Abstract
Objective
High FiO2s may augment lung damage to exacerbate lung injury in patients with ARDS. Participants enrolled in ARDS Network Trials had a goal PaO2 range of 55–80 mmHg, yet the effect of oxygen exposure above this arterial oxygen tension range on clinical outcomes is unknown. We sought to determine if oxygen exposure that resulted in a PaO2s above goal (>80 mmHg) was associated with worse outcomes in patients with ARDS.
Design
Longitudinal analysis of data collected in these trials.
Setting
Ten clinical trials conducted at ARDS Network hospitals between 1996 and 2013.
Subjects
Critically ill patients with ARDS.
Measurements and Main Results
Each day, if the PaO2 >80 mmHg and FiO2 > 0.5, we determined above goal oxygen exposure, defined as the difference between the administered FiO2 and 0.5, and summed these values over the first five days. We determined the effect of a cumulative five-day above goal oxygen exposure on mortality prior to discharge home at 90 days. Among 2994 participants (mean age 51.3 years, 54% male) with a study-entry PaO2/FiO2 that met ARDS criteria, average cumulative above goal oxygen exposure was 0.24 FiO2-days (interquartile range 0 to 0.38). Participants with above goal oxygen exposure were more likely to die (adjusted interquartile-range OR=1.20, 95% CI 1.11 to 1.31), and have lower ventilator-free days (adjusted interquartile-range mean difference of −0.83, −1.18 to −0.48) and lower hospital-free days (adjusted interquartile-range mean difference of −1.38, −2.09 to −0.68). We observed a dose-response relationship between the cumulative above goal oxygen exposure and worsened clinical outcomes for participants with mild, moderate, or severe ARDS, suggesting that the observed relationship is not primarily influenced by severity of illness.
Conclusions
Oxygen exposure resulting in arterial oxygen tensions above the protocol goal occurred frequently and was associated with worse clinical outcomes at all levels of ARDS severity.
Keywords: ARDS, oxygen therapy, clinical outcomes
INTRODUCTION
ARDS is a critical illness syndrome associated with a risk factor that induces acute hypoxemic respiratory failure with a PaO2/FiO2≤300 mmHg while receiving PEEP≥5 cm H2O (1). Despite beneficial interventions, ARDS mortality remains high at 30%–40% (2–6), suggesting that other variables may affect clinical outcomes. Oxygen is a first-line therapy for hypoxemia in ARDS, with the goal to achieve acceptable arterial oxygenation and maintain tissue viability. However, it is not known whether targeting a specified oxygenation goal affects clinical outcomes in ARDS.
Mechanically ventilated patients are frequently exposed to higher FiO2s than necessary to achieve adequate arterial oxygenation, and often for prolonged periods. In an analysis of ARDS patients, Rachmale et al found excessive oxygen use, defined as a FiO2≥0.5 when SpO2>92%, in 74% of patients for a median 17 of the first 48 hoursof ventilator support (7). Similarly, De Graaf et al reported that among mechanically ventilated patients with a PaO2>120 mmHg, the FiO2 was reduced in only 25% of instances over a 24-hour period (8).
Excess oxygen is detrimental in several acute, life-threatening illnesses. A meta-analysis of critically-ill patients following cardiac arrest, traumatic brain injury, stroke, and post-cardiac surgery found that above normal arterial PaO2 values correlated with higher mortality (9), with the strongest association following cardiac arrest (10). Helmerhorst et al found that ICU patients exposed to severe hyperoxia (PaO2>200 mmHg) had higher mortality rates and fewer ventilator-free days when compared to mild hyperoxia (PaO2 121–200 mmHg) or normoxia (PaO2 60–100 mmHg) (11). Potential mechanisms of damage induced by high levels of oxygen include an excessive pro-inflammatory response that can impede innate immunity (12) and augment lung injury (13), generation of reactive oxygen species that damage cells, and vasoconstriction to vital organs (14, 15). Pre-existent lung damage in ARDS may impair anti-oxidant enzyme production and other adaptive responses, rendering patients particularly susceptible to oxygen-induced injury (16).
We analyzed if cumulative effect of excess oxygen contributed towards worst clinical outcomes despite enrollment into ARDS clinical trials with a protocol targeting a PaO2 goal range (55–80 mmHg). We quantified excess (above goal) oxygen exposure for any FiO2>0.5 when PaO2>80 mmHg.
METHODS
Description of studies
We used data of ARDS patients enrolled in randomized clinical trials (RCTs) (17–25), excluding those assigned to receive targeted tidal volumes of 12 mL/kg PBW (17). All trials required that PEEP or FiO2 be titrated to a common target of 55–80 mmHg or SpO2 of 88–95%. When both PaO2 and SpO2 were available, PaO2 took precedence. Adults aged ≥18 years were enrolled from 1996–2013 at participating hospitals, and were eligible if intubated, were receiving mechanical ventilation, and met criteria for acute lung injury (26). We included 10 trials enrolled participants within 36 (17, 22–24) or 48 hours (18, 21, 25) after inclusion criteria were met. Data collection followed common protocols (17–25). This analysis was approved by the institutional review board of the Johns Hopkins School of Medicine in Baltimore, USA.
Outcomes
The primary outcome was mortality prior to discharge home at 90 days (17–25). Secondary outcomes included ventilator-free (VFDS) and hospital-free days (HFDS) scores (27).
