Abstract
Background/Objective
Insulin-like growth factor binding protein 2 (IGFBP-2) regulates blood glucose levels, facilitates hippocampal synaptic plasticity and may have a predictive value for Alzheimer’s disease (AD) diagnosis.
Methods
IGFBP-2 levels were studied in plasma in 566 subjects and in cerebrospinal fluid (CSF) in 245 subjects across the AD spectrum from the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Variants in the IGFBP-2 gene were examined. Linear mixed modeling in SPSS tested main effects of IGFBP-2 and interactions with APOE4 on neurocognitive indices and biomarkers. Voxel-wise regression was used to gauge IGFBP-2 and regional gray matter and glucose metabolism associations.
Results
Each point increase in IGFBP-2 corresponded to a 3 times greater likelihood of having mild cognitive impairment (MCI) or AD. IGFBP-2 showed beneficial associations with respect to cognitive scores in individuals with two APOE4 alleles. Higher IGFBP-2 predicted higher insulin resistance, but not CSF amyloid or tau. Voxel-wise analyses showed that plasma IGFBP-2 predicted lower grey matter volume and FDG metabolism in a large area spanning the frontal, temporal, and occipital lobes. CSF IGFBP-2 levels showed similar voxel-wise analysis results, but were uniquely associated with CSF amyloid and tau. Analysis of single nucleotide polymorphisms (SNPs) in IGFBP-2 showed that subjects carrying risk alleles versus common alleles had increased risk of AD and lower memory scores. Voxel-wise analyses of these SNPs also implicated the hippocampus and prefrontal cortex.
Conclusions
IGFBP-2 is associated with AD risk and outcomes; plasma IGFBP-2 provides stronger predictive power for brain outcomes, while CSF IGFBP-2 provides improved predictive accuracy for AD CSF biomarkers.
Keywords: Insulin Resistance, Diabetes Mellitus, Fluorodeoxyglucose F18, MRI, Biomarkers, Neuroimaging, Memory, Polymorphism, Single Nucleotide
Introduction
Insulin-like growth factor binding proteins (IGFBP) serve to bind insulin-like growth factors (IGF) to regulate their concentrations and consequently metabolic activity [1]. IGFBPs are central for the transport of IGF to its receptor site, and have the ability to enhance binding to the receptor site or inhibit the access of IGF to its receptors by creating strong bonds, lowering binding potential [2]. IGFBP is centrally important in regulating blood glucose levels, although in excess can be a marker of disease. IGFBP-2, in particular, binds to both IGF-1 and IGF-2, and can inhibit IGF functions such as DNA synthesis, cell proliferation, and cell death, as well as glucose and amino acid uptake in cells [3, 4]. For example, transgenic mice that overexpressed IGFBP-2 showed significantly higher blood glucose levels than controls at 30, 60 and 90 minutes during an oral glucose tolerance test, as well as lower levels of GLUT4–the insulin-dependent glucose transporter–at cell surfaces compared to controls [5]. Abnormally high IGFBP-2 levels are often an indicator of severe catabolic events, such as gastric cancer, anorexia nervosa and renal failure [6-8]. In a study of 625 men and women aged 70 and older, plasma IGFBP-2 significantly predicted mortality from all causes after adjusting for markers of body composition, as well as fasting glucose and insulin [9].
Alzheimer’s disease (AD) has been associated with defects in the insulin signaling pathway. Higher levels of insulin resistance (IR) were correlated with increased regional amyloid deposition and atrophy in frontal and temporal areas of the brain in late middle-aged adults [10, 11]. In addition, higher IR was associated with decreased cerebral glucose metabolic rate and worse memory scores in cognitively normal adults with pre-diabetes or type 2 diabetes [12]. Animal models provide evidence that IGFBP-2 may play a role in AD. Over-expression of IGFBP-2 in mice led to decreased weights of the hippocampus, cerebellum, olfactory bulb and prefrontal cortex at 12 weeks, compared to controls [13]. An important study in humans utilized ex vivo tissue from individuals across the AD spectrum, and showed that IGF resistance was related to beta amyloid (Aβ) plaques, other markers of AD, and worse ante-mortem cognition [14]. Some associations may be compartment-specific, as CSF but not plasma IGFBP-2 levels were higher in 92 AD patients versus 72 healthy controls [15], where CSF IGFBP-2 levels were positively correlated with total tau and phosphorylated-181-tau (p-tau). Also, APOE4 alleles have been shown to play a role in insulin metabolism and cognitive function [16]. Cognitively normal (CN) and AD participants who were APOE4 homozygous showed improvements in memory with lower doses of intranasal insulin as compared to individuals who were not APOE4 homozygous [16]. Additionally, a study using euglycemic hyperinsulinemic clamps in individuals with impaired glucose tolerance and healthy controls showed that IGFBP-2 was the only insulin signaling molecule assayed to independently predict bioactive levels of IGF-1 in both groups; this study showed a negative correlation between IGFBP-2 and IGF-1 [17].
As biomarkers become increasingly more important in tracking AD diagnosis and progression, and metabolic markers have gained more interest, additional research is needed to explore the role of IGFBP-2 in AD. We hypothesize that IGFBP-2 levels would be positively associated with more brain atrophy, less neuronal glucose uptake, and impaired cognitive function. In this study, we utilized data from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) to examine plasma levels of IGFBP-2 among 566 individuals that were cognitively normal (CN), or had mild cognitive impairment (MCI) or AD. This is the first study, to our knowledge, to systematically study the relationship between peripheral IGFBP-2 levels and related single nucleotide polymorphisms (SNPs) with neural, cognitive, and biomarker outcomes relevant to AD, including peripheral inflammatory markers like Interleukin-6 (IL-6) receptor and C-peptide. On an exploratory basis in a subset of 245 subjects, we also examined how CSF mass spectrometry IGFBP-2 peptide was related to these outcomes as compared to plasma IGFBP-2.
Materials and Methods
Participants
Data from middle-aged to aged adults were obtained from the ADNI database (http://adni.loni.usc.edu). The ADNI was launched in 2003 as a public-private partnership, led by Principal Investigator Michael W. Weiner, MD. The primary goal of ADNI has been to test whether serial magnetic resonance imaging (MRI), positron emission tomography (PET), other biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of MCI and early AD. For up-to-date information, see http://www.adni-info.org. Written informed consent was obtained from all ADNI participants at their respective ADNI sites. The ADNI protocol was approved by site-specific institutional review boards. All analyses used in this report only included baseline data. Baseline plasma data for IGFBP-2 was available for 566 participants: 58 CN, 396 MCI, and 112 AD. Baseline CSF data for IGFBP-2 was available for 245 subjects: 45 CN, 134 MCI, and 66 AD. Baseline genomic data was available for 756 participants: 229 CN, 354 MCI, and 173 AD.
