Abstract
Recent advancement in mitochondrial research has significantly extended our knowledge on the role and regulation of mitochondria in health and disease. One important breakthrough is the delineation of how mitochondrial morphological changes, termed mitochondrial dynamics, are coupled to the bioenergetics and signaling functions of mitochondria. In general, it is believed that fusion leads to an increased mitochondrial respiration efficiency and resistance to stress-induced dysfunction while fission does the contrary. This concept seems not applicable to adult cardiomyocytes. The mitochondria in adult cardiomyocytes exhibit fragmented morphology (tilted towards fission) and show less networking and movement as compared to other cell types. However, being the most energy-demanding cells, cardiomyocytes in the adult heart possess vast number of mitochondria, high level of energy flow, and abundant mitochondrial dynamics proteins. This apparent discrepancy could be explained by recently identified new functions of the mitochondrial dynamics proteins. These “non-canonical” roles of mitochondrial dynamics proteins range from controlling inter-organelle communication to regulating cell viability and survival under metabolic stresses. Here, we summarize the newly identified non-canonical roles of mitochondrial dynamics proteins. We focus on how these fission and fusion independent roles of dynamics proteins regulate mitochondrial bioenergetics. We also discuss potential molecular mechanisms, unique intracellular location, and the cardiovascular disease relevance of these non-canonical roles of the dynamics proteins. We propose that future studies are warranted to differentiate the canonical and non-canonical roles of dynamics proteins and to identify new approaches for the treatment of heart diseases.
Keywords: Mitochondrial dynamics proteins, Cardiac bioenergetics, Mitochondria associated membranes, Metabolic heart disease
1. Introduction
In recent years, we have witnessed the renaissance of mitochondrial research featured by exciting developments on the identity, structure and organization of mitochondrial related proteins such as ion channels [1]. Conceptually, a key advancement is the integration of mitochondria into the cellular signaling mechanisms through ATP, reactive oxygen species (ROS) and Ca2+, which makes mitochondria the major player for cell regulation, in addition to their traditional role as a cell powerhouse, in eukaryotes [2]. Moreover, we now know that mitochondria are dynamic organelles, which means their size, shape and location are constantly changing [3]. Mitochondrial dynamics are controlled by a group of dynamin-related GTPase, such as mitofusin 1 and 2 (Mfn1/2) and optic atrophy 1 (Opa1) for fusion and dynamin-related protein 1 (Drp1) for fission [4]. Mitochondrial dynamics are essential for normal mitochondrial function, transportation and communication as well as their biogenesis and quality control via mitophagy [5]. There are known human diseases such as the Charcot-Marie-Tooth neuropathy type 2A and dominant optic atrophy that are caused by mutations in the fission and fusion proteins (Table 1) [5–8].
Table 1.
The canonical and non-canonical roles, metabolic and cardiac phenotypes, and related human diseases of the major fission and fusion proteins.
| Drp1 | Mfn1/2 | Opa1 | |
|---|---|---|---|
| Canonical roles | Mitochondrial fission | Outer membrane fusion | Inner membrane fusion |
| Non-canonical roles |
|
|
|
| Mitochondrial and metabolic phenotypes after inhibiting the dynamics proteins (e.g., non-functional mutations, KD or KO models)* |
|
|
|
| Cardiac phenotypes in KO/KD models# |
KO in adult heart:
|
Cardiac Mfn2 KO:
|
Heterogeneous Opa1 KD:
|
| Human diseases related to mutations of the dynamics proteins |
|
|
|
| Small molecule Regulators | Mdivi-1, P110 | S3 | N/A |
The model in which a specific phenotype is found is shown in the parentheses. The inhibition of Mfn1 has no metabolic phenotype and the listed phenotypes are from Mfn2 KO or Mfn1/2 double KO.
The phenotypes are from the specific mouse models indicated. Germline Drp1 KO, Mfn1/2 double KO or Opa1 KO is embryonically lethal. Mfn1 KO has normal cardiac phenotype.
KO: knockout; DK: knockdown; N/A: not available
Mitochondria in the heart are particularly important since they provide 95% of the total of ~250 grams of ATP generated and utilized hourly in the beating human heart. The established paradigm to explain how the heart can cope with the huge energy demand centers on Ca2+ transportation between sarcoplasmic/endoplasmic reticulum (SR/ER) and mitochondria. In this paradigm, a small amount of Ca2+ enters mitochondria through the channels such as the mitochondrial Ca2+ uniporter (MCU) during excitation-contraction (EC) coupling to activate enzymes in TCA cycle and electron transport chain (ETC) complexes [9]. During mitochondrial respiration and ATP production, ROS can be generated through electron leakage along the ETC. The concept that ATP, ROS and Ca2+ signals are integrated in cardiac mitochondria is now well recognized [2]. Recent discoveries have added another dimension for the integration of mitochondrial function: the canonical and non-canonical roles of mitochondrial dynamics proteins. On one hand, the canonical roles of these proteins allow them to control the division/fusion of mitochondria in the developing and adult heart, and through which impact mitochondrial biogenesis, repair and quality control [10, 11]. On the other hand, the abundant amount of fission and fusion proteins in the adult heart are strategically located at mitochondrial micro-domains and bear non-canonical roles such as tethering SR/ER with mitochondria, facilitating mitochondrial Ca2+ handling and modulating energy production [12–15]. In this review, we will briefly summarize the newly identified non-canonical roles of fission and fusion proteins and discuss how they regulate bioenergetics in the healthy heart and participate in the development of stress-induced heart diseases.
2. Mitochondrial dynamics and bioenergetics are reciprocally coupled
The first evidence suggesting that mitochondrial shape and bioenergetics are intimately associated comes from the observation made almost 50 years ago that mitochondria shrink at state 3 respiration [16]. More recently, electron tomography studies indicate that the swelling and shrinking of the mitochondrial matrix is accompanied, not by passive unfolding and folding, but by fission and fusion of the inner membrane [17]. It is well known that mitochondrial toxins, metabolic perturbations and oxidative stress impair the function of mitochondria (decreased ATP production, increased ROS generation and loss of membrane potential) and, at the same time, alter mitochondrial morphology (mostly towards fission or fragmentation) [5]. In many cell types, elongated mitochondria are linked to more efficient ATP production and distribution and better sustaining stress-induced cell damage while fragmented (through fission) mitochondria are associated with declined ATP production and increased susceptibility for injury [18, 19]. Accumulating evidence supports that mitochondrial dynamics and bioenergetics may reciprocally influence each other depending on the experimental condition or initial stimulation. However, it is a convolute picture as to whether mitochondrial dynamics and bioenergetics are causally linked and, if so, in which direction and through which molecular mechanism.
Mitophagy is one of the well-established mechanisms that link compromised mitochondrial bioenergetics to altered mitochondrial morphology. Mitophagy is a physiological process in the cell to remove damaged mitochondria and/or use them as additional fuels during the hardship of lacking exogenous substrates [10, 20, 21]. Damaged mitochondria exhibit compromised metabolism and energy production and dissipated membrane potential, which is a hallmark for mitochondrial dysfunction and the initial trigger for mitophagy. Both fission and fusion proteins have been reported to play a key role in mitophagy regulation [22]. The major fission protein, Drp1 is recruited to mitochondria by AMPK pathway in response to mitochondrial toxins to promote fission [23]. Fission is thought to specifically split a mitochondrion into a healthy portion and a damaged portion and facilitate the damaged portion to be degraded via mitophagy [20]. However, the exact roles of Drp1 in mitophagy regulation in the heart is still elusive [24] with reports showing knockout of Drp1 can lead to either decreased [25], partially blocked [26] or progressively increased [27] mitophagy in the heart. The decreased membrane potential also leads to phosphorylation of the fusion protein Mfn2 at T111 and S442 via increased stability of the outer membrane located serine threonine protein kinase, PTEN-induced putative kinase-1 (PINK1) [28]. Mfn2 phosphorylation promotes recruitment of the E3 ubiquitin ligase Parkin to mitochondria, which is also phosphorylated and activated by PINK1 and initiates mitophagy [28]. Thus, Mfn2 is a central mediator for PINK/Parkin-dependent mitophagy [29, 30]. Here, the role of Mfn2 in mitophagy is serving as the receptor of Parkin on the outer membrane and likely independent to its canonical role in promoting fusion. A support for this notion is that knockout Mfn2 ameliorates Parkin recruitment and mitophagy but at the same time causes enlarged rather than smaller mitochondria in the heart [31, 32]. Interestingly, Parkin not only tags the mitochondria for mitophagy elimination but also ubiquitinates Mfn1/2 [33], which can enhance their activity [34] suggesting a positive feedback mechanism. It should be noted that the above reports show that mitophagy plays a role in connecting the compromised mitochondrial bioenergetics to altered mitochondrial dynamics proteins and/or morphological changes. Since the fission and fusion proteins are mostly GTP hydrolyzing enzymes, it is likely that normal mitochondrial energy production could also impact the activity of the dynamics proteins. However, whether and how mitochondrial bioenergetics under normal conditions can determine or impact mitochondrial morphology and dynamics is largely unknown.
On the other hand, altered mitochondrial morphology and dynamics can influence bioenergetics. This notion is supported by genetic models, in which one or more of the key fission and fusion proteins are knocked out or knocked down. For instance, knocking out the fission protein Drp1 is embryonic lethal, which is accompanied by drastically altered mitochondrial morphology and compromised respiration [35]. In the heart specific Drp1 knockout (KO) mice, increased mitochondrial size, decreased mitochondrial respiration and energy production, and severe cardiomyopathy are reported [25–27]. Similarly, genetic models of Mfn1/2 or Opa1 inhibition also exhibit compromised mitochondrial respiration along with significant morphological changes [27, 31, 32, 36–38]. For instance, cardiac specific knockout of Mfn2 in mice leads to decreased respiration, lower mitochondrial membrane potential and cardiomyopathy despite increased mitochondrial size [13]. The in-depth analyses and comparisons of the metabolic and cardiac phenotypes of the genetic models of dynamics proteins are summarized in Table 1 and have been extensively reviewed before [13, 24]. Besides the genetic models, studies using small chemicals or peptides that manipulate the activity of mitochondrial dynamics proteins have also suggested that mitochondrial dynamics can impact mitochondrial respiration [34, 39, 40]. Collectively, these studies support that shifting the balance of mitochondrial dynamics toward either fission or fusion will compromise mitochondrial bioenergetics in cardiomyocytes. This implies that a balanced mitochondrial dynamics could be critical for normal mitochondrial bioenergetics. It should be noted that these studies manipulated dynamics proteins, which cannot provide absolute evidence on whether morphological changes per se play a causal role in regulating mitochondrial respiration. Indeed, the above manipulations could modulate respiration independent to morphological changes and through the non-canonical roles of dynamics proteins (see next Section).
