Abstract
Introduction
Chronic Fatigue Syndrome (CFS) is a poorly understood illness that is characterized by diverse somatic symptoms, hypothalamic pituitary adrenal (HPA) axis dysfunction and heightened inflammatory indicators. These symptoms are often exacerbated and accompanied by psychological distress states and depression. Since depression is known to be associated with HPA axis dysfunction and greater inflammation, a psychoneuroendocrinological (PNE) model of inflammation was examined in persons diagnosed with CFS in order to uncover underlying biopsychosocial mechanisms in this poorly understood chronic illness.
Methods
Baseline data were drawn from two randomized controlled trials testing the efficacy of different forms of psychosocial intervention, and included psychological questionnaires, di-urnal salivary cortisol, and blood samples. Data were analyzed with structural equation modeling (SEM).
Results
The sample (N=257) was mostly middle-aged (Mage=49.41 ± 10.9, range=20–73 years), White non-Hispanic (69.3%), female (84.8%), highly educated (87.5% completed some college, college, or graduate program), and depressed (CES-D M =23.87 ± 12.02, range 2–57). The SEM supporting a psychoneuroendocrinological model of immune dysregulation in CFS fit the data χ2 (12) = 17.725, p=0.1243, RMSEA= 0.043, CFI=0.935, SRMR=0.036. Depression was directly related to evening salivary cortisol and inflammation, such that higher evening cortisol predicted greater depressive symptoms (β=0.215, p<0.01) and higher pro-inflammatory cytokines (interleukin-2 [IL-2], IL-6, and tumor necrosis factor-alpha [TNF-α] levels (β=0.185, p<0.05), when controlling for covariates.
Discussion
Results highlight the role of depression, cortisol and inflammation in possible biological mechanisms involved in the pathophysiology of CFS. Time-lagged, longitudinal analyses are needed to fully explore these relationships.
Keywords: depression, evening cortisol, chronic fatigue syndrome (CFS), inflammation, structural equation modeling
1. INTRODUCTION
Chronic fatigue syndrome (CFS) is a chronic unremitting condition with an estimated worldwide prevalence of 0.8–3.5% (Bhui et al., 2011), and is overrepresented among women (Klimas and Koneru, 2007). The disorder is a mysterious and debilitating inflammatory illness with no known etiology or cure. CFS symptoms include debilitating fatigue, post-exertional malaise, sore throat, and unrefreshing sleep, among other varied somatic symptoms (Fukuda et al., 1994). Research has revealed physiological manifestations of the disease, such as dysregulated cortisol awakening response (CAR) and cytokine expression imbalance, which are both shown to be associated with psychological distress in otherwise healthy individuals and those suffering from similar disorders such as fibromyalgia (Marques et al., 2009; Menzies et al., 2013a; Powell et al., 2013; Sutcigil et al., 2007; Vreeburg et al., 2013). However, there is less known about how cortisol relates to inflammatory cytokine levels in this patient population.
Cortisol dysregulation has been shown in CFS (Nater et al., 2008), which is significant because chronically dysregulated cortisol can result in poorer inflammatory control (Cohen et al., 2012). Periods of increased perceived stress or depression can have deleterious effects on immune functioning (Anderson et al., 2014; Silverman and Sternberg, 2012; Wright et al., 2015). The concept of allostatic load, which was put forth by McEwen and colleagues (McEwen, 2000) may be relevant here (Arroll, 2013). One of the concepts in allostatic load is the idea that cortisol is immunosuppressive under short term, acute conditions. However, with chronic increased cortisol secretion, the cells and tissues of the body become less responsive to cortisol (i.e., glucocorticoid resistance, GR) (Cohen et al., 2012; Marques et al., 2009; McEwen, 2000). Once glucocorticoid resistance occurs, immune cells are less responsive to the immunosuppressive actions of cortisol; therefore, pro-inflammatory cytokine production is relatively uninhibited (Cohen et al., 2012; Marques et al., 2009; McEwen, 2000; Silverman and Sternberg, 2012).
Hypothalamic pituitary adrenal (HPA) axis functioning can be evaluated in many ways. The cortisol awakening response (CAR) either with respect to baseline or increased levels (area under the curve with respect to ground [AUCg] and increase [AUCi], respectively), cortisol diurnal slope, and evening cortisol levels are all indicators of HPA functioning and have been implicated in chronic stress and depression (Herbert, 2013; Hsiao et al., 2010; Powell et al., 2013). Given the putative role of inflammatory signaling in CFS (Broderick et al., 2010; Fletcher et al., 2009; Jason et al., 2011; Klimas and Koneru, 2007; Morris et al., 2016; Peterson et al., 2015), it is important to evaluate the effect of HPA functioning on inflammatory markers in CFS patients, yet little work exists, especially using structural modeling techniques.
Because links between psychological adversity and neuroimmune processes (i.e. glucocorticoid resistance and inflammation) have been established in many conditions (Cohen et al., 2012; Marques et al., 2009; Pace et al., 2007), it is important to consider the role of psychological distress states such as depression in CFS (Jason et al., 2011). CFS is commonly comorbid with depression. In a study comparing CFS, fibromyalgia and irritable bowel syndrome, CFS sufferers experienced more mood and anxiety disorders (ORs=2.00–4.08 and 1.63–2.32, respectively) than the other groups (Janssens et al., 2015). The interaction of HPA dysregulation, depression, and inflammation may account for the debilitating fatigue and somatic symptoms of CFS/ME by way of sickness behavior processes (Dantzer et al., 2008; Maes, 2011). There is evidence that HPA dysregulation predicts depression and inflammation, and that depression itself is also directly linked to inflammation; however, a model simultaneously examining the direct and indirect effects of depression on cortisol variables and inflammation has not yet been tested in the CFS population using statistical approaches such as structural equation modeling (SEM) (Maes, 2011; Menzies et al., 2013b; Morris et al., 2015). Analytical approaches such as SEM are particularly useful for simultaneously estimating indirect and direct effects of factors that are possibly involved in the dysregulation of homeostatic systems evident in this heterogeneous disorder. The present study aims to test a pyschoneuroendocrinological model in this patient population to examine the inter-relationships among depressive symptoms and multiple indicators of HPA axis function and inflammation.