Assessment of excessive oxygen exposure
We defined above goal oxygen exposure a priori as any value above a FiO2>0.5 among participants with a PaO2>80 mmHg from altitude-adjusted morning ABGs (17). With a PaO2>80 mmHg and a corresponding FiO2>0.5, excess oxygen (FiO2-days) was calculated as FiO2 – 0.5. Using this definition, study participants with a higher relative FiO2 at the same arterial oxygen tension had more above goal exposure for that time interval. We calculated a cumulative exposure as the sum of above goal oxygen exposures over the first five days because data points were collected each day during that interval. Participants may have not had an ABG during that 5-day interval either because it was not taken or because the participant was extubated or died. In those cases, we divided the cumulative above goal oxygen exposure by the number of days when an ABG was available and multiplied by five, and conducted sensitivity analyses with subsets of data for participants with ≥4 ABGs. The average number of ABGs per participant was 4.1, so we believe that this assumption is likely to have had a small effect on our analysis.
Definitions
We analyzed all participants with ARDS on day of study entry and used Berlin criteria to define ARDS severity (1). We calculated tidal volumes by mL/kg PBW using standard equations (28) and static compliance as tidal volume/(inspiratory plateau pressure–PEEP).
Biostatistical methods
We evaluated the association between cumulative above goal oxygen exposure at five days after enrollment and in-hospital death at 90 days. We calculated octiles of cumulative above goal oxygen exposure for values above zero and visually examined the dose-response relationship between categories of above goal oxygen exposure (ranging from none to octiles of cumulative exposure) and either the probability or log odds of in-hospital death. We used logistic regression to model the odds of in-hospital death at 90 days as a function of the cumulative above goal oxygen exposure at five days, age, sex, APACHE III, PEEP, and baseline ARDS severity [14]. We reported odds ratios of mortality for observed values of the cumulative above goal oxygen exposure in the interquartile range. We conducted severity-stratified analyses to determine if baseline severity modified the association between cumulative above goal oxygen exposure and in-hospital death at 90 days, and included indicator variables for each trial in our models to account for potential differences among trials. As sensitivity analyses, we modified the definition of above goal oxygen exposure for different thresholds of FiO2 (0.3, 0.4, 0.6) and PaO2 (85, 90, 95,100 mmHg).
We also evaluated the association between cumulative above goal oxygen exposure at five days after enrollment and either VFDS or HFDS. We used linear regression to model free-days as a function of the cumulative above goal oxygen exposure at five days, age, sex, APACHE III, PEEP, and ARDS severity at study entry. We used analysis-of-variance to compare means of continuous variables between subgroups, and chi-square tests to compare proportions of dichotomous variables. We conducted analyses in R (www.r-project.org).
RESULTS
Participant characteristics
4361 participants were enrolled in 10 RCTs in 1996–2013. Of these, 4243 (97%) had at least one ABG in the first five days, 3815 (87%) were managed with protocols that targeted tidal volumes of 6 mL/kg PBW, and 2994 (69%) had an ABG on day 0 to define severity. Among 2994 participants, average age±SD was 51.3±16.2 years, average APACHE III was 91.8±29.9, and 54% were male. A total of 23% (687), 55% (1659), and 22% (648) had mild, moderate, and severe ARDS on day 0, respectively. No differences in age (mean 51.4 vs. 52.6 years; p=0.07), sex (53.4% vs. 52.3%; p=0.63), or APACHE III (mean 92.7 vs. 91.5; p=0.40) were found between participants who did not have a day 0 ABG and those who did; however, tidal volumes (7.1 vs. 7.6 mL/kg PBW; p<0.001) and PEEP (9.0 vs. 9.4; p<0.01) were lower. Static compliance was also not different (34.2 vs. 33.1 mL/cm H2O; p=0.23).
We summarized differences in participant characteristics by categories of cumulative above goal oxygen exposure at five days (Table 1). Disease severity was greater with higher categories of above goal oxygen exposure, as evidenced by higher APACHE III, higher minute ventilation, higher plateau pressure, higher PEEP, lower pH, and lower systolic blood pressure.
Table 1.
Participant characteristics by categories of above goal oxygen exposure.
