Abstract
Recent research indicates that the expression of long non-coding and endogenous retroviral RNAs is coordinated with the activity of immune molecules often dysregulated in schizophrenia. We measured the expression of TMEVPG1, NRON, HERV-W env and HERV-W gag in blood cells from participants with schizophrenia and controls. We report that a) expression levels of these non-coding RNAs are correlated with proinflammatory cytokine mRNA expression in all participants, b) HERV-W transcripts are negatively correlated with atypical antipsychotic use in participants with schizophrenia, and c) that these RNAs are transcribed in response to proinflammatory stimuli in a THP-1 monocyte cell line.
Keywords: lncRNA, HERV-W, cytokine
1. Introduction
Persistent sub-clinical inflammation, primarily characterized by elevated expression of proinflammatory cytokines, including IL-6, TNF-α and IFN- γ, is a well replicated finding in the schizophrenia literature (Chase et al., 2016, 2015; Miller et al., 2011). Additionally, we and others have shown that corresponding inflammatory cell signaling pathways (JAK-STAT1 and NF-κB) are also affected (Sharma et al., 2016; Song et al., 2009). However, the explanation for and potential pathophysiological consequences of inflammation in this disorder remain to be established.
Altered activity of long non-coding RNAs (lncRNAs) has been hypothesized to contribute to neuropsychiatric disorders, including schizophrenia (Huang et al., 2016), and lncRNAs are shown to play critical roles in the regulation of immune function (Satpathy and Chang, 2015). For example, the lncRNA TMEVPG1 enhances the expression of IFN-γ (Collier et al., 2012), and the lncRNA NRON regulates the transcription factor NFAT, which in turn controls the expression of a number of cytokines including IL-6 and IFN-γ (Fenimore and Young, 2016; Fric et al., 2012; Willingham et al., 2005). Reciprocally, inflammatory cytokines and stimuli can alter the expression of lncRNAs (Ilott et al., 2014; Kambara et al., 2014). Endogenous retroviral transcripts are another type of predominantly non-coding RNA that have been implicated in inflammation, and elevated expression of human endogenous retrovirus type W (HERV-W) has been repeatedly reported in individuals with schizophrenia (Li and Karlsson, 2016 for review).
To our knowledge, expression analyses of lncRNAs related to immune function have not been previously reported in the schizophrenia literature, nor have HERV transcripts been measured in relation to cytokine gene expression. We hypothesized an induction of non-coding RNAs as a consequence of chronically elevated immune activity in schizophrenia and to investigate this we used several approaches. Firstly, we examined for a diagnostic effect on non-coding RNA expression. Secondly, we determined whether there was an association between long non-coding (TMEVPG1 and NRON) and endogenous retroviral (HERV-W env and HERV-W gag) RNA levels with markers of inflammation in this human sample. We selected IL-6 and TNF-α based on our previous findings of elevated mRNA expression in schizophrenia (Chase et al, 2015; Chase et al, 2016), and IFN-γ because of its interaction with the selected non-coding RNAs and previously reported increased levels in schizophrenia (Miller et al 2011). Finally, we examined the effects of stimuli that activate immune signaling pathways shown to be altered in schizophrenia on expression of these RNAs using a human monocyte cell line.
2. Method
2.1 Participant information and clinical measures
The study was approved by the IRB of the University of Illinois, and signed consent was obtained prior to the initiation of study procedures. Participants with schizophrenia (n=17) and control (n=16) groups were matched for age (36±13.9 and 39±10.4), race and BMI (31.5±9.9 and 31.9±9.2). There were more males in the group of participants with schizophrenia (12 males and 5 females) and more females in the control participant group (12 females and 4 males), however we found no effect of sex on any of the variables measured. With regards to nicotine consumption, 3 control participants and 8 participants with schizophrenia were self-reported smokers. Again, we found no effect of smoking status on non-coding RNA expression. All but 2 participants with schizophrenia were taking antipsychotic medication (2 typical and 12 atypical), and dosage was converted to chlorpromazine (CPZ) equivalents (Danivas and Venkatasubramanian 2013; Gardner et al. 2010).
2.2 Sample Collection, Processing and qPCR
Collection of blood samples, PBMC isolation, RNA extraction, DNase treatment and quantitative real-time PCR (qPCR) were carried out according to previously described protocols (Chase et al., 2016). Relative quantification values were calculated for TMEVPG1, NRON, HERV-W env, HERV-W gag and IFN-γ using the geometric mean of the housekeeping genes GAPDH and ACTB. Primer sequences are available from the authors on request.
