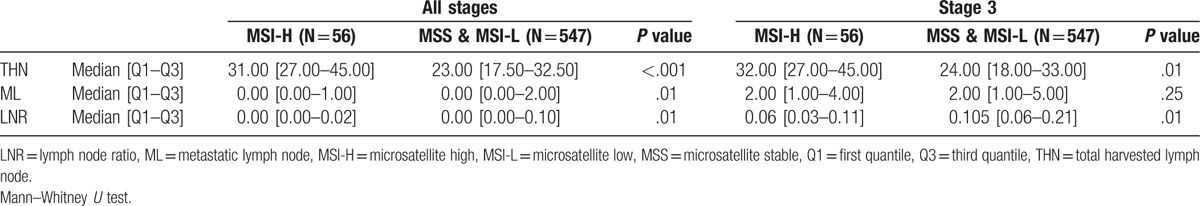
Figure 1.
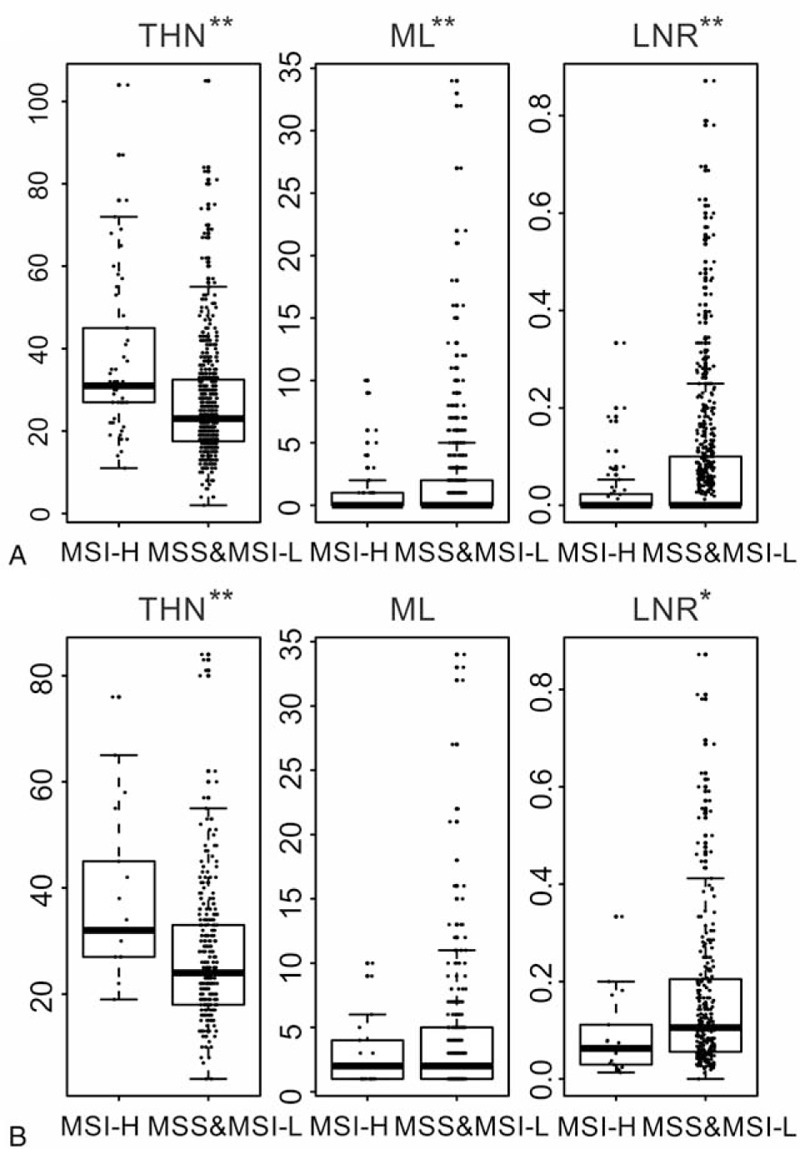
A comparison of lymph node features according to MSI status. (A) All stages, (B) stage 3. LNR = lymph node ratio, ML = metastatic lymph node, THN = total harvested lymph nodes (∗ P value < .05, ∗∗ P value < .01).