2. METHODS
2.1. Participants
Participants in this study were participating in one of two trials evaluating the effects of psychosocial interventions. Baseline data using in this analysis were derived from the women who participated in the trials. Recruitment procedures for one of these trials are described elsewhere, and reflect the procedures used in the second trial (Hall et al., 2014; Lattie et al., 2012b). All participants received a physician-determined CFS diagnosis, as defined by the CDC criteria (Fukuda et al., 1994). Recruitment methods included physician referral, support groups, CFS conferences and advertisements in CFS-related websites. Participants were eligible if they were fluent in English, and were between the ages of 21 and 75 years.
Potential participants were excluded from the study if they met criteria for schizophrenia, bipolar disorder, substance abuse, or if they were actively suicidal, as assessed by a brief screening measure adapted from the Structured Clinical Interview for the DSM-IV (First et al., 1997). Participants were also excluded if they showed markedly diminished cognitive capabilities, as evidenced by making four or more errors on the Short Portable Mental Status Questionnaire (Pfeiffer, 1975). Presence of another condition (e.g. AIDS, lupus, rheumatoid arthritis) that might influence biological processes associated with CFS symptomatology, or taking medications that would modulate immune or neuroendocrine functioning excluded participants from the study. Potential participants were also excluded from the study if they were suffering from untreated obstructive sleep apnea (OSA). A total of approximately 220 screened participants were excluded from the two studies.
Participants who met criteria signed an informed consent form and were administered a battery of self-report measures. Salivary cortisol samples were collected for two consecutive weekdays, and a blood sample was drawn by a certified phlebotomist, all within a span of 10 days. After completing these assessments participants were compensated with $50.
2.2. Center for Epidemiologic Studies Depression Scale (CES-D)
The CES-D (Radloff, 1977) is a 20-item measure that assesses depressive symptomatology over the past week. Participants were asked questions such as “I felt sad” and responded on a 4 point scale ranging from “Rarely or none of the time (<1 day)” to “Most or All of the Time (5–7 days).” A score of 16 or above indicates clinically significant depressive symptoms. The reliability coefficient for the CES-D is 0.85 in the general population, 0.90 in an in-patient psychiatric population (Radloff, 1977), and 0.88 in a chronically ill population of HIV patients (Gay et al., 2016).
2.3. Salivary Cortisol
We provided the participants with 8 Salivette® tubes, as described previously (Hall et al., 2014; Lattie et al., 2012a). Participants provided saliva samples from two consecutive weekdays, on which they were asked to take a sample at awakening, 30 minutes after awakening, at 4 pm and at 9 pm. Participants were instructed to abstain from eating or drinking before and between the first two samples each day, and to avoid eating a large meal an hour before the afternoon and evening samples. Participants were also asked to avoid alcohol for at least 12 hours prior to sample collection and to avoid vigorous exercise on sample collection days. Following the collection of samples, participants were instructed to freeze the Salivette® tubes in their home freezers in order to keep the salivary cortisol stable until it was retrieved by a member of the study staff or mailed back to the lab. Once received, saliva samples were frozen at −20°C until enough samples were accumulated to be assayed in batches. On the day of the assay, saliva samples were thawed, vortexed and centrifuged at 1500 RPM for 15 minutes. Samples were then assayed using the Salimetrics high sensitivity ELISA kit (State College, PA). The present study only focused on awakening and 30 minutes post awakening to calculate the AUC values. In addition evening (9 pm) averaged cortisol values were computed separately. Area under the curve with respect to ground (AUCg) and increase (AUCi) were also calculated (Powell et al., 2013). AUC values were used to approximate the cortisol awakening response (CAR), while evening cortisol was analyzed separately.
2.4. Circulating Pro-Inflammatory Cytokines
Blood was centrifuged and plasma stored at −80°C within 4 hours of collection until the samples were assayed in batches and in duplicate. Circulating pro-inflammatory cytokines interleukin-2 (IL-2), IL-6 and tumor necrosis factor-alpha (TNF-α) were measured from blood plasma as previously described (Fletcher et al., 2009) using the an ELISA-based test (Q-Plex™ Human Cytokine –Screen, Quansys Biosciences Logan, Utah). Images of the ELISA plate were captured using Quansys Imager, driven by an 8.4 megapixel Canon 20D digital SLR camera, and analyzed using Quansys Software. In order to assure compatibility with measurements of cytokines in previously published studies in the field (Chiswick et al., 2012; Trune et al., 2011; Wong et al., 2008), the antigen standard concentrations used by Quansys (R&D) were referenced to “gold standard” for each cytokine represented on the multiplex plate as previously described (Lattie et al., 2012b).
2.5. Statistical Analyses
Descriptive analysis of the sample was run using IBM SPSS Statistics software version 24. Structural equation modeling (SEM) analyses were performed using Mplus software version 7.4 (Muthen and Muthen, Los Angeles, CA). Cross-sectional direct and indirect associations between observed measures and a hypothesized latent construct were estimated simultaneously using maximum likelihood estimation (Markus, 2012). According to traditional SEM practices, the analyses were performed in two steps. First, a confirmatory factor analysis (CFA) was conducted to establish construct validity (Markus, 2012). The latent construct Inflammation was hypothesized to represent the common variance among selected indicators including circulating serum pro-inflammatory IL-2, IL-6, and TNF-α. The latent construct HPA Dysregulation was similarly composed of indicators including area under the curve with respect to increase (AUCi), area under the curve with respect to ground (AUCg), and evening cortisol concentration. These indicators were chosen because of their relevance to the factor and also to this patient population (Fletcher et al., 2009; Gupta et al., 1997; Moss et al., 1999; Pandi-Perumal et al., 2007; Powell et al., 2013). Then, path analysis was performed to analyze direct associations between validated latent constructs and other measured variables (Markus, 2012). Cortisol and cytokine data were winsorized and/or log- transformed (i.e. ln(IL-6+1)) to meet assumptions of normality. Inflammation was regressed on evening cortisol and depression, while controlling for participants’ age, education level, and gender, as those variables have been shown to affect both HPA functioning and inflammation (O'Connor et al., 2009). Model fit indices were compared against Root Mean Square Error of Approximation (RMSEA) ≤ 0.05, Comparative Fit Index (CFI) ≥ 0.95, and Standardized Root Mean Square Residual (SRMR) ≤ 0.08 (Kline, 1998). Chisquare tests were also used to confirm model fit at α=0.05 (Kline, 1998).
