Abstract
Objective
Oxygenation measured 24 hours after acute respiratory distress syndrome (ARDS) onset more accurately stratifies risk, relative to oxygenation at onset, in both children and adults. However, waiting 24 hours is problematic, especially for interventions that are more efficacious early in the disease course. We aimed to delineate whether oxygenation measured at timepoints earlier than 24 hours would retain predictive validity in pediatric ARDS.
Design
Observational cohort study.
Setting
Two large, academic pediatric intensive care units.
Patients
Invasively ventilated children with ARDS.
Interventions
None.
Measurements and Main Results
PaO2/FIO2 and oxygenation index (OI; mean airway pressure × FIO2 × 100)/PaO2) were measured at ARDS onset, at 6, 12, 18, and 24 hours after in 459 children at the Children’s Hospital of Philadelphia. Neither PaO2/FIO2 nor OI at ARDS onset discriminated outcome. Between 6 and 24 hours, both PaO2/FIO2 (area under receiver operating curve [AUROC] for mortality between 0.57 to 0.62, p = 0.049 to 0.002) and OI (AUROC 0.60 to 0.62, p = 0.006 to 0.001) showed good discrimination and calibration across multiple outcomes, including mortality, ventilator-free days at 28 days, ventilator days in survivors, and probability of extubation given competing risk of death. The utility of oxygenation at 12 hours was confirmed in an independent cohort from the Children’s Hospital of Los Angeles.
Conclusion
Oxygenation measured between 6 and 12 hours of ARDS onset accurately stratified outcomes in children. Our results have critical implications for the design of trials, especially for interventions with greater impact in early ARDS.
Keywords: pediatric, acute respiratory distress syndrome, ARDS, PARDS, oxygenation index, OI
INTRODUCTION
The 1994 American-European Consensus Conference (AECC)(1) and 2012 Berlin (2) definitions of acute respiratory distress syndrome (ARDS) were developed without pediatric considerations. In 2015, the Pediatric Acute Lung Injury Consensus Conference (PALICC) proposed a definition for pediatric ARDS (PARDS)(3). PALICC uses oxygenation index (OI) instead of PaO2/FIO2 (mild OI 4 to < 8; moderate 8 to < 16; severe ≥ 16), has alternative stratification SpO2-based when PaO2 is unavailable (oxygen saturation index, OSI; mild OSI 5 to < 7.5; moderate 7.5 to < 12.3; severe ≥ 12.3)(4, 5), and has less restrictive radiographic criteria (unilateral versus bilateral). Undefined in all definitions, however, is specifically when clinicians should measure hypoxemia. PaO2/FIO2 and SpO2/FIO2 are susceptible to ventilator settings (6, 7), venous admixture (8), and FIO2 (9, 10). OI and OSI adjust for airway pressure, but are affected by treatment response (11). Berlin does not specify when the PaO2/FIO2 should be measured; PALICC suggests measuring oxygenation at PARDS onset and at 24, 48, and 72 hours after, without comment regarding superiority.
We (12, 13) and others have demonstrated in pediatric (14) and adult (6, 7) ARDS that oxygenation 24 hours after ARDS onset more accurately discriminates mortality, relative to oxygenation at onset. Thus, 24-hour oxygenation is a better metric for trial enrollment and risk stratification. However, waiting 24 hours is problematic from both a clinical and research perspective, particularly for interventions with greater impact early in ARDS. Therefore, we performed a secondary analysis of an ongoing prospective cohort examining PaO2/FIO2 and OI during the first 24 hours of ARDS to delineate which timepoint before 24 hours would retain utility. Results were validated in an independent PARDS cohort screened using OI or OSI.
METHODS
Derivation Cohort
This was secondary retrospective analysis of an ongoing prospective cohort, approved by the Children’s Hospital of Philadelphia’s (CHOP) Institutional Review Board (IRB), with requirement for informed consent waived. The cohort has previously been described in detail (12). Briefly, intubated children meeting AECC criteria for acute lung injury (two consecutive PaO2/FIO2 ≤ 300 separated by ≥ 1 hour with bilateral infiltrates) admitted to the CHOP pediatric intensive care unit (PICU) between July 1, 2011 and June 30, 2016 were enrolled. The CHOP PICU is a 55-bed unit which admits >3500 patients annually, with a 2.7% prevalence of ARDS (12). As the study was initiated prior to the Berlin definition (2), minimum positive end-expiratory pressure (PEEP) was not specified; however, CHOP PICU does not utilize PEEP < 5 cmH2O. Thus, all patients met Berlin criteria. Similarly, as the study was initiated prior to the PALICC definition of PARDS (3), we did not screen using OI; however, all but one patient met PARDS criteria by OI. Ventilation and use of ancillary therapies were not protocolized.