Participants with MCI had the following diagnostic criteria: 1) memory complaint identified by the participant or their study partner; 2) abnormal memory as assessed by the Logical Memory II subscale from the Wechsler Memory Scale- Revised, with varying criteria based on years of education; 3) Mini-Mental State Exam (MMSE) score between 24 and 30; 4) Clinical dementia rating of 0.5; 5) Deficits not severe enough for the participant to be diagnosed with Alzheimer’s disease by the site physician at screening. Participants with AD met similar criteria, however, were required to have an MMSE score between 20 and 26, a clinical dementia rating of 0.5 or 1.0, and NINCDS/ADRDA criteria for probable AD.
Mass Spectrometry and Fasting Glucose
Data were downloaded from the Biomarkers Consortium CSF Proteomics MRM dataset and the Biomarkers Consortium Plasma Proteomics Project RBM multiplex. As described previously [18], the ADNI Biomarkers Consortium Project investigated the extent to which selected peptides, measured with mass spectrometry, could discriminate among disease states. Briefly, Multiple Reaction Monitoring-MS (MRMMS) was used for targeted quantitation of 567 peptides representing 221 proteins in a single run (Caprion Proteome Inc., Montreal, QC, Canada). Fasting insulin was assayed using a plasma multiplex immunoassay panel (http://adni.loni.usc.edu/), which we note produce consistently lower insulin values than a standard ELISA kit. Fasting glucose was derived from a standard lab test. Insulin and glucose were used to calculate the homeostatic model assessment, HOMA-IR. Analyses for this report focused on IGFBP-2 levels, which were assayed in the plasma multiplex panel and CSF proteomics panel, for which the peptide LIQGAPTIR was chosen, which performed better in most analyses (data not shown). For proinflammatory markers, C-peptide and IL-6 receptor levels were derived from the plasma multiplex.
APOE Genotype
The ADNI Biomarker Core at the University of Pennsylvania conducted APOE ε4 genotyping. We characterized participants as having zero APOE4 alleles, one APOE4 allele, or two APOE ε4 alleles.
Amyloid and Tau CSF Biomarkers
CSF sample collection, processing, and quality control of p-tau, total tau, and Aβ1-42 are described in the ADNI1 protocol manual (http://adni.loni.usc.edu/) and Shaw et al [19].
Neuropsychological Assessment
ADNI utilizes an extensive battery of assessments to examine cognitive functioning with particular emphasis on domains relevant to AD. A full description is available at http://www.adni-info.org/Scientists/CognitiveTesting.aspx. All subjects underwent clinical and neuropsychological assessment at the time of scan acquisition. Neuropsychological assessments included: The Clinical Dementia Rating sum of boxes (CDR-sob), Mini-Mental Status Exam (MMSE), Auditory Verbal Learning Test (RAVLT), and AD Assessment Schedule - Cognition (ADAS-Cog). A composite memory score encompassing the RAVLT, ADAS-COG, MMSE, and Logical Memory assessments was also utilized [20]. Additionally, a composite executive function score comprising Category Fluency—animals, Category Fluency—vegetables, Trails A and B, Digit span backwards, WAIS-R Digit Symbol Substitution, Number Cancellation and 5 Clock Drawing items was used[21]. These composite scores were used in formal analyses to represent global memory and executive function among subjects.
Magnetic Resonance Imaging (MRI) Acquisition and Pre-Processing
T1-weighted MRI scans were acquired within 10-14 days of the screening visit following a back-to-back 3D magnetization prepared rapid gradient echo (MP-RAGE) scanning protocol described elsewhere [22]. Images were pre-processed using techniques previously described [11]. Briefly, the SPM12 “New Segmentation” tool was used to extract modulated gray matter (GM) volume maps. Maps were smoothed with an 8mm Gaussian kernel and then used for voxel-wise analyses.
FDG-PET
FDG-PET acquisition and pre-processing details have been described previously [22]. Briefly, 185 MBq of [18-153-F]-FDG was injected intravenously. After 30 minutes, six 5-minute frames were acquired. Frames of each baseline image series were co-registered to the first frame and combined into dynamic image sets. Each set was averaged, reoriented to a standard 160 × 160 × 96 voxel spatial matrix of resliced 1.5 mm3 voxels, normalized for intensity, and smoothed with an 8 mm FWHM kernel. In order to derive the standardized uptake value ratio (SUVR), pixel intensity was normalized according to the pons since it demonstrates preserved glucose metabolism in AD [23]. Normalization to the pons removed inter-individual tracer metabolism variability. The Montreal Neurological Institute (MNI) template space was used to spatially normalize images using SPM12 (http://www.fil.ion.ucl.ac.uk/spm/software/spm12/). A subset of subjects underwent FDG-PET scans and analyses included in this report.
Statistical Analysis
All analyses were conducted using SPSS 24 (IBM Corp., Armonk, NY) or SPM12 (http://www.fil.ion.ucl.ac.uk/spm/software/spm12/). Linear mixed effects models tested the main effects of plasma or CSF IGFBP-2 on cognitive scores, biomarkers, Baseline Diagnosis, and their outcomes of interest in SPSS 24. Main effects and interactions with APOE or Baseline Diagnosis were tested in a single model. Covariates included age at baseline, sex, and baseline Diagnosis in all models. An additional covariate, years of education, was included when analyzing memory and cognitive performance. Outcomes included neuropsychological performance, modulated GM maps and FDG maps, and CSF biomarkers including Aβ1-42, t-tau and p-tau. Binomial logistic regression was also used to assess the odds ratio of a given participant being diagnosed as MCI or AD versus the CN reference group. Linear regression was used in PLINK to assess genetic associations.
To correct for type 1 error in non voxel-wise analyses, as described previously [24, 25], Holm-Bonferroni correction was used for each set of analyses. This closed test procedure maintains a family-wise P value = 0.05 by requiring unadjusted P values of 0.05 divided by x, x being the number of null hypotheses tested. For 4 cognitive tests, for example, P values of .0125, .025, .0375, and .050 are successively needed among any test to proceed with testing in the closed set. For sets that were not robust under Holm-Bonferroni correction, a less strict form of correction was used. Specifically, omnibus testing using MANCOVA incorporating all dependent variables of the set was conducted, where a significant main effect or Baseline Diagnosis interaction allowed further testing of all outcomes as follow-up tests. A family-wise error rate of .05 is maintained using this approach [26].