3. RESULTS
The sample (N=257) was mostly middle-aged (Mage=49.41 ± 10.9, range=20–73 years), White non-Hispanic (69.3%), female (84.8%), highly educated (87.5% completed some college, college, or graduate program), and depressed (CES-D M =23.87 ± 12.02, range 2–57), as shown in Table 1. Two latent factors were operationalized: 1) HPA Dysregulation indicated by AUCg, AUCi, and evening cortisol; and 2) Inflammation indicated by IL-2, IL-6, and TNF-α. The CFA yielded a measurement model of adequate fit according to previously established standards (Markus, 2012) χ2(8)= 13.306, p=0.1017, RMSEA= 0.052, CFI=0.957, SRMR=0.048. Standardized and unstandardized factor loadings, which were shown to have statistically significant associations between each measured indicator and respective latent construct, are shown in Table 2.
Table 1.
Demographic and Symptom-Related Characteristics of the Sample
| Participant Characteristics M ± SD |
|
|---|---|
| Age in years | 49.36 ± 10.90 |
| BMI (kg/in2) | 27.52 ± 6.50 |
| Depression Severity (CES-D) | 23.78 ± 12.09 |
| Fatigue Severity | 25.80 ± 6.40 |
| Fatigue Interference | 47.18± 14.51 |
| CFS/ME Symptom Severity | 2.33 ± .78 |
|
| |
| Demographics n (%) | |
|
| |
| Male | 39 (15.2%) |
| Female | Female 218 (84.8%) |
| Attended at least part of college | 225 (87.5%) |
| White non-Hispanic | 178 (69.3 %) |
Table 2.
Means and Standardized and Unstandardized Regression Coefficients and Significance of HPA Dysregulation and Inflammation Indicators
| Means | SD | Standardized β | Unstandardized B | P | R2 | |
|---|---|---|---|---|---|---|
| AUCg | 2.385 | 0.636 | 1.013 | 1.00 | - | - |
| AUCi | 0.890 | 0.883 | 0.453 | 0.626 | 0.038 | 0.205 |
| Evening cortisola | 0.090 | 0.096 | 0.319 | 0.048 | 0.041 | 0.102 |
|
| ||||||
| IL-6b | 0.631 | 0.368 | 0.475 | 1.00 | - | 0.225 |
| IL-2b | 0.808 | 0.369 | 0.477 | 0.241 | >0.01 | 0.228 |
| TNF-αb | 0.970 | 0.436 | 0.722 | 0.522 | >0.01 | 0.522 |
measured in ug/dL;
measured in ln([x+1]) pg/mL
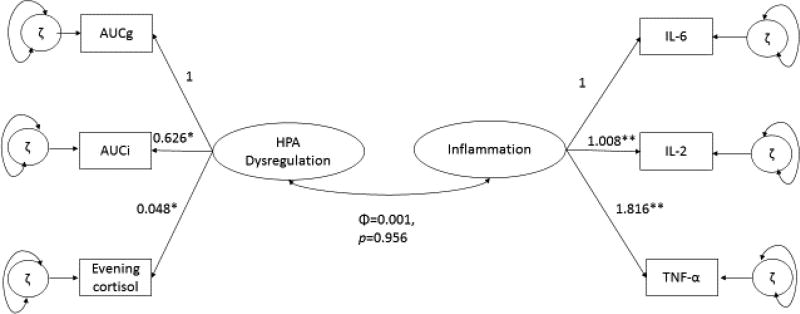
Evening cortisol did not load as strongly onto the HPA Dysregulation factor as the other indicators pertaining to cortisol awakening response (Table 2). The normalized residual between evening cortisol and circulating IL-6 was 2.426. The correlation between the two latent constructs was not significant (Φ=−0.005, se=0.084, p=0.956). The results are presented in Figure 1 (unstandardized loadings).
Figure 1.
Confirmatory Factor Analysis (CFA) of Latent Constructs HPA Dysregulation and Inflammation
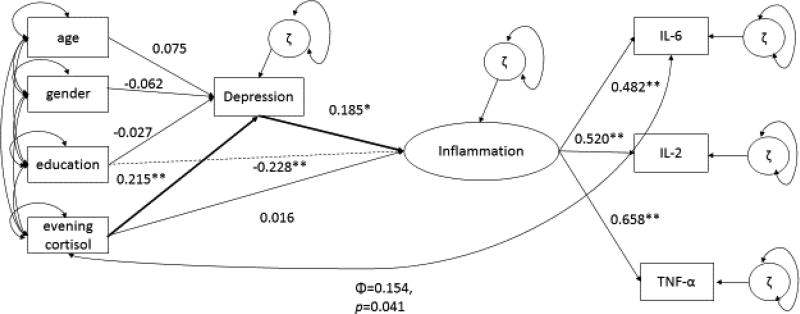
The results of the CFA showed no correlation between the two latent constructs HPA Dysregulation and Inflammation, and implied that evening cortisol should be considered as a separate indicator of diurnal cortisol abnormalities (i.e. as an indicator of flattened diurnal cortisol slope). Because prior literature showed that evening cortisol was related to cytokine levels that peak during the night (i.e. IL-6) (Logan and Sarkar, 2012; Nakamura et al., 2010; Vgontzas et al., 2002), and that evening cortisol levels are associated with depression (Hsiao et al., 2010; Kabia et al., 2015), a new model was tested that used only (observed) evening cortisol as an indicator of HPA functioning. The latent construct Inflammation was regressed on depression and evening cortisol (Figure 2) while controlling for possible confounding variables age, educational status (as a measure of socio-economic status) and gender (O'Connor et al., 2009). Taking into account the results from the CFA, and modification indices of the first model, evening cortisol and IL-6 were covaried. The resulting mixed structural regression model fit the data and supported that evening cortisol predicts greater depressive symptoms, which predict greater inflammation (χ2 (12) = 17.725, p=0.1243, RMSEA= 0.043, CFI=0.935, SRMR=0.036). Table 3 summarizes the structural and regression-based results.