Demographics, ventilator settings, PaO2/FIO2 and OI at ARDS onset and at 24 hours, and treatments for the first 3 days were recorded prospectively. ARDS onset was defined by time of initial PaO2/FIO2. We retrospectively abstracted PaO2/FIO2 and OI at 6, 12, and 18 hours after ARDS onset. All subjects had arterial catheters to be eligible (12), and blood gases were available at least every 6 hours.
Validation Cohort
A separate cohort of children meeting PALICC PARDS criteria by either OI or OSI from the Children’s Hospital of Los Angeles (CHLA) between March 1, 2009, and April 30, 2013, were used for validation (13). This was approved by the CHLA IRB, and requirement for informed consent waived. PARDS onset was defined by time of initial OI or OSI. Oxygenation was abstracted at PARDS onset, and at 6, 12, 18 and 24 hours after. Because of extensive (> 25%) missing data, we restricted analyses to patients with available oxygenation data at PARDS onset, and at 12 and 24 hours after.
Equations and Definitions
Oxygenation was measured using PaO2/FIO2, OI (mean airway pressure [mPaw] × FIO2 × 100)/PaO2), or OSI (mPaw × FIO2 × 100)/SpO2) ensuring SpO2 ≤ 97%, as previously described (5, 13). Vasopressor score (15) was: dopamine (μg/kg/min) × 1 + dobutamine (μg/kg/min) × 1 + epinephrine (μg/kg/min) × 100 + norepinephrine (μg/kg/min) × 100 + phenylephrine (μg/kg/min) × 100 + vasopressin (U/kg/min) × 10,000 + milrinone (μg/kg/min) × 10. Non-pulmonary organ failures were identified using accepted definitions in children (16). The designation “immunocompromised” required presence of an immunocompromising diagnosis (oncologic, immunologic, rheumatologic, or transplant) and active immunosuppressive chemotherapy, or a congenital immunodeficiency (12, 17). Severity of illness score used was the Pediatric Risk of Mortality (PRISM) III at 12 hours.
Outcomes
Primary outcome was PICU mortality. Duration of ventilation and ventilator-free days (VFD) at 28 days were also reported. All mention of “ventilation” implies invasive ventilation; non-invasive support was not counted. “Day 1” was initiation of invasive ventilation. Liberation from invasive ventilation ≥ 24 hours defined duration of ventilation. Patients requiring re-initiation of invasive ventilation had the extra days counted towards total ventilator days. VFD was determined by subtracting total ventilator days from 28 in survivors. Patients with ≥ 28 ventilator days and PICU non-survivors were assigned VFD = 0.
Statistical Analysis
Data are expressed as percentages, means (± standard deviation), or medians [interquartile range]. A generalized estimating equation using auto-regressive correlation tested change in oxygenation over time between survivors and non-survivors. In some analyses, VFD was dichotomized to ≤ 14 or > 14 to compute areas under the receiver operating characteristic (AUROC) curve. AUROC tested discriminative ability of Berlin and PALICC definitions using dummy variables for oxygenation categories. A non-parametric test of trend tested if mortality, VFD, or ventilator days in survivors increased across worsening oxygenation categories at different timepoints. Calibration of Berlin and PALICC categories for mortality was assessed by three metrics (18). First, we performed regression testing association of Berlin or PALICC categories at different timepoints with outcomes. Mortality calibration was assessed with Hosmer-Lemeshow (p < 0.05 implies deviation from prediction, and poor calibration). Next, we plotted predicted (x-axis) versus observed (y-axis) mortality, VFD, or ventilator days in survivors, and reported the slope (perfect calibration = 1, assuming linear relationship between categories and outcomes) and variance explained (r2; perfect calibration = 1).
Because VFD incorporates both mortality and length of ventilation, it is a problematic endpoint from which to identify variables associated predominantly with ventilator duration. Therefore, in addition to analyzing VFD and ventilator days in survivors, competing risk regression was used to test the association of Berlin or PALICC severity categories with duration of ventilation at different timepoints after ARDS onset, using extubation as primary outcome, and death as a competing risk. Observations were censored at 28 days. Fine and Gray competing risk regression (19) calculates a subdistribution hazard ratio for risk of extubation, accounting for competing risk of death. Trend analysis for competing risk regression was not available. Analyses were performed using Stata 14.2 SE. Significance was considered p < 0.05.