3. Non-canonical roles of mitochondrial dynamics proteins regulate cardiac bioenergetics
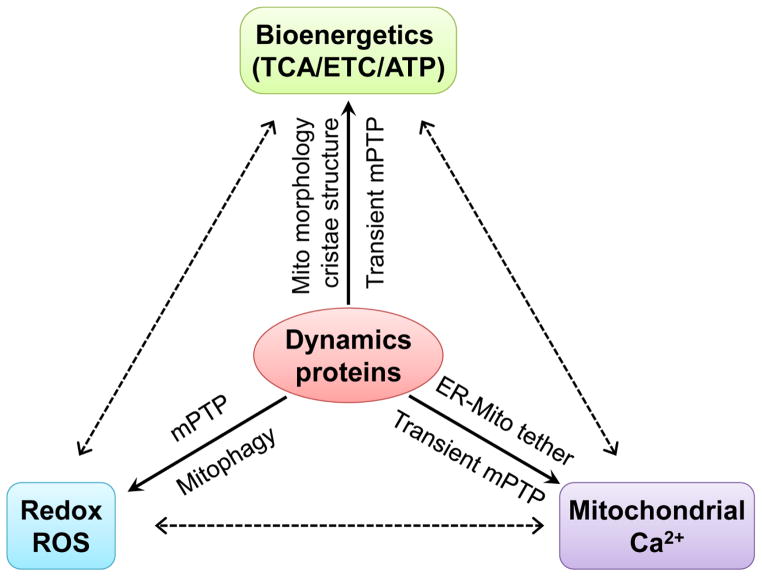
The mitochondria in adult cardiomyocytes are static and have low levels of mobility and networking [41], which raises the possibility that the highly expressed dynamics proteins in the heart may bear non-canonical functions besides mitochondrial morphology regulation. In mammalian tissues, Northern blot analyses show that Drp1 and Mfn1/2 mRNA are detected ubiquitously with the highest levels found in energy demanding tissues like the heart, skeletal muscles, and brain [42, 43]. The high level of Drp1 and Mfn1/2 in the heart seems to be at odds with numerous studies showing that mitochondrial dynamics in adult ventricular myocytes occur rather infrequently compared to many other cell types, such as neurons, murine embryonic fibroblasts (MEF) and the widely used H9C2 cardiac myoblast cells [44]. In fact, the mitochondria in many cell types and cell lines are constantly moving and their shape changes within seconds (Supplementary movie 1). However, mitochondria in adult cardiomyocytes, which occupy ~30–40% of the cell volume and are tightly packed between bundles of myofilaments (inter-myofibril mitochondria), under the sarcolemma (sub-sarcolemmal mitochondria), and around the nucleus (perinuclear mitochondria), exhibit undetectable morphological changes within the same time frame (Supplementary movie 2). It is estimated that the mitochondrial fusion and fission cycle may take up to 2 weeks in adult mouse heart [12, 45]. Beside the lack of constant morphological changes, the inter-myofibrillar mitochondria in adult ventricular myocytes appear to be “fragmented” and with limited networking [46, 47], in contrast to neurons, cell lines and even skeletal muscles [48]. One piece of evidence showing the lack of networking among cardiac mitochondria comes from the monitoring of mitochondrial flash, which is an integrated single mitochondrial event in live cells. We often observe flashes from a network of mitochondria in neurons, cell lines and skeletal muscles [49–51]. However, the flash events are almost exclusively restricted in individual mitochondrion in adult murine cardiomyocytes [49, 50, 52] suggesting lack of functional networking. Finally, genetic ablation of Mfn1/2 or Drp1 provokes only a modest, less than 2-fold changes in mitochondrial size in adult ventricular myocytes compared to complete mitochondrial fragmentation or hyper-fusion in MEF cells undergoing the same manipulations [27, 39, 53]. Taken together, the dichotomy between the abundance of Drp1 and Mfn1/2 and the scarcity of morphological dynamics and networking in adult cardiac mitochondria – in cells that have both the greatest mitochondrial density and the highest respiratory capacity of any mammalian cells – has led to the discovery of non-canonical roles of the fission and fusion proteins in cardiac muscles (Fig. 1 and Table 1) [12, 14].
Fig. 1.
Mitochondrial dynamics proteins orchestrate bioenergetics, redox and Ca2+ signaling in cardiac mitochondria.
Drp1 regulates mitochondrial energetics through mitophagy, mitochondrial permeability transition pore (mPTP) opening and respiration stimulation. First, recent reports show that Drp1-mediated mitophagy is not strictly related to morphometric remodeling in adult cardiomyocytes [25, 27]. Moreover, genetic (over expression of dominant negative Drp1 K38A mutation or Drp1 shRNA) or pharmacologic (mitochondrial division inhibitor, Mdivi-1[54]) inhibition of endogenous Drp1 significantly decreases mitochondrial respiration and ATP levels with a modest increase in mitochondrial enlargement in adult cardiomyocytes [39]. Drp1 inhibition also leads to a decrease in the frequency of transient and stochastic opening of mPTP which triggers mitochondrial flash – a biomarker for cellular energetic state [39, 52, 55, 56]. Moreover, the effect of Mdivi-1 (a Drp1 inhibitor) on respiration is absent in adult cardiomyocytes from cyclophilin D (a mPTP regulator) null mice [39]. These findings lead to an intriguing hypothesis that Drp1, through its induction of transient or subconductance flickering of mPTP openings, may cause physiological oscillations of mitochondrial membrane potential (ΔΨm). This, in turn, can trigger the oscillatory bursts of mitochondrial respiration [57–59]. It is however, yet to be determined how Drp1, which locates on the outer membrane, can interact with mPTP, which encompasses the inner and outer mitochondrial membranes. Nevertheless, physiological relevance of the role of Drp1 in regulating mPTP and respiration is further exemplified by the finding that Drp1 inhibition also suppresses mitochondrial ROS production and Ca2+ handling [39]. Since we previously reported that increased cytosolic Ca2+ transients promote translocation of Drp1 to mitochondria [60], it is likely that Drp1 could regulate physiological heart function through orchestrating cytosolic Ca2+ signaling and mitochondrial energy metabolism (Fig. 1). Pathologically, excessive activation of Drp1 may participate in heart dysfunction and cardiomyocyte death via prolonged mPTP opening. For instance, during cardiac ischemia reperfusion (I/R), inhibiting Drp1 by its dominant negative mutation (K38A), Drp1 shRNA knockdown, or Mdivi-1 significantly ameliorates cardiomyocyte death and myocardial infarction and these effects are dependent on mPTP [25, 61]. In addition, chronic β-adrenergic receptor (β-AR) stimulation also induces heart hypertrophy via activation of Drp1, which persistently increases mPTP opening [56].
Mfn2 may regulate mitochondrial metabolism in the heart through its non-canonical role in regulating mitophagy, which is independent of mitochondrial fusion (Fig. 1) [10, 12]. A strong support for this notion comes from the phenotypes of cardiac specific Mfn2 KO mice. Mfn2 KO mice develop cardiac hypertrophy, mitochondrial dysfunction and increased sensitivity to stress at an older age (e.g., > 4 month old) (Table 1). However, these pathological changes are unlikely stem from decreased mitochondrial fusion. This is because increased rather than decreased mitochondrial size is found in the Mfn2 KO hearts [13, 31, 32], which is counterintuitive to its primary role in mitochondrial fusion and contrary to the fragmented phenotype in Mfn2 KO cell lines [62, 63]. In addition, while Mfn1/2 double KO leads to fragmented mitochondria and embryonic lethality [64], embryonic knockout of Mfn1 in the heart decreases mitochondrial size but has no impact on cardiac function or pressure overload-induced heart dysfunction [37], which suggests that Mfn1 and Mfn2 have overlapping roles in mitochondrial fusion through the binding of their heptad repeat domains [53]. Finally, Mfn2 but not Mfn1 KO alters mitophagy and oxidative stress-related cardiomyocyte death [28, 65]. Taken together, Mfn2 may play a more prominent role in regulating mitophagy rather than mitochondrial morphology in the heart. In addition to Parkin recruitment, Mfn2 may also facilitate the fusion between autophagosome and lysosome and through which regulate general autophagy in cardiomyocytes, a mechanism also controls mitochondrial quality [66]. Through regulating mitophagy, Mfn2 participates in mitochondrial maturation in the developing heart [67] and the maintenance of a healthy mitochondrial population [10], both of which are critical for normal cardiac bioenergetics.
Another non-canonical role of Mfn2 is the tethering of mitochondria to ER or SR forming the ER/SR-mitochondria contact sites, which are unique structures critical for mitochondrial bioenergetics (Fig. 1) [68, 69]. The ER/SR-mitochondria contact sites, defined as mitochondria associated membranes (MAM) contain many mitochondrial and ER/SR proteins. One of the major functions of MAM is to create microdomains with high Ca2+ concentration during Ca2+ release from ER/SR to facilitate mitochondrial Ca2+ uptake [70]. Mfn2 is found on both ER/SR and mitochondrial membranes and Mfn1 only on mitochondrial membrane. The homologous binding between two Mfn2 proteins on either membrane or heterologous binding between Mfn2 on ER/SR and Mfn1 on mitochondrial membranes can set the appropriate distance between the two organelles [62, 71], which is around 10–30 nm [72]. The function of Mfn2 in tethering ER/SR with mitochondria and promoting mitochondrial Ca2+ uptake is important for mitochondrial bioenergetics, since mitochondrial Ca2+ is a known factor for the activity of enzymes in TCA cycle and ETC. The role of Mfn2 in ER-mitochondrial tethering has been well defined in cell lines or other cell types [69], but there are discrepancies regarding whether this mechanism exists in adult cardiomyocytes [13].
The inner membrane fusion protein Opa1 bears non-canonical roles in regulating the cristae structure of mitochondrial inner membrane (Fig. 1) [73, 74], which is essential for the assembly and function of ETC complexes [75]. Consistent with this role, Opa1 transgenic mice exhibit non-pathological cardiac hypertrophy and are resistant to stress-induced dysfunction in multiple organs [76]. Increased Opa1 levels also partially rescue the phenotypes of mitochondrial diseases caused by defects in ETC components [77]. Conversely, inhibiting Opa1 leads to mitochondrial damage, increased sensitivity to stress and heart failure [36, 38]. Opa1 knockdown also more significantly inhibits respiration compared to Mfn1/2 double knockdown [78]. Opa1 is expressed in eight different splice variants, which are grouped into long or short forms [79, 80]. Both the long and short forms of Opa1 can regulate the cristae structure and through which maintain mitochondrial metabolism [81]. However, only the long form is capable of regulating inner membrane fusion [38, 81, 82].
There are reports suggesting other mechanisms for the dynamics proteins to regulate cardiac bioenergetics. First, the Mfn2 has been reported to maintain mitochondrial coenzyme Q pool and through which regulate bioenergetics. Mfn2 KO exhibits impaired mitochondrial respiration and reduced ATP production in adult cardiomyocytes and MEF cells. These outcomes are originated from depleted mitochondrial coenzyme Q pool [63]. Although morphological change is ruled out as a potential mechanism, whether mitophagy and/or ER-mitochondria tethering mediates the effects of Mfn2 in maintaining the coenzyme Q pool is not known [63]. In addition, Mfn1/2 or Opa1 can regulate gene expression profile and intracellular signaling pathways and through which modulate cardiomyocyte differentiation and heart development [64]. Ablation of Mfn1 and Mfn2 in the embryonic mouse heart arrested mouse heart development and gene-trapping of Opa1 in mouse embryonic stem cells impaired their differentiation into cardiomyocytes [64]. These effects are linked to increased cytosolic Ca2+ release that activates calcineurin, which upregulates Notch1 signaling leading to changes of a whole profile of genes regulating cardiac development [64]. However, how the lack of Mfn1/2 or Opa1 leads to increased cytosolic Ca2+ is not clear. It is possible that the non-canonical roles of Mfn1/2 (on mitochondria-ER tethering) or Opa1 (on respiration via maintaining cristae structure) maybe involved.
4. The MAM location of mitochondrial dynamics proteins underlies their non-canonical roles
The mitochondrial dynamics proteins, including Drp1, Mfn1/2 and Opa1, are resided or enriched in or near MAM, which suggests that they may be deliberately positioned to this microdomain through cross interactions with localized Ca2+ and ROS to carry out non-canonical roles (Fig. 2B). As mentioned above, the MAM is a specific inter organelle structure formed by the ER/SR and mitochondrial membranes and plays a critical role in mitochondrial respiration and energetics [70, 83]. The proximity (e.g., 10–30 nm) of the ER/SR membrane and the mitochondrial outer membrane sets the stage for fast and precise communication between the two organelles. One of the well-established functions of MAM is to facilitate Ca2+ transportation from its storage, the ER/SR, to its workplace, the mitochondria [69, 70]. Although it has been known for decades that mitochondria take up Ca2+ for their bioenergetics and for buffering cytosolic Ca2+, it remains a mystery how Ca2+ is transported via the MCUs that have low Ca2+ affinity (e.g., Kd = 20–30 μM) [84]. The negative ΔΨm (~180 mV) could facilitate the import of cations across mitochondrial inner membrane, but this mechanism is not specific for Ca2+. In this regard, the demonstration of MAM has juxtaposed mitochondria with ER/SR to create the microdomains that retain a high Ca2+ concentration (e.g., more than 10 μM). Besides providing the local Ca2+ hotspots, MAM also processes multiple ER/SR proteins, chaperones and mitochondrial proteins that are associated with ER/SR Ca2+ release and mitochondrial Ca2+ uptake [69, 83]. In most cell types, the major ER Ca2+ release channel, IP3 receptor is located at MAM and tethered with mitochondrial outer membrane channel, voltage dependent anion channel (VDAC) via chaperones such as GRP75 [69]. MCU is also enriched at the MAM [69] and, together with its key gatekeeper, EMRE, colocalized with ryanodine receptor 2 (RyR2), the major SR Ca2+ release channel in cardiac muscles [85]. Taken together, by creating a “highway” for Ca2+ transportation into mitochondria, the MAM plays a central role in regulating Ca2+-induced mitochondrial metabolism and energy production [86].