2.3 THP-1 Cell Culture and Treatment
THP-1 cells (ATCC) were maintained in RPMI 1640 medium (Gibco) supplemented with 10% FBS (Gibco), l. glutamine and penicillin/streptomycin and incubated at 37°C with 5% CO2. Wells of 1×106 cells were treated with a) 10 ng/ml recombinant IFN-γ (BD Pharmingen) b) 100 ng/ml lipopolysaccharide (LPS; e. coli; 0111:B4; Sigma) or c) vehicle, and harvested at 1, 6 and 24 hour time points. RNA extraction and qPCR were carried out as above.
2.4 Data Analysis
SPSS (version 22.0 for Windows) was used for all statistical analyses. Independent samples t-tests and multiple regressions controlling for covariates (age and sex) were used to examine expression data for differences in lncRNA and HERV transcript levels in control participants and those with schizophrenia. Spearman’s correlations were carried out to determine the association of lncRNA and HERV transcript expression with IL-6, TNF-α and IFN-γ mRNA expression.
3. Results
3.1 No significant difference between diagnostic groups for TMEVPG1, NRON or HERV-W transcripts
There was no effect of diagnosis (control vs participants with schizophrenia), age, or sex, on RNA expression levels of TMEVPG1, NRON, HERV-W env or HERV-W gag when examined using t-tests or multiple regression (Table 1). Additionally, there was no significant difference in IFN-γ mRNA expression (p=.35) in participants with schizophrenia (M=37.79, SD=35.34) compared to controls (M=51.13, SD=42.44). IL-6 and TNF-α mRNA expression levels had been previously collected and reported and are presented here for completeness (Chase et al., 2016, 2015); IL-6 (p=.05) and TNF-α (p=.004) mRNA expression levels as earlier reported are significantly elevated in participants with schizophrenia (IL-6: M=4.14, SD=2.42; TNF-α: M=2.12, SD=.64) compared to controls (IL-6: M=2.77, SD=1.23; TNF-α: M=1.55, SD=.37).
Table 1.
Mean, standard deviation and p-value from t-tests for non-coding RNAs in each diagnostic group. Spearman’s rho correlation results for associations with inflammatory cytokines and atypical antipsychotic CPZ equivalents (*p<0.05, **p<0.01). CON: control; SCZ: schizophrenia; AACPZE: atypical antipsychotic CPZ equivalent.
| TMEVPG1 | NRON | HERV-W | HERV-W | ||
|---|---|---|---|---|---|
| M ± SD | M ± SD | M ± SD | M ± SD | ||
| Relative mRNA expression | CON (n=16) SCZ (n=17) p-value |
3.71±1.76 4.95±2.84 .15 |
2.21±0.95 2.59±0.92 .25 |
1.78±0.43 env 2.08±0.67 .15 |
1.88±0.42 gag 2.27±0.74 .09 |
| rs, p | rs, p | rs, p | rs, p | ||
| IL-6 | All (n=33) CON (n=16) SCZ (n=17) |
.56, .001** .30, .26 .66, .007** |
.67, .000** .69, .003** .63, .007** |
.47, .006** .68, .006** .39, .12 |
.49, .005** .17, .55 .63, .009** |
| TNF-α | All (n=33) CON (n=16) SCZ (n=17) |
−.01, .96 −.14, .61 −.17, .52 |
.03, .85 −.34, .19 .14, .61 |
.01, .98 −.14, .63 −.06, .83 |
.00, .99 −.34, .22 .04, .89 |
| IFN-γ | All (n=33) CON (n=16) SCZ (n=17) |
.22, .25 .45, .11 .31, .24 |
.29, .11 .36, .21 .33, .20 |
.17, .39 .13, .68 .34, .18 |
.38, .04* .80, .001** .36, .18 |
| AACPZE (n=12) | −.46, .13 | −.42, .17 | −.65, .02* | −.70, .01* | |
3.2 Correlation of TMEVPG1, NRON and HERV-W transcripts with inflammatory cytokine mRNA expression
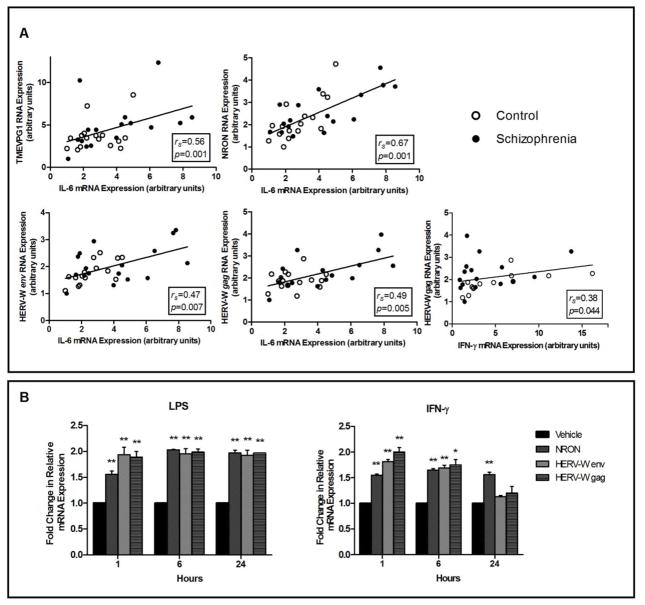