| Characteristic or factor | Cumulative above goal oxygen exposure at five days | ||||
|---|---|---|---|---|---|
| None | 0·02 – 0·24 | 0·25 – 0·49 | 0·5 – 2·50 | p-value | |
| Sample size | 1549 | 527 | 330 | 588 | |
| Age in years, mean (SD) | 52·4 (16·4) | 50·4 (15·6) | 51·2 (15·6) | 49·4 (16·7) | <0·001 |
| % Male (n) | 55% (847) | 54% (286) | 55% (181) | 50% (292) | 0·20 |
| APACHE III, mean (SD) | 87·8 (29·7) | 90·9 (29·0) | 96·4 (28·5) | 100·5 (30·0) | <0·001 |
| Body mass index in kg/m2, mean (SD) | 29·0 (7·6) | 29·5 (8·8) | 28·8 (8·1) | 28·9 (8·6) | 0·57 |
| Tidal volume per kg PBW, mean (SD) | 7·6 (1·9) | 7·7 (2·0) | 7·5 (2·0) | 7·5 (2·2) | 0·21 |
| Minute ventilation in L/min, mean (SD) | 11·6 (3·8) | 12·1 (3·6) | 12·4 (4·0) | 12·6 (3·9) | <0·001 |
| Plateau pressure in cm H2O, mean (SD) | 25·2 (7·0) | 26·1 (6·7) | 27·5 (7·1) | 28·3 (7·9) | <0·001 |
| PEEP in cm H2O, mean (SD) | 8·5 (3·5) | 9·7 (3·5) | 11·0 (4·1) | 11·6 (4·4) | <0·001 |
| pH, mean (SD) | 7·38 (0·08) | 7·37 (0·09) | 7·36 (0·08) | 7·34 (0·10) | <0·001 |
| FiO2, mean (SD) | 0·54 (0·17) | 0·63 (0·11) | 0·73 (0·13) | 0·87 (0·16) | <0·001 |
| PaO2 in mmHg, mean (SD) | 79·6 (18·3) | 92·7 (26·1) | 99·3 (33·8) | 110·2 (46·5) | <0·001 |
| Systolic blood pressure, mean (SD) | 115·3 (20·8) | 113·0 (20·3) | 112·0 (20·3) | 110·8 (20·3) | <0·001 |
| 90-day mortality % | 25% | 23% | 29% | 37% | <0·001 |
| Ventilator-free days score, mean (SD) | 15·2 (14·2) | 14·2 (10·1) | 12·6 (10·6) | 10·4 (10·5) | <0·001 |
| Hospital-free days score, mean (SD) | 30·6 (21·6) | 29·8 (20·5) | 26·9 (21·4) | 23·4 (21·7) | <0·001 |
Patterns of above goal oxygen exposure
1549 (48%) study participants had a cumulative above goal oxygen exposure above 0. Among 2994 participants, average±SD cumulative above goal oxygen exposure at five days was 0.24±0.41 FiO2-days. Daily mean excess among all participants decreased from 0.09 (±0.16) on day 0 to 0.02 (±0.09) on day 4., and the proportion of above goal oxygen exposure decreased from 32% on day 0 to 10% on day 4, We summarized the distribution of cumulative above goal oxygen exposure at five days stratified by ARDS severity (Figure 1). Participants with mild ARDS had a larger proportion of at goal oxygen exposure days when compared to participants with moderate or severe ARDS (71% vs. 46% vs. 46%; p<0.001). Cumulative above goal oxygen exposure in severe ARDS was higher at any percentile when compared to those with moderate ARDS, followed by those with mild ARDS (Figure 1). Average cumulative above goal oxygen exposure increased (p<0.001) but the proportion of participants with severe ARDS decreased over the time period of eligible clinical trials (p<0.001; Online Supplement, e-Figure 1).
Figure 1.
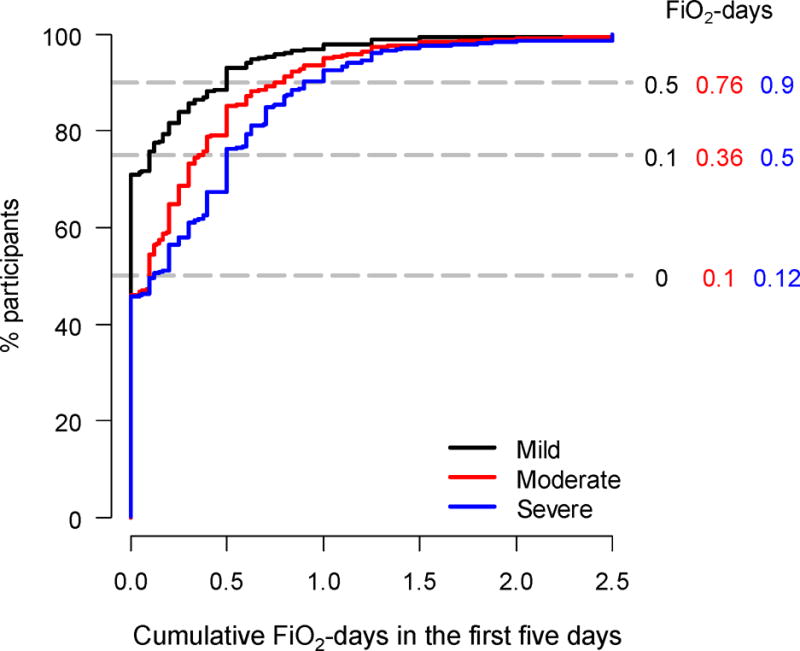
Empirical cumulative distribution of above goal oxygen exposure at five days stratified by severity of ARDS. The 50th, 75th, and 90th percentiles of cumulative above goal oxygen exposure are shown by the horizontal dashed lines. Exposures of FiO2-days for each of these percentiles are indicated to the right of the horizontal dashed lines according to ARDS severity.
Association between above goal oxygen exposure and clinical outcomes
In-hospital mortality by 90 days was greater with higher categories of above goal oxygen exposure (Figure 2). The distribution across categories of cumulative above goal oxygen exposure, ranging from 0.1–0.2 to 1–2.5, was fairly even. The slope of the relationship between cumulative above goal oxygen exposure and the log odds of mortality was approximately linear, thus supporting the use of a single slope in our regression analyses to model this relationship.
Figure 2.