RESULTS
CHOP Cohort
Four hundred fifty-nine children had Berlin-defined ARDS (Supplementary Tables 1 and 2). All but 6 met PALICC PARDS criteria simultaneously, 5 of whom met OI criteria within 6 hours (4 mild, 1 moderate PARDS) of meeting Berlin. Oxygenation improved over 24 hours in both survivors and non-survivors (Supplementary Figure 1), with no difference in trajectory (p = 0.233 for PaO2/FIO2; p = 0.083 for OI). Most patients shifted into less severe categories over time (Supplementary Figure 2). Redistribution using Berlin categories appeared unchanged between 12 and 24 hours; no clear comparable timepoint was seen for PALICC categories. Of the 67 non-survivors (14.6% mortality), 13 (19.4% of 67) died of hypoxemia, 24 (35.8%) of multisystem organ failure (MSOF), and 30 (44.8%) died secondary to poor neurologic prognosis.
Discriminative Ability of Oxygenation Metrics
Berlin and PALICC categories at ARDS onset did not discriminate mortality, whereas categories at 6, 12, 18, and 24 hours did (Table 1, Supplementary Figure 3). Berlin and PALICC categories at all timepoints discriminated VFD ≤ 14. AUROC improved from 6 to 24 hours for both mortality and VFD ≤ 14 for both PaO2/FIO2 and OI, but comparisons between AUROC between 6 and 24 hours were not significant for mortality (p between 0.256 and 0.974). AUROC for VFD ≤ 14 were better for Berlin categories at 18 (p = 0.041) and 24 hours (p < 0.001), relative to 6 hours; AUROC for PALICC categories at 24 hours was significantly better than at 6 hours (p < 0.001). Comparisons between Berlin and PALICC categories at the same timepoints were not different for either mortality or VFD ≤ 14 (p between 0.114 and 0.899).
Table 1.
Areas under the receiver operating characteristic (AUROC) curve testing the discriminative ability of Berlin (PaO2/FIO2) and PALICC (oxygenation index) categories at different timepoints after ARDS onset in the CHOP, CHLA, and combined cohorts
| AUROC (95% CI) p value |
||||
|---|---|---|---|---|
| Berlin (PaO2/FIO2) | PALICC (oxygenation index) | |||
|
| ||||
| Mortality | VFD ≤ 14 days | Mortality | VFD ≤ 14 days | |
| CHOP cohort (n = 459) | ||||
| Onset | 0.55 (0.47 to 0.63) | 0.55 (0.50 to 0.60) | 0.57 (0.49 to 0.64) | 0.57 (0.52 to 0.62) |
| p = 0.200 | p = 0.038 | p = 0.105 | p = 0.006 | |
| 6 hours | 0.58 (0.52 to 0.66) | 0.58 (0.53 to 0.63) | 0.61 (0.54 to 0.69) | 0.61 (0.56 to 0.66) |
| p = 0.017 | p = 0.002 | p = 0.002 | p < 0.001 | |
| 12 hours | 0.57 (0.50 to 0.64) | 0.61 (0.56 to 0.66) | 0.61 (0.54 to 0.68) | 0.62 (0.58 to 0.67) |
| p = 0.049 | p < 0.001 | p = 0.002 | p < 0.001 | |
| 18 hours | 0.58 (0.50 to 0.65) | 0.62 (0.58 to 0.67) | 0.60 (0.53 to 0.67) | 0.64 (0.59 to 0.69) |
| p = 0.043 | p < 0.001 | p = 0.006 | p < 0.001 | |
| 24 hours | 0.62 (0.54 to 0.70) | 0.67 (0.62 to 0.72) | 0.62 (0.55 to 0.70) | 0.70 (0.65 to 0.74) |
| p = 0.002 | p < 0.001 | p = 0.001 | p < 0.001 | |
|
| ||||
| CHLA cohort (n = 182) | ||||
| Onset | 0.56 (0.47 to 0.66) | 0.59 (0.52 to 0.68) | ||
| p = 0.160 | p = 0.015 | |||
| 12 hours | 0.61 (0.51 to 0.71) | 0.62 (0.54 to 0.70) | ||
| p = 0.033 | p = 0.003 | |||
| 24 hours | 0.68 (0.58 to 0.78) | 0.68 (0.70 to 0.75) | ||
| p < 0.001 | p < 0.001 | |||
|
| ||||
| Combined (n = 615) | ||||
| Onset | 0.58 (0.51 to 0.64) | 0.58 (0.54 to 0.62) | ||
| p = 0.015 | p < 0.001 | |||
| 12 hours | 0.62 (0.56 to 0.67) | 0.63 (0.58 to 0.67) | ||
| p < 0.001 | p < 0.001 | |||
| 24 hours | 0.65 (0.59 to 0.71) | 0.69 (0.65 to 0.73) | ||
| p < 0.001 | p < 0.001 | |||
CHOP: Children’s Hospital of Philadelphia; CHLA: Children’s Hospital of Los Angeles; PALICC: Pediatric Acute Lung Injury Consensus Conference; VFD: ventilator free days
Calibration of Oxygenation Metrics for Outcomes