Figure 2.
Mixed Structural Regression Model of the Indirect Effect of Depression on Evening Cortisol Levels and Inflammation
Table 3.
Standardized and Unstandardized Regression Coefficients and Significance for Revised Model Relating Evening Cortisol, Depressive Symptoms and Inflammation in Persons with CFS/ME
| Criterion Measure | Predictor | Standardized β | Unstandardized B | SE | P |
|---|---|---|---|---|---|
| Inflammation | IL-6 | 0.482 | 1.000 | 0.000 | - |
| IL-2 | 0.520 | 1.082 | 0.267 | >0.01 | |
| TNF- α | 0.658 | 1.628 | 0.386 | >0.01 | |
|
| |||||
| Inflammation | Evening cortisol | 0.016 | 0.029 | 0.173 | 0.865 |
| Depression | 0.185 | 0.003 | 0.001 | 0.048 | |
| Education | −0.228 | −0.029 | 0.011 | 0.011 | |
| Age | −0.145 | −0.002 | 0.001 | 0.097 | |
| Gender | −0.101 | −0.050 | 0.042 | 0.237 | |
|
| |||||
| Depression | Evening cortisol | 0.215 | 27.086 | 8.102 | 0.001 |
| Education | −0.027 | −0.229 | 0.527 | 0.664 | |
| Age | −0.062 | −0.068 | 0.068 | 0.317 | |
| Gender | 0.075 | 2.501 | 2.077 | 0.228 | |
This model includes the direct effect of evening cortisol on depression (β=0.215, se= 0.063, p=0.001), such that a one unit increase in evening cortisol relates to a 0.215 standardized unit increase in depression severity (as measured by the CES-D), when controlling for age, gender, and education. The model also supports the direct association between depression and inflammation (β=0.185, SEM=0.086, p=0.048), when controlling for age, gender, and education, such that a one unit increase in depression results in a 0.185 standardized unit increase in inflammation. There is a positive indirect effect of evening cortisol on inflammation, via depression (β=0.185, SEM=0.086, p=0.048), such that a one unit increase in evening cortisol relates to a 0.185 standardized unit increase in inflammation, with respect to a one unit increase in depression, when controlling for the effects of age and gender. There is no significant direct effect of evening cortisol on inflammation (β=0.016, SEM=0.093, p=0.865). There are no indirect effects of evening cortisol on the relationship between depression and inflammation (no support of evening cortisol as a mediator, β=0.00). The model supports the indirect path between evening cortisol, depression, and inflammation (with depression as the intermediate in this pathway) (β=0.039). Among the variables entered as covariates, only education exerted a negative direct effect on inflammation (β= −0.228, se=0.080, p=0.004), such that a one unit increase in education results in a −0.228 standardized unit decrease in inflammation (Figure 2, path shown). All other covariates (age and gender) did not show significant associations with depression or inflammation (all p’s>0.05). Finally, the covariance between evening cortisol and IL-6 was significant (ϕ= 0.154, se= 0.075, p=0.041).
4. DISCUSSION
The present study tested a structural model examining the relationships between HPA dysregulation, depression and inflammation in persons diagnosed with chronic fatigue syndrome (CFS). A CFA showed that the two latent factors HPA Dysregulation and Inflammation adequately captured shared variance of indicators AUCg, AUCi and evening cortisol; and IL-6, IL-2, and TNF-α, respectively. A mixed structural regression model fit the data, and supported the theory that greater evening salivary cortisol predicts greater depression, which predicts greater circulating pro-inflammatory cytokines (IL-2, IL-6, TNF-α). Interestingly, results showed that evening cortisol levels were not directly associated with inflammation, as defined.
That evening cortisol levels were associated with depression corroborates findings from studies conducted in adolescents and older adults (Kabia et al., 2015; Van den Bergh and Van Calster, 2009). There is also evidence that cortisol awakening response (CAR) area under the curve (AUC) measurements are not consistently related to depression in other work (Adam et al., 2010; Carnegie et al., 2014). In a sample of healthy individuals, trait hopelessness reactivity, which was related to the likelihood of developing depression in other studies, was correlated with an increased CAR(van Santen et al., 2011); therefore, our use of the CES-D did not capture this subtle facet of depression, which may explain our negative results. In a sample of otherwise healthy participants suffering from burnout and healthy controls, the CAR was not significantly different between the control and burnout groups, nor was depression related to the CAR in the burnout group (Mommersteeg et al., 2006). Burnout syndrome is characterized by many of the same symptoms of CFS, including but not limited to exhaustion, muscle aches and pains, concentration and memory problems, and tension headaches. Perhaps, the CAR more easily reflects variance in trait-like negative attitudes in healthy individuals versus that of individuals with depressive and comorbid somatic symptoms. The mechanism behind these myriad apparent distinctions is an extremely complicated area of psychoneuroendocrinology that warrants further study (Saxbe, 2008).
The CAR has been studied extensively in CFS; however, the sample characteristics across studies differ with respect to whether or not depression status is an exclusionary factor (Amel Kashipaz et al., 2003). The results of the present study underscore the importance of examining evening cortisol levels in a sample of CFS individuals who also present with varying levels of depressive symptoms. Depression was also shown to predict increased inflammation in healthy and chronically ill individuals (Berk et al., 2013; Dantzer et al., 2008). The results presented here add to the literature relating greater depressive symptoms to greater inflammation, and extend these findings to men and women with CFS.
The results showing that evening cortisol or HPA Dysregulation did not correlate with inflammation (as defined in our study) did not support the theory that cortisol would affect inflammatory cytokine levels either by acting as an immunosuppressive agent or would reflect glucocorticoid resistance (possibly due to CFS and/or chronic stress). The idea of glucocorticoid resistance in CFS is controversial. Indeed, there is some recent evidence of glucocorticoid hypersensitivity in CFS (de Vega et al., 2017; Papadopoulos et al., 2009; Vangeel et al., 2015).Using evening cortisol as the index of HPA functioning in our model might not have adequately captured the hypo- or hyper-sensitivity to glucocorticoids that is sensitive to mood fluctuation or intrinsic in CFS patient phenotypes. Moreover, our study did not use DST or LPS challenge experimental paradigms which would better capture HPA dysregulation dynamically in vivo or in-vitro, respectively (Gaab et al., 2005; Zhang et al., 2016). Our results, among others, solicit the need for further research exploring psycho-neuro-endocrine-immune relationships, especially in this patient population and particularly when taking into account the inconsistent results seen currently in the literature (Light et al., 2012).