Results of the correlation analyses are reported in Table 1. RNA expression of TMEVPG1, NRON, HERV-W env and HERV-W gag demonstrated a positive correlation with IL-6 mRNA expression in the entire participant sample (Figure 1A). Within the control participant group RNA expression of NRON and HERV-W env were positively correlated with IL-6 mRNA expression, and within the group of participants with schizophrenia RNA expression of TMEVPG1, NRON and HERV-W gag were positively correlated with IL-6 mRNA levels. Across the entire sample IFN-γ mRNA expression was positively correlated with HERV-W gag transcript expression. This relationship was present in the control participants, but not participants with schizophrenia. There was no correlation of any of the transcripts measured with TNF-α mRNA expression, either combined or split by diagnostic category. Because this exploratory study with a small sample is concerned with controlling the proportion of actually falsely rejected null hypotheses, we applied the False Discovery Rate. Using an FDR threshold of 0.05 and utilizing all p-values generated, the Benjamini-Hochberg calculation executed by SPSS allows us to call any raw unadjusted p-value above 0.009 as a likely rejection of a false null hypothesis (Benajmini and Hochberg, 1995). This increases confidence for several key findings in Table 1.
Figure 1. Long non-coding and endogenous retroviral RNA levels correlate with proinflammatory gene expression, and are responsive to proinflammatory stimuli.
A) RNA expression levels of TMEVPG1, NRON, HERV-W env and HERV-W gag are positively correlated with mRNA expression of IL-6 in PBMCs from the entire participant sample (2-tailed; n=33). RNA expression of HERV-W gag is positively correlated with mRNA expression of IFN-γ. Data points for participants with schizophrenia and controls are displayed as filled and hollow points, respectively. B) Fold change relative to vehicle of non-coding and endogenous retroviral transcript expression following IFN-γ and LPS treatment at 1, 8 and 24 hour time points in THP-1 cells (n=2). One-way ANOVA with Tukey’s post-hoc **p<0.01, *p<0.05
3.3 Correlation of HERV-W transcript expression with atypical antipsychotic use
Atypical antipsychotic CPZ equivalents demonstrated a negative correlation with RNA expression of HERV-W env and HERV-W gag (Table 1). There was no association of atypical antipsychotic CPZ equivalents with TMEVPG1 or NRON.
3.4 NRON and HER-W transcript expression increases following IFN-γ and endotoxin stimulation in a THP-1 monocyte cell line
NRON, HERV-W env and HERV-W gag RNA expression increased following stimulation with both the proinflammatory cytokine IFN-γ, which activates the JAK-STAT1 signaling pathway, and the bacterial endotoxin LPS, which activates the NF-κB signaling pathway (Figure 1B). TMEVPG1 was not expressed in this cell line at baseline or after stimulation.
4. Discussion
To our knowledge, this is the first study to demonstrate an association of lncRNA (TMEVPG1 and NRON) and HERV-W (env and gag) transcript expression with IL-6 and IFN-γ mRNA expression in primary cells from human participants. IL-6 expression is elevated in schizophrenia and has been associated with positive symptom severity as well as cognitive dysfunction (Chase et al. 2016; Frydecka et al. 2015). We did not demonstrate an association with TNF-α mRNA expression, another proinflammatory cytokine that is elevated in individuals with schizophrenia.