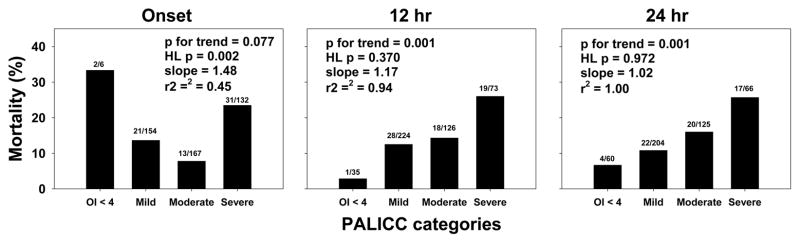
Mortality (Figure 1), VFD, ventilator days in survivors, and probability of extubation were plotted against oxygenation categories (Supplementary Figures 4 and 5). At ARDS onset, neither Berlin nor PALICC categories were associated with mortality (p for trend > 0.05 and Hosmer-Lemeshow p < 0.05 for both). From 6 to 24 hours, both Berlin and PALICC categories had increasing mortality with worsening oxygenation. Calibration was best for PALICC categories at 24 hours (Hosmer-Lemeshow p = 0.972, slope = 1.02, r2 = 1.00). PALICC demonstrated more consistent calibration after ARDS onset for mortality, with r2 > 0.90 at all timepoints and similar slopes. Comparable results were seen for VFD, ventilator days in survivors, and probability of extubation: poor calibration and no association between categories and outcome at ARDS onset, with stepwise improvement from 6 to 24 hours (Supplementary Figures 4 and 5). VFD and ventilator days in survivors ≥ 12 hours all had r2 > 0.90.
Figure 1.
Mortality rates stratified by PALICC (OI) oxygenation categories at ARDS onset, and at 12 and 24 hours after. PALICC categories did not demonstrate any trend with mortality at ARDS onset, with subsequent timepoints showing a significant trend (see also Supplementary Figures 4 and 5 for all timepoints). Numbers above bars represent non-survivors and total number of patients in category. P values for a non-parametric test of trend, Hosmer-Lemeshow (HL) p values, and slopes and r2 of calibration curves are provided for each graph. Because of the low number of subjects (n = 6) in PALICC OI < 4 at ARDS onset, we removed this category for calibration tests.
CHLA Cohort
Two hundred fifty-four children met PALICC criteria for PARDS, of whom 182 had oxygenation recorded at onset, and at 12 and 24 hours (Supplementary Table 3). Eighty-nine subjects (49%) met PALICC criteria using OSI, and 93 (51%) using OI. PRISM III (10 [5, 17]) was similar to CHOP (11 [5, 17]; p = 0.882). PALICC categories at onset did not discriminate mortality (Table 1, Supplementary Figure 3), whereas categories at 12 and 24 hours did.
Mortality, VFD, ventilator days in survivors, and probability of extubation (Supplementary Figure 6) were plotted against PALICC categories at onset, 12, and 24 hours. At onset, PALICC categories were poorly calibrated. At 12 and 24 hours, calibration for mortality was poor in the CHLA cohort, with r2 ≤ 0.70 at all timepoints. However, VFD, ventilator days in survivors, and probability of extubation all demonstrated improved association with oxygenation severity at 12 and 24 hours, with improved calibration.
Combined Analysis
We analyzed 635 subjects from both cohorts meeting PALICC criteria for PARDS at onset (Supplementary Table 4); 6 subjects not meeting PALICC criteria at CHOP (only met Berlin initially) were excluded. In this cohort (107 non-survivors, 16.9%), PALICC categories discriminated mortality and VFD ≤ 14 at all timepoints (Table 1, Supplementary Figure 3). As in prior analyses, calibration was poor for all outcomes at PARDS onset, with improved calibration at 12 and 24 hours.
DISCUSSION
Oxygenation at ARDS onset did not discriminate outcomes. By 6 hours, both Berlin and PALICC categories discriminated mortality and demonstrated improved calibration across all outcomes tested. Given the greater consistency of OI (relative to PaO2/FIO2), we suggest that OI 6 to 12 hours after ARDS onset is an appropriate metric for enrollment criteria in clinical trials, and is a reliable biomarker for risk stratification. This reduces by more than half the previous timepoint of 24 hours after ARDS for prognostication. Our data have implications for the design of future trials in pediatric ARDS.