Although this study generated some provocative findings there are several limitations that must be kept in mind when interpreting the results. The model tested was based on secondary analyses of cross-sectional, baseline data from previously conducted psychosocial intervention trials. As in many secondary analyses, not all key information is available for examining the role of competing or confounding variables. For instance, the study did not examine the effects of BMI, which is usually included as a covariate in endocrinological and immunological research (O'Connor et al., 2009), because the data was not available on all cases. There are also other indicators of endocrine dysfunction or inflammation that were not measured in this study (e.g. GC receptor or inflammatory gene expression), and those variables might better measure relevant phenomena such underlying proposed associations between glucocorticoid receptor resistance and poorer inflammatory control (Antoni et al., 2012; Cohen et al., 2012). While the CES-D has high reliability for depressed and chronically ill individuals, caution is warranted in interpreting these results, as the CES-D has two items that might overlap with CFS symptoms (i.e. trouble concentrating, restless sleep).
Additionally, many of the mechanisms posited in this area of research are bidirectional and are potentially affected by related variables, such as sleep (Lorton et al., 2006; Milrad et al., 2017; Pace et al., 2007). We have reported elsewhere that greater sleep disruption is associated with greater inflammation in CFS patients (Milrad et al., 2017). Inflammation can directly cause depressive symptoms, as pro-inflammatory cytokines can cross the blood-brain barrier to induce sickness behavior and depressed mood (Dantzer, 2009). Inflammatory cytokines can also negatively impact glucocorticoid signaling (Marques et al., 2009). Importantly, relatively little is known about the ways that the clinical symptoms of CFS fluctuate in response to variability in the psychological, endocrine and inflammatory measures tested in the present study, though there is some preliminary evidence linking CFS symptom exacerbation to psychological adversity (Antoni et al., 1994; Bhui et al., 2011; Chan et al., 2014; Lattie et al., 2012b; Lopez et al., 2011). However most of these studies are cross-sectional.
Therefore, future studies using longitudinal designs and employing structural equation modeling and latent growth modeling should be applied using these variables to further assess the biobehavioral processes that were tested in this study in relation to the waxing and waning of CFS symptoms over time. These findings shed light specifically on important associations between individual differences in depression, one HPA axis dysregulatory indicator, and inflammatory markers in CFS, a poorly understood condition that is likely maintained and exacerbated as a function of psychological adversity and accompanying neuroimmune processes. Further research should be aimed at assessing mechanisms of change of these variables and at improving these interacting variables synergistically. If depression plays a key role in determining neuroimmune processes that underlie the symptomology of CFS then behavioral and pharmacologic approaches to managing depression and other forms of adversity can be examined as part of a comprehensive treatment approach.
In a more general sense, fatigue, inflammation, depression, and HPA dysregulation is implicated in a wide variety of disorders that affect physical and mental well-being. While all variables can theoretically affect one another, the mechanism by which that happens differs for disorders. As an example, in a sample of people diagnosed with multiple sclerosis, proinflammatory cytokine levels are correlated with fatigue but not HPA axis dysfunction, while HPA dysfunction is correlated with cognitive impairment (Heesen et al., 2006). In contrast, breast cancer survivors with persistent fatigue show altered cortisol responses (Bower et al., 2005). Therefore, the type of analysis used in this study might be helpful in determining the psychoneuroendocrinological mechanisms of action of other fatigue-related disorders in order to more completely understand the etiology of the symptoms and optimize its treatment.
Highlights.
A structural regression model of chronic fatigue syndrome (CFS) is presented.
The psychoneuroendocrinological model of immune dysregulation in CFS fit the data.
Results highlight the role of depression, cortisol and inflammation in CFS.
Acknowledgments
Sources of Funding:
This research was supported by the NIH grant 1R01 NS055672.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
The authors report no conflicts of interest.
References
- Adam EK, Doane LD, Zinbarg RE, Mineka S, Craske MG, Griffith JW. Prospective prediction of major depressive disorder from cortisol awakening responses in adolescence. Psychoneuroendocrinology. 2010;35:921–931. doi: 10.1016/j.psyneuen.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amel Kashipaz MR, Swinden D, Todd I, Powell RJ. Normal production of inflammatory cytokines in chronic fatigue and fibromyalgia syndromes determined by intracellular cytokine staining in short-term cultured blood mononuclear cells. Clinical & Experimental Immunology. 2003;132:360–365. doi: 10.1046/j.1365-2249.2003.02149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson G, Berk M, Maes M. Biological phenotypes underpin the physiosomatic symptoms of somatization, depression, and chronic fatigue syndrome. Acta psychiatrica Scandinavica. 2014;129:83–97. doi: 10.1111/acps.12182. [DOI] [PubMed] [Google Scholar]