The association of TMEVPG1 and NRON lncRNA levels with IL-6 mRNA expression is consistent with the current literature as both lncRNAs are shown to be involved in the regulation of proinflammatory cytokine gene expression (Satpathy and Chang, 2015). While TMEVPG1 is thought to play a role in IFN-γ expression, we did not find an association of expression of these RNAs in our participant sample. The association of HERV-W transcript and IL-6 and IFN-γ mRNA expression supports findings of altered HERV-W expression in diseases with an inflammatory component (Li and Karlsson, 2016). Whether HERV-W expression may be a cause and/or consequence of inflammation is still the subject of debate. At least in vitro, our THP-1 cell culture results support the hypothesis that these transcripts can be secondary to inflammation, as the expression of HERV-W env and gag transcripts increased following treatment with two independent pro-inflammatory stimuli, IFN-γ and LPS, which activate the JAK-STAT1 and NF-κB signaling pathways shown to be dysregulated in individuals with schizophrenia (Sharma et al., 2016; Song et al., 2009). Interestingly, direct binding of transcription factors to HERV promoter elements following cytokine stimulation, and a subsequent increase in expression, has recently been demonstrated for the HERV-K family of endogenous retroviruses (Manghera et al., 2016).
Based on the finding that IL-6 mRNA expression is significantly greater in participants with schizophrenia (Chase et al. 2015), and the positive correlation between IL-6 mRNA expression and TMEVPG1, NRON and HERV-W transcript expression that we demonstrate here, we might expect that these RNAs would also be elevated in the group of participants with schizophrenia. The lack of a significant diagnostic effect is possibly due to the small sample size. The negative correlation of HERV-W env and gag with atypical antipsychotic CPZ equivalents indicates that these medications may have an inhibitory effect on HERV-W transcript expression, and like immune function, has important implications in the clinical course of schizophrenia.
There are clear limitations to this preliminary and exploratory study, including the small number of RNA molecules investigated, sample size, and the lack of treatment naïve participants with schizophrenia. However, given the well documented findings of altered immune activity at multiple stages of illness in individuals with schizophrenia as well as the already active literature with regards to the critical involvement of lncRNAs and endogenous retroviruses in immune function, a larger genome-wide approach is warranted, focused on treatment naïve participants as well as those with long-term illness. Furthermore, the specific effects of psychotropic medication on the expression of these RNAs will be a future research objective.
Highlights.
Expression of lncRNAs and cytokines are correlated in human blood cells.
Expression of HERV transcripts and cytokines are correlated in human blood cells.
These lncRNAs/HERVs are responsive to inflammatory stimuli in a monocyte cell line.
Acknowledgments
The authors would like to thank all the individuals who participated in this study. This work was supported in part by PHS grant (NIH) R01MH094358 (R.P.S.).
Funding
This work was supported in part by PHS grant (NIH) R01MH094358 (R.P.S.).
Footnotes
Conflict of interest statement
There are no conflicts of interest.
Financial disclosure statement
There are no financial interests to disclose.
Ethical Approval
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000. The study was approved by university institutional review board (IRB).