For voxel-wise analysis, 2nd-level mixed models tested main effects of IGFBP-2 on regional GM volume and FDG, controlling for age, sex, education, and Baseline Diagnosis. The thresholds were set at P < .005 (uncorrected) and P < .05 (corrected) for voxels and clusters respectively. Results were considered significant at the cluster level. As described previously [24], in order to reduce type 1 error, we utilized a GM threshold of 0.2 to ensure that voxels with <20% likelihood of being GM were not analyzed. For GM, Monte Carlo simulations in ClusterSim (http://afni.nimh.nih.gov/afni/doc/manual/3dClustSim) were used to estimate that 462 contiguous voxels were needed for such a cluster to occur at P < 0.05. For FDG voxel-wise analyses, Monte Carlo simulations in ClusterSim were used to estimate that 224 contiguous voxels were needed for such a cluster to occur at P < 0.05.
Genomic Data Processing and Quality Control
Genomic data underwent stringent quality control (QC) by assessing concordance with Hardy-Weinberg equilibrium (HWE) scrutinized data for Mendelian inheritance errors. Single Nucleotide Polymorphisms (SNPs) were filtered based on HWE P-value > 0.00001 and MAF >0.05% and a call rate of 95%. Samples with greater than 5% missingness were removed. Sample genotypes were imputed using 1000Genomes data with Shapeit/Impute2 software following the protocol outlined here [27]. SNPS with call rates <95 % or R2 ≤ 0.3 were removed leaving 2,976,223 imputed and genotyped SNPs after quality control. All analyses were conducted using PLINK v1.9 (http:// www.cog-genomics.org/plink2).
Results
Data Summary
Clinical, demographic, and CSF data for subjects with plasma IGFBP-2 are presented in Table 1. There were no differences based on years of education or age at baseline between CN, MCI or AD subjects. As expected for this ADNI sub-population, there was a significant difference in the percentage of APOE4 carriers and in cognitive function. Subsequently, analyses were conducted with plasma IGFBP-2. Demographic data for subjects with CSF IGFBP-2 are presented in Supplemental Table 1. See Supplemental Text 1 for analyses that used CSF IGFBP-2 in a subset of ADNI subjects.
Table 1.
Demographic Data for Subjects with Plasma IGFBP-2
| CN (N=58) | MCI (N=396) | AD (N=112) | |
|---|---|---|---|
| Age | 76.27 ± 5.73 | 74.91 ± 7.49 | 75.23 ± 8.39 |
| Education (years) | 15.67 ± 2.78 | 15.64 ± 3.0 | 15.1 ± 3.2 |
| Sex % Female | 48 | 35 | 42 |
| % APOE 4 carriers*** | 9% | 49% | 64% |
| Plasma IGFBP2 (ng/mL)*** | 1.88 ± 0.20 | 1.99 ± 0.23 | 1.91 ± 0.12 |
| C Peptide (ng/mL) | 0.37 ± 0.21 | 0.39±.21 | 0.36±.18 |
| IL-6 Receptor (ng/mL)* | 1.51 ± 0.12 | 1.46 ± 0.14 | 1.47 ± 0.11 |
| Glucose (mg/dL) | 102.33 ± 23.47 | 101.42 ± 27.25 | 99.06 ± 22.48 |
| Insulin (uIU/mL) | 3.00 ± 2.34 | 2.64 ± 2.73 | 2.38 ± 1.43 |
| HOMA-IR | 0.82 ± 0.80 | 0.68 ± 0.75 | 0.59 ± 0.39 |
| CSF Total Tau (pg/mL)*** | 63.62 ± 21.76 | 102.66 ± 59.81 | 120.30 ± 56.33 |
| Ptau (pg/mL)*** | 21.07 ± 8.43 | 36.19 ± 19.28 | 41.92 ± 19.90 |
| Abeta 42 (pg/mL)*** | 250.85 ± 21.08 | 163.97 ± 53.15 | 142.69 ± 39.15 |
| CDR-SOB*** | 0.03 ± 0.11 | 1.61 ± 0.88 | 4.31 ± 1.61 |
| MMSE*** | 28.93 ± 1.16 | 27.02 ± 1.78 | 23.59 ± 1.96 |
| Immediate RAVLT*** | 40.98 ± 7.19 | 30.73 ± 9.05 | 23.54 ± 7.42 |
| Delayed RAVLT* | 3.73 ± 3.13 | 4.72 ± 2.25 | 4.47 ± 1.94 |
| ADAS-COG*** | 6.37 ± 2.76 | 11.52 ± 4.38 | 18.27 ± 6.37 |
| ADNI_MEM Score*** | 0.46 ± 0.72 | 0.03 ± 1.04 | −0.35 ± 0.85 |
| Executive Function*** | 0.71 ± 0.58 | −0.03 ± 0.78 | −0.93 ± 0.83 |
Values are mean ± SD,
indicates ANOVA p<0.05;
indicates p<0.01;
indicates ANOVA p<0.001.
The ADNI memory factor values are Z-scored with mean 0 and a standard deviation of 1, based on the 810 subjects with baseline memory data [20].
Clinical Characteristics and Alzheimer’s Disease Risk
Logistic regression was used to examine if plasma IGFBP-2 expression predicted an increased likelihood of being MCI or AD. The reference group was CN. The likelihood ratio statistic [X2=74.450, p<.001] indicated that higher IGFBP-2 levels predicted a higher Odds Ratio for being MCI or AD [Wald=9.938, β=3.003, p=0.002]. These results suggest that a per point increase in IGFBP-2 corresponded to a roughly 3 times likelihood of having some degree of clinically relevant memory impairment. No significant associations were shown with IGFBP-2 and MCI conversion. No significant interaction was shown between IGFBP-2 and APOE with regards to predicting an odds ratio for being MCI or AD.
Global Cognition, Memory, Visual Spatial, and Executive Function
Linear mixed models showed a non-significant main effect for IGFBP-2 on global indices including CDR-sob, ADAS-cog and MMSE. Among neuropsychological indices depicted in Figure 1, the number of APOE4 alleles modified how plasma IGFBP2 was related to global cognition. Specifically, for CDR-sob, a IGFBP-2* APOE interaction was significant (β±SE= −2.825±0.983, p=.034). Similarly for ADAS-cog, interaction analyses between IGFBP-2* APOE were significant (β±SE= −6.607±3.469, p=.020), as well as for MMSE (β±SE= 1.407±0.699, p=.045). These results indicate that among subjects with 1 or 2 APOE4 alleles, higher IGFBP-2 predicted better function on global assessments.