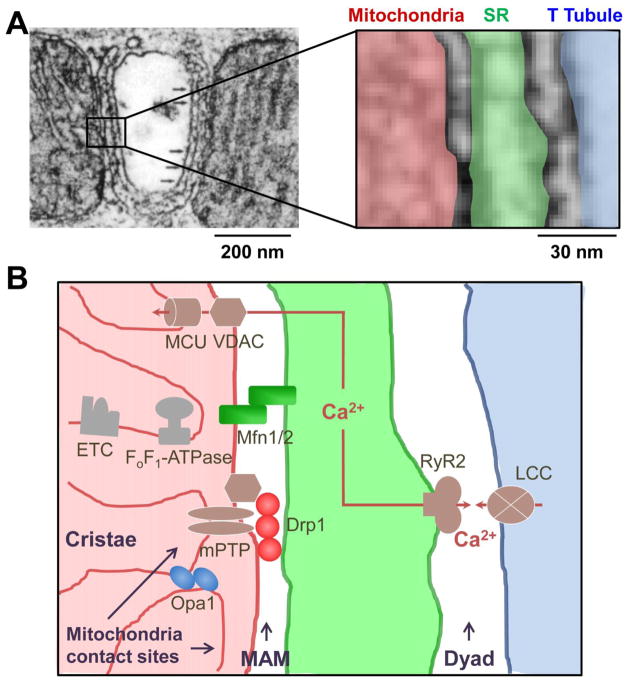
Fig. 2.
Location of the dynamics proteins at or near the MAM in adult cardiomyocyte. A, Left: electron microscopic (EM) image of a portion of an adult cardiomyocyte. Right: enlarged area showing the juxtaposition of mitochondria (red), SR (green) and T Tubule (blue). B, Diagram based on the structural proximity of the organelles shown in A to highlight the enrichment of mitochondrial dynamics proteins at or near MAM. The MAM is within 10–30 nm from the dyad (for EC coupling) and mitochondrial contact site (for mPTP and respiration). The major pathways involving MAM located dynamics proteins are: (1) Drp1 interaction with mPTP components to regulate transient mPTP opening, which stimulates respiration; (2) Mfn1/2 tether mitochondria with SR at MAM to facilitate mitochondrial Ca2+ uptake via VDAC and MCU to activate metabolism; and (3) Opa1 maintains the cristae structure at mitochondrial contact site adjacent to MAM, which allows the appropriate assembly and function of ETC complexes. The EM image in A is adopted from a previous report (Ref #68) with permission. Ca2+: calcium, Drp1: dynamin related protein 1, ETC: electron transport chain, LCC: L-type Ca2+ channel, MAM: mitochondrial associated membrane, MCU: mitochondrial Ca2+ uniporter, Mfn1/2: mitofusion 1/2, mPTP: mitochondrial permeability transition pore, Opa1: optic atrophy 1, RyR2: ryanodine receptor 2, VDAC: voltage dependent anion channel.
Mfn2 is enriched at MAM and plays a key role in tethering mitochondrial outer membrane with ER/SR membrane (Fig. 2B) [71]. This function may be important to keep the appropriate distance between the two membranes to ensure efficient ER-mitochondria Ca2+ transfer. Supporting this, Mfn2 KO increases the distance between ER and mitochondrial membranes, decreases SR-mitochondrial contact length, and decreases mitochondrial Ca2+ uptake without affecting ER Ca2+ release and mitochondrial Ca2+ uptake machineries [32, 71, 87]. A mitochondrial ubiquitin ligase, MITOL is shown to regulate ER-mitochondria interaction via ubiquitination of Mfn2 [88]. There are reports showing Mfn2 KO increases rather than decreases mitochondrial surface juxtaposed to ER and Ca2+ transfer between the two organelles, which challenge Mfn2 as a MAM tether [62, 89]. Similar discrepancies also exist regarding the role of Mfn2 in SR-mitochondria tethering in the heart. In adult cardiomyocytes, SR and mitochondria are in close contact with each other [90] and Mfn2 KO has been shown to decrease their contact by 30% [32]. However, another group, using a similar Mfn2 KO model, does not report any changes [31]. It should be noted that, since both studies show increased mitochondrial size and heart dysfunction after Mfn2 KO, it is possible that deranged mitophagy, another non-canonical role of Mfn2 could be responsible for the outcome. Future studies are needed to clarify the exact role of Mfn2 in tethering MAM in cell lines and adult cardiomyocytes. It is also important to differentiate the relative contribution of the multiple roles of Mfn2 (e.g., ER-mitochondria tether and mitophagy) in different cell types/tissues and under different circumstances. Whether the preferential location of Mfn2 at MAM mediates its role in PINK/Parkin-dependent mitophagy needs to be explored as well.
There is evidence that Drp1 may be enriched at or closely associated with MAM. First, the ER-mitochondria contact sites are critical for mitochondrial fission [3]. The two Drp1 receptors, MiD49/MiD51 are found enriched at the MAM in a Drp1-dependent manner to regulate fission and cristae remodeling during apoptosis independent of Opa1 in Hela cells [91]. It has been shown that ER can wrap the mitochondrion at the fission site and facilitate Drp1 recruitment by actin [92] to physically split the mitochondrion [93, 94]. These sequential steps may occur transiently during fission and the recruited Drp1 may stay at MAM for its non-canonical roles. In cardiomyocytes, whether these sequential steps of SR and Drp1 working in concert mediate mitochondrial fission has not been demonstrated. Second, Ca2+ can activate Drp1 and promote its mitochondrial translocation and fission. We have shown that mitochondrial translocation of Drp1 in cardiomyocytes is triggered by increased cytosolic Ca2+ when SR Ca2+ pump, SERCA is inhibited by thapsigargin or when Ca2+ influx through L-type Ca2+ channels (LCC) is increased by high KCl depolarization of cell membrane potential [60]. Since the cytosolic Ca2+ release machinery (e.g., LCC on T tubules and RyR2 on SR membrane at the dyad [95]) are adjacent to MAM in adult cardiomyocytes (Fig. 2B), it is conceivable that the activation of Drp1 and its recruitment to mitochondria would happen within or near the small space that contains the dyad and MAM. Additional evidence that implies Drp1’s association with MAM comes from the role of Drp1 in regulating mPTP [39, 61]. Drp1 promotes the oligomerization of Bax/Bak [54, 96], which mediate outer membrane permeabilization, cytochrome C release and apoptosis [97, 98]. The outer membrane permeabilization is thought to be the first step of mPTP opening [99]. Although the molecular identity of mPTP is still unknown, recent studies have identified several potential components of mPTP such as VDAC, CypD, Bax/Bak and FoF1-ATP synthase [99–103], many of which are enriched at MAM. For instance, VDAC regulates mPTP and apoptosis [103] and is enriched at MAM [69]. CypD is an essential regulator of mPTP [100], it mediates the effect of Drp1 on mPTP and respiration [39], and it is found residing at MAM [69]. Since the physiological function of mPTP is to regulate mitochondrial ROS and Ca2+, it is likely that mPTP and Drp1 may be clustered at MAM (Fig. 2B). Finally, our unpublished results using purified subcellular fractions of murine heart tissues indicate that majority of mitochondria-associated Drp1 is in the MAM fraction but not the purified mitochondria fraction that has minimal SR membrane “contamination” (unpublished). Thus, just like Mfn1/2, Drp1 could be strategically located at MAM to regulate bioenergetics during heartbeats. It should be further determined how Drp1 is recruited to MAM and how it interacts with mPTP and other MAM proteins to conduct its non-canonical roles in mPTP and respiration regulation.
There is evidence that the inner membrane protein Opa1 could be located adjacent to MAM. Opa1 is associated with the mitochondrial contact site and cristae organizing system (MICOS) [104, 105] located at the sites where mitochondrial outer and inner membranes are in contact (mitochondrial contact sites) [106, 107]. These are unique structures that facilitate the interaction between outer and inner membrane proteins and shape the cristae structure [106, 108]. Intriguingly, these sites are often close to the SR-mitochondria junctions (Fig. 2). Furthermore, the mPTP multi-protein complexes also exist most abundantly in the mitochondrial contact sites [109–111]. In addition, Opa1-mediated cristae opening is Bax/Bak dependent suggesting close functional coupling between cristae structure and mPTP [112]. Whether and how MAM and mitochondrial contact sites are spatially arranged and functionally integrated are not clear and need to be investigated.
Taken together, the fission and fusion proteins are enriched at or near MAM. This location is associated with their canonical and non-canonical roles in regulating mitochondrial dynamics, ER/SR-mitochondrial tethering, mPTP, and cristae structure. Furthermore, the non-canonical roles of Drp1, Mfn1/2 and Opa1 may synergistically regulate energy production in mitochondria (Fig. 2B). Drp1 is activated by cytosolic Ca2+ and clustered at MAM to regulate transient mPTP opening, which stimulates respiration. Mfn1/2 tether mitochondria with SR at MAM to facilitate mitochondrial Ca2+ uptake, which activates metabolism. Opa1 maintains cristae structure at the mitochondrial contact site adjacent to MAM to allow the appropriate assembly and function of ETC complexes. The MAM location of these dynamics proteins is particularly important for the heart. This is because MAM is positioned within 10–30 nm in between the dyad (for Ca2+ handling which consumes energy) and mitochondrial contact site (for respiration which generates energy) in adult cardiomyocytes (Fig. 2). Thus, the dynamics proteins could be strategically located at this microdomain in order to bridge energy demand with its production in cardiomyocytes. In another word, MAM, together with dyad and mitochondrial contact site, serves as the platform for energy homeostasis regulation in the adult heart. The advantage for this synergistic regulation at MAM could be two folds for the beating heart: to effectively integrate the various signals and to promptly and precisely respond to workload fluctuations and stresses.
5. Non-canonical roles of mitochondrial dynamics proteins in heart diseases
Cardiac bioenergetics is often compromised under stresses that eventually lead to heart dysfunction. In fact, a major common defect in the failing heart is imbalanced energy demand and supply, which renders the heart unable to sustain its pumping function. Thus, understanding how cardiac bioenergetics is adversely impacted under stress conditions will gain insights into effective interventions for heart disease [113]. Many factors, such as fuel supply, Ca2+ signaling, redox, bioactive lipids and exercise can regulate the metabolic and energetic homeostasis in the heart. The non-canonical roles of dynamics proteins may represent a new mechanism for cardiac metabolism regulation, and as such could participate in stress-induced heart dysfunction. Indeed, there is evidence that Drp1, Mfn1/2 and Opa1 are involved in stress-induced heart diseases, because their expression, modification and activity are altered under pathological conditions of the heart [114]. Here, we summarize how the non-canonical roles of dynamics proteins participate in the major types of heart diseases induced by adrenergic, ischemic and metabolic stresses.
Chronic β-adrenergic receptor (β-AR) stimulation can lead to heart failure (HF) and β-blockers have been frequently prescribed to treat this disease [115, 116]. Although the key mechanisms of HF center on Ca2+ mediated contractile dysfunction, arrhythmias, excessive oxygen consumption, and energy failure, little is known about the role of dynamics proteins in the pathogenesis of β-AR mediated HF. We recently reported that, in mice chronically infused with isoproterenol (ISO), Drp1 phosphorylation is increased at serine 616 (S616), but not the PKA-dependent S637 site. ISO infusion also promotes Drp1 translocation to mitochondria. Both effects are dependent on Ca2+/calmodulin-dependent kinase II (CaMKII), the downstream signaling of β-AR [56]. These effects are recapitulated in cultured adult cardiomyocytes and are responsible for chronic ISO-stimulation induced mitochondrial flashes, mPTP opening and cell death [56]. In vivo, blocking Drp1 activity ameliorated ISO-induced heart hypertrophy. Drp1 can be modified by phosphorylation, S-nitrosylation, SUMOylation, O-GlcNAcylation, and ubiquitination [4, 117]. Its phosphorylation/de-phosphorylation at the inhibitory S637 site [118, 119] has been shown to participate in muscle energy metabolism [120] and I/R induced neonatal cardiomyocyte apoptosis [121]. The phosphorylation at S616 site has been shown to promote fission and participate in mitosis in Hela and vascular smooth muscle cells [122, 123]. However, in adult cardiomyocytes, no obvious morphological change is observed when S616 phosphorylation is altered. Rather, the major changes are related to the non-canonical roles of Drp1 in mPTP and respiration regulation. Finally, in human failing heart samples, Drp1 phosphorylation at S616 site is significantly increased [56] suggesting the clinical relevance of altered Drp1 activity and/or its non-canonical roles in human heart failure. Besides Drp1, Opa1 level is found to decrease in rat heart failure model and human failing hearts, while Mfn1/2 levels only increased in human failing hearts despite the fragmented mitochondrial morphology [124]. Whether and how the non-canonical roles of Opa1 and Mfn1/2 contribute to adrenergic stress-induced heart failure has not been explored.