Multiple studies have demonstrated inadequacy of initial oxygenation in ARDS, relative to oxygenation at 24 hours (6, 7, 12–14). Our novel contribution is shortening the timeframe for screening and trial recruitment. This is significant for interventions with greater impact early in ARDS, such as PEEP, recruitment maneuvers, and prone positioning. Oxygenation at PARDS onset did discriminate mortality in the combined cohort, suggesting that lack of discrimination at onset in the CHOP and CHLA cohorts individually was potentially due to infrequent deaths and low power. However, calibration for mortality and ventilator duration was poor at PARDS onset even in the combined cohort, and improved by 12 hours, consistent with our primary analysis.
Although the CHOP cohort was screened using PaO2/FIO2, OI-based categories demonstrated more consistent calibration. This is potentially due to OI accounting for mPaw, making it more independent of ventilator management, whereas PaO2/FIO2 is highly dependent on ventilator strategy. Our study validates the decision by PALICC to use OI, rather than PaO2/FIO2, as a metric of oxygenation in PARDS (3). As the CHLA cohort was categorized using either OI or OSI, non-invasive measurements would potentially stratify as well as OI in future studies. This is critical for comparisons between units with differing practices regarding frequency of blood gas measurements, or in placement of arterial catheters.
The CHLA cohort was poorly calibrated for mortality at 12 and 24 hours, although discrimination improved. As this cohort was restricted to the 182 subjects with available oxygenation at onset, 12, and 24 hours, there is possible selection bias towards sicker subjects (more oxygenation measurements). This is suggested by the higher mortality at CHLA (23.1%), relative to CHOP (14.6%, p = 0.014), which potentially affects the reliability of conclusions regrading mortality in the CHLA cohort. Additionally, etiology of death may also contribute to the poor calibration for mortality. The epidemiology of death in pediatric ARDS is undescribed, but the frequency of withdrawal for neurologic reasons was comparable to adult data (20), where 29% of adult ARDS patients had neurologic dysfunction as the primary organ failure precipitating death. Despite heterogeneous causes of death, there was improved calibration for both Berlin and PALICC categories from 6 to 24 hours after ARDS for outcomes based primarily on duration of ventilation in both cohorts.
The lack of a reproducible phenotype, with reliance on measurements prone to variability such as PaO2/FIO2 and radiographs, has hampered ARDS research (21). Interventions like higher PEEP (22) and prone positioning (23, 24) may only be efficacious in severe ARDS, and inclusion of less severe phenotypes rendered initial trials negative (25–28). Our study suggests OI measured 6 to 12 hours after pediatric ARDS onset improves reliability of the phenotype, as this metric appropriately discriminates multiple outcomes and is well-calibrated across increasing severity classes. Improved classification based on timing has already proven advantageous in adult trials. PROne Positioning in SEVere ARDS (PROSEVA) limited enrollment to patients with PaO2/FIO2 < 150 and required an additional 12–24 hours of stabilization, after which PaO2/FIO2 < 150 was confirmed (29). PROSEVA demonstrated an unprecedented 42% relative reduction in mortality with proning.
Our study has limitations. The two-center nature may limit generalizability, although ARDS etiologies and severity were comparable to others (11, 14, 30–32). Ventilator management, sedation, and fluid management was not protocolized, and it is uncertain whether oxygenation measured on standardized settings would improve risk stratification. Our study did not identify an “optimum” timepoint for risk stratification, as we did not measure oxygenation hourly and assess test characteristics. Oxygenation only modestly discriminated mortality, and was outperformed by PRISM III (AUROC 0.74, 95% CI 0.68 to 0.79, p = 0.004 relative to OI at 12 hours). However, PRISM III is not designed for risk stratification in ARDS, and is generally unavailable at bedside. Additionally, PRISM III was only calibrated for mortality, and had no utility for duration of ventilation (median ventilator days of survivors: 10, 9, 11, 10 across increasing quartiles of PRISM score; p for trend = 0.323). Finally, eligibility at CHOP was based on PaO2/FIO2, not OI, as the study was initiated prior to publication of PALICC, precluding us from making firm statements regarding utility of PALICC categories based on timing. However, 453 of 459 (99%) children met PALICC criteria by OI simultaneous with meeting Berlin criteria, and 5 of the 6 not initially meeting PALICC criteria met OI criteria within 6 hours, making this unlikely to invalidate our findings.
Our study has several strengths. The derivation cohort was a large, prospective ARDS cohort from a large PICU, with detailed data collection. Results were validated in a separate large quaternary PICU. We tested association of Berlin and PALICC categories with a range of outcomes. The association with multiple outcomes is necessary in pediatric ARDS, where mortality is low, and often unrelated to lung injury. The utility of OI as early as 6 hours after ARDS onset significantly improves upon the previously reported 24 hours. These results have critical implications for the design of trials in pediatric ARDS.