- Antoni MH, Brickman A, Lutgendorf S, Klimas N, Imia-Fins A, Ironson G, Quillian R, Miguez MJ, van Riel F, Morgan R. Psychosocial correlates of illness burden in chronic fatigue syndrome. Clinical Infectious Diseases. 1994;18:S73–S78. doi: 10.1093/clinids/18.supplement_1.s73. [DOI] [PubMed] [Google Scholar]
- Antoni MH, Lutgendorf SK, Blomberg B, Carver CS, Lechner S, Diaz A, Stagl J, Arevalo JM, Cole SW. Cognitive-behavioral stress management reverses anxiety-related leukocyte transcriptional dynamics. Biol Psychiatry. 2012;71:366–372. doi: 10.1016/j.biopsych.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroll MA. Allostatic overload in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) Med. Hypotheses. 2013;81:506–508. doi: 10.1016/j.mehy.2013.06.023. [DOI] [PubMed] [Google Scholar]
- Berk M, Williams LJ, Jacka FN, O’Neil A, Pasco JA, Moylan S, Allen NB, Stuart AL, Hayley AC, Byrne ML, Maes M. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013;11:200. doi: 10.1186/1741-7015-11-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhui KS, Dinos S, Ashby D, Nazroo J, Wessely S, White PD. Chronic fatigue syndrome in an ethnically diverse population: the influence of psychosocial adversity and physical inactivity. BMC Med. 2011;9:26. doi: 10.1186/1741-7015-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Aziz N. Altered cortisol response to psychologic stress in breast cancer survivors with persistent fatigue. Psychosom Med. 2005;67:277–280. doi: 10.1097/01.psy.0000155666.55034.c6. [DOI] [PubMed] [Google Scholar]
- Broderick G, Fuite J, Kreitz A, Vernon SD, Klimas N, Fletcher MA. A Formal Analysis of Cytokine Networks in Chronic Fatigue Syndrome. Brain Behav. Immun. 2010;24:1209–1217. doi: 10.1016/j.bbi.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnegie R, Araya R, Ben-Shlomo Y, Glover V, O'Connor TG, O'Donnell KJ, Pearson R, Lewis G. Cortisol awakening response and subsequent depression: prospective longitudinal study. The British journal of psychiatry : the journal of mental science. 2014;204:137–143. doi: 10.1192/bjp.bp.113.126250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan JSM, Ho RTH, Chung K-F, Wang C-W, Yao T-J, Ng S-M, Chan CLW. Qigong exercise alleviates fatigue, anxiety, and depressive symptoms, improves sleep quality, and shortens sleep latency in persons with chronic fatigue syndrome-like illness. Evid Based Complement Alternat Med. 2014;2014:106048. doi: 10.1155/2014/106048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiswick EL, Duffy E, Japp B, Remick D. Detection and quantification of cytokines and other biomarkers. Methods Mol. Biol. 2012;844:15–30. doi: 10.1007/978-1-61779-527-5_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Janicki-Deverts D, Doyle WJ, Miller GE, Frank E, Rabin BS, Turner RB. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc Natl Acad Sci U S A. 2012;109:5995–5999. doi: 10.1073/pnas.1118355109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R. Cytokine, sickness behavior, and depression. Immunology and allergy clinics of North America. 2009;29:247–264. doi: 10.1016/j.iac.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vega WC, Herrera S, Vernon SD, McGowan PO. Epigenetic modifications and glucocorticoid sensitivity in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) BMC medical genomics. 2017;10:11. doi: 10.1186/s12920-017-0248-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL. User's Guide for the Structured Clinical Interview for DSM-IV Axis II Personality Disorders: SCID-II. American Psychiatric Pub.; 1997. [Google Scholar]
- Fletcher MA, Zeng XR, Barnes Z, Levis S, Klimas NG. Plasma cytokines in women with chronic fatigue syndrome. J Transl Med. 2009;7:96. doi: 10.1186/1479-5876-7-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann. Intern. Med. 1994;121:953–959. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- Gaab J, Rohleder N, Heitz V, Engert V, Schad T, Schurmeyer TH, Ehlert U. Stress-induced changes in LPS-induced pro-inflammatory cytokine production in chronic fatigue syndrome. Psychoneuroendocrinology. 2005;30:188–198. doi: 10.1016/j.psyneuen.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Gay CL, Kottorp A, Lerdal A, Lee KA. Psychometric limitations of the Center for Epidemiologic Studies-Depression Scale for assessing depressive symptoms among adults with HIV/AIDS: a Rasch analysis. Depression research and treatment. 2016;2016 doi: 10.1155/2016/2824595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Aggarwal S, See D, Starr A. Cytokine production by adherent and non-adherent mononuclear cells in chronic fatigue syndrome. Journal of Psychiatric Research. 1997;31:149–156. doi: 10.1016/s0022-3956(96)00063-5. [DOI] [PubMed] [Google Scholar]
- Hall DL, Lattie EG, Antoni MH, Fletcher MA, Czaja S, Perdomo D, Klimas NG. Stress management skills, cortisol awakening response, and post-exertional malaise in Chronic Fatigue Syndrome. Psychoneuroendocrinology. 2014;49:26–31. doi: 10.1016/j.psyneuen.2014.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heesen C, Nawrath L, Reich C, Bauer N, Schulz K, Gold S. Fatigue in multiple sclerosis: an example of cytokine mediated sickness behaviour? Journal of Neurology, Neurosurgery & Psychiatry. 2006;77:34–39. doi: 10.1136/jnnp.2005.065805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert J. Cortisol and depression: three questions for psychiatry. Psychological medicine. 2013;43:449–469. doi: 10.1017/S0033291712000955. [DOI] [PubMed] [Google Scholar]
- Hsiao FH, Yang TT, Ho RT, Jow GM, Ng SM, Chan CL, Lai YM, Chen YT, Wang KC. The self-perceived symptom distress and health-related conditions associated with morning to evening diurnal cortisol patterns in outpatients with major depressive disorder. Psychoneuroendocrinology. 2010;35:503–515. doi: 10.1016/j.psyneuen.2009.08.019. [DOI] [PubMed] [Google Scholar]