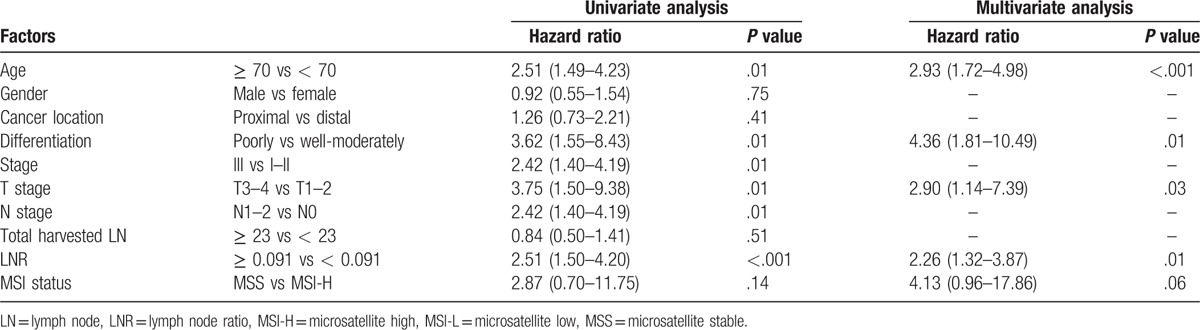
3.3. Survival analysis and identifying the risk factors by Cox hazard regression model
The plots of the survival analysis according to MSI status across all stages are shown in Figure 2. The median follow-up duration was 47.76 months. There was a better prognosis of patients with MSI-H colorectal cancer. However, it did not reach a statistically significant difference (P = .13). Cox hazard regression was performed to identify the clinicopathological factors affecting the survival rate. The LNR was divided into 2 groups based on the median value of 0.091 (Table 3). In univariate analysis, old age, poor differentiation, advanced T stage and N stage, high stage, and higher LNR values were found to be statistically significant predictors of poor outcomes. One interesting finding is that LNR was a better predictor of prognosis than N stage (N stage vs LNR; AIC: 697.16 vs 696.03, BIC: 699.22 vs 698.09). In this analysis, MSI status was not identified as a statistically significant prognostic factor (P = .14). Multivariate analysis was performed on MSI status and the factors proved statistically significant in the univariate analysis. Age, differentiation, T stage, and LNR were identified as significant prognostic factors in multivariate analysis. In this analysis, MSI was still not statistically significant.
Figure 2.
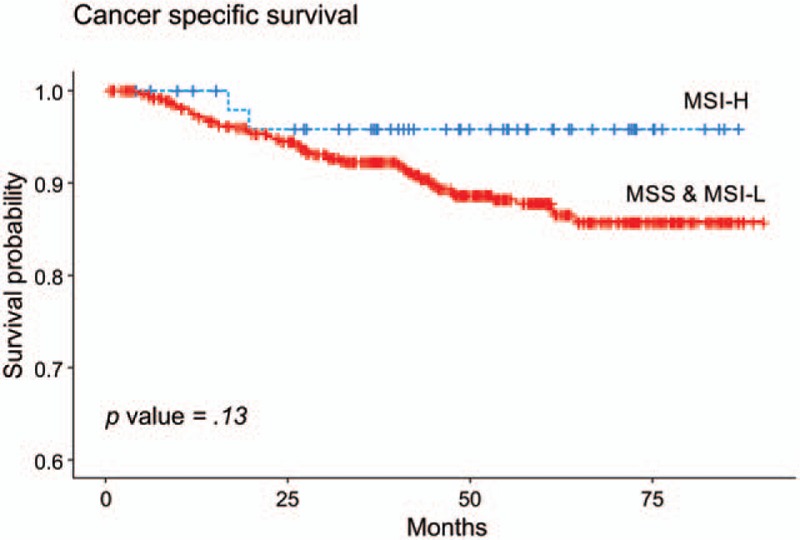
Cancer-specific survival plots by MSI status. MSI = microsatellite instability.
Table 3.
Cox proportional hazard regression for clinical characteristics.