Supplementary Material
Change in PaO2/FIO2 and oxygenation index over the first 24 hours after ARDS onset, stratified by survival status (mean ± SD). Oxygenation improved in both survivors (white) and non-survivors (black), with no significant difference in trajectory. P values represent results of a generalized estimating equation using an auto-regressive correlation structure for the interaction term (group × time).
Re-classification of patients based on their initial and subsequent Berlin and PALICC oxygenation categories. Patients generally shifted into less severe oxygenation categories over the first 24 hours after ARDS onset. Initial Berlin and PALICC severity categories are provided on the x-axis. At every timepoint, subjects were assigned the severity category at that timepoint: no longer meeting Berlin or PALICC criteria (yellow), mild (orange), moderate (red), or severe (purple).
Receiver operating characteristic (ROC) curves for mortality and ventilator-free days (VFD) ≤ 14 days. Curves are shown for the CHOP cohort (both Berlin and PALICC categories), the CHLA cohort (PALICC categories), and both cohorts combined (PALICC categories). Timepoints are at onset (red), 6 hours (orange), 12 hours (green), 18 hours (blue), and 24 hours (black). The area under the ROC (AUROC) curve is provided in each individual graph legend (AUROC = A).
Mortality, VFD at 28 days, ventilator days in survivors, and probability of extubation given competing risk of death stratified by Berlin (PaO2/FIO2) categories at ARDS onset, and at 6, 12, 18, and 24 hours after. Numbers above mortality bars represent non-survivors and total number of patients in category. Numbers above VFD and ventilator days are medians. P values for a non-parametric test of trend, Hosmer-Lemeshow (HL) p values (for mortality), and slopes and r2 of calibration curves are provided for each graph.
Mortality, VFD at 28 days, ventilator days in survivors, and probability of extubation given competing risk of death stratified by PALICC (OI) categories at ARDS onset, and at 6, 12, 18, and 24 hours after. Numbers above mortality bars represent non-survivors and total number of patients in category. Numbers above VFD and ventilator days are medians. P values for a non-parametric test of trend, Hosmer-Lemeshow (HL) p values (for mortality), and slopes and r2 of calibration curves are provided for each graph. Because of the low number of subjects (n = 6) in PALICC OI < 4 at ARDS onset, we removed this category for calibration tests.
Mortality, VFD at 28 days, ventilator days in survivors, and probability of successful extubation (given the competing risk of death) at PARDS onset, and at 12 and 24 hours after, in the CHLA cohort with complete data at all timepoints (n = 182). Patients were classified using PALICC oxygenation categories based on either OI or OSI. Numbers above mortality bars represent non-survivors and total number of patients in category. Hosmer-Lemeshow (HL) p values are provided for mortality. P values for a non-parametric test of trend, and slopes and r2 of calibration curves are provided for each graph. Medians are provided within each bar for VFD and ventilator days in survivors.
Acknowledgments
Financial support:
NIH K12-HL109009; K23-HL136688 (NY)
NIH K23-HD075069 (RGK)
Footnotes
Institution: Children’s Hospital of Philadelphia
Reprints Planned: No
Copyright form disclosure: Dr. Yehya’s institution received funding from the National Heart, Lung, and Blood Institute, and he received support for article research from the National Institutes of Health. Dr. Thomas received funding from Therabron, CareFusion, and GeneFluidics. Dr. Khemani received funding from Orangemed.