Figure 1.
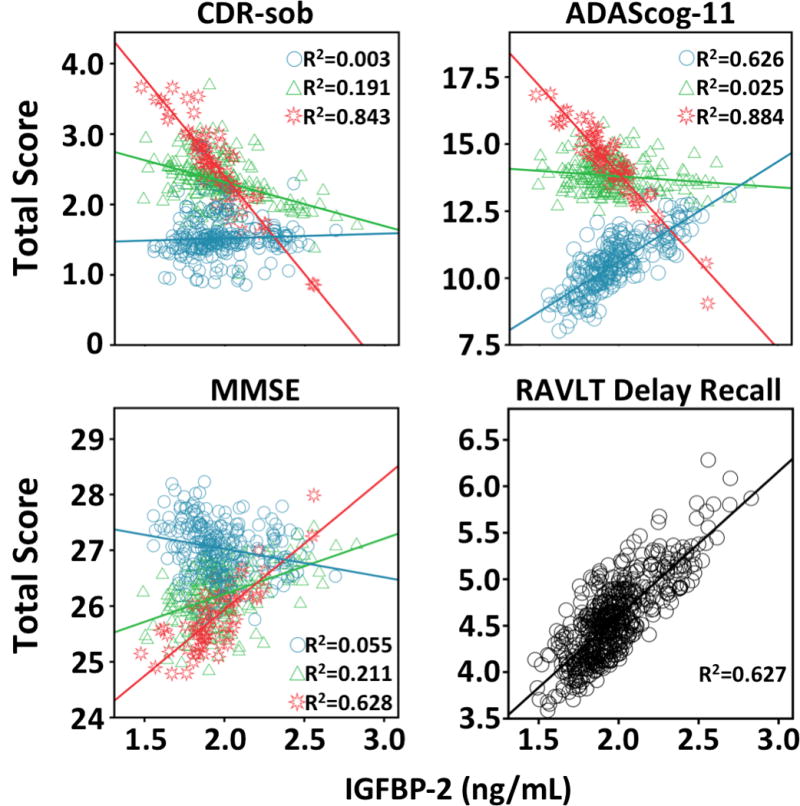
Cognitive scores and IGFBP-2 analyses. The association between plasma IGFBP-2 and the Clinical Dementia Rating sum of boxes (CDR-sob), Mini-Mental Status Exam (MMSE), Rey Auditory Verbal Learning Test (RAVLT), AD Assessment Schedule - Cognition (ADAS-Cog). “Blue circle” indicates 0 APOE4 alleles, “green triangle” indicate 1 APOE4 allele, and “red star” indicates 2 APOE4 alleles.
For memory, higher IGFBP2 was also related to higher scores on the RAVLT Delay (β±SE= 1.652±0.482, p=<.001). No main effects or interactions with APOE4 were shown with respect to a derived memory factor.
There were no IGFBP-2 by Baseline Diagnosis interactions. However, on an exploratory basis, when split by diagnosis, AD patients showed that IGFBP-2 was a significant predictor of worse scores on the constructional praxis portion of ADAS-cog, representing visual spatial abilities (β±SE= 1.189±0.580, p=.043). There were no significant associations between plasma IGFBP-2 and executive function.
AD CSF Biomarkers and Markers of Inflammation
Plasma IGFBP-2 was not associated with total tau, p-tau-181 or Aβ1-42. However, higher levels of IGFBP-2 were related to higher expression of IL-6 receptor (β±SE = 0.071±0.029, F=5.940, p=0.15) and lower C-peptide (β±SE=−0.156±0.043, F=13.289, p<.001). No significant differences were seen with respect to IGFBP-2 and APOE4 or Baseline Diagnosis interactions. Importantly, as noted in Supplementary Text 1, CSF IGFBP-2 was conversely related to all AD biomarkers but not peripheral inflammation.
Glucose, Insulin and HOMA-IR
Higher plasma IGFBP-2 was significantly associated with lower HOMA-IR (β=-.441, F=6.810, p=0.009) and insulin (β=−1.593, F=6.986, p=0.009), but not glucose (F=0.564, p=0.453). Subsequent IGFBP-2* APOE interactions were non significant. 150 of the participants met fasting blood glucose requirements for pre-diabetes diagnosis (between 100 and 125 mg/dL), and 57 participants met fasting blood glucose requirements for diabetes diagnosis (>125 mg/dL). Both plasma and CSF IGFBP-2 were not predictive of whether or not a person met pre-diabetes or type 2 diabetes fasting blood glucose criteria. As noted in Supplementary Text 1, CSF IGFBP-2 was only related to higher glucose.
Regional Grey Matter Volume
Next, voxel-wise analysis was used to regress plasma IGFBP-2 concentrations against regional GM at baseline for 325 participants who had structural MRI data, demographic, and biological data. Higher plasma IGFBP-2 was related to less GM in a large cluster of voxels (k=128,608) across the right and left inferior parietal gyri, right frontal superior lobe, left postcentral gyrus, right cerebellum and right fusiform gyrus, with the maximum voxel located in the right frontal superior lobe (Figure 2; Supplementary Table 2). Smaller clusters included the cuneus, hippocampus, precuneus, and left and right superior prefrontal cortices. A similar pattern of results was found when regressing CSF IGFBP-2 against regional GM. Higher CSF IGFBP-2 was related to less GM primarily spanning the left and right amygdala, parahippocampus and hippocampus, among 186 participants who had structural MRI data, demographic, and biological data (Supplementary Text 1).
Figure 2.

Brain areas showing less grey matter corresponding to higher plasma IGFBP-2. The graph depicts the relationship at a sub-maximal voxel in dorsal prefrontal cortex.
Regional FDG Metabolism
Higher plasma IGFBP-2 was related to less FDG glucose uptake in one cluster of 5419 voxels primarily spanning the superior dorsolateral prefrontal cortex, among 266 participants who had FDG data, demographic, and biological data (Figure 3).
Figure 3.