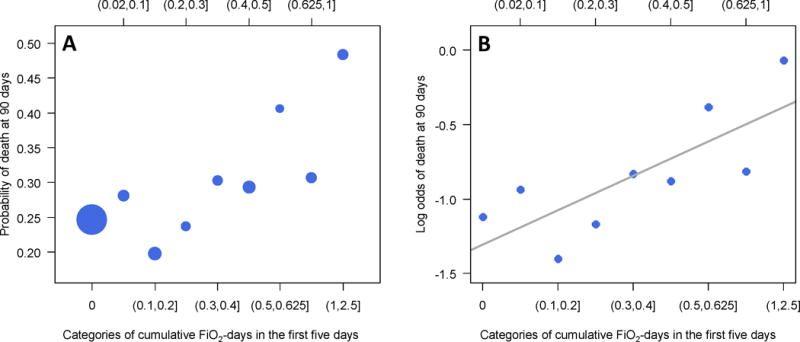
Probability (Panel A) and log odds (Panel B) of hospital mortality at 90 days by categories of cumulative above goal oxygen exposure at five days. In panel A, the sizes of filled circles are proportional to the sample size in each category. This graph could mean either that above goal oxygen exposure is detrimental, or that participants with more severe ARDS are more likely to die and also receive more above goal oxygen exposure.
We summarized regression results for clinical outcomes by cumulative above goal oxygen exposure and other a priori selected variables (Table 2). Participants with cumulative above goal oxygen exposure were more likely to die in-hospital (adjusted interquartile-range [AIQR] OR=1.20, 95% CI 1.11 to 1.31), have a lower VFDS (AIQR mean difference of −0.83, −1.18 to −0.48) and HFDS (AIQR mean difference of −1.38, −2.09 to −0.68). In sensitivity analyses, modifying the FiO2 threshold to a lower (0.3 or 0.4) or higher value (0.6) did not affect the direction of the association and, in most cases, the statistical significance (Online Supplement, e-Table 1). Modifying the PaO2 threshold to a higher value (85, 90, 95, or 100 mmHg) also did not affect the direction of the association; however, the magnitude of the association was weakened (Online Supplement, e-Table 2). The relationship between above goal oxygen exposure and mortality do not appear to be affected by residual confounding after accounting for potential differences in hospital mortality by clinical trial (AIQR OR=1.21, 95% CI 1.11 to 1.32). In subset analyses, the association between cumulative above goal oxygen exposure and mortality was not different for participants with either ≥4 ABGs (AIQR OR=1.34, 95% CI 1.19 to 1.52) or 5 ABGs (AIQR OR=1.25, 95% CI 1.09 to 1.44).
Table 2.
Single variable and multivariable regression analyses of clinical outcomes as a function of multiple factors including cumulative above goal oxygen exposure.
| Factor | Interquartile range or % | In-hospital mortality at 90 days, Odds ratio (95% CI) | Ventilator-free days score, absolute difference (95% CI) | Hospital-free days score, absolute difference (95% CI) | |||
|---|---|---|---|---|---|---|---|
| Single variable | Multivariable | Single variable | Multivariable | Single variable | Multivariable | ||
| Age in years, interquartile range | 39 – 63 | 2·21 (1·95 to 2·52) | 2·01 (1·75 to 2·31) | −2·72 (−3·28 to −2·16) | −2·13 (−2·68 to −1·58) | −6·06 (−7·18 to −4·93) | −4·34 (−5·45 to −3·23) |
| Being female (male is reference) | 46% | 0·84 (0·71 to 0·99) | 0·82 (0·68 to 0·97) | 0·81 (0·03 to 1·58) | 0·90 (0·19 to 1·62) | 2·62 (1·06 to 4·18) | 2·82 (1·37 to 4·27) |
| APACHE III, interquartile difference | 70 – 111 | 3·03 (2·67 to 3·44) | 2·75 (2·41 to 3·15) | −5·24 (−5·74 to −4·74) | −4·31 (−4·82 to −3·79) | −10·7 (−11·7 to −9·7) | −9·30 (−10·34 to −8·26) |
| Cumulative above goal oxygen exposure at 5 days interquartile range | 0 – 0·38 | 1·25 (1·16 to 1·34) | 1·20 (1·11 to 1·31) | −1·45 (−1·80 to −1·10) | −0·83 (−1·17 to −0·48) | −2·48 (−3·22 to −1·74) | −1·38 (−2·09 to −0·68) |
| PEEP in cm H2O, interquartile range | 5 – 12 | 1·14 (0·99 to 1·31) | 0·89 (0·74 to 1·07) | −2·44 (−3·13 to −1·77) | −0·70 (−1·42 to 0·02) | −2·43 (−3·81 to −1·06) | 0·61 (−0·85 to 2·06) |
| Severity (mild is reference) | |||||||
| Moderate | 55% | 1·43 (1·16 to 1·77) | 1·25 (0·99 to 1·58) | −2·54 (−3·48 to −1·59) | −1·45 (−2·35 to −0·53) | −3·71 (−5·62 to −1·80) | −2·01 (−3·84 to −0·18) |
| Severe | 22% | 1·98 (1·55 to 2·53) | 1·51 (1·13 to 2·01) | −5·90 (−7·04 to −4·76) | −3·47 (−4·63 to −2·31) | −8·69 (−10·99 to −6·38) | −5·00 (−7·35 to −2·65) |
Effect modification by severity of ARDS
We assessed if above goal oxygen exposure was associated with hospital mortality at 90 days among different strata of ARDS severity (Figure 3). We also calculated the percentage of participants who met or exceeded each of the thresholds of cumulative above goal oxygen exposure (0.1, 0.25, and 0.5 FiO2-days). At least 10% of participants in each stratum of ARDS severity were exposed to at least 0.5 FiO2-days (i.e., an average of 0.1 FiO2 excess each day), and within the 0.5 FiO2-days above goal oxygen exposure group, the odds ratio of death was increased similarly in mild ARDS as in either moderate or severe ARDS. We found a dose-response relationship between cumulative above goal oxygen exposure at five days and greater mortality at 90 days, and this relationship held true for mild, moderate, or severe ARDS.