Myocardial infarction followed by reperfusion is the major stress leading to heart failure. Mitochondria play a central role in I/R injury-induced cardiomyocyte death via Ca2+ overload, excessive ROS production, and mPTP opening [125]. It has been first observed in HL-1 cardiac cell lines that hypoxia can induce mitochondrial fission [126]. More recently, the regulation and roles of Drp1 in cardiac I/R injury have been extensively studied [14]. Simulated ischemia induces mitochondrial translocation of Drp1 in neonatal cardiomyocytes [121, 127] and perfused adult hearts [128]. I/R also decreases Drp1 phosphorylation at S637 (inhibitory) site in HL-1 cells [129] and perfused hearts [128], and increases its phosphorylation at S616 (activating) site [129]. Importantly, chemical (Mdivi-1), small peptide (P110) or genetic inhibition (K38A mutation or shRNA) of Drp1 protects against I/R-induced cardiomyocyte death [61, 128, 130], decreases infarct size [61, 127, 130, 131], improves cardiac diastolic and systolic function acutely [128, 130], and prevents long-term heart decompensation [132]. Since inhibiting Drp1 by Mdivi-1, dominant negative K38A or Drp1 shRNA decreases mitochondrial respiration (OCR) and ATP production as well as preventing prolonged mPTP opening in MEF cells, neonatal and adult cardiomyocytes [25, 26, 39, 130], the non-canonical roles of Drp1 are responsible for I/R-induced cardiomyocyte death and heart dysfunction. Interestingly, while acute inhibition of Drp1 by small molecules or shRNA ameliorates I/R injury in the heart [61, 128, 132], chronic inhibition of Drp1 in the heterozygous knockout hearts increases infarct size after I/R [25]. It is proposed that chronic inhibition of Drp1 impairs mitophagy and renders cardiomyocytes prone to I/R injury. Besides increased Drp1 and fission in the heart or cardiomyocytes after I/R, there are reports showing unchanged Mfn2 level [133], decreased Mfn1 [134], and decreased Opa1 level after I/R [133]. Although overexpression of Opa1 protects against cardiac ischemic injury [76], hearts deficient in both Mfn1 and Mfn2 are protected against acute myocardial infarction due to lesser degree of mitochondrial Ca2+ overload as a result of impaired SR-mitochondria tethering [135]. Thus, the exact regulation and roles of fusion proteins in heart I/R injury are multifactorial and require quantitative and integrative assessments on the relative contributions of individual proteins.
The regulation and roles of mitochondrial dynamics proteins in metabolic syndrome such as diabetes and obesity have been extensively studied in tissues other than the heart, including pancreatic islets, liver, blood vessels, adipose tissues and skeletal muscles [136, 137]. Nutritional stress can influence mitophagy, ER-mitochondria tethering and cristae remodeling, which are regulated by the non-canonical roles of Mfn1/2 and Opa1. For instance, food intake can enhance ER-mitochondrial contact through activation of Mfn2, which in turn promotes the cleavage of Opa1 and changes cristae morphology in the liver [138]. Excessive nutrition during obesity can also enhance ER-mitochondria tethering and leads to mitochondrial Ca2+ overload and dysfunction in the liver [139]. In skeletal muscles, palmitate induces fission and upregulates Drp1. Inhibiting Drp1 rescues palmitate-induced mitochondrial fission, dysfunction and insulin resistance [140]. The same study also shows that high-fat diet-fed increases Drp1 and Fis1 levels but not Mfn2 and Opa1 levels in skeletal muscles [140]. Fewer reports, however, investigated the impact of metabolic stresses, such as hyperglycemia, obesity and insulin resistance, on mitochondrial morphology and dynamics proteins in the heart or cardiomyocyte. It is also elusive as to whether altered dynamics proteins or mitochondrial morphology contribute to the development of heart dysfunction in metabolic syndrome [141–143]. In the heart of diabetic patients, only Mfn1 protein level is significantly decreased together with a smaller mitochondrial size [144]. In high glucose incubated H9C2 cells, Drp1 protein level is increased [145]. High glucose also stimulates fission, respiration, ROS production and mPTP opening in H9C2 cells in a Drp1 dependent manner [146, 147]. Our unpublished results suggest that high palmitate treatment or feeding the mice with a high fat diet can increase Drp1 protein level in adult cardiomyocyte and in mouse heart, respectively, which leads to decreased mitochondrial size, mPTP opening and cell death (Unpublished). Hyperglycemia also increases O-GlcNAcylation of Drp1 and fission in cardiomyocytes [148]. Transgenic mice overexpressing the dominant negative Drp1 mutation, Drp1 K38A, ameliorated diabetic oxidative stress and excessive fission in hepatocytes [149]. Another report shows, however, that Mfn1, Opa1 and Drp1 protein levels are all decreased after high glucose incubation in neonatal cardiomyocytes and preventing O-GlcNAcylation of Opa1 ameliorates mitochondrial fission and dysfunction [150]. Consistent with these findings, Opa1 has been shown as a target downstream of insulin receptor in neonatal cardiomyocytes to mediate insulin’s effects on mitochondrial metabolism [151]. Although almost all the studies on metabolic stress-induced diabetic cardiomyopathy show compromised mitochondrial respiration, mitochondrial fission is observe only in some studies [142] suggesting morphological changes and bioenergetics are not always coupled and the functional outcomes may be more associated with the non-canonical roles of dynamics proteins.
6. Therapeutic potential of targeting mitochondrial dynamics proteins in the heart
As shown above, increased Drp1 expression and activity participate in several types of heart diseases. Thus, there is tremendous interest in developing Drp1 inhibitors. One of the most widely used Drp1 inhibitors is Mdivi-1, which is a derivative of quinazolinone. Mdivi-1 is the short term for mitochondrial division inhibitor 1 and is identified by a yeast-based screening of 23,000 compounds [54]. It inhibits the GTPase activity of purified Drp1 and acutely and effectively promotes mitochondrial network formation in yeast [54] and HL-1 cells [61]. Several groups have applied Mdivi-1 in HL-1 cells, neonatal cardiomyocytes and rodent hearts and reported its protective effects on I/R-induced cardiomyocyte death, myocardial infarction and contractile dysfunction [61, 128, 131]. However, the specificity of Mdivi-1 as a Drp1 inhibitor has been challenged recently. The short term administration of Mdivi-1 exerts cell protective effects in neonatal cardiomyocytes that do not mimic the effects of Drp1 shRNA. However, long term Mdivi-1 application mimics the effects of Drp1 knockdown [25]. Another recent report shows that Mdivi-1 inhibits Complex I activity and reverse electron flow-induced ROS production in COS-7 and MEF cells [152]. These effects are not Drp1 dependent, since they are not coupled with mitochondrial elongation and are found in Drp1-deficient fibroblasts. This study further shows that Mdivi-1 does not inhibit the GTPase activity of human Drp1 [152]. Thus, it remains to be fully tested whether Mdivi-1’s effect is Drp1 dependent, partially dependent or independent in cardiomyocyte and the heart. This will also call for new compound screening experiments to preferably use mammalian systems for identifying new Drp1 inhibitors. Based on Drp1’s binding with one of its receptors, Fis1, a small peptide named P110 has been developed and initially used to ameliorate neuronal dysfunction [40]. When used in the heart I/R model, P110 effectively decreases infarction, acute heart dysfunction and long-term remodeling [132]. There are several other Drp1 receptors and they may play various and independent roles in Drp1 recruitment to mitochondria [153, 154]. Thus, they could be new targets for developing receptor specific inhibitors/modulators for Drp1. However, whether and how these receptors mediate Drp1’s effects on fission and non-canonical roles in mPTP and respiration regulation are unknown.
Efforts for manipulating Mfn1/2 focus on enhancing their fusion activity. A diterpenoid derivative 15-oxospiramilactone, named S3 has been shown to inhibit the deubiquitinase, USP30 and through which increase the non-degradative ubiquitination of Mfn1/2. S3 enhances Mfn1/2 activity, promotes mitochondrial fusion, and restores the morphology and function of mitochondria in fusion-deficient human and mouse cells in vitro [34]. Based on the intramolecular binding interactions of Mfn2, a small cell permeant peptide has been engineered to destabilize fusion-constrained conformation of Mfn2 to promote fusion. This peptide reverses mitochondrial abnormalities in cultured fibroblasts and neurons expressing mutated Mfn2 [155]. Given the important role of Mfn2 in ER-mitochondria tethering, this approach could be tested to modulate the communication between the two organelles. Drug-inducible inter-organelle linkers have been tested to maintain the distance between ER and mitochondria and the local Ca2+ transferring between them [156]. Such an approach to enhance mitochondrial Ca2+ uptake via Mfn1/2 could promote mitochondrial energy production, buffer cytosolic Ca2+ overload, and benefit the failing heart, where the T-tubule and SR systems are deranged [157].
Small molecules for Opa1 function are yet to be developed. Two factors should be considered when developing effective Opa1 modulators. One is the inner membrane location of Opa1 that could adversely influence the efficiency of the compound. Another is the multiple splicing variants and their different roles in fusion and cristae organization, which may complicate the specificity of Opa1 modulators.
In summary, a key consideration when developing or testing the new compounds targeting fission and fusion proteins is the original defects or the initial direction of disturbances in mitochondrial dynamics. Because the vitality of cells hinges on a well-balanced mitochondrial dynamics, the ultimate goal is to identify candidate molecules that can maintain or restore this balance through preventing or suppressing the adverse activity (e.g., excessive mPTP opening) or enhancing the beneficial effects (e.g., cristae organization and ER-mitochondria tethering).
7. Conclusion and future perspectives
Increasing evidence indicates that mitochondrial dynamics proteins are abundant in the adult heart and carry out roles other than mitochondrial morphology regulation. These new or “non-canonical” roles of mitochondrial dynamics proteins include tethering mitochondria with ER/SR, modulating respiration chain organization, regulating mitophagy, and controlling mPTP opening and bursting mitochondrial respiration events (Fig. 1 and Table 1). Moreover, these dynamics proteins are preferentially located or enriched at the microdomain in between SR and mitochondria, named MAM, which could be a strategic design of nature to ensure the seamless integration of multiple mitochondrial functions including Ca2+ transportation, redox, mPTP and respiration (Fig. 2). Recent studies have shown that the non-canonical roles of mitochondrial dynamics proteins and their location at or near the MAM play crucial roles in maintaining energy homeostasis and sustaining contractile function of the working heart. Under pathological conditions, the recruitment of mitochondrial dynamics proteins to MAM and/or their non-canonical roles are impacted, which contribute to the deteriorated heart performance. Therefore, targeting the non-canonical roles and/or the MAM location of dynamics proteins could be a promising new strategy for heart disease therapy. In this regard, small molecule inhibitors of Drp1, such as Mdivi-1 and P110, have been tested in in vitro and animal models of myocardial infarction and I/R injury. Small molecules manipulating Mfn1/2 or Opa1 functions are scarce. The S3 compound, which indirectly enhances the function of Mfn1/2, has not been tested in heart disease models. Future studies are warranted to develop and test new agents that regulate the non-canonical roles of dynamics proteins and/or their MAM locations for treating heart disease.
To facilitate translational application of the non-canonical roles of mitochondrial dynamics proteins, many fundamental questions still need to be answered. One of them is how to differentiate the canonical (e.g., fission and fusion) and non-canonical roles. The MAM location could be a determining factor. For example, in cell lines, Drp1 is recruited to the ER-mitochondria contact sites to perform fission. In the heart, Drp1 is highly clustered at the MAM under resting conditions but without clear signs of inducing mitochondrial division. Thus, the MAM location of Drp1 could be important for mPTP regulation rather than fission in cardiomyocytes. The other question is how the dynamics proteins are recruited to MAM and activated. For instance, how the MAM-enriched Drp1 interacts with mPTP components needs to be determined. One interesting finding is that Drp1 can bind with different phospholipids, which restrain the activity of Drp1 [158]. Also for Drp1, its polymerization is needed for GTPase activity and fission, but whether polymers or monomers of Drp1 regulate mPTP opening and respiration needs to be determined. It is still unknown what other partner proteins are associated with this Drp1 oligomer and what are their functional implications. For Mfn, recent studies reveal that conformational changes induced by GTP binding to Mfn1 controls its membrane tethering function and conformational plasticity within the domains of Mfn2 determines its fusion activity [155, 159]. Since many of the non-canonical roles of dynamics proteins are identified in non-cardiac mitochondria, whether they also apply to cardiac mitochondria is another question needs to be answered. Controversies exist regarding the role of Mfn2 in tethering SR and mitochondria in the cells [160, 161]. Finally, cardiac mitochondria can be divided into different sub-populations based on their location in the cell. It would be interesting to know whether the non-canonical roles of dynamics proteins differ among the sub-populations of mitochondria that exhibit vastly different morphology, proximity to high Ca2+ domain during EC coupling, and location in relation to other cellular constituencies (e.g. nucleus, plasma membrane and ER/SR). Future studies are needed to fully uncover the unique regulatory mechanisms underlying each of the non-canonical roles of fission and fusion proteins to aid the development of more specific and effective treatments for related heart diseases.