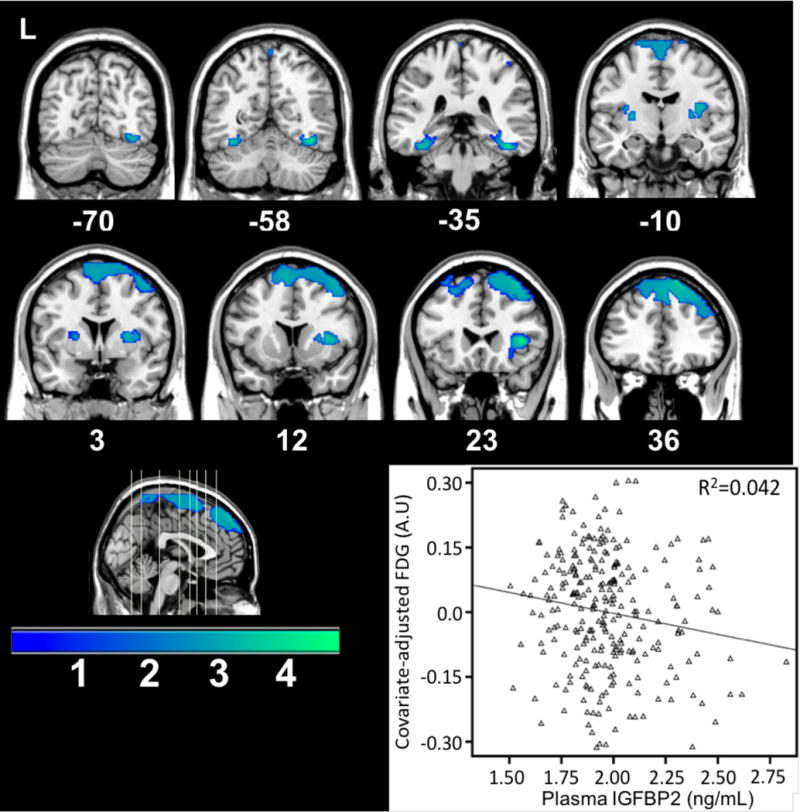
Brain areas showing less FDG metabolism corresponding to increased plasma IGFBP-2. The graph depicts the relationship at a sub-maximal voxel in the dorsal prefrontal cortex.
Genetic Analysis of IGFBP-2
Linear regression in PLINK tested the additive genetic model of each SNP for association with cognitive scores while controlling for age, gender, and Baseline Diagnosis as covariates. Four variants in the IGFBP-2 locus on Chromosome 7 were nominally associated with worse cognitive function on the MMSE. The most significant SNP was rs4619, an intron missense A to G mutation resulting in an isoleucine replaced with a methionine, also showed increased risk of developing AD (Odds Ratio=1.22, P=0.045). Results from association analyses are reported in Table 2, along with genotype distributions and odds ratios in Table 3. RS4619 was nominally associated with MMSE (F=5.645, P=0.015), and the ADNI latent memory factor (F=6.115, P=0.013). Additionally, the relationship between these SNPs and baseline regional FDG and grey matter were assessed using voxel-wise analysis. Result maps indicated carriers of minor alleles in these SNPs showed less grey matter and FDG metabolism in a small region spanning the right frontal superior lobe. There was strong overlap between this result and what was found for IGFBP-2 gray matter and FDG result maps (Figure 4); however, the voxel wise result maps for individual SNPs marginally surpassed the ClusterSim statistical significance threshold of 462 voxels.
Table 2.
IGFBP-2 SNP Genotype Distributions and Odds Ratios for Developing AD
| Variant ID | Genomic Position | Molecular Consequence | Genotype | Controls (n) | Cases (n) | OR 95% (CI) | P Value |
|---|---|---|---|---|---|---|---|
| rs4619 | Chr7:45893070 | 759A>G – Ile253Met | AA | 152 | 322 | 1.22 (0.96–1.67) | 0.045 |
| GA + GG | 77 | 205 | |||||
| rs138105891 | Chr7:45890641 | 443A>G – His148Arg | AA | 142 | 303 | 1.08 (0.75–1.33) | 0.031 |
| GA + GG | 87 | 224 | |||||
| rs41258845 | Chr7:45888992 | 340C>G – His114Asp | CC | 128 | 338 | 1.12 (0.82–1.53) | 0.026 |
| GC + GG | 101 | 189 | |||||
| rs1065782 | Chr7:45825939 | 840G>A – Val183Ile | GG | 203 | 350 | 1.04 (0.84–1.11) | 0.048 |
| GA + AA | 26 | 177 |
Table 3.
Association of IGFBP-2 SNPs with Mini-Mental Status Exam (MMSE) scores and the ADNI Memory composite score
| SNP | MMSE | ADNI Memory Factor | ||||||
|---|---|---|---|---|---|---|---|---|
| Common Allele | Effect Allele | Beta | Beta P value | Common Allele | Effect Allele | Beta | Beta P value | |
| rs4619 AA vs GA + GG |
29.1 ± 1.55 | 24.9 ± 2.02 | −0.32 | P = 0.089 | 1.01 ± 0.59 | −0.14 ± 0.64 | −0.12 | P = 0.047 |
|
| ||||||||
| rs138105891 AA vs GA + GG |
28.5 ± 1.31 | 25.4 ± 0.57 | −0.21 | P = 0.044 | 0.95 ± 0.44 | 0.33 ± 0.39 | −0.30 | P = 0.068 |
|
| ||||||||
| rs41258845 CC vs GC + GG |
29.2 ± 1.17 | 25.6 ± 1.02 | −0.34 | P = 0.199 | 1.03 ± 0.67 | −0.04 ± 0.77 | −0.11 | P = 0.041 |
|
| ||||||||
| rs1065782 GG vs GA + AA |
27.2 ± 1.75 | 24.5 ± 1.09 | −0.17 | P = 0.441 | 1.10 ± 0.48 | −0.02 ± 0.58 | −0.22 | P = 0.052 |
Beta values represent the difference in the predicted value of either MMSE or the ADNI memory factor based on an increase from no risk allele, one risk allele, or two risk alleles.
Figure 4.
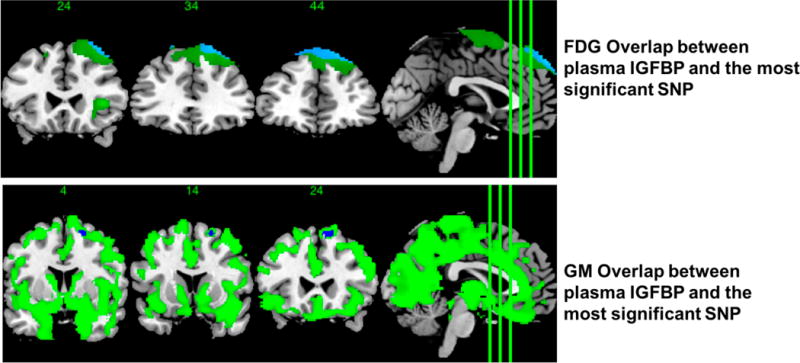
Brain areas showing overlap between either the GM or FDG and IGFBP2 result maps and the result map of the strongest SNP association for GM or FDG.