Figure 3.
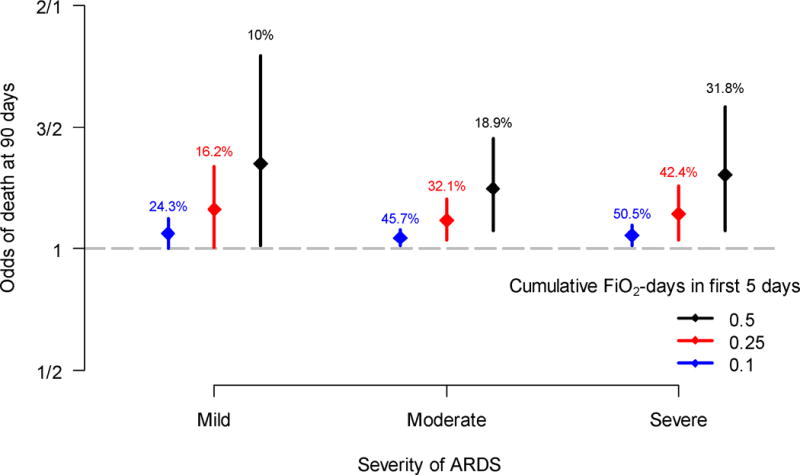
Odds of hospital mortality at 90 days by levels of cumulative above goal oxygen exposure at five days (0·1, 0·25, and 0·5, respectively) stratified by severity of ARDS. The circles represent odds ratios, and the vertical segments are 95% confidence intervals. The percentages above the vertical segments indicate the proportion of participants with values greater or equal to selected levels of cumulative above goal oxygen exposure. These data suggest that above goal oxygen exposure is detrimental even in patients with mild ARDS.
DISCUSSION
In our analysis of participants enrolled in 10 RCTs, we found a positive and dose-dependent association between oxygen exposure above the protocol goal and higher mortality, and lower VFDS and HFDS. Above goal oxygen exposure was associated with higher mortality irrespective of severity of ARDS at enrollment, suggesting that this association is less likely affected by reverse causality. As little as 2% of above goal oxygen exposure per day was sufficient to influence clinical outcomes. Observation of higher mortality with lower VFDS in the group with above goal oxygen exposure suggests the possibility that excess oxygen can exacerbate lung injury and thus prolong the need for mechanical ventilation. Although only correlative in humans, experimental animal models have also demonstrated synergistic lung injury using hyperoxia and ventilation with larger tidal volumes (29).
Other studies support the concept that above goal oxygen exposure may have adverse effects in acute respiratory failure. De Jonge et al found a positive association between hospital mortality and higher FiO2 values in the first 24 hours of mechanical ventilation, including the subset of patients with high PaO2s (30). In a study of mechanically ventilated ARDS patients, excess oxygen exposure was associated with longer ICU and hospital length of stays (7); however, lower PEEP levels in the excessive oxygen group may have confounded those results. In a single-center RCT, Girardis et al compared controlled normoxia (goal PaO2 70–100 mmHg) versus usual care oxygen therapy (goal PaO2 up to 150 mmHg), and found lower ICU mortality in the controlled normoxia group (31), although subjects with moderate or severe ARDS were excluded and the conservative oxygen group was healthier at baseline. When Asfar et al randomized mechanically ventilated septic patients to non-titrated 100% oxygen for 24 hours versus oxygen titrated to an oxygen saturation of 88–95%, the trial was stopped due to a possible harm signal in the 100% oxygen group (32). However, not all studies suggest that exposure to high levels of oxygen are detrimental. Eastwood et al did not find an association between higher than necessary oxygen exposure in the first 24 hours and higher hospital mortality (33).
Our study demonstrates a dose-response association between above goal oxygen exposure and mortality in patients with mild, moderate, and severe ARDS, and is important for the following reasons. First, we determined the cumulative dose of above goal oxygen exposure over a five day period, which integrates longitudinal data on oxygen exposure and contrasts single exposure assessments in prior studies (9, 34). We found that above goal oxygen exposure was an important patient-related factor and a longitudinal variable for which the cumulative dose effect was significant. Second, all of the analyzed data is from a large number of participants enrolled in trials where ventilation parameters were managed using defined protocols with a pre-specified target PaO2 range. PEEP levels were also adjusted according to protocol, and unlike the findings by Rachmale et al (7), were higher in participants exposed to oxygen above protocol goals in our analysis. Yet, PEEP was not associated with any clinical outcomes. Third, because we analyzed data over two decades of multi-center ARDS Network trials, we are confident that above goal oxygen exposure was associated with worse outcomes. Interestingly, although the severity of ARDS at enrollment is somewhat reduced in trials conducted in recent years (2009–2013), cumulative above goal oxygen exposure increased. In early ARDS Network trials (22, 23), there was more focus on ventilator management rules with protocol-compliance reports provided to investigators. As such, investigators may have been more inclined to reduce FiO2 when arterial oxygenation exceeded the goal range during early trials.