Highlights.
Mitochondrial dynamics proteins have non-canonical roles in the heart
The non-canonical roles of dynamics proteins regulates cardiac bioenergetics
Drp1, Mfn1/2 and Opa1 are localized at or near mitochondria-ER contact sites (MAM)
The non-canonical roles and MAM location of dynamics proteins participate in heart disease
Acknowledgments
This work is supported in part by National Institute of Health grants HL114760 to WW, NIH HL093671 and HL122124 to SSS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Picard M, Wallace DC, Burelle Y. The rise of mitochondria in medicine. Mitochondrion. 2016;30:105–116. doi: 10.1016/j.mito.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287:C817–833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 3.Friedman JR, Nunnari J. Mitochondrial form and function. Nature. 2014;505:335–343. doi: 10.1038/nature12985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall AR, Burke N, Dongworth RK, Hausenloy DJ. Mitochondrial fusion and fission proteins: novel therapeutic targets for combating cardiovascular disease. Br J Pharmacol. 2014;171:1890–1906. doi: 10.1111/bph.12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan DC. Fusion and fission: interlinked processes critical for mitochondrial health. Annu Rev Genet. 2012;46:265–287. doi: 10.1146/annurev-genet-110410-132529. [DOI] [PubMed] [Google Scholar]
- 6.Waterham HR, Koster J, van Roermund CW, Mooyer PA, Wanders RJ, Leonard JV. A lethal defect of mitochondrial and peroxisomal fission. N Engl J Med. 2007;356:1736–1741. doi: 10.1056/NEJMoa064436. [DOI] [PubMed] [Google Scholar]
- 7.Ranieri M, Brajkovic S, Riboldi G, Ronchi D, Rizzo F, Bresolin N, Corti S, Comi GP. Mitochondrial fusion proteins and human diseases. Neurol Res Int. 2013;2013:293893. doi: 10.1155/2013/293893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delettre C, Lenaers G, Griffoin JM, Gigarel N, Lorenzo C, Belenguer P, Pelloquin L, Grosgeorge J, Turc-Carel C, Perret E, Astarie-Dequeker C, Lasquellec L, Arnaud B, Ducommun B, Kaplan J, Hamel CP. Nuclear gene OPA1, encoding a mitochondrial dynamin-related protein, is mutated in dominant optic atrophy. Nat Genet. 2000;26:207–210. doi: 10.1038/79936. [DOI] [PubMed] [Google Scholar]
- 9.Balaban RS. Cardiac energy metabolism homeostasis: role of cytosolic calcium. J Mol Cell Cardiol. 2002;34:1259–1271. doi: 10.1006/jmcc.2002.2082. [DOI] [PubMed] [Google Scholar]
- 10.Tong M, Sadoshima J. Mitochondrial autophagy in cardiomyopathy. Curr Opin Genet Dev. 2016;38:8–15. doi: 10.1016/j.gde.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dorn GW, 2nd, Vega RB, Kelly DP. Mitochondrial biogenesis and dynamics in the developing and diseased heart. Genes Dev. 2015;29:1981–1991. doi: 10.1101/gad.269894.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song M, Dorn GW., 2nd Mitoconfusion: noncanonical functioning of dynamism factors in static mitochondria of the heart. Cell Metab. 2015;21:195–205. doi: 10.1016/j.cmet.2014.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorn GW, 2nd, Song M, Walsh K. Functional implications of mitofusin 2-mediated mitochondrial-SR tethering. J Mol Cell Cardiol. 2015;78:123–128. doi: 10.1016/j.yjmcc.2014.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ong SB, Kalkhoran SB, Hernandez-Resendiz S, Samangouei P, Ong SG, Hausenloy DJ. Mitochondrial-Shaping Proteins in Cardiac Health and Disease - the Long and the Short of It! Cardiovasc Drugs Ther. 2017;31:87–107. doi: 10.1007/s10557-016-6710-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang W, Karamanlidis G, Tian R. Novel targets for mitochondrial medicine. Sci Transl Med. 2016;8:326rv323. doi: 10.1126/scitranslmed.aac7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hackenbrock CR. Ultrastructural bases for metabolically linked mechanical activity in mitochondria. II. Electron transport-linked ultrastructural transformations in mitochondria. J Cell Biol. 1968;37:345–369. doi: 10.1083/jcb.37.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mannella CA, Pfeiffer DR, Bradshaw PC, Moraru, Slepchenko B, Loew LM, Hsieh CE, Buttle K, Marko M. Topology of the mitochondrial inner membrane: dynamics and bioenergetic implications. IUBMB Life. 2001;52:93–100. doi: 10.1080/15216540152845885. [DOI] [PubMed] [Google Scholar]
- 18.Schrepfer E, Scorrano L. Mitofusins, from Mitochondria to Metabolism. Molecular cell. 2016;61:683–694. doi: 10.1016/j.molcel.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 19.Liesa M, Shirihai OS. Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab. 2013;17:491–506. doi: 10.1016/j.cmet.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Twig G, Shirihai OS. The interplay between mitochondrial dynamics and mitophagy. Antioxid Redox Signal. 2011;14:1939–1951. doi: 10.1089/ars.2010.3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shirihai OS, Song M, Dorn GW., 2nd How mitochondrial dynamism orchestrates mitophagy. Circulation research. 2015;116:1835–1849. doi: 10.1161/CIRCRESAHA.116.306374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Twig G, Elorza A, Molina AJ, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, Alroy J, Wu M, Py BF, Yuan J, Deeney JT, Corkey BE, Shirihai OS. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27:433–446. doi: 10.1038/sj.emboj.7601963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toyama EQ, Herzig S, Courchet J, Lewis TL, Jr, Loson OC, Hellberg K, Young NP, Chen H, Polleux F, Chan DC, Shaw RJ. Metabolism. AMP-activated protein kinase mediates mitochondrial fission in response to energy stress. Science (New York, NY) 2016;351:275–281. doi: 10.1126/science.aab4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dorn GW., 2nd Gone fission...: diverse consequences of cardiac Drp1 deficiency. Circulation research. 2015;116:225–228. doi: 10.1161/CIRCRESAHA.114.305672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ikeda Y, Shirakabe A, Maejima Y, Zhai P, Sciarretta S, Toli J, Nomura M, Mihara K, Egashira K, Ohishi M, Abdellatif M, Sadoshima J. Endogenous Drp1 mediates mitochondrial autophagy and protects the heart against energy stress. Circulation research. 2015;116:264–278. doi: 10.1161/CIRCRESAHA.116.303356. [DOI] [PubMed] [Google Scholar]
- 26.Kageyama Y, Hoshijima M, Seo K, Bedja D, Sysa-Shah P, Andrabi SA, Chen W, Hoke A, Dawson VL, Dawson TM, Gabrielson K, Kass DA, Iijima M, Sesaki H. Parkin-independent mitophagy requires Drp1 and maintains the integrity of mammalian heart and brain. EMBO J. 2014;33:2798–2813. doi: 10.15252/embj.201488658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song M, Mihara K, Chen Y, Scorrano L, Dorn GW., 2nd Mitochondrial fission and fusion factors reciprocally orchestrate mitophagic culling in mouse hearts and cultured fibroblasts. Cell Metab. 2015;21:273–285. doi: 10.1016/j.cmet.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Y, Dorn GW., 2nd PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science (New York, NY) 2013;340:471–475. doi: 10.1126/science.1231031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Narendra DP, Jin SM, Tanaka A, Suen DF, Gautier CA, Shen J, Cookson MR, Youle RJ. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010;8:e1000298. doi: 10.1371/journal.pbio.1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Billia F, Hauck L, Konecny F, Rao V, Shen J, Mak TW. PTEN-inducible kinase 1 (PINK1)/Park6 is indispensable for normal heart function. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:9572–9577. doi: 10.1073/pnas.1106291108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Papanicolaou KN, Khairallah RJ, Ngoh GA, Chikando A, Luptak I, O’Shea KM, Riley DD, Lugus JJ, Colucci WS, Lederer WJ, Stanley WC, Walsh K. Mitofusin-2 maintains mitochondrial structure and contributes to stress-induced permeability transition in cardiac myocytes. Mol Cell Biol. 2011;31:1309–1328. doi: 10.1128/MCB.00911-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Y, Csordas G, Jowdy C, Schneider TG, Csordas N, Wang W, Liu Y, Kohlhaas M, Meiser M, Bergem S, Nerbonne JM, Dorn GW, 2nd, Maack C. Mitofusin 2-containing mitochondrial-reticular microdomains direct rapid cardiomyocyte bioenergetic responses via interorganelle Ca(2+) crosstalk. Circulation research. 2012;111:863–875. doi: 10.1161/CIRCRESAHA.112.266585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valente EM, Abou-Sleiman PM, Caputo V, Muqit MM, Harvey K, Gispert S, Ali Z, Del Turco D, Bentivoglio AR, Healy DG, Albanese A, Nussbaum R, Gonzalez-Maldonado R, Deller T, Salvi S, Cortelli P, Gilks WP, Latchman DS, Harvey RJ, Dallapiccola B, Auburger G, Wood NW. Hereditary early-onset Parkinson’s disease caused by mutations in PINK1. Science (New York, NY) 2004;304:1158–1160. doi: 10.1126/science.1096284. [DOI] [PubMed] [Google Scholar]
- 34.Yue W, Chen Z, Liu H, Yan C, Chen M, Feng D, Wu H, Du L, Wang Y, Liu J, Huang X, Xia L, Liu L, Wang X, Jin H, Wang J, Song Z, Hao X, Chen Q. A small natural molecule promotes mitochondrial fusion through inhibition of the deubiquitinase USP30. Cell Res. 2014;24:482–496. doi: 10.1038/cr.2014.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishihara N, Nomura M, Jofuku A, Kato H, Suzuki SO, Masuda K, Otera H, Nakanishi Y, Nonaka I, Goto Y, Taguchi N, Morinaga H, Maeda M, Takayanagi R, Yokota S, Mihara K. Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nature cell biology. 2009;11:958–966. doi: 10.1038/ncb1907. [DOI] [PubMed] [Google Scholar]
- 36.Piquereau J, Caffin F, Novotova M, Prola A, Garnier A, Mateo P, Fortin D, Huynh le H, Nicolas V, Alavi MV, Brenner C, Ventura-Clapier R, Veksler V, Joubert F. Down-regulation of OPA1 alters mouse mitochondrial morphology, PTP function, and cardiac adaptation to pressure overload. Cardiovasc Res. 2012;94:408–417. doi: 10.1093/cvr/cvs117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Papanicolaou KN, Ngoh GA, Dabkowski ER, O’Connell KA, Ribeiro RF, Jr, Stanley WC, Walsh K. Cardiomyocyte deletion of mitofusin-1 leads to mitochondrial fragmentation and improves tolerance to ROS-induced mitochondrial dysfunction and cell death. American journal of physiology. Heart and circulatory physiology. 2012;302:H167–179. doi: 10.1152/ajpheart.00833.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wai T, Garcia-Prieto J, Baker MJ, Merkwirth C, Benit P, Rustin P, Ruperez FJ, Barbas C, Ibanez B, Langer T. Imbalanced OPA1 processing and mitochondrial fragmentation cause heart failure in mice. Science (New York, NY) 2015;350:aad0116. doi: 10.1126/science.aad0116. [DOI] [PubMed] [Google Scholar]
- 39.Zhang H, Wang P, Bisetto S, Yoon Y, Chen Q, Sheu SS, Wang W. A novel fission-independent role of dynamin-related protein 1 in cardiac mitochondrial respiration. Cardiovasc Res. 2017;113:160–170. doi: 10.1093/cvr/cvw212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qi X, Qvit N, Su YC, Mochly-Rosen D. A novel Drp1 inhibitor diminishes aberrant mitochondrial fission and neurotoxicity. Journal of cell science. 2013;126:789–802. doi: 10.1242/jcs.114439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jayashankar V, Rafelski SM. Integrating mitochondrial organization and dynamics with cellular architecture. Current opinion in cell biology. 2014;26:34–40. doi: 10.1016/j.ceb.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 42.Imoto M, Tachibana I, Urrutia R. Identification and functional characterization of a novel human protein highly related to the yeast dynamin-like GTPase Vps1p. Journal of cell science. 1998;111(Pt 10):1341–1349. doi: 10.1242/jcs.111.10.1341. [DOI] [PubMed] [Google Scholar]