Discussion
In this study, we hypothesized that IGFBP-2 may serve as a useful biomarker for predicting AD outcomes. Importantly, higher IGFBP-2 corresponded to less grey matter in AD-sensitive brain regions such as superior frontal gyrus and angular gyrus, and significantly less glucose uptake in the superior medial prefrontal cortex. Strikingly, for every point increase in IGFBP-2 levels, there was a 3 times increased risk of being diagnosed as MCI or AD when compared to CN. Curiously, however, higher IGFBP-2 corresponded to better cognitive performance in the RAVLT Delay, as well as global indices for APOE4 carriers only. These conflicting results may be due to a progression of insulin resistance, where the body is at first able to compensate for insulin resistance by releasing additional IGF to keep up with glucose demands of neuronal cells. Although much of glucose transport in the brain is insulin-independent via GLUT-1 and GLUT-3, insulin is necessary for GLUT-4 actions, which in rats has been detected in the cerebellum, olfactory bulb, and dentate gyrus of the hippocampus [28]. Although further research is necessary to translate these findings to humans, Craft et al. showed that intranasal insulin reduced progression of neuronal hypometabolism in individuals with MCI and AD, suggesting that insulin does play an important role in glucose uptake in the brain [29]. We hypothesize that central insulin resistance progresses to a point where GLUT-4 receptors are unable to respond to insulin and cells are delivered a suboptimal level of glucose. We suggest that the cognitive score results in our study are representative of the early compensatory response, which may in part be related to APOE4 status, while the grey matter, FDG, and diagnosis results are indicative of a post-compensatory state. In general, having one or more E4 alleles corresponded to higher IGFBP2 predicting better cognitive performance, whereas for non-APOE4s either no association or a detrimental pattern was observed, such as for ADAS-cog. Additionally, previous research has shown that individuals who are APOE4 positive have lower expression of insulin degrading enzyme, thus potentially amplifying the early cognitive benefits of higher IGFBP-2 levels [30].
IGF-1 appears to be a key modulator of IGFBP-2 associations with AD outcomes. Because IGFBP is fundamental in regulating IGF bioavailability, the two proteins are intertwined in determining insulin expression and glucose levels in the periphery and brain, which this report illustrates at least in plasma. IGF-1 importantly determines glucose and lipid handling in the brain, myelin expression, and remodeling after neuronal injury [1]. Mice that under-expressed IGF-1 showed an accumulation of Aβ plaques, which were subsequently decreased after infusing the mice with IGF-1 [31].
To validate IGFBP-2 as a useful biomarker of AD and central IR, higher plasma IGFBP-2 corresponded with higher expression of plasma markers of inflammation including IL-6 receptor and C-peptide. Previous research has shown many correlations between inflammation in the brain and neuronal damage [32]. Obesity and insulin resistance are also associated with higher levels of inflammatory biomarkers [33].
We also compared and contrasted the utility of plasma IGFBP-2 to CSF IGFBP-2 for AD-related outcomes. CSF-based markers are often thought to provide better diagnostic accuracy in understanding the progression of diseases that cause cognitive impairment and dementia; however, collecting a CSF sample is more invasive. Our data indicate that when considering IGFBP-2, the plasma concentration was a better predictor of brain structure and cognition, while only CSF concentrations reflected CSF amyloid and tau. Future studies should take into account the differing clinical utility of blood-based and CSF biomarkers against the practicality of sample collection, in addition to their predictive utility of disease progression. Improving how biomarkers are used in clinical trials will likely provide more precise diagnoses to patients with AD.
Subsequently, we examined how IGFBP SNPs influenced AD-related outcomes. Since population stratification can result in erroneous genetic associations, we restricted our analyses to only subjects of Northern and Western European heritage. While the exact mechanism has yet to be revealed, our data show that genetic variation in IGFBP-related genes modified cognitive decline. This may be in part due to modifying IGF-1 regulation of glucose in the brain, ultimately increasing neuronal vulnerability to induce cognitive decline. Genetic variation negatively affecting IGFBP-2 signaling may decrease the protective effects that circulating IGFBP-2 exerts on cognition described above, whereby these SNPs may predispose individuals to develop AD. Previous large-scale genome-wide association studies (GWAS) examining genetic association with AD have not implicated SNPs at IGFBP loci because these variants commonly do not surpass genome wide significance of P < 1×10^-8. Performing targeted genomic association analysis in combination with neuroimaging analysis provides a unified approach to understand how genomic variation may contribute to AD symptoms.
The limitations of this study should be addressed. This study included a modest sample size, where the exploratory nature of this study design would benefit from a larger sample. It is worth noting that these analyses did not show any significant correlations between plasma or CSF IGFBP-2 and MCI conversion. However, a larger sample size of individuals with MCI may be needed to see if IGFBP-2 can predict if an individual with MCI converts to AD. Furthermore, since this is a cross-sectional study using baseline data from the ADNI cohort, it was beyond the scope of the project to determine the causal effects between IGFBP-2 on longitudinal CSF biomarkers and disease progression. Additionally, we could not correlate IGFBP-2 with all of the disease states throughout the literature such as gastric cancer or eating disorders because this was beyond the scope of ADNI’s mission. Like all genetic studies, these results should be validated using additional independent and larger cohorts. Finally, other unrecognized cellular pathways, not related to neurodegeneration, may be disrupted that influence IGF-1 signaling and AD progression.
Conclusion
This study provides evidence that plasma IGFBP-2 is associated with AD risk, brain atrophy and less glucose metabolism in regions sub-serving memory and global cognitive function. We also demonstrated that plasma IGFBP-2 served as a more comprehensive predictor of AD-related outcomes than CSF IGFBP-2. These results illustrate the potential that integration of genomic, biologic and neuroimaging data may lead to identification of novel targets for treatment of AD while improving the overall understanding of potential mechanisms underlying the pathophysiology of AD. In conclusion, insulin signaling mechanisms such as IGFBP-2 may be important for future therapeutics to target via genetic modification or regulating circulating IGFBP-2 to delay the onset of AD by improving neural metabolism and cognitive function.