Informed consent
Informed consent was obtained from all participants before beginning any study procedures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Benajmini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J R Stat Soc B. 1995;57:289–300. Author (s): Yoav Benjamini and Yosef Hochberg Source: Journal of the Royal Statistical Society. Series B (Methodological), Vol. 57, No. 1. [Google Scholar]
- Chase KA, Cone JJ, Rosen C, Sharma RP. The value of interleukin 6 as a peripheral diagnostic marker in schizophrenia. BMC Psychiatry. 2016;16:152. doi: 10.1186/s12888-016-0866-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase KA, Rosen C, Gin H, Bjorkquist O, Feiner B, Marvin R, Conrin S, Sharma RP. Metabolic and inflammatory genes in schizophrenia. Psychiatry Res. 2015;225:208–11. doi: 10.1016/j.psychres.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier SP, Collins PL, Williams CL, Boothby MR, Aune TM. Cutting edge: influence of Tmevpg1, a long intergenic noncoding RNA, on the expression of Ifng by Th1 cells. J Immunol. 2012;189:2084–8. doi: 10.4049/jimmunol.1200774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danivas V, Venkatasubramanian G. Current perspectives on chlorpromazine equivalents: Comparing apples and oranges! Indian J Psychiatry. 2013;55:207–208. doi: 10.4103/0019-5545.111475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenimore J, Young HA. Regulation of Cytokine Gene Expression in Immunity and Diseases. 2016 doi: 10.1007/978-94-024-0921-5. [DOI] [Google Scholar]
- Fric J, Zelante T, Wong AYW, Mertes A, Yu H, Dc W, Fric J, Zelante T, Wong AYW, Mertes A, Yu H, Ricciardi-castagnoli P. NFAT control of innate immunity. Blood. 2012;120:1380–1389. doi: 10.1182/blood-2012-02-404475. [DOI] [PubMed] [Google Scholar]
- Frydecka D, Misiak B, Pawlak-Adamska E, Karabon L, Tomkiewicz A, Sedlaczek P, Kiejna A, Beszłej JA. Interleukin-6: The missing element of the neurocognitive deterioration in schizophrenia? The focus on genetic underpinnings, cognitive impairment and clinical manifestation. Eur Arch Psychiatry Clin Neurosci. 2015;265:449–459. doi: 10.1007/s00406-014-0533-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner DM, Murphy AL, O’Donnell H, Centorrino F, Baldessarini RJ. International consensus study of antipsychotic dosing. Am J Psychiatry. 2010;167:686–693. doi: 10.1176/appi.ajp.2009.09060802. [DOI] [PubMed] [Google Scholar]
- Huang X, Luo YL, Mao YS, Ji JL. The link between long noncoding RNAs and depression. Prog Neuro-Psychopharmacology Biol Psychiatry. 2016;73:73–78. doi: 10.1016/j.pnpbp.2016.06.004. [DOI] [PubMed] [Google Scholar]
- Ilott NE, Heward JA, Roux B, Tsitsiou E, Fenwick PS, Lenzi L, Goodhead I, Hertz-Fowler C, Heger A, Hall N, Donnelly LE, Sims D, Lindsay MA. Long non-coding RNAs and enhancer RNAs regulate the lipopolysaccharide-induced inflammatory response in human monocytes. Nat Commun. 2014:5. doi: 10.1038/ncomms4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambara H, Niazi F, Kostadinova L, Moonka DK, Siegel CT, Post AB, Carnero E, Barriocanal M, Fortes P, Anthony DD, Valadkhan S. Negative regulation of the interferon response by an interferon-induced long non-coding RNA. Nucleic Acids Res. 2014;42:10668–10681. doi: 10.1093/nar/gku713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Karlsson H. Expression and regulation of human endogenous retrovirus W elements. Apmis. 2016;124:52–66. doi: 10.1111/apm.12478. [DOI] [PubMed] [Google Scholar]
- Manghera M, Ferguson-Parry J, Lin R, Douville RN. NF-κB and IRF1 Induce Endogenous Retrovirus-K Expression via Interferon-Stimulated Response Elements in its 5’ Long Terminal Repeat. J Virol. 2016:90. doi: 10.1128/JVI.01503-16. JVI.01503-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: Clinical status and antipsychotic effects. Biol Psychiatry. 2011;70:663–671. doi: 10.1016/j.biopsych.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satpathy AT, Chang HY. Long Noncoding RNA in Hematopoiesis and Immunity. Immunity. 2015 doi: 10.1016/j.immuni.2015.05.004. [DOI] [PubMed] [Google Scholar]
- Sharma RP, Rosen C, Melbourne JK, Feiner B, Chase KA. Activated Phosphorylated STAT1 Levels as a Biologically Relevant Immune Signal in Schizophrenia. Neuroimmunomodulation. 2016;23:224–229. doi: 10.1159/000450581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song XQ, Lv LX, Li WQ, Hao YH, Zhao JP. The interaction of nuclear factor-kappa B and cytokines is associated with schizophrenia. Biol Psychiatry. 2009;65:481–488. doi: 10.1016/j.biopsych.2008.10.018. [DOI] [PubMed] [Google Scholar]
- Willingham AT, Orth aP, Batalov S, Peters EC, Wen BG, Aza-Blanc P, Hogenesch JB, Schultz PG. A strategy for probing the function of noncoding RNAs finds a repressor of NFAT. Science. 2005;309:1570–3. doi: 10.1126/science.1115901. [DOI] [PubMed] [Google Scholar]