- 43.Santel A, Frank S, Gaume B, Herrler M, Youle RJ, Fuller MT. Mitofusin-1 protein is a generally expressed mediator of mitochondrial fusion in mammalian cells. Journal of cell science. 2003;116:2763–2774. doi: 10.1242/jcs.00479. [DOI] [PubMed] [Google Scholar]
- 44.Dorn GW., 2nd Mitochondrial dynamism and heart disease: changing shape and shaping change. EMBO molecular medicine. 2015;7:865–877. doi: 10.15252/emmm.201404575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen Y, Liu Y, Dorn GW., 2nd Mitochondrial fusion is essential for organelle function and cardiac homeostasis. Circulation research. 2011;109:1327–1331. doi: 10.1161/CIRCRESAHA.111.258723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang X, Sun L, Ji S, Zhao T, Zhang W, Xu J, Zhang J, Wang Y, Wang X, Franzini-Armstrong C, Zheng M, Cheng H. Kissing and nanotunneling mediate intermitochondrial communication in the heart. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:2846–2851. doi: 10.1073/pnas.1300741110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eisner V, Cupo RR, Gao E, Csordas G, Slovinsky WS, Paillard M, Cheng L, Ibetti J, Chen SR, Chuprun JK, Hoek JB, Koch WJ, Hajnoczky G. Mitochondrial fusion dynamics is robust in the heart and depends on calcium oscillations and contractile activity. Proceedings of the National Academy of Sciences of the United States of America. 2017;114:E859–E868. doi: 10.1073/pnas.1617288114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Glancy B, Hartnell LM, Malide D, Yu ZX, Combs CA, Connelly PS, Subramaniam S, Balaban RS. Mitochondrial reticulum for cellular energy distribution in muscle. Nature. 2015;523:617–620. doi: 10.1038/nature14614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang W, Fang H, Groom L, Cheng A, Zhang W, Liu J, Wang X, Li K, Han P, Zheng M, Yin J, Mattson MP, Kao JP, Lakatta EG, Sheu SS, Ouyang K, Chen J, Dirksen RT, Cheng H. Superoxide flashes in single mitochondria. Cell. 2008;134:279–290. doi: 10.1016/j.cell.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang W, Gong G, Wang X, Wei-LaPierre L, Cheng H, Dirksen R, Sheu SS. Mitochondrial Flash: Integrative Reactive Oxygen Species and pH Signals in Cell and Organelle Biology. Antioxid Redox Signal. 2016;25:534–549. doi: 10.1089/ars.2016.6739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fang H, Chen M, Ding Y, Shang W, Xu J, Zhang X, Zhang W, Li K, Xiao Y, Gao F, Shang S, Li JC, Tian XL, Wang SQ, Zhou J, Weisleder N, Ma J, Ouyang K, Chen J, Wang X, Zheng M, Wang W, Cheng H. Imaging superoxide flash and metabolism-coupled mitochondrial permeability transition in living animals. Cell Res. 2011;21:1295–1304. doi: 10.1038/cr.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gong G, Liu X, Zhang H, Sheu SS, Wang W. Mitochondrial flash as a novel biomarker of mitochondrial respiration in the heart. American journal of physiology. Heart and circulatory physiology. 2015;309:H1166–1177. doi: 10.1152/ajpheart.00462.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Koshiba T, Detmer SA, Kaiser JT, Chen H, McCaffery JM, Chan DC. Structural basis of mitochondrial tethering by mitofusin complexes. Science (New York, NY) 2004;305:858–862. doi: 10.1126/science.1099793. [DOI] [PubMed] [Google Scholar]
- 54.Cassidy-Stone A, Chipuk JE, Ingerman E, Song C, Yoo C, Kuwana T, Kurth MJ, Shaw JT, Hinshaw JE, Green DR, Nunnari J. Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Dev Cell. 2008;14:193–204. doi: 10.1016/j.devcel.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wei L, Salahura G, Boncompagni S, Kasischke KA, Protasi F, Sheu SS, Dirksen RT. Mitochondrial superoxide flashes: metabolic biomarkers of skeletal muscle activity and disease. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2011;25:3068–3078. doi: 10.1096/fj.11-187252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu S, Wang P, Zhang H, Gong G, Gutierrez Cortes N, Zhu W, Yoon Y, Tian R, Wang W. CaMKII induces permeability transition through Drp1 phosphorylation during chronic beta-AR stimulation. Nat Commun. 2016;7:13189. doi: 10.1038/ncomms13189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cortassa S, Aon MA, Winslow RL, O’Rourke B. A mitochondrial oscillator dependent on reactive oxygen species. Biophysical journal. 2004;87:2060–2073. doi: 10.1529/biophysj.104.041749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial ROS-induced ROS release: an update and review. Biochimica et biophysica acta. 2006;1757:509–517. doi: 10.1016/j.bbabio.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 59.Ichas F, Jouaville LS, Mazat JP. Mitochondria are excitable organelles capable of generating and conveying electrical and calcium signals. Cell. 1997;89:1145–1153. doi: 10.1016/s0092-8674(00)80301-3. [DOI] [PubMed] [Google Scholar]
- 60.Hom J, Yu T, Yoon Y, Porter G, Sheu SS. Regulation of mitochondrial fission by intracellular Ca2+ in rat ventricular myocytes. Biochimica et biophysica acta. 2010;1797:913–921. doi: 10.1016/j.bbabio.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation. 2010;121:2012–2022. doi: 10.1161/CIRCULATIONAHA.109.906610. [DOI] [PubMed] [Google Scholar]
- 62.Filadi R, Greotti E, Turacchio G, Luini A, Pozzan T, Pizzo P. Mitofusin 2 ablation increases endoplasmic reticulum-mitochondria coupling. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:E2174–2181. doi: 10.1073/pnas.1504880112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mourier A, Motori E, Brandt T, Lagouge M, Atanassov I, Galinier A, Rappl G, Brodesser S, Hultenby K, Dieterich C, Larsson NG. Mitofusin 2 is required to maintain mitochondrial coenzyme Q levels. J Cell Biol. 2015;208:429–442. doi: 10.1083/jcb.201411100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kasahara A, Cipolat S, Chen Y, Dorn GW, 2nd, Scorrano L. Mitochondrial fusion directs cardiomyocyte differentiation via calcineurin and Notch signaling. Science (New York, NY) 2013;342:734–737. doi: 10.1126/science.1241359. [DOI] [PubMed] [Google Scholar]
- 65.Song M, Chen Y, Gong G, Murphy E, Rabinovitch PS, Dorn GW., 2nd Super-suppression of mitochondrial reactive oxygen species signaling impairs compensatory autophagy in primary mitophagic cardiomyopathy. Circulation research. 2014;115:348–353. doi: 10.1161/CIRCRESAHA.115.304384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao T, Huang X, Han L, Wang X, Cheng H, Zhao Y, Chen Q, Chen J, Xiao R, Zheng M. Central role of mitofusin 2 in autophagosome-lysosome fusion in cardiomyocytes. J Biol Chem. 2012;287:23615–23625. doi: 10.1074/jbc.M112.379164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gong G, Song M, Csordas G, Kelly DP, Matkovich SJ, Dorn GW., 2nd Parkin-mediated mitophagy directs perinatal cardiac metabolic maturation in mice. Science (New York, NY) 2015;350:aad2459. doi: 10.1126/science.aad2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sharma VK, Ramesh V, Franzini-Armstrong C, Sheu SS. Transport of Ca2+ from sarcoplasmic reticulum to mitochondria in rat ventricular myocytes. J Bioenerg Biomembr. 2000;32:97–104. doi: 10.1023/a:1005520714221. [DOI] [PubMed] [Google Scholar]
- 69.Tubbs E, Rieusset J. Metabolic signaling functions of ER-mitochondria contact sites: role in metabolic diseases. J Mol Endocrinol. 2017;58:R87–R106. doi: 10.1530/JME-16-0189. [DOI] [PubMed] [Google Scholar]
- 70.Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science (New York, NY) 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- 71.de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- 72.Csordas G, Renken C, Varnai P, Walter L, Weaver D, Buttle KF, Balla T, Mannella CA, Hajnoczky G. Structural and functional features and significance of the physical linkage between ER and mitochondria. J Cell Biol. 2006;174:915–921. doi: 10.1083/jcb.200604016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cogliati S, Frezza C, Soriano ME, Varanita T, Quintana-Cabrera R, Corrado M, Cipolat S, Costa V, Casarin A, Gomes LC, Perales-Clemente E, Salviati L, Fernandez-Silva P, Enriquez JA, Scorrano L. Mitochondrial cristae shape determines respiratory chain supercomplexes assembly and respiratory efficiency. Cell. 2013;155:160–171. doi: 10.1016/j.cell.2013.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Frezza C, Cipolat S, Martins de Brito O, Micaroni M, Beznoussenko GV, Rudka T, Bartoli D, Polishuck RS, Danial NN, De Strooper B, Scorrano L. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell. 2006;126:177–189. doi: 10.1016/j.cell.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 75.Quintana-Cabrera R, Mehrotra A, Rigoni G, Soriano ME. Who and how in the regulation of mitochondrial cristae shape and function. Biochem Biophys Res Commun. 2017 doi: 10.1016/j.bbrc.2017.04.088. [DOI] [PubMed] [Google Scholar]
- 76.Varanita T, Soriano ME, Romanello V, Zaglia T, Quintana-Cabrera R, Semenzato M, Menabo R, Costa V, Civiletto G, Pesce P, Viscomi C, Zeviani M, Di Lisa F, Mongillo M, Sandri M, Scorrano L. The opa1-dependent mitochondrial cristae remodeling pathway controls atrophic, apoptotic, and ischemic tissue damage. Cell Metabolism. 2015;21:834–844. doi: 10.1016/j.cmet.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Civiletto G, Varanita T, Cerutti R, Gorletta T, Barbaro S, Marchet S, Lamperti C, Viscomi C, Scorrano L, Zeviani M. Opa1 overexpression ameliorates the phenotype of two mitochondrial disease mouse models. Cell Metabolism. 2015;21:845–854. doi: 10.1016/j.cmet.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen H, Chomyn A, Chan DC. Disruption of fusion results in mitochondrial heterogeneity and dysfunction. J Biol Chem. 2005;280:26185–26192. doi: 10.1074/jbc.M503062200. [DOI] [PubMed] [Google Scholar]
- 79.Song Z, Chen H, Fiket M, Alexander C, Chan DC. OPA1 processing controls mitochondrial fusion and is regulated by mRNA splicing, membrane potential, and Yme1L. J Cell Biol. 2007;178:749–755. doi: 10.1083/jcb.200704110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Delettre C, Griffoin JM, Kaplan J, Dollfus H, Lorenz B, Faivre L, Lenaers G, Belenguer P, Hamel CP. Mutation spectrum and splicing variants in the OPA1 gene. Hum Genet. 2001;109:584–591. doi: 10.1007/s00439-001-0633-y. [DOI] [PubMed] [Google Scholar]
- 81.Lee H, Smith SB, Yoon Y. The short variant of the mitochondrial dynamin OPA1 maintains mitochondrial energetics and cristae structure. J Biol Chem. 2017;292:7115–7130. doi: 10.1074/jbc.M116.762567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ishihara N, Fujita Y, Oka T, Mihara K. Regulation of mitochondrial morphology through proteolytic cleavage of OPA1. EMBO J. 2006;25:2966–2977. doi: 10.1038/sj.emboj.7601184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Marchi S, Patergnani S, Pinton P. The endoplasmic reticulum-mitochondria connection: one touch, multiple functions. Biochimica et biophysica acta. 2014;1837:461–469. doi: 10.1016/j.bbabio.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 84.Marchi S, Pinton P. The mitochondrial calcium uniporter complex: molecular components, structure and physiopathological implications. J Physiol. 2014;592:829–839. doi: 10.1113/jphysiol.2013.268235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.De La Fuente S, Fernandez-Sanz C, Vail C, Agra EJ, Holmstrom K, Sun J, Mishra J, Williams D, Finkel T, Murphy E, Joseph SK, Sheu SS, Csordas G. Strategic Positioning and Biased Activity of the Mitochondrial Calcium Uniporter in Cardiac Muscle. J Biol Chem. 2016;291:23343–23362. doi: 10.1074/jbc.M116.755496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lopez-Crisosto C, Pennanen C, Vasquez-Trincado C, Morales PE, Bravo-Sagua R, Quest AFG, Chiong M, Lavandero S. Sarcoplasmic reticulum-mitochondria communication in cardiovascular pathophysiology. Nat Rev Cardiol. 2017;14:342–360. doi: 10.1038/nrcardio.2017.23. [DOI] [PubMed] [Google Scholar]