- Janssens KA, Zijlema WL, Joustra ML, Rosmalen JG. Mood and Anxiety Disorders in Chronic Fatigue Syndrome, Fibromyalgia, and Irritable Bowel Syndrome: Results From the LifeLines Cohort Study. Psychosom Med. 2015;77:449–457. doi: 10.1097/PSY.0000000000000161. [DOI] [PubMed] [Google Scholar]
- Jason LA, Sorenson M, Porter N, Belkairous N. An Etiological Model for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Neuroscience and medicine. 2011;2:14–27. doi: 10.4236/nm.2011.21003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabia FM, Rhebergen D, van Exel E, Stek ML, Comijs HC. The predictive value of cortisol levels on 2-year course of depression in older persons. Psychoneuroendocrinology. 2015;63:320–326. doi: 10.1016/j.psyneuen.2015.10.006. [DOI] [PubMed] [Google Scholar]
- Klimas NG, Koneru AOB. Chronic fatigue syndrome: inflammation, immune function, and neuroendocrine interactions. Curr Rheumatol Rep. 2007;9:482–487. doi: 10.1007/s11926-007-0078-y. [DOI] [PubMed] [Google Scholar]
- Kline RB. Structural equation modeling. New York: Guilford; 1998. [Google Scholar]
- Lattie EG, Antoni MH, Fletcher MA, Penedo F, Czaja S, Lopez C, Perdomo D, Sala A, Nair S, Fu SH, Klimas N, et al. Stress management skills, neuroimmune processes and fatigue levels in persons with chronic fatigue syndrome. Brain Behav Immun. 2012a;26:849–858. doi: 10.1016/j.bbi.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattie EG, Antoni MH, Fletcher MA, Penedo F, Czaja S, Lopez C, Perdomo D, Sala A, Nair S, Fu SH, Klimas N, et al. Stress management skills, neuroimmune processes and fatigue levels in persons with chronic fatigue syndrome. Brain Behav. Immun. 2012b;26:849–858. doi: 10.1016/j.bbi.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light KC, White AT, Tadler S, Iacob E, Light AR. Genetics and Gene Expression Involving Stress and Distress Pathways in Fibromyalgia with and without Comorbid Chronic Fatigue Syndrome. Pain Research and Treatment. 2012;2012:427869. doi: 10.1155/2012/427869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan RW, Sarkar DK. Circadian nature of immune function. Mol Cell Endocrinol. 2012;349:82–90. doi: 10.1016/j.mce.2011.06.039. [DOI] [PubMed] [Google Scholar]
- Lopez C, Antoni M, Penedo F, Weiss D, Cruess S, Segotas MC, Helder L, Siegel S, Klimas N, Fletcher MA. A pilot study of cognitive behavioral stress management effects on stress, quality of life, and symptoms in persons with chronic fatigue syndrome. J Psychosom Res. 2011;70:328–334. doi: 10.1016/j.jpsychores.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorton D, Lubahn CL, Estus C, Millar BA, Carter JL, Wood CA, Bellinger DL. Bidirectional communication between the brain and the immune system: implications for physiological sleep and disorders with disrupted sleep. Neuroimmunomodulation. 2006;13:357–374. doi: 10.1159/000104864. [DOI] [PubMed] [Google Scholar]
- Maes M. An intriguing and hitherto unexplained co-occurrence: Depression and chronic fatigue syndrome are manifestations of shared inflammatory, oxidative and nitrosative (IO&NS) pathways. Progress in neuro-psychopharmacology & biological psychiatry. 2011;35:784–794. doi: 10.1016/j.pnpbp.2010.06.023. [DOI] [PubMed] [Google Scholar]
- Markus KA. Principles and Practice of Structural Equation Modeling by Rex B Kline. Structural Equation Modeling: A Multidisciplinary Journal. 2012;19:509–512. [Google Scholar]
- Marques AH, Silverman MN, Sternberg EM. Glucocorticoid Dysregulations and Their Clinical Correlates: From Receptors to Therapeutics. Annals of the New York Academy of Sciences. 2009;1179:1–18. doi: 10.1111/j.1749-6632.2009.04987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Allostasis and Allostatic Load: Implications for. Neuropsychopharmacology. 2000;22:108–124. doi: 10.1016/S0893-133X(99)00129-3. [DOI] [PubMed] [Google Scholar]
- Menzies V, Lyon DE, Elswick RK, Jr, Montpetit AJ, McCain NL. Psychoneuroimmunological relationships in women with fibromyalgia. Biol Res Nurs. 2013a;15:219–225. doi: 10.1177/1099800411424204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies V, Lyon DE, Elswick RK, Montpetit AJ, McCain NL. Psychoneuroimmunological Relationships in Women with Fibromyalgia. Biological research for nursing. 2013b;15:219–225. doi: 10.1177/1099800411424204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milrad SF, Hall DL, Jutagir DR, Lattie EG, Ironson GH, Wohlgemuth W, Nunez MV, Garcia L, Czaja SJ, Perdomo DM, Fletcher MA, Klimas N, Antoni MH. Poor sleep quality is associated with greater circulating pro-inflammatory cytokines and severity and frequency of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) symptoms in women. J Neuroimmunol. 2017;303:43–50. doi: 10.1016/j.jneuroim.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mommersteeg PM, Heijnen CJ, Verbraak MJ, van Doornen LJ. Clinical burnout is not reflected in the cortisol awakening response, the day-curve or the response to a low-dose dexamethasone suppression test. Psychoneuroendocrinology. 2006;31:216–225. doi: 10.1016/j.psyneuen.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Morris G, Anderson G, Maes M. Hypothalamic-Pituitary-Adrenal Hypofunction in Myalgic Encephalomyelitis (ME)/Chronic Fatigue Syndrome (CFS) as a Consequence of Activated Immune-Inflammatory and Oxidative and Nitrosative Pathways. Mol. Neurobiol. 2016:1–14. doi: 10.1007/s12035-016-0170-2. [DOI] [PubMed] [Google Scholar]
- Morris G, Berk M, Galecki P, Walder K, Maes M. The Neuro-Immune Pathophysiology of Central and Peripheral Fatigue in Systemic Immune-Inflammatory and Neuro-Immune Diseases. Mol Neurobiol. 2015 doi: 10.1007/s12035-015-9090-9. [DOI] [PubMed] [Google Scholar]