Allowing arterial oxygenation to exceed targets frequently leads to above goal oxygen exposure as we defined it for this study. This permissiveness may be due to a reluctance to titrate oxygen in critically-ill patients to maintain a margin of safety against hypoxia, especially when the set FiO2 ≤0.6 (35), as was demonstrated by Suzuki et al when they assessed physician responses to SpO2 ≥ 99% (36). In our study, more frequent above goal oxygen exposure occurred in moderate and severe ARDS as compared to mild ARDS, supporting the hypothesis that ICU physicians tend to favor higher arterial oxygenation goals with increasing severity of disease. Recent prospective studies, however, suggest that targeting a lower arterial oxygen saturation goal is feasible and safe among mechanically ventilated patients (37, 38). Helmerhorst et al implemented training and feedback protocols regarding conservative oxygen thresholds, resulting in less hyperoxia, reduced mechanical ventilator time and hospital mortality compared to pre-implementation ICU data (39).
Our analysis has some shortcomings. First, it was conducted retrospectively, and therefore cannot establish causal relationships. Second, some participants did not have an ABG on each of the five days following enrollment, necessitating an approximation to determine the cumulative five-day exposure. Since above goal oxygen exposure was similar each day, we likely did not over- or under-estimate the cumulative exposure. Third, we did not have any information on whether physicians titrated FiO2 and PEEP according to the ARDS Network FiO2/PEEP table. Fourth, we cannot determine if clinicians primarily used SpO2 instead of PaO2 to titrate FiO2. In ARDS patients, a wide range of PaO2 values can be measured for a given SpO2 and vice-versa (40). If clinicians also used SpO2 to titrate FiO2, it may have affected the actual above goal exposure time determined by daily PaO2. Fifth, we used a fixed threshold of FiO2 at 0.5 to define the amount of oxygen delivered when PaO2 was above goal (>80 mmHg) that was not adjusted for severity. While 0.5 may not be the best threshold for FiO2, sensitivity analyses demonstrated that our findings were robust to the choice of FiO2 threshold (between 0.3 and 0.6). Moreover, it was not clear if 80 mmHg was an appropriate threshold to define above goal oxygen exposure; however, our findings were robust across a range of PaO2 thresholds (80 - 100 mmHg). Moving the threshold of PaO2 to higher value may have weakened the association because higher PaO2 values are likely reflective of a less sick study population. Finally, residual confounding or reverse causality due to severity of illness may affect our results; however, above oxygen exposure effect sizes were similar regardless of ARDS severity.
In contrast to our findings of negative clinical outcomes associated with above goal arterial oxygen tensions, Mikkelson et al found an increased incidence of long-term cognitive impairment in ARDS survivors who had a lower average PaO2 (71 vs. 86 mmHg) during the study period (41). A study of preterm newborns demonstrated a higher risk of death in participants randomized to a lower oxygen saturation target of 85–89% (42). As such, there appears to be equipoise for a prospective, randomized study in adults with ARDS to determine the short- and long-term clinical impact of adjusting oxygen exposure to target a lower PaO2 goal vs. a higher PaO2 goal.
In summary, above goal oxygen exposure was associated with worse clinical outcomes including death and length of stay in ARDS patients. This association was consistent across categories of ARDS severity and was robust to varying thresholds of oxygen exposure that could be considered unsafe. Future research needs to evaluate these associations in RCTs of oxygen management strategies, and determine if they extend to the general population of mechanically ventilated patients.
Supplementary Material
Acknowledgments
Supported by NHLBI Contracts NO1-HR-46054 through 46064 and NO1-HR 56165 through 56179 with the National Institutes of Health, National Heart, Lung, and Blood Institute. William Checkley was supported by a Pathway to Independence Award (R00HL096955) from the National Heart, Lung and Blood Institute, National Institutes of Health. Neil Aggarwal was supported by a Fellow-to-Faculty Award (11FTF7280014) from the American Heart Association. The funding agencies had no role in study design or conduct, or in the writing of this report.
Copyright form disclosure: Drs. Aggarwal, Thompson, Shanholtz, and Checkley received support for article research from the National Institutes of Health (NIH). Dr. Brower received funding from Applied Clinical Intelligence and Global Blood Therapeutics. Dr. Thompson received funding from consultancy for Alexion, Asahi Kasei, Boehringer Ingelheim, Bristol-Myers Squibb, GlaxoSmithKline, Vertex, and Regeneron unrelated to the current work. Dr. Shanholtz’s institution received funding from NIH National Heart, Lung, and Blood Institute ARDS Clinical Trials Network.
Footnotes
Contributorship: Conception and design (NA, RB, and WC); Analysis and interpretation (NA, WC); Drafting the manuscript for important intellectual content (NA, RB, DH, BT, GB, CS, AG, WC).
The remaining authors have disclosed that they do not have any potential conflicts of interest.
Disclosure: Dr. Aggarwal contributed to this article as an employee of Johns Hopkins University. The views expressed are his own and those of Johns Hopkins University School of Medicine, and do not necessarily represent the views of the National Institutes of Health or the United States Government.