- 87.Naon D, Zaninello M, Giacomello M, Varanita T, Grespi F, Lakshminaranayan S, Serafini A, Semenzato M, Herkenne S, Hernandez-Alvarez MI, Zorzano A, De Stefani D, Dorn GW, 2nd, Scorrano L. Critical reappraisal confirms that Mitofusin 2 is an endoplasmic reticulum-mitochondria tether. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:11249–11254. doi: 10.1073/pnas.1606786113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sugiura A, Nagashima S, Tokuyama T, Amo T, Matsuki Y, Ishido S, Kudo Y, McBride HM, Fukuda T, Matsushita N, Inatome R, Yanagi S. MITOL regulates endoplasmic reticulum-mitochondria contacts via Mitofusin2. Molecular cell. 2013;51:20–34. doi: 10.1016/j.molcel.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 89.Cosson P, Marchetti A, Ravazzola M, Orci L. Mitofusin-2 independent juxtaposition of endoplasmic reticulum and mitochondria: an ultrastructural study. PLoS One. 2012;7:e46293. doi: 10.1371/journal.pone.0046293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yoshikane H, Nihei T, Moriyama K. Three-dimensional observation of intracellular membranous structures in dog heart muscle cells by scanning electron microscopy. J Submicrosc Cytol. 1986;18:629–636. [PubMed] [Google Scholar]
- 91.Otera H, Miyata N, Kuge O, Mihara K. Drp1-dependent mitochondrial fission via MiD49/51 is essential for apoptotic cristae remodeling. J Cell Biol. 2016;212:531–544. doi: 10.1083/jcb.201508099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ji WK, Hatch AL, Merrill RA, Strack S, Higgs HN. Actin filaments target the oligomeric maturation of the dynamin GTPase Drp1 to mitochondrial fission sites. Elife. 2015;4:e11553. doi: 10.7554/eLife.11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Friedman JR, Lackner LL, West M, DiBenedetto JR, Nunnari J, Voeltz GK. ER tubules mark sites of mitochondrial division. Science (New York, NY) 2011;334:358–362. doi: 10.1126/science.1207385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Korobova F, Ramabhadran V, Higgs HN. An actin-dependent step in mitochondrial fission mediated by the ER-associated formin INF2. Science (New York, NY) 2013;339:464–467. doi: 10.1126/science.1228360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Scriven DR, Asghari P, Moore ED. Microarchitecture of the dyad. Cardiovasc Res. 2013;98:169–176. doi: 10.1093/cvr/cvt025. [DOI] [PubMed] [Google Scholar]
- 96.Montessuit S, Somasekharan SP, Terrones O, Lucken-Ardjomande S, Herzig S, Schwarzenbacher R, Manstein DJ, Bossy-Wetzel E, Basanez G, Meda P, Martinou JC. Membrane remodeling induced by the dynamin-related protein Drp1 stimulates Bax oligomerization. Cell. 2010;142:889–901. doi: 10.1016/j.cell.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Antignani A, Youle RJ. How do Bax and Bak lead to permeabilization of the outer mitochondrial membrane? Current opinion in cell biology. 2006;18:685–689. doi: 10.1016/j.ceb.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 98.Scorrano L, Oakes SA, Opferman JT, Cheng EH, Sorcinelli MD, Pozzan T, Korsmeyer SJ. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science (New York, NY) 2003;300:135–139. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- 99.Karch J, Kwong JQ, Burr AR, Sargent MA, Elrod JW, Peixoto PM, Martinez-Caballero S, Osinska H, Cheng EH, Robbins J, Kinnally KW, Molkentin JD. Bax and Bak function as the outer membrane component of the mitochondrial permeability pore in regulating necrotic cell death in mice. Elife. 2013;2:e00772. doi: 10.7554/eLife.00772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 101.Giorgio V, von Stockum S, Antoniel M, Fabbro A, Fogolari F, Forte M, Glick GD, Petronilli V, Zoratti M, Szabo I, Lippe G, Bernardi P. Dimers of mitochondrial ATP synthase form the permeability transition pore. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:5887–5892. doi: 10.1073/pnas.1217823110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Alavian KN, Beutner G, Lazrove E, Sacchetti S, Park HA, Licznerski P, Li H, Nabili P, Hockensmith K, Graham M, Porter GA, Jr, Jonas EA. An uncoupling channel within the c-subunit ring of the F1FO ATP synthase is the mitochondrial permeability transition pore. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:10580–10585. doi: 10.1073/pnas.1401591111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Naghdi S, Hajnoczky G. VDAC2-specific cellular functions and the underlying structure. Biochimica et biophysica acta. 2016 doi: 10.1016/j.bbamcr.2016.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ding C, Wu Z, Huang L, Wang Y, Xue J, Chen S, Deng Z, Wang L, Song Z. Mitofilin and CHCHD6 physically interact with Sam50 to sustain cristae structure. Scientific reports. 2015;5:16064. doi: 10.1038/srep16064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Darshi M, Mendiola VL, Mackey MR, Murphy AN, Koller A, Perkins GA, Ellisman MH, Taylor SS. ChChd3, an inner mitochondrial membrane protein, is essential for maintaining crista integrity and mitochondrial function. J Biol Chem. 2011;286:2918–2932. doi: 10.1074/jbc.M110.171975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.van der Laan M, Horvath SE, Pfanner N. Mitochondrial contact site and cristae organizing system. Current opinion in cell biology. 2016;41:33–42. doi: 10.1016/j.ceb.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 107.Wollweber F, von der Malsburg K, van der Laan M. Mitochondrial contact site and cristae organizing system: A central player in membrane shaping and crosstalk. Biochimica et biophysica acta. 2017 doi: 10.1016/j.bbamcr.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 108.Brdiczka DG, Zorov DB, Sheu SS. Mitochondrial contact sites: their role in energy metabolism and apoptosis. Biochimica et biophysica acta. 2006;1762:148–163. doi: 10.1016/j.bbadis.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 109.Brenner C, Grimm S. The permeability transition pore complex in cancer cell death. Oncogene. 2006;25:4744–4756. doi: 10.1038/sj.onc.1209609. [DOI] [PubMed] [Google Scholar]
- 110.Shanmughapriya S, Rajan S, Hoffman NE, Higgins AM, Tomar D, Nemani N, Hines KJ, Smith DJ, Eguchi A, Vallem S, Shaikh F, Cheung M, Leonard NJ, Stolakis RS, Wolfers MP, Ibetti J, Chuprun JK, Jog NR, Houser SR, Koch WJ, Elrod JW, Madesh M. SPG7 Is an Essential and Conserved Component of the Mitochondrial Permeability Transition Pore. Molecular cell. 2015;60:47–62. doi: 10.1016/j.molcel.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rasola A, Sciacovelli M, Pantic B, Bernardi P. Signal transduction to the permeability transition pore. FEBS letters. 2010;584:1989–1996. doi: 10.1016/j.febslet.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yamaguchi R, Lartigue L, Perkins G, Scott RT, Dixit A, Kushnareva Y, Kuwana T, Ellisman MH, Newmeyer DD. Opa1-mediated cristae opening is Bax/Bak and BH3 dependent, required for apoptosis, and independent of Bak oligomerization. Molecular cell. 2008;31:557–569. doi: 10.1016/j.molcel.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Brown DA, Perry JB, Allen ME, Sabbah HN, Stauffer BL, Shaikh SR, Cleland JG, Colucci WS, Butler J, Voors AA, Anker SD, Pitt B, Pieske B, Filippatos G, Greene SJ, Gheorghiade M. Expert consensus document: Mitochondrial function as a therapeutic target in heart failure. Nat Rev Cardiol. 2017;14:238–250. doi: 10.1038/nrcardio.2016.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nan J, Zhu W, Rahman MS, Liu M, Li D, Su S, Zhang N, Hu X, Yu H, Gupta MP, Wang J. Molecular regulation of mitochondrial dynamics in cardiac disease. Biochimica et biophysica acta. 2017;1864:1260–1273. doi: 10.1016/j.bbamcr.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 115.Ho D, Yan L, Iwatsubo K, Vatner DE, Vatner SF. Modulation of beta-adrenergic receptor signaling in heart failure and longevity: targeting adenylyl cyclase type 5. Heart Fail Rev. 2010;15:495–512. doi: 10.1007/s10741-010-9183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Grimm M, Brown JH. Beta-adrenergic receptor signaling in the heart: role of CaMKII. J Mol Cell Cardiol. 2010;48:322–330. doi: 10.1016/j.yjmcc.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Marin-Garcia J, Akhmedov AT. Mitochondrial dynamics and cell death in heart failure. Heart Fail Rev. 2016;21:123–136. doi: 10.1007/s10741-016-9530-2. [DOI] [PubMed] [Google Scholar]
- 118.Chang CR, Blackstone C. Cyclic AMP-dependent protein kinase phosphorylation of Drp1 regulates its GTPase activity and mitochondrial morphology. J Biol Chem. 2007;282:21583–21587. doi: 10.1074/jbc.C700083200. [DOI] [PubMed] [Google Scholar]
- 119.Cereghetti GM, Stangherlin A, Martins de Brito O, Chang CR, Blackstone C, Bernardi P, Scorrano L. Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:15803–15808. doi: 10.1073/pnas.0808249105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pfluger PT, Kabra DG, Aichler M, Schriever SC, Pfuhlmann K, Garcia VC, Lehti M, Weber J, Kutschke M, Rozman J, Elrod JW, Hevener AL, Feuchtinger A, Hrabe de Angelis M, Walch A, Rollmann SM, Aronow BJ, Muller TD, Perez-Tilve D, Jastroch M, De Luca M, Molkentin JD, Tschop MH. Calcineurin Links Mitochondrial Elongation with Energy Metabolism. Cell Metab. 2015;22:838–850. doi: 10.1016/j.cmet.2015.08.022. [DOI] [PubMed] [Google Scholar]
- 121.Din S, Mason M, Volkers M, Johnson B, Cottage CT, Wang Z, Joyo AY, Quijada P, Erhardt P, Magnuson NS, Konstandin MH, Sussman MA. Pim-1 preserves mitochondrial morphology by inhibiting dynamin-related protein 1 translocation. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:5969–5974. doi: 10.1073/pnas.1213294110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Taguchi N, Ishihara N, Jofuku A, Oka T, Mihara K. Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J Biol Chem. 2007;282:11521–11529. doi: 10.1074/jbc.M607279200. [DOI] [PubMed] [Google Scholar]
- 123.Marsboom G, Toth PT, Ryan JJ, Hong Z, Wu X, Fang YH, Thenappan T, Piao L, Zhang HJ, Pogoriler J, Chen Y, Morrow E, Weir EK, Rehman J, Archer SL. Dynamin-related protein 1-mediated mitochondrial mitotic fission permits hyperproliferation of vascular smooth muscle cells and offers a novel therapeutic target in pulmonary hypertension. Circulation research. 2012;110:1484–1497. doi: 10.1161/CIRCRESAHA.111.263848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen L, Gong Q, Stice JP, Knowlton AA. Mitochondrial OPA1, apoptosis, and heart failure. Cardiovasc Res. 2009;84:91–99. doi: 10.1093/cvr/cvp181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Murphy E, Ardehali H, Balaban RS, DiLisa F, Dorn GW, 2nd, Kitsis RN, Otsu K, Ping P, Rizzuto R, Sack MN, Wallace D, Youle RJ. Mitochondrial Function, Biology, and Role in Disease: A Scientific Statement From the American Heart Association. Circulation research. 2016;118:1960–1991. doi: 10.1161/RES.0000000000000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Brady NR, Hamacher-Brady A, Gottlieb RA. Proapoptotic BCL-2 family members and mitochondrial dysfunction during ischemia/reperfusion injury, a study employing cardiac HL-1 cells and GFP biosensors. Biochimica et biophysica acta. 2006;1757:667–678. doi: 10.1016/j.bbabio.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 127.Wang JX, Jiao JQ, Li Q, Long B, Wang K, Liu JP, Li YR, Li PF. miR-499 regulates mitochondrial dynamics by targeting calcineurin and dynamin-related protein-1. Nat Med. 2011;17:71–78. doi: 10.1038/nm.2282. [DOI] [PubMed] [Google Scholar]