Supplementary Material
Acknowledgments
The authors would like to thank the undergraduate students working in Dr. Willette’s lab for their hard work and dedication. Without their contributions, this work would not have been possible. This study was funded in part by the College of Human Sciences at Iowa State University and NIH grant AG047282. Neither funding source had any involvement in the report. Data collection and sharing for this project were funded by the ADNI (National Institutes of Health Grant U01-AG-024904) and Department of Defense ADNI (award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the Alzheimer’s Association and the Alzheimer’s Drug Discovery Foundation. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private-sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Disease Cooperative Study at the University of California, San Diego. ADNI data are disseminated by the Laboratory for Neuroimaging at the University of Southern California. The data used in the preparation of this article were obtained from the ADNI database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report.
Footnotes
Conflict of Interest/Disclosure Statement: The authors have no conflict of interest to report.
References
- 1.Fernandez AM, Torres-Aleman I. The many faces of insulin-like peptide signalling in the brain. Nat Rev Neurosci. 2012;13:225–239. doi: 10.1038/nrn3209. [DOI] [PubMed] [Google Scholar]
- 2.Hoeflich A, Russo VC. Physiology and pathophysiology of IGFBP-1 and IGFBP-2 - consensus and dissent on metabolic control and malignant potential. Best Pract Res Clin Endocrinol Metab. 2015;29:685–700. doi: 10.1016/j.beem.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Jones JI, Clemmons DR. Insulin-like growth-factors and their binding-proteins - biological actions. Endocrine Reviews. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- 4.Carrick FE, Forbes BE, Wallace JC. BIAcore analysis of bovine insulin-like growth factor (IGF)-binding protein-2 identifies major IGF binding site determinants in both the amino- and carboxyl-terminal domains. J Biol Chem. 2001;276:27120–27128. doi: 10.1074/jbc.M101317200. [DOI] [PubMed] [Google Scholar]
- 5.Reyer A, Schindler N, Ohde D, Walz C, Kunze M, Tuchscherer A, Wirthgen E, Brenmoehl J, Hoeflich A. The RGD sequence present in IGFBP-2 is required for reduced glucose clearance after oral glucose administration in female transgenic mice. Am J Physiol Endocrinol Metab. 2015;309:E409–417. doi: 10.1152/ajpendo.00168.2015. [DOI] [PubMed] [Google Scholar]
- 6.Subbannayya Y, Mir SA, Renuse S, Manda SS, Pinto SM, Puttamallesh VN, Solanki HS, Manju HC, Syed N, Sharma R, Christopher R, Vijayakumar M, Veerendra Kumar KV, Keshava Prasad TS, Ramaswamy G, Kumar RV, Chatterjee A, Pandey A, Gowda H. Identification of differentially expressed serum proteins in gastric adenocarcinoma. J Proteomics. 2015;127:80–88. doi: 10.1016/j.jprot.2015.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Counts DR, Gwirtsman H, Carlsson LM, Lesem M, Cutler GB., Jr The effect of anorexia nervosa and refeeding on growth hormone-binding protein, the insulin-like growth factors (IGFs), and the IGF-binding proteins. J Clin Endocrinol Metab. 1992;75:762–767. doi: 10.1210/jcem.75.3.1381372. [DOI] [PubMed] [Google Scholar]
- 8.Narayanan RP, Fu B, Heald AH, Siddals KW, Oliver RL, Hudson JE, Payton A, Anderson SG, White A, Ollier WE, Gibson JM. IGFBP2 is a biomarker for predicting longitudinal deterioration in renal function in type 2 diabetes. Endocr Connect. 2012;1:95–102. doi: 10.1530/EC-12-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu D, Pawlikowska L, Kanaya A, Hsueh WC, Colbert L, Newman AB, Satterfield S, Rosen C, Cummings SR, Harris TB, Ziv E, Health A, Body Composition S. Serum insulin-like growth factor-1 binding proteins 1 and 2 and mortality in older adults: the health, aging, and body composition study. J Am Geriatr Soc. 2009;57:1213–1218. doi: 10.1111/j.1532-5415.2009.02318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willette AA, Bendlin BB, Starks EJ, Birdsill AC, Johnson SC, Christian BT, Okonkwo OC, La Rue A, Hermann BP, Koscik RL, Jonaitis EM, Sager MA, Asthana S. Association of Insulin Resistance With Cerebral Glucose Uptake in Late Middle-Aged Adults at Risk for Alzheimer Disease. JAMA Neurol. 2015;72:1013–1020. doi: 10.1001/jamaneurol.2015.0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willette AA, Xu G, Johnson SC, Birdsill AC, Jonaitis EM, Sager MA, Hermann BP, La Rue A, Asthana S, Bendlin BB. Insulin Resistance, Brain Atrophy, and Cognitive Performance in Late Middle–Aged Adults. Diabetes Care. 2013;36:443–449. doi: 10.2337/dc12-0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baker LD, Cross D, Minoshima S, Belongia D, Watson GS, Craft S. Insulin resistance is associated with Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with pre-diabetes or early type 2 diabetes. Arch Neurol. 2011;68:51–57. doi: 10.1001/archneurol.2010.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schindler N, Mayer J, Saenger S, Gimsa U, Walz C, Brenmoehl J, Ohde D, Wirthgen E, Tuchscherer A, Russo VC, Frank M, Kirschstein T, Metzger F, Hoeflich A. Phenotype analysis of male transgenic mice overexpressing mutant IGFBP-2 lacking the Cardin-Weintraub sequence motif: reduced expression of synaptic markers and myelin basic protein in the brain and a lower degree of anxiety-like behaviour. Growth Horm IGF Res. 2016;33:1–8. doi: 10.1016/j.ghir.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Talbot K, Wang HY, Kazi H, Han LY, Bakshi KP, Stucky A, Fuino RL, Kawaguchi KR, Samoyedny AJ, Wilson RS, Arvanitakis Z, Schneider JA, Wolf BA, Bennett DA, Trojanowski JQ, Arnold SE. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest. 2012;122:1316–1338. doi: 10.1172/JCI59903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hertze J, Nagga K, Minthon L, Hansson O. Changes in cerebrospinal fluid and blood plasma levels of IGF-II and its binding proteins in Alzheimer’s disease: an observational study. BMC Neurol. 2014;14:64. doi: 10.1186/1471-2377-14-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Craft S, Asthana S, Cook DG, Baker LD, Cherrier M, Purganan K, Wait C, Petrova A, Latendresse S, Watson GS, Newcomer JW, Schellenberg GD, Krohn AJ. Insulin dose-response effects on memory and plasma amyloid precursor protein in Alzheimer’s disease: interactions with apolipoprotein E genotype. Psychoneuroendocrinology. 2003;28:809–822. doi: 10.1016/s0306-4530(02)00087-2. [DOI] [PubMed] [Google Scholar]