3.4. Propensity score matching of the significant clinicopathological factors
Among the significant prognostic factors identified by univariate Cox analysis, the degree of differentiation, stage, T stage, N stage, and LNR were factors related to MSI status (Table 1). The poor differentiation, advanced T stage, early N stage and low LNR were associated with MSI-H. Among these factors, poor differentiation, and advanced T stage were associated with a poor prognosis, and the other factors correlated with MSI-H were associated with a good prognosis.
To reduce the differences due to these factors, 1:1 patient matching was performed between the MSI-H and MSS/MSI-L group by propensity score matching. The matching variables were pathologic factors including differentiation, T stage, N stage, and LNR. After matching, there was no significant difference in pathological factors between the 2 groups (Table 4). The median follow-up period of the matched patients was 37.53 months. Kaplan Meier survival analysis was performed according to MSI status (Fig. 3). The 5-year survival rate was 95.8% (95% CI: 90.3%–100%) in the MSI-H group and 74.5% (95% CI: 56.1%–98.8%) in the MSS/MSI-L group. Surprisingly, the difference in survival rate was greater than before matching, and achieved statistical significance (P value = .045).
Table 4.
Clinical and pathological features after propensity score matching.
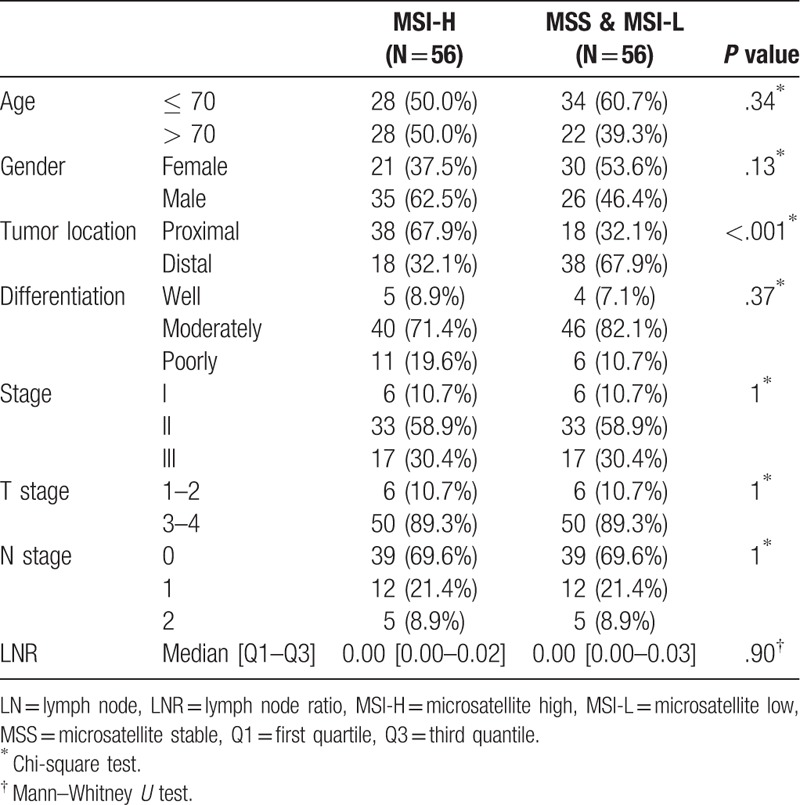
Figure 3.
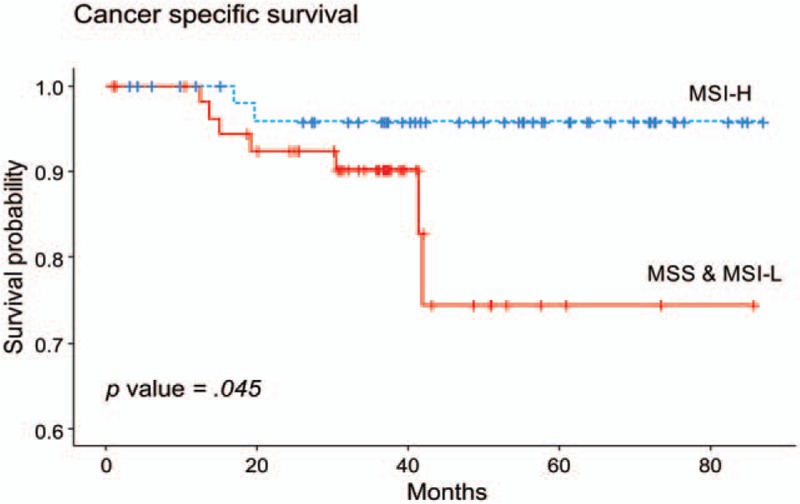
Cancer-specific survival after propensity score matching based on MSI status. MSI = microsatellite instability.