- 128.Sharp WW, Fang YH, Han M, Zhang HJ, Hong Z, Banathy A, Morrow E, Ryan JJ, Archer SL. Dynamin-related protein 1 (Drp1)-mediated diastolic dysfunction in myocardial ischemia-reperfusion injury: therapeutic benefits of Drp1 inhibition to reduce mitochondrial fission. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2014;28:316–326. doi: 10.1096/fj.12-226225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zaja I, Bai X, Liu Y, Kikuchi C, Dosenovic S, Yan Y, Canfield SG, Bosnjak ZJ. Cdk1, PKCdelta and calcineurin-mediated Drp1 pathway contributes to mitochondrial fission-induced cardiomyocyte death. Biochem Biophys Res Commun. 2014;453:710–721. doi: 10.1016/j.bbrc.2014.09.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zepeda R, Kuzmicic J, Parra V, Troncoso R, Pennanen C, Riquelme JA, Pedrozo Z, Chiong M, Sanchez G, Lavandero S. Drp1 loss-of-function reduces cardiomyocyte oxygen dependence protecting the heart from ischemia-reperfusion injury. J Cardiovasc Pharmacol. 2014;63:477–487. doi: 10.1097/FJC.0000000000000071. [DOI] [PubMed] [Google Scholar]
- 131.Ishikita A, Matoba T, Ikeda G, Koga J, Mao Y, Nakano K, Takeuchi O, Sadoshima J, Egashira K. Nanoparticle-Mediated Delivery of Mitochondrial Division Inhibitor 1 to the Myocardium Protects the Heart From Ischemia-Reperfusion Injury Through Inhibition of Mitochondria Outer Membrane Permeabilization: A New Therapeutic Modality for Acute Myocardial Infarction. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.003872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Disatnik MH, Ferreira JC, Campos JC, Gomes KS, Dourado PM, Qi X, Mochly-Rosen D. Acute inhibition of excessive mitochondrial fission after myocardial infarction prevents long-term cardiac dysfunction. J Am Heart Assoc. 2013;2:e000461. doi: 10.1161/JAHA.113.000461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Le Page S, Niro M, Fauconnier J, Cellier L, Tamareille S, Gharib A, Chevrollier A, Loufrani L, Grenier C, Kamel R, Sarzi E, Lacampagne A, Ovize M, Henrion D, Reynier P, Lenaers G, Mirebeau-Prunier D, Prunier F. Increase in Cardiac Ischemia-Reperfusion Injuries in Opa1+/− Mouse Model. PLoS One. 2016;11:e0164066. doi: 10.1371/journal.pone.0164066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Li J, Li Y, Jiao J, Wang J, Qin D, Li P. Mitofusin 1 is negatively regulated by microRNA 140 in cardiomyocyte apoptosis. Mol Cell Biol. 2014;34:1788–1799. doi: 10.1128/MCB.00774-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hall AR, Burke N, Dongworth RK, Kalkhoran SB, Dyson A, Vicencio JM, Dorn GW, II, Yellon DM, Hausenloy DJ. Hearts deficient in both Mfn1 and Mfn2 are protected against acute myocardial infarction. Cell Death Dis. 2016;7:e2238. doi: 10.1038/cddis.2016.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yoon Y, Galloway CA, Jhun BS, Yu T. Mitochondrial dynamics in diabetes. Antioxid Redox Signal. 2011;14:439–457. doi: 10.1089/ars.2010.3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zorzano A, Liesa M, Palacin M. Role of mitochondrial dynamics proteins in the pathophysiology of obesity and type 2 diabetes. Int J Biochem Cell Biol. 2009;41:1846–1854. doi: 10.1016/j.biocel.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 138.Sood A, Jeyaraju DV, Prudent J, Caron A, Lemieux P, McBride HM, Laplante M, Toth K, Pellegrini L. A Mitofusin-2-dependent inactivating cleavage of Opa1 links changes in mitochondria cristae and ER contacts in the postprandial liver. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:16017–16022. doi: 10.1073/pnas.1408061111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Arruda AP, Pers BM, Parlakgul G, Guney E, Inouye K, Hotamisligil GS. Chronic enrichment of hepatic endoplasmic reticulum-mitochondria contact leads to mitochondrial dysfunction in obesity. Nat Med. 2014;20:1427–1435. doi: 10.1038/nm.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jheng HF, Tsai PJ, Guo SM, Kuo LH, Chang CS, Su IJ, Chang CR, Tsai YS. Mitochondrial fission contributes to mitochondrial dysfunction and insulin resistance in skeletal muscle. Mol Cell Biol. 2012;32:309–319. doi: 10.1128/MCB.05603-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Westermeier F, Navarro-Marquez M, Lopez-Crisosto C, Bravo-Sagua R, Quiroga C, Bustamante M, Verdejo HE, Zalaquett R, Ibacache M, Parra V, Castro PF, Rothermel BA, Hill JA, Lavandero S. Defective insulin signaling and mitochondrial dynamics in diabetic cardiomyopathy. Biochimica et biophysica acta. 2015;1853:1113–1118. doi: 10.1016/j.bbamcr.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Galloway CA, Yoon Y. Mitochondrial dynamics in diabetic cardiomyopathy. Antioxid Redox Signal. 2015;22:1545–1562. doi: 10.1089/ars.2015.6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation. 2007;115:3213–3223. doi: 10.1161/CIRCULATIONAHA.106.679597. [DOI] [PubMed] [Google Scholar]
- 144.Montaigne D, Marechal X, Coisne A, Debry N, Modine T, Fayad G, Potelle C, El Arid JM, Mouton S, Sebti Y, Duez H, Preau S, Remy-Jouet I, Zerimech F, Koussa M, Richard V, Neviere R, Edme JL, Lefebvre P, Staels B. Myocardial contractile dysfunction is associated with impaired mitochondrial function and dynamics in type 2 diabetic but not in obese patients. Circulation. 2014;130:554–564. doi: 10.1161/CIRCULATIONAHA.113.008476. [DOI] [PubMed] [Google Scholar]
- 145.Yu T, Jhun BS, Yoon Y. High-glucose stimulation increases reactive oxygen species production through the calcium and mitogen-activated protein kinase-mediated activation of mitochondrial fission. Antioxid Redox Signal. 2011;14:425–437. doi: 10.1089/ars.2010.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yu T, Sheu SS, Robotham JL, Yoon Y. Mitochondrial fission mediates high glucose-induced cell death through elevated production of reactive oxygen species. Cardiovasc Res. 2008;79:341–351. doi: 10.1093/cvr/cvn104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yu T, Robotham JL, Yoon Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:2653–2658. doi: 10.1073/pnas.0511154103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gawlowski T, Suarez J, Scott B, Torres-Gonzalez M, Wang H, Schwappacher R, Han X, Yates JR, 3rd, Hoshijima M, Dillmann W. Modulation of dynamin-related protein 1 (DRP1) function by increased O-linked-beta-N-acetylglucosamine modification (O-GlcNAc) in cardiac myocytes. J Biol Chem. 2012;287:30024–30034. doi: 10.1074/jbc.M112.390682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Galloway CA, Lee H, Nejjar S, Jhun BS, Yu T, Hsu W, Yoon Y. Transgenic control of mitochondrial fission induces mitochondrial uncoupling and relieves diabetic oxidative stress. Diabetes. 2012;61:2093–2104. doi: 10.2337/db11-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Makino A, Suarez J, Gawlowski T, Han W, Wang H, Scott BT, Dillmann WH. Regulation of mitochondrial morphology and function by O-GlcNAcylation in neonatal cardiac myocytes. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1296–1302. doi: 10.1152/ajpregu.00437.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Parra V, Verdejo HE, Iglewski M, Del Campo A, Troncoso R, Jones D, Zhu Y, Kuzmicic J, Pennanen C, Lopez-Crisosto C, Jana F, Ferreira J, Noguera E, Chiong M, Bernlohr DA, Klip A, Hill JA, Rothermel BA, Abel ED, Zorzano A, Lavandero S. Insulin stimulates mitochondrial fusion and function in cardiomyocytes via the Akt-mTOR-NFkappaB-Opa-1 signaling pathway. Diabetes. 2014;63:75–88. doi: 10.2337/db13-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bordt EA, Clerc P, Roelofs BA, Saladino AJ, Tretter L, Adam-Vizi V, Cherok E, Khalil A, Yadava N, Ge SX, Francis TC, Kennedy NW, Picton LK, Kumar T, Uppuluri S, Miller AM, Itoh K, Karbowski M, Sesaki H, Hill RB, Polster BM. The Putative Drp1 Inhibitor mdivi-1 Is a Reversible Mitochondrial Complex I Inhibitor that Modulates Reactive Oxygen Species. Dev Cell. 2017;40:583–594. e586. doi: 10.1016/j.devcel.2017.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Osellame LD, Singh AP, Stroud DA, Palmer CS, Stojanovski D, Ramachandran R, Ryan MT. Cooperative and independent roles of the Drp1 adaptors Mff, MiD49 and MiD51 in mitochondrial fission. Journal of cell science. 2016;129:2170–2181. doi: 10.1242/jcs.185165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Loson OC, Song Z, Chen H, Chan DC. Fis1, Mff, MiD49, and MiD51 mediate Drp1 recruitment in mitochondrial fission. Mol Biol Cell. 2013;24:659–667. doi: 10.1091/mbc.E12-10-0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Franco A, Kitsis RN, Fleischer JA, Gavathiotis E, Kornfeld OS, Gong G, Biris N, Benz A, Qvit N, Donnelly SK, Chen Y, Mennerick S, Hodgson L, Mochly-Rosen D, Dorn GW., II Correcting mitochondrial fusion by manipulating mitofusin conformations. Nature. 2016;540:74–79. doi: 10.1038/nature20156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Csordas G, Varnai P, Golenar T, Roy S, Purkins G, Schneider TG, Balla T, Hajnoczky G. Imaging interorganelle contacts and local calcium dynamics at the ER-mitochondrial interface. Molecular cell. 2010;39:121–132. doi: 10.1016/j.molcel.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Song LS, Sobie EA, McCulle S, Lederer WJ, Balke CW, Cheng H. Orphaned ryanodine receptors in the failing heart. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:4305–4310. doi: 10.1073/pnas.0509324103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Adachi Y, Itoh K, Yamada T, Cerveny KL, Suzuki TL, Macdonald P, Frohman MA, Ramachandran R, Iijima M, Sesaki H. Coincident Phosphatidic Acid Interaction Restrains Drp1 in Mitochondrial Division. Molecular cell. 2016;63:1034–1043. doi: 10.1016/j.molcel.2016.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Cao YL, Meng S, Chen Y, Feng JX, Gu DD, Yu B, Li YJ, Yang JY, Liao S, Chan DC, Gao S. MFN1 structures reveal nucleotide-triggered dimerization critical for mitochondrial fusion. Nature. 2017;542:372–376. doi: 10.1038/nature21077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Filadi R, Greotti E, Turacchio G, Luini A, Pozzan T, Pizzo P. On the role of Mitofusin 2 in endoplasmic reticulum-mitochondria tethering. Proceedings of the National Academy of Sciences of the United States of America. 2017;114:E2266–E2267. doi: 10.1073/pnas.1616040114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Naon D, Zaninello M, Giacomello M, Varanita T, Grespi F, Lakshminaranayan S, Serafini A, Semenzato M, Herkenne S, Hernandez-Alvarez MI, Zorzano A, De Stefani D, Dorn GW, 2nd, Scorrano L. Reply to Filadi et al.: Does Mitofusin 2 tether or separate endoplasmic reticulum and mitochondria? Proceedings of the National Academy of Sciences of the United States of America. 2017;114:E2268–E2269. doi: 10.1073/pnas.1618610114. [DOI] [PMC free article] [PubMed] [Google Scholar]