- Moss RB, Mercandetti A, Vojdani A. TNF-alpha and chronic fatigue syndrome. J. Clin. Immunol. 1999;19:314–316. doi: 10.1023/a:1020595709352. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Schwander SK, Donnelly R, Ortega F, Togo F, Broderick G, Yamamoto Y, Cherniack NS, Rapoport D, Natelson BH. Cytokines across the night in chronic fatigue syndrome with and without fibromyalgia. Clin. Vaccine Immunol. 2010;17:582–587. doi: 10.1128/CVI.00379-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nater UM, Maloney E, Boneva RS, Gurbaxani BM, Lin JM, Jones JF, Reeves WC, Heim C. Attenuated morning salivary cortisol concentrations in a population-based study of persons with chronic fatigue syndrome and well controls. J Clin Endocrinol Metab. 2008;93:703–709. doi: 10.1210/jc.2007-1747. [DOI] [PubMed] [Google Scholar]
- O'Connor M-F, Bower JE, Cho HJ, Creswell JD, Dimitrov S, Hamby ME, Hoyt MA, Martin JL, Robles TF, Sloan EK, Thomas KS, Irwin MR. To assess, to control, to exclude: effects of biobehavioral factors on circulating inflammatory markers. Brain Behav. Immun. 2009;23:887–897. doi: 10.1016/j.bbi.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace TW, Hu F, Miller AH. Cytokine-effects on glucocorticoid receptor function: relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain Behav Immun. 2007;21:9–19. doi: 10.1016/j.bbi.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandi-Perumal SR, Cardinali DP, Chrousos GP. Neuroimmunology of sleep. Springer; 2007. [Google Scholar]
- Papadopoulos A, Ebrecht M, Roberts AD, Poon L, Rohleder N, Cleare AJ. Glucocorticoid receptor mediated negative feedback in chronic fatigue syndrome using the low dose (0.5 mg) dexamethasone suppression test. J Affect Disord. 2009;112:289–294. doi: 10.1016/j.jad.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Peterson D, Brenu EW, Gottschalk G, Ramos S, Nguyen T, Staines D, Marshall-Gradisnik S. Cytokines in the Cerebrospinal Fluids of Patients with Chronic Fatigue Syndrome/Myalgic Encephalomyelitis. Mediators Inflamm. 2015;2015 doi: 10.1155/2015/929720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23:433–441. doi: 10.1111/j.1532-5415.1975.tb00927.x. [DOI] [PubMed] [Google Scholar]
- Powell DJH, Liossi C, Moss-Morris R, Schlotz W. Unstimulated cortisol secretory activity in everyday life and its relationship with fatigue and chronic fatigue syndrome: a systematic review and subset meta-analysis. Psychoneuroendocrinology. 2013;38:2405–2422. doi: 10.1016/j.psyneuen.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Saxbe DE. A field (researcher's) guide to cortisol: tracking HPA axis functioning in everyday life. Health Psychology Review. 2008;2:163–190. [Google Scholar]
- Silverman MN, Sternberg EM. Glucocorticoid regulation of inflammation and its functional correlates: from HPA axis to glucocorticoid receptor dysfunction. Annals of the New York Academy of Sciences. 2012;1261:55–63. doi: 10.1111/j.1749-6632.2012.06633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcigil L, Oktenli C, Musabak U, Bozkurt A, Cansever A, Uzun O, Sanisoglu SY, Yesilova Z, Ozmenler N, Ozsahin A, Sengul A. Pro- and Anti-Inflammatory Cytokine Balance in Major Depression: Effect of Sertraline Therapy. Clin Dev Immunol. 2007;2007 doi: 10.1155/2007/76396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trune DR, Larrain BE, Hausman FA, Kempton JB, MacArthur CJ. Simultaneous measurement of multiple ear proteins with multiplex ELISA assays. Hear. Res. 2011;275:1–7. doi: 10.1016/j.heares.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bergh BR, Van Calster B. Diurnal cortisol profiles and evening cortisol in post-pubertal adolescents scoring high on the Children's Depression Inventory. Psychoneuroendocrinology. 2009;34:791–794. doi: 10.1016/j.psyneuen.2008.12.008. [DOI] [PubMed] [Google Scholar]
- van Santen A, Vreeburg SA, Van der Does AW, Spinhoven P, Zitman FG, Penninx BW. Psychological traits and the cortisol awakening response: results from the Netherlands Study of Depression and Anxiety. Psychoneuroendocrinology. 2011;36:240–248. doi: 10.1016/j.psyneuen.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Vangeel E, Van Den Eede F, Hompes T, Izzi B, Del Favero J, Moorkens G, Lambrechts D, Freson K, Claes S. Chronic Fatigue Syndrome and DNA Hypomethylation of the Glucocorticoid Receptor Gene Promoter 1F Region: Associations With HPA Axis Hypofunction and Childhood Trauma. Psychosom Med. 2015;77:853–862. doi: 10.1097/PSY.0000000000000224. [DOI] [PubMed] [Google Scholar]
- Vgontzas AN, Zoumakis M, Papanicolaou DA, Bixler EO, Prolo P, Lin HM, Vela-Bueno A, Kales A, Chrousos GP. Chronic insomnia is associated with a shift of interleukin-6 and tumor necrosis factor secretion from nighttime to daytime. Metab. Clin. Exp. 2002;51:887–892. doi: 10.1053/meta.2002.33357. [DOI] [PubMed] [Google Scholar]
- Vreeburg SA, Hoogendijk WJ, DeRijk RH, van Dyck R, Smit JH, Zitman FG, Penninx BW. Salivary cortisol levels and the 2-year course of depressive and anxiety disorders. Psychoneuroendocrinology. 2013;38:1494–1502. doi: 10.1016/j.psyneuen.2012.12.017. [DOI] [PubMed] [Google Scholar]
- Wong H-L, Pfeiffer RM, Fears TR, Vermeulen R, Ji S, Rabkin CS. Reproducibility and correlations of multiplex cytokine levels in asymptomatic persons. Cancer Epidemiol. Biomarkers Prev. 2008;17:3450–3456. doi: 10.1158/1055-9965.EPI-08-0311. [DOI] [PubMed] [Google Scholar]
- Wright KP, Drake AL, Frey DJ, Fleshner M, Desouza CA, Gronfier C, Czeisler CA. Influence of sleep deprivation and circadian misalignment on cortisol, inflammatory markers, and cytokine balance. Brain Behav. Immun. 2015 doi: 10.1016/j.bbi.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZT, Du XM, Ma XJ, Zong Y, Chen JK, Yu CL, Liu YG, Chen YC, Zhao LJ, Lu GC. Activation of the NLRP3 inflammasome in lipopolysaccharide-induced mouse fatigue and its relevance to chronic fatigue syndrome. Journal of neuroinflammation. 2016;13:71. doi: 10.1186/s12974-016-0539-1. [DOI] [PMC free article] [PubMed] [Google Scholar]