References
- 1.Force ADT. Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. Jama. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 2.Rubenfeld GD, Caldwell E, Peabody E, et al. Incidence and outcomes of acute lung injury. The New England journal of medicine. 2005;353(16):1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 3.Brun-Buisson C, Minelli C, Bertolini G, et al. Epidemiology and outcome of acute lung injury in European intensive care units. Results from the ALIVE study Intensive Care Med. 2004;30(1):51–61. doi: 10.1007/s00134-003-2022-6. [DOI] [PubMed] [Google Scholar]
- 4.Estenssoro E, Dubin A, Laffaire E, et al. Incidence, clinical course, and outcome in 217 patients with acute respiratory distress syndrome. Crit Care Med. 2002;30(11):2450–2456. doi: 10.1097/00003246-200211000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Hudson LD, Milberg JA, Anardi D, et al. Clinical risks for development of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1995;151(2 Pt 1):293–301. doi: 10.1164/ajrccm.151.2.7842182. [DOI] [PubMed] [Google Scholar]
- 6.Luhr OR, Antonsen K, Karlsson M, et al. Incidence and mortality after acute respiratory failure and acute respiratory distress syndrome in Sweden, Denmark, and Iceland. The ARF Study Group. Am J Respir Crit Care Med. 1999;159(6):1849–1861. doi: 10.1164/ajrccm.159.6.9808136. [DOI] [PubMed] [Google Scholar]
- 7.Rachmale S, Li G, Wilson G, et al. Practice of excessive F(IO(2)) and effect on pulmonary outcomes in mechanically ventilated patients with acute lung injury. Respiratory care. 2012;57(11):1887–1893. doi: 10.4187/respcare.01696. [DOI] [PubMed] [Google Scholar]
- 8.de Graaff AE, Dongelmans DA, Binnekade JM, et al. Clinicians’ response to hyperoxia in ventilated patients in a Dutch ICU depends on the level of FiO2. Intensive Care Med. 2011;37(1):46–51. doi: 10.1007/s00134-010-2025-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Helmerhorst HJ, Roos-Blom MJ, van Westerloo DJ, et al. Association Between Arterial Hyperoxia and Outcome in Subsets of Critical Illness: A Systematic Review, Meta-Analysis, and Meta-Regression of Cohort Studies. Crit Care Med. 2015;43(7):1508–1519. doi: 10.1097/CCM.0000000000000998. [DOI] [PubMed] [Google Scholar]
- 10.Kilgannon JH, Jones AE, Shapiro NI, et al. Association between arterial hyperoxia following resuscitation from cardiac arrest and in-hospital mortality. Jama. 2010;303(21):2165–2171. doi: 10.1001/jama.2010.707. [DOI] [PubMed] [Google Scholar]
- 11.Helmerhorst HJ, Arts DL, Schultz MJ, et al. Metrics of Arterial Hyperoxia and Associated Outcomes in Critical Care. Crit Care Med. 2017;45(2):187–195. doi: 10.1097/CCM.0000000000002084. [DOI] [PubMed] [Google Scholar]
- 12.Baleeiro CE, Wilcoxen SE, Morris SB, et al. Sublethal hyperoxia impairs pulmonary innate immunity. J Immunol. 2003;171(2):955–963. doi: 10.4049/jimmunol.171.2.955. [DOI] [PubMed] [Google Scholar]
- 13.Aggarwal NR, D’Alessio FR, Tsushima K, et al. Moderate oxygen augments lipopolysaccharide-induced lung injury in mice. Am J Physiol Lung Cell Mol Physiol. 2010;298(3):L371–381. doi: 10.1152/ajplung.00308.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cornet AD, Kooter AJ, Peters MJ, et al. The potential harm of oxygen therapy in medical emergencies. Crit Care. 2013;17(2):313. doi: 10.1186/cc12554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farquhar H, Weatherall M, Wijesinghe M, et al. Systematic review of studies of the effect of hyperoxia on coronary blood flow. American heart journal. 2009;158(3):371–377. doi: 10.1016/j.ahj.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 16.Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest. 2012;122(8):2731–2740. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. The New England journal of medicine. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 18.National Heart L, Blood Institute Acute Respiratory Distress Syndrome Clinical Trials N. Wheeler AP, et al. Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. The New England journal of medicine. 2006;354(21):2213–2224. doi: 10.1056/NEJMoa061895. [DOI] [PubMed] [Google Scholar]
- 19.National Heart L, Blood Institute ACTN. Truwit JD, et al. Rosuvastatin for sepsis-associated acute respiratory distress syndrome. The New England journal of medicine. 2014;370(23):2191–2200. doi: 10.1056/NEJMoa1401520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Heart L, Blood Institute Acute Respiratory Distress Syndrome Clinical Trials N. Matthay MA, et al. Randomized, placebo-controlled clinical trial of an aerosolized beta(2)-agonist for treatment of acute lung injury. Am J Respir Crit Care Med. 2011;184(5):561–568. doi: 10.1164/rccm.201012-2090OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Heart L, Blood Institute Acute Respiratory Distress Syndrome Clinical Trials N. Rice TW, et al. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. Jama. 2012;307(8):795–803. doi: 10.1001/jama.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brower RG, Lanken PN, MacIntyre N, et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. The New England journal of medicine. 2004;351(4):327–336. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 23.Randomized, placebo-controlled trial of lisofylline for early treatment of acute lung injury and acute respiratory distress syndrome. Crit Care Med. 2002;30(1):1–6. doi: 10.1097/00003246-200201000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Ketoconazole for early treatment of acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. The ARDS Network. Jama. 2000;283(15):1995–2002. doi: 10.1001/jama.283.15.1995. [DOI] [PubMed] [Google Scholar]