- 17.Arafat AM, Weickert MO, Frystyk J, Spranger J, Schofl C, Mohlig M, Pfeiffer AF. The role of insulin-like growth factor (IGF) binding protein-2 in the insulin-mediated decrease in IGF-I bioactivity. J Clin Endocrinol Metab. 2009;94:5093–5101. doi: 10.1210/jc.2009-0875. [DOI] [PubMed] [Google Scholar]
- 18.Spellman DS, Wildsmith KR, Honigberg LA, Tuefferd M, Baker D, Raghavan N, Nairn AC, Croteau P, Schirm M, Allard R, Lamontagne J, Chelsky D, Hoffmann S, Potter WZ, Alzheimer’s Disease Neuroimaging I, Foundation for NIHBCCSFPPT Development and evaluation of a multiplexed mass spectrometry based assay for measuring candidate peptide biomarkers in Alzheimer’s Disease Neuroimaging Initiative (ADNI) CSF. Proteomics Clin Appl. 2015;9:715–731. doi: 10.1002/prca.201400178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaw LM, Vanderstichele H, Knapik-Czajka M, Figurski M, Coart E, Blennow K, Soares H, Simon AJ, Lewczuk P, Dean RA, Siemers E, Potter W, Lee VMY, Trojanowski JQ, Alzheimer’s Disease Neuroimaging I Qualification of the analytical and clinical performance of CSF biomarker analyses in ADNI. Acta neuropathologica. 2011;121:597–609. doi: 10.1007/s00401-011-0808-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crane PK, Carle A, Gibbons LE, Insel P, Mackin RS, Gross A, Jones RN, Mukherjee S, Curtis SM, Harvey D, Weiner M, Mungas D, Alzheimer’s Disease Neuroimaging I Development and assessment of a composite score for memory in the Alzheimer’s Disease Neuroimaging Initiative (ADNI) Brain Imaging Behav. 2012;6:502–516. doi: 10.1007/s11682-012-9186-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gibbons LE, Carle AC, Mackin RS, Harvey D, Mukherjee S, Insel P, Curtis SM, Mungas D, Crane PK. A composite score for executive functioning, validated in Alzheimer’s Disease Neuroimaging Initiative (ADNI) participants with baseline mild cognitive impairment. Brain imaging and behavior. 2012;6:517–527. doi: 10.1007/s11682-012-9176-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jagust WJ, Bandy D, Chen K, Foster NL, Landau SM, Mathis CA, Price JC, Reiman EM, Skovronsky D, Koeppe RA, Alzheimer’s Disease Neuroimaging I The Alzheimer’s Disease Neuroimaging Initiative positron emission tomography core. Alzheimers Dement. 2010;6:221–229. doi: 10.1016/j.jalz.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dowling NM, Hermann B, La Rue A, Sager MA. Latent structure and factorial invariance of a neuropsychological test battery for the study of preclinical Alzheimer’s disease. Neuropsychology. 2010;24:742–756. doi: 10.1037/a0020176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Willette AA, Bendlin BB, Starks EJ, Birdsill AC, Johnson SC, Christian BT, Okonkwo OC, La Rue A, Hermann BP, Koscik RL, Jonaitis EM, Sager MA, Asthana S. Association of insulin resistance with cerebral glucose uptake in late middle-aged adults at risk for Alzheimer disease. Jama Neurology. 2015;72:1013–1020. doi: 10.1001/jamaneurol.2015.0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holland BS, Copenhaver MD. An improved sequentially rejective Bonferroni test procedure. Biometrics. 1987;43:417–423. [Google Scholar]
- 26.Wilkinson L. Response variable hypotheses in multivariate-analysis of variance. Psychological Bulletin. 1975;82:408–412. [Google Scholar]
- 27.van Leeuwen EM, Kanterakis A, Deelen P, Kattenberg MV, Genome of the Netherlands C. Slagboom PE, de Bakker PI, Wijmenga C, Swertz MA, Boomsma DI, van Duijn CM, Karssen LC, Hottenga JJ. Population-specific genotype imputations using minimac or IMPUTE2. Nat Protoc. 2015;10:1285–1296. doi: 10.1038/nprot.2015.077. [DOI] [PubMed] [Google Scholar]
- 28.Vannucci SJ, Koehler-Stec EM, Li K, Reynolds TH, Clark R, Simpson IA. GLUT4 glucose transporter expression in rodent brain: effect of diabetes. Brain Res. 1998;797:1–11. doi: 10.1016/s0006-8993(98)00103-6. [DOI] [PubMed] [Google Scholar]
- 29.Craft S, Baker LD, Montine TJ, Minoshima S, Watson GS, Claxton A, Arbuckle M, Callaghan M, Tsai E, Plymate SR, Green PS, Leverenz J, Cross D, Gerton B. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch Neurol. 2012;69:29–38. doi: 10.1001/archneurol.2011.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Keeney JT, Ibrahimi S, Zhao L. Human ApoE Isoforms Differentially Modulate Glucose and Amyloid Metabolic Pathways in Female Brain: Evidence of the Mechanism of Neuroprotection by ApoE2 and Implications for Alzheimer’s Disease Prevention and Early Intervention. J Alzheimers Dis. 2015;48:411–424. doi: 10.3233/JAD-150348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carro E, Trejo JL, Gomez-Isla T, LeRoith D, Torres-Aleman I. Serum insulin-like growth factor I regulates brain amyloid-beta levels. Nat Med. 2002;8:1390–1397. doi: 10.1038/nm1202-793. [DOI] [PubMed] [Google Scholar]
- 32.Leonard BE. Inflammation, depression and dementia: are they connected? Neurochem Res. 2007;32:1749–1756. doi: 10.1007/s11064-007-9385-y. [DOI] [PubMed] [Google Scholar]
- 33.Ribe EM, Lovestone S. Insulin signalling in Alzheimer’s disease and diabetes: from epidemiology to molecular links. J Intern Med. 2016;280:430–442. doi: 10.1111/joim.12534. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