4. Discussion
The results of this study showed that colon cancer patients with MSI-H have a mixture of good and bad clinicopathological characteristics, which is consistent with the findings of previous studies,[9,24] such as more common in the right colon, early stage, poor differentiation, and higher LN harvest. The survival rate was better in the MSI-H CRC group, but the difference was not statistically significant. However, after correcting for the pathologic factors including differentiation, T stage, N stage, and LNR by propensity score matching, the survival rate became statistically significant, indicating a better prognosis in MSI-H patients, which is supported by many studies.[10,11,25]
The MSI-H phenotype was reported as a factor associated with improved prognosis, which is supported by many studies.[10,11,25] However, its mechanism has not been discovered yet. One of the mechanisms was thought to be a relationship between the immunological reaction of patients with MSI-H and improved prognosis.[26] Buckowitz et al[27] reported that enhanced lymphocytic infiltration accompanied by a reaction similar to that in Crohn disease increased host immunity, which could prevent metastasis. This increases local immunity can help the immune response against the cancer by presenting more neoantigens.[28] This study confirmed the higher total number of harvested LNs in the MSI-H phenotype group, which has been previously shown to be a predictor of a good prognosis.[12] These results match those observed in earlier studies. Søreide et al[9] suggested the Crohn disease-like reaction as a reason for the higher total number of harvested LNs from the specimens of MSI-H CRCs. In addition, a Dutch study found that high LN yields were significantly associated with the MSI-H phenotype in stage III colon cancer.[11] The relationship between MSI-H and a high total number of harvested LNs is a possible explanation for the better survival rates of patients with MSI-H CRCs.
This study has been unable to demonstrate a relationship between MSI status and adequate LN harvest (harvested LN > 12). Adequate LN harvest was recommended by the NCCN guidelines to improve survival and provide guidelines for adjuvant therapy. Some studies found a positive association between the MSI-H phenotype and adequate LN retrieval.[13,14] This inconsistency may be due to the variability in adequate node harvest proportions during surgery. A previous study demonstrated sufficient node harvest operations in just 67% of patients.[14] Adequate LN harvest in colon cancer surgery was achieved in only 50% to 60% of surgeries prior to 2005, after which it has been increasing annually.[29] In this study, it was up to 95%. Many factors influence adequate node harvest in surgery, including patient age, race, specialized surgeon and pathologist, and hospital volume.[29,30] The patients enrolled in our study had several factors that could increase the proportion of adequate LN harvest, such as Asian race, large hospital volume, and a colon specialized surgeon. These factors are likely to be related to the sufficient LNs harvest. Thus, the current study showed that MSI status was not associated with adequate node harvest under optimal LN harvest conditions.
This study provides new insights into LNR according to MSI status. Low LNR was proven to be a strong predictor of a good outcome.[12,31,32] Although some studies have evaluated the clinical characteristics of CRC with MSI-H, there is little published data on LNR correlated with MSI. If MSI status is associated with the total number of harvested LNs, we can consider the possibility of a relationship between MSI status and LNR. Ferri et al[33] reported no association between MSI status and LNR. Contrary to the previous study, this study found a significant relationship of MSI-H with low LNR in both all stage and stage III. These findings may help us to understand why MSI-H CRC patients show better survival.
Consistent with the result of this study, most previous studies have reported that MSI-H is more commonly observed in proximal colon cancers than in distal colon cancers.[34–36] Although the significant difference of Cox hazard ratio between proximal colon cancer and distal colon cancer was not shown in this study, proximal colon cancer is generally known to have poor prognosis compared to distal colon cancer,[35,37] and the molecular feature of proximal colon cancer differs from that of distal colon cancer. The proximal colon cancer exhibits more frequent BRAF mutation and CpG island methylation,[36,38] which is associated with poorer prognosis. Therefore, the higher incidence of MSI-H in proximal colon cancer is likely to be one of the factors that makes it difficult to determine the value of MSI as a prognostic factor.