References
- 1.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149(3 Pt 1):818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 2.Force ADT, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 3.Group PALICC. Pediatric acute respiratory distress syndrome: consensus recommendations from the Pediatric Acute Lung Injury Consensus Conference. Pediatr Crit Care Med. 2015;16(5):428–439. doi: 10.1097/PCC.0000000000000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thomas NJ, Shaffer ML, Willson DF, et al. Defining acute lung disease in children with the oxygenation saturation index. Pediatr Crit Care Med. 2010;11(1):12–17. doi: 10.1097/PCC.0b013e3181b0653d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khemani RG, Thomas NJ, Venkatachalam V, et al. Comparison of SpO2 to PaO2 based markers of lung disease severity for children with acute lung injury. Crit Care Med. 2012;40(4):1309–1316. doi: 10.1097/CCM.0b013e31823bc61b. [DOI] [PubMed] [Google Scholar]
- 6.Villar J, Perez-Mendez L, Lopez J, et al. An early PEEP/FIO2 trial identifies different degrees of lung injury in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2007;176(8):795–804. doi: 10.1164/rccm.200610-1534OC. [DOI] [PubMed] [Google Scholar]
- 7.Villar J, Perez-Mendez L, Blanco J, et al. A universal definition of ARDS: the PaO2/FiO2 ratio under a standard ventilatory setting--a prospective, multicenter validation study. Intensive Care Med. 2013;39(4):583–592. doi: 10.1007/s00134-012-2803-x. [DOI] [PubMed] [Google Scholar]
- 8.Gowda MS, Klocke RA. Variability of indices of hypoxemia in adult respiratory distress syndrome. Crit Care Med. 1997;25(1):41–45. doi: 10.1097/00003246-199701000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Douglas ME, Downs JB, Dannemiller FJ, et al. Change in pulmonary venous admixture with varying inspired oxygen. Anesthesia and analgesia. 1976;55(5):688–695. doi: 10.1213/00000539-197609000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Karbing DS, Kjaergaard S, Smith BW, et al. Variation in the PaO2/FiO2 ratio with FiO2: mathematical and experimental description, and clinical relevance. Crit Care. 2007;11(6):R118. doi: 10.1186/cc6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trachsel D, McCrindle BW, Nakagawa S, et al. Oxygenation index predicts outcome in children with acute hypoxemic respiratory failure. Am J Respir Crit Care Med. 2005;172(2):206–211. doi: 10.1164/rccm.200405-625OC. [DOI] [PubMed] [Google Scholar]
- 12.Yehya N, Servaes S, Thomas NJ. Characterizing degree of lung injury in pediatric acute respiratory distress syndrome. Crit Care Med. 2015;43(5):937–946. doi: 10.1097/CCM.0000000000000867. [DOI] [PubMed] [Google Scholar]
- 13.Parvathaneni K, Belani S, Leung D, et al. Evaluating the Performance of the Pediatric Acute Lung Injury Consensus Conference Definition of Acute Respiratory Distress Syndrome. Pediatr Crit Care Med. 2017;18(1):17–25. doi: 10.1097/PCC.0000000000000945. [DOI] [PubMed] [Google Scholar]
- 14.Lopez-Fernandez Y, Azagra AM, de la Oliva P, et al. Pediatric Acute Lung Injury Epidemiology and Natural History study: Incidence and outcome of the acute respiratory distress syndrome in children. Crit Care Med. 2012;40(12):3238–3245. doi: 10.1097/CCM.0b013e318260caa3. [DOI] [PubMed] [Google Scholar]
- 15.Gaies MG, Gurney JG, Yen AH, et al. Vasoactive-inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr Crit Care Med. 2010;11(2):234–238. doi: 10.1097/PCC.0b013e3181b806fc. [DOI] [PubMed] [Google Scholar]
- 16.Goldstein B, Giroir B, Randolph A, et al. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6(1):2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- 17.Yehya N, Topjian AA, Thomas NJ, et al. Improved oxygenation 24 hours after transition to airway pressure release ventilation or high-frequency oscillatory ventilation accurately discriminates survival in immunocompromised pediatric patients with acute respiratory distress syndrome*. Pediatr Crit Care Med. 2014;15(4):e147–156. doi: 10.1097/PCC.0000000000000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steyerberg EW, Vickers AJ, Cook NR, et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology. 2010;21(1):128–138. doi: 10.1097/EDE.0b013e3181c30fb2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association. 1999;94(446):496–509. [Google Scholar]
- 20.Stapleton RD, Wang BM, Hudson LD, et al. Causes and timing of death in patients with ARDS. Chest. 2005;128(2):525–532. doi: 10.1378/chest.128.2.525. [DOI] [PubMed] [Google Scholar]
- 21.Pham T, Rubenfeld GD. Fifty Years of Research in ARDS. The Epidemiology of Acute Respiratory Distress Syndrome. A 50th Birthday Review. Am J Respir Crit Care Med. 2017;195(7):860–870. doi: 10.1164/rccm.201609-1773CP. [DOI] [PubMed] [Google Scholar]
- 22.Goligher EC, Kavanagh BP, Rubenfeld GD, et al. Oxygenation response to positive end-expiratory pressure predicts mortality in acute respiratory distress syndrome. A secondary analysis of the LOVS and ExPress trials. Am J Respir Crit Care Med. 2014;190(1):70–76. doi: 10.1164/rccm.201404-0688OC. [DOI] [PubMed] [Google Scholar]