- 25.National Heart L, Blood Institute Acute Respiratory Distress Syndrome Clinical Trials N. Wiedemann HP, et al. Comparison of two fluid-management strategies in acute lung injury. The New England journal of medicine. 2006;354(24):2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 26.Steinberg KP, Hudson LD, Goodman RB, et al. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. The New England journal of medicine. 2006;354(16):1671–1684. doi: 10.1056/NEJMoa051693. [DOI] [PubMed] [Google Scholar]
- 27.Schoenfeld DA, Bernard GR, Network A Statistical evaluation of ventilator-free days as an efficacy measure in clinical trials of treatments for acute respiratory distress syndrome. Crit Care Med. 2002;30(8):1772–1777. doi: 10.1097/00003246-200208000-00016. [DOI] [PubMed] [Google Scholar]
- 28.Linares-Perdomo O, East TD, Brower R, et al. Standardizing Predicted Body Weight Equations for Mechanical Ventilation Tidal Volume Settings. Chest. 2015;148(1):73–78. doi: 10.1378/chest.14-2843. [DOI] [PubMed] [Google Scholar]
- 29.Sinclair SE, Altemeier WA, Matute-Bello G, et al. Augmented lung injury due to interaction between hyperoxia and mechanical ventilation. Crit Care Med. 2004;32(12):2496–2501. doi: 10.1097/01.ccm.0000148231.04642.8d. [DOI] [PubMed] [Google Scholar]
- 30.de Jonge E, Peelen L, Keijzers PJ, et al. Association between administered oxygen, arterial partial oxygen pressure and mortality in mechanically ventilated intensive care unit patients. Critical care. 2008;12(6):R156. doi: 10.1186/cc7150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Girardis M, Busani S, Damiani E, et al. Effect of Conservative vs Conventional Oxygen Therapy on Mortality Among Patients in an Intensive Care Unit: The Oxygen-ICU Randomized Clinical Trial. Jama. 2016;316(15):1583–1589. doi: 10.1001/jama.2016.11993. [DOI] [PubMed] [Google Scholar]
- 32.Asfar P, Schortgen F, Boisrame-Helms J, et al. Hyperoxia and hypertonic saline in patients with septic shock (HYPERS2S): a two-by-two factorial, multicentre, randomised, clinical trial. The Lancet Respiratory medicine. 2017 doi: 10.1016/S2213-2600(17)30046-2. [DOI] [PubMed] [Google Scholar]
- 33.Eastwood G, Bellomo R, Bailey M, et al. Arterial oxygen tension and mortality in mechanically ventilated patients. Intensive Care Med. 2012;38(1):91–98. doi: 10.1007/s00134-011-2419-6. [DOI] [PubMed] [Google Scholar]
- 34.Eastwood GM, Peck L, Young H, et al. Intensive care clinicians’ opinion of conservative oxygen therapy (SpO(2) 90–92%) for mechanically ventilated patients. Australian critical care: official journal of the Confederation of Australian Critical Care Nurses. 2014;27(3):120–125. doi: 10.1016/j.aucc.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 35.Aggarwal NR, Brower RG. Targeting normoxemia in acute respiratory distress syndrome may cause worse short-term outcomes because of oxygen toxicity. Annals of the American Thoracic Society. 2014;11(9):1449–1453. doi: 10.1513/AnnalsATS.201407-297PS. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki S, Eastwood GM, Peck L, et al. Current oxygen management in mechanically ventilated patients: a prospective observational cohort study. Journal of critical care. 2013;28(5):647–654. doi: 10.1016/j.jcrc.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki S, Eastwood GM, Glassford NJ, et al. Conservative oxygen therapy in mechanically ventilated patients: a pilot before-and-after trial. Crit Care Med. 2014;42(6):1414–1422. doi: 10.1097/CCM.0000000000000219. [DOI] [PubMed] [Google Scholar]
- 38.Panwar R, Hardie M, Bellomo R, et al. Conservative versus Liberal Oxygenation Targets for Mechanically Ventilated Patients. A Pilot Multicenter Randomized Controlled Trial Am J Respir Crit Care Med. 2016;193(1):43–51. doi: 10.1164/rccm.201505-1019OC. [DOI] [PubMed] [Google Scholar]
- 39.Helmerhorst HJ, Schultz MJ, van der Voort PH, et al. Effectiveness and Clinical Outcomes of a Two-Step Implementation of Conservative Oxygenation Targets in Critically Ill Patients: A Before and After Trial. Crit Care Med. 2016;44(3):554–563. doi: 10.1097/CCM.0000000000001461. [DOI] [PubMed] [Google Scholar]
- 40.Rice TW, Wheeler AP, Bernard GR, et al. Comparison of the SpO2/FIO2 ratio and the PaO2/FIO2 ratio in patients with acute lung injury or ARDS. Chest. 2007;132(2):410–417. doi: 10.1378/chest.07-0617. [DOI] [PubMed] [Google Scholar]
- 41.Mikkelsen ME, Christie JD, Lanken PN, et al. The adult respiratory distress syndrome cognitive outcomes study: long-term neuropsychological function in survivors of acute lung injury. Am J Respir Crit Care Med. 2012;185(12):1307–1315. doi: 10.1164/rccm.201111-2025OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Network SSGotEKSNNR. Carlo WA, Finer NN, et al. Target ranges of oxygen saturation in extremely preterm infants. The New England journal of medicine. 2010;362(21):1959–1969. doi: 10.1056/NEJMoa0911781. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