A positive correlation between poor differentiation and MSI-H was confirmed again in the present study. Several investigators have reported that MSI-H CRC is correlated with poor differentiation.[4,39,40] However, it is unclear how this affects the survival rate. Xiao et al[17] showed a better prognosis for poorly differentiated MSI than MSS CRC, but it was not statistically significant. In contrast to LNs, there is much less information about poor differentiation in MSI CRC. However, it is believed that this factor could confuse the true direction of MSI in evaluating the prognosis of CRC patients, and thus, an evaluation of it without the confounding factors is necessary. In addition, the advanced T stage in MSI-H patients is another confounding factor for MSI-H to be a good prognostic factor. In this study, advanced T stage was observed in MSI-H patients although the difference of frequency was not large. The advanced T stage in MSI-H has not been reported in the other study groups. However, considering that advanced T stage is associated with proximal colon cancer,[41] this may be related to cancer location rather than MSI status. To determine the value of MSI as a prognostic factor, these confounding factors should be reduced.
After propensity score matching was used, the stage of the MSS/MSI-L group shifted in the lower direction and showed exactly the same distribution with the MSI-H group. Poor differentiation and proximal colon cancer were still more common in the MSI-H group, because the incidence of those cases in the MSS/MSI-L groups was very low. Nonetheless, contrary to expectations, the survival rate differed more than before matching. The MSS/MSI-L group showed a significantly lower survival rate than MSI-H. This means that MSI-H is an independent prognostic factor after correcting for clinicopathological factors, and it suggests that there might be a molecular biologic mechanism associated with the good prognosis of MSI-H CRC regardless of clinical features.
There are several limitations of this study. First, it is a single-center retrospective study and thus carries the possibility of selection bias. To decrease this bias, we enrolled all consecutive patients for the study population just after routine MSI testing was begun for patients with CRCs who underwent surgery. Second, this study did not consider the exact effect of chemotherapy. CRC with MSI-H has been reported to be less responsive to 5-FU chemotherapy than MSS.[42,43] However, most of the patients in all groups received 5-FU-based chemotherapy, and thus, the effect of chemotherapy on the outcomes would be expected to be minimal. However, further studies should be carried out to investigate this point. Third, 1: 1 propensity score matching would lead selection bias by exclusion of a large number of MSS/MSI-L groups. This is due to the significantly lower incidence of MSI-H than MSS / MSS-L in colorectal cancer. So, we tried to minimize the selection bias by balancing the difference between the 2 groups in Table 4. However, ideally further studies with higher number of MSI-H patients are needed to overcome this limitation.
In conclusion, CRC with MSI-H has a positive relationship with a high LN harvest, low LNR, and poor differentiation. MSI-H showed a trend toward a better prognosis. Moreover, after correcting for the same clinicopathological factors, the survival difference between the 2 groups reached a statistically significant level. MSI-H is a definitive prognostic factor associated with good outcomes, and it is necessary to elucidate the undiscovered molecular biologic mechanism of this.
Footnotes
Abbreviations: AIC = Akaike information criterion, BIC = Bayesian information criterion, CRC = colorectal cancer, DFS = disease-free survival, LNR = lymph node ratio, LNs = lymph nodes, MSI = microsatellite instability, MSI-H = microsatellite instability high, MSI-L = microsatellite instability low, MSS = microsatellite stable, NCCN = National Comprehensive Cancer Network, OS = overall survival, THN = total harvest of lymph nodes.
The authors have no conflicts of interest to disclose.