- 23.Hu SL, He HL, Pan C, et al. The effect of prone positioning on mortality in patients with acute respiratory distress syndrome: a meta-analysis of randomized controlled trials. Crit Care. 2014;18(3):R109. doi: 10.1186/cc13896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee JM, Bae W, Lee YJ, et al. The efficacy and safety of prone positional ventilation in acute respiratory distress syndrome: updated study-level meta-analysis of 11 randomized controlled trials. Crit Care Med. 2014;42(5):1252–1262. doi: 10.1097/CCM.0000000000000122. [DOI] [PubMed] [Google Scholar]
- 25.Brower RG, Lanken PN, MacIntyre N, et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351(4):327–336. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 26.Mercat A, Richard JC, Vielle B, et al. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299(6):646–655. doi: 10.1001/jama.299.6.646. [DOI] [PubMed] [Google Scholar]
- 27.Gattinoni L, Tognoni G, Pesenti A, et al. Effect of prone positioning on the survival of patients with acute respiratory failure. N Engl J Med. 2001;345(8):568–573. doi: 10.1056/NEJMoa010043. [DOI] [PubMed] [Google Scholar]
- 28.Guerin C, Gaillard S, Lemasson S, et al. Effects of systematic prone positioning in hypoxemic acute respiratory failure: a randomized controlled trial. JAMA. 2004;292(19):2379–2387. doi: 10.1001/jama.292.19.2379. [DOI] [PubMed] [Google Scholar]
- 29.Guerin C, Reignier J, Richard JC, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368(23):2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 30.Flori HR, Glidden DV, Rutherford GW, et al. Pediatric acute lung injury: prospective evaluation of risk factors associated with mortality. Am J Respir Crit Care Med. 2005;171(9):995–1001. doi: 10.1164/rccm.200404-544OC. [DOI] [PubMed] [Google Scholar]
- 31.Erickson S, Schibler A, Numa A, et al. Acute lung injury in pediatric intensive care in Australia and New Zealand: a prospective, multicenter, observational study. Pediatr Crit Care Med. 2007;8(4):317–323. doi: 10.1097/01.PCC.0000269408.64179.FF. [DOI] [PubMed] [Google Scholar]
- 32.Khemani RG, Rubin S, Belani S, et al. Pulse oximetry vs. PaO2 metrics in mechanically ventilated children: Berlin definition of ARDS and mortality risk. Intensive Care Med. 2015;41(1):94–102. doi: 10.1007/s00134-014-3486-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Change in PaO2/FIO2 and oxygenation index over the first 24 hours after ARDS onset, stratified by survival status (mean ± SD). Oxygenation improved in both survivors (white) and non-survivors (black), with no significant difference in trajectory. P values represent results of a generalized estimating equation using an auto-regressive correlation structure for the interaction term (group × time).
Re-classification of patients based on their initial and subsequent Berlin and PALICC oxygenation categories. Patients generally shifted into less severe oxygenation categories over the first 24 hours after ARDS onset. Initial Berlin and PALICC severity categories are provided on the x-axis. At every timepoint, subjects were assigned the severity category at that timepoint: no longer meeting Berlin or PALICC criteria (yellow), mild (orange), moderate (red), or severe (purple).
Receiver operating characteristic (ROC) curves for mortality and ventilator-free days (VFD) ≤ 14 days. Curves are shown for the CHOP cohort (both Berlin and PALICC categories), the CHLA cohort (PALICC categories), and both cohorts combined (PALICC categories). Timepoints are at onset (red), 6 hours (orange), 12 hours (green), 18 hours (blue), and 24 hours (black). The area under the ROC (AUROC) curve is provided in each individual graph legend (AUROC = A).
Mortality, VFD at 28 days, ventilator days in survivors, and probability of extubation given competing risk of death stratified by Berlin (PaO2/FIO2) categories at ARDS onset, and at 6, 12, 18, and 24 hours after. Numbers above mortality bars represent non-survivors and total number of patients in category. Numbers above VFD and ventilator days are medians. P values for a non-parametric test of trend, Hosmer-Lemeshow (HL) p values (for mortality), and slopes and r2 of calibration curves are provided for each graph.
Mortality, VFD at 28 days, ventilator days in survivors, and probability of extubation given competing risk of death stratified by PALICC (OI) categories at ARDS onset, and at 6, 12, 18, and 24 hours after. Numbers above mortality bars represent non-survivors and total number of patients in category. Numbers above VFD and ventilator days are medians. P values for a non-parametric test of trend, Hosmer-Lemeshow (HL) p values (for mortality), and slopes and r2 of calibration curves are provided for each graph. Because of the low number of subjects (n = 6) in PALICC OI < 4 at ARDS onset, we removed this category for calibration tests.
Mortality, VFD at 28 days, ventilator days in survivors, and probability of successful extubation (given the competing risk of death) at PARDS onset, and at 12 and 24 hours after, in the CHLA cohort with complete data at all timepoints (n = 182). Patients were classified using PALICC oxygenation categories based on either OI or OSI. Numbers above mortality bars represent non-survivors and total number of patients in category. Hosmer-Lemeshow (HL) p values are provided for mortality. P values for a non-parametric test of trend, and slopes and r2 of calibration curves are provided for each graph. Medians are provided within each bar for VFD and ventilator days in survivors.