References
- [1].Poynter JN, Siegmund KD, Weisenberger DJ, et al. Molecular characterization of MSI-H colorectal cancer by MLHI promoter methylation, immunohistochemistry, and mismatch repair germline mutation screening. Cancer Epidemiol Biomarkers Prev 2008;17:3208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Halvorsen TB, Seim E. Association between invasiveness, inflammatory reaction, desmoplasia and survival in colorectal cancer. J Clin Pathol 1989;42:162–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Koopman M, Kortman GA, Mekenkamp L, et al. Deficient mismatch repair system in patients with sporadic advanced colorectal cancer. Br J Cancer 2009;100:266–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gryfe R, Kim H, Hsieh ET, et al. Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. N Engl J Med 2000;342:69–77. [DOI] [PubMed] [Google Scholar]
- [5].Popat S, Hubner R, Houlston R. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol 2005;23:609–18. [DOI] [PubMed] [Google Scholar]
- [6].Azzoni C, Bottarelli L, Cecchini S, et al. Sporadic colorectal carcinomas with low-level microsatellite instability: a distinct subgroup with specific clinicopathological and molecular features. Int J Colorectal Dis 2011;26:445–53. [DOI] [PubMed] [Google Scholar]
- [7].Kim HR, Kim HC, Yun H-R, et al. An alternative pathway in colorectal carcinogenesis based on the mismatch repair system and p53 expression in Korean patients with sporadic colorectal cancer. Ann Surg Oncol 2013;20:4031–40. [DOI] [PubMed] [Google Scholar]
- [8].Kim H, Jen J, Vogelstein B, et al. Clinical and pathological characteristics of sporadic colorectal carcinomas with DNA replication errors in microsatellite sequences. Am J Pathol 1994;145:148–56. [PMC free article] [PubMed] [Google Scholar]
- [9].Søreide K, Nedrebø BS, Søreide JA, et al. Lymph node harvest in colon cancer: influence of microsatellite instability and proximal tumor location. World J Surg 2009;33:2695–703. [DOI] [PubMed] [Google Scholar]
- [10].Eveno C, Nemeth J, Soliman H, et al. Association between a high number of isolated lymph nodes in T1 to T4 N0M0 colorectal cancer and the microsatellite instability phenotype. Arch Surg 2010;145:12–7. [DOI] [PubMed] [Google Scholar]
- [11].Belt EJT, Velde EAT, Krijgsman O, et al. High lymph node yield is related to microsatellite instability in colon cancer. Ann Surg Oncol 2012;19:1222–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Chang GJ, Rodriguez-Bigas MA, Skibber JM, et al. Lymph node evaluation and survival after curative resection of colon cancer: systematic review. J Natl Cancer Inst 2007;99:433–41. [DOI] [PubMed] [Google Scholar]
- [13].Merok MA, Ahlquist T, Royrvik EC, et al. Microsatellite instability has a positive prognostic impact on stage II colorectal cancer after complete resection: results from a large, consecutive Norwegian series. Ann Oncol 2013;24:1274–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Berg M, Guriby M, Nordgård O, et al. Influence of microsatellite instability and KRAS and BRAF mutations on lymph node harvest in stage I–III colon cancers. Mol Med 2013;19:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Benson AB, 3rd, Venook AP, Bekaii-Saab T, et al. Colon cancer, version 3.2014. J Natl Compr Canc Netw 2014;12:1028–59. [DOI] [PubMed] [Google Scholar]
- [16].Kazama Y, Watanabe T, Kanazawa T, et al. Microsatellite instability in poorly differentiated adenocarcinomas of the colon and rectum: relationship to clinicopathological features. J Clin Pathol 2007;60:701–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Xiao H, Yoon YS, Hong SM, et al. Poorly differentiated colorectal cancers: correlation of microsatellite instability with clinicopathologic features and survival. Am J Clin Pathol 2013;140:341–7. [DOI] [PubMed] [Google Scholar]
- [18].Xiao Y, Freeman GJ. The microsatellite instable subset of colorectal cancer is a particularly good candidate for checkpoint blockade immunotherapy. Cancer Discov 2015;5:16–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Sugai T, Yoshida M, Eizuka M, et al. Analysis of the DNA methylation level of cancer-related genes in colorectal cancer and the surrounding normal mucosa. Clin Epigenetics 2017;9:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ahn YJ, Kwon HY, Park YA, et al. Contributing factors on lymph node yield after surgery for mid-low rectal cancer. Yonsei Med J 2013;54:389–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Pawlik TM, Raut CP, Rodriguez-Bigas MA. Colorectal carcinogenesis: MSI-H versus MSI-L. Dis Markers 2004;20:199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011;46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ho DE, Imai K, King G, et al. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Software 2006;42: [Google Scholar]
- [24].Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology 2010;138:2073–87. e2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kim CG, Ahn JB, Jung M, et al. Effects of microsatellite instability on recurrence patterns and outcomes in colorectal cancers. Br J Cancer 2016;115:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Schwitalle Y, Kloor M, Eiermann S, et al. Immune response against frameshift-induced neopeptides in HNPCC patients and healthy HNPCC mutation carriers. Gastroenterology 2008;134:988–97. [DOI] [PubMed] [Google Scholar]
- [27].Buckowitz A, Knaebel HP, Benner A, et al. Microsatellite instability in colorectal cancer is associated with local lymphocyte infiltration and low frequency of distant metastases. Br J Cancer 2005;92:1746–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kim WK, Park M, Park M, et al. Identification and selective degradation of neopeptide-containing truncated mutant proteins in the tumors with high microsatellite instability. Clin Cancer Res 2013;19:3369–82. [DOI] [PubMed] [Google Scholar]
- [29].Nathan H, Shore AD, Anders RA, et al. Variation in lymph node assessment after colon cancer resection: patient, surgeon, pathologist, or hospital? J Gastrointest Surg 2011;15:471–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wong SL, Ji H, Hollenbeck BK, et al. Hospital lymph node examination rates and survival after resection for colon cancer. JAMA 2007;298:2149–54. [DOI] [PubMed] [Google Scholar]
- [31].Tepper JE, O’Connell MJ, Niedzwiecki D, et al. Impact of number of nodes retrieved on outcome in patients with rectal cancer. J Clin Oncol 2001;19:157–63. [DOI] [PubMed] [Google Scholar]
- [32].Ceelen W, Van Nieuwenhove Y, Pattyn P. Prognostic value of the lymph node ratio in stage III colorectal cancer: a systematic review. Ann Surg Oncol 2010;17:2847–55. [DOI] [PubMed] [Google Scholar]
- [33].Ferri M, Lorenzon L, Onelli MR, et al. Lymph node ratio is a stronger prognotic factor than microsatellite instability in colorectal cancer patients: results from a 7 years follow-up study. Int J Surg 2013;11:1016–21. [DOI] [PubMed] [Google Scholar]
- [34].Sinicrope FA, Rego RL, Halling KC, et al. Prognostic impact of microsatellite instability and DNA ploidy in human colon carcinoma patients. Gastroenterology 2006;131:729–37. [DOI] [PubMed] [Google Scholar]
- [35].Minoo P, Zlobec I, Peterson M, et al. Characterization of rectal, proximal and distal colon cancers based on clinicopathological, molecular and protein profiles. Int J Oncol 2010;37:707–18. [DOI] [PubMed] [Google Scholar]
- [36].Yamauchi M, Morikawa T, Kuchiba A, et al. Assessment of colorectal cancer molecular features along bowel subsites challenges the conception of distinct dichotomy of proximal versus distal colorectum. Gut 2012;61:847–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hemminki K, Santi I, Weires M, et al. Tumor location and patient characteristics of colon and rectal adenocarcinomas in relation to survival and TNM classes. BMC Cancer 2010;10:688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Missiaglia E, Jacobs B, D’Ario G, et al. Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. Ann Oncol 2014;25:1995–2001. [DOI] [PubMed] [Google Scholar]
- [39].Emterling A, Wallin A, Arbman G, et al. Clinicopathological significance of microsatellite instability and mutated RIZ in colorectal cancer. Ann Oncol 2004;15:242–6. [DOI] [PubMed] [Google Scholar]
- [40].Ward R, Meagher A, Tomlinson I, et al. Microsatellite instability and the clinicopathological features of sporadic colorectal cancer. Gut 2001;48:821–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Warschkow R, Sulz MC, Marti L, et al. Better survival in right-sided versus left-sided stage I–III colon cancer patients. BMC Cancer 2016;16:554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med 2003;349:247–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Sargent DJ, Marsoni S, Monges G, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol 2010;28:3219–26. [DOI] [PMC free article] [PubMed] [Google Scholar]


