Abstract
Objective
Transfusion-associated circulatory overload (TACO) is characterized by hydrostatic pulmonary edema following blood transfusion. Restrictive transfusion practice may affect the incidence and severity of TACO in critically ill patients. We sought to examine contemporary risk factors and outcomes for TACO.
Design
Case-control study
Setting
Four tertiary care hospitals
Patients
We prospectively enrolled 200 patients with TACO identified by active surveillance and 405 transfused controls matched by transfusion intensity.
Interventions
None
Measurements and Main Results
Among 20,845 transfused patients who received 128,263 blood components from May 2015 until July 2016, TACO incidence was 1 case per 100 transfused patients. In addition to cardiovascular comorbidities, multivariable analysis identified the following independent predictors of TACO: acute kidney injury, emergency surgery, pre-transfusion diuretic use, and plasma transfusion – the latter especially in females. Compared to matched controls, TACO cases were more likely to require mechanical ventilation (71% vs. 49%; p < 0.001), experienced longer intensive care and hospital lengths of stay following transfusion, and had higher mortality (21% vs. 11%; p=0.02) even after adjustment for other potentially confounding variables.
Conclusions
Despite restrictive transfusion practice, TACO remains a frequent complication of transfusion and is an independent risk factor for in-hospital morbidity and mortality. In addition to cardiovascular and renal risk factors, plasma transfusion was associated with TACO after controlling for other covariates. Additional research is needed to examine the benefit of reduced erythrocyte or plasma exposure in patients at high risk for TACO.
Keywords: blood component transfusion, transfusion reaction, pulmonary edema, risk factors, outcomes
INTRODUCTION
Increased awareness of potential infectious, cardiopulmonary, and immunomodulatory complications of blood transfusion has stimulated the development of patient blood management programs.(1–4) These strategies focus on optimization of hemoglobin levels before surgery, minimization of blood loss, and more restrictive transfusion practice. As a result of these programs, there has been widespread reduction in blood utilization in the United States and elsewhere.(5–11)
With the growing emphasis on blood safety, pulmonary transfusion reactions are receiving greater attention as potentially preventable medical complications.(12, 13) Systematic data gathering has played an important role in understanding the incidence and outcomes of transfusion reactions such as transfusion-related acute lung injury (TRALI) and transfusion-associated circulatory overload (TACO) in critically ill patients.(14–18) The impact of hemovigilance efforts is best illustrated by the identification of risk factors for TRALI and implementation of mitigation strategies leading to dramatic declines in its incidence.(15, 19–22)
While the incidence of TACO has been difficult to assess; it is known to occur frequently in critically ill patients and is a leading cause of transfusion-related morbidity and mortality.(23–25) With the rapid expansion of electronic health records, algorithms to identify severe transfusion reactions allow us to study changes in transfusion practice and patient outcomes in the era of patient blood management programs.(15, 17, 26) In this multicenter case-control study, we utilized an automated screening algorithm to estimate TACO incidence and describe risk factors and outcomes associated with contemporary transfusion practice.(27).
METHODS
Study design and subjects
As part of the NHLBI Recipient Epidemiology and Donor Evaluation Study-III (REDS-III), a case control study of risk factors and outcomes of TACO was conducted between May 2015 and July 2016 at four university and community tertiary care hospitals.(28) Cases of TACO and other pulmonary transfusion reactions were identified by active surveillance of all adult hospitalized patients who received a blood transfusion. The protocol was approved by institutional review boards at the participating sites (Aurora St. Luke’s Medical Center, University of California San Francisco, University of Pittsburgh Medical Center, and Yale New Haven Hospital). The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines were followed in the conduct of the study and reporting of its results.(29)
As previously described (27), TACO cases were identified using four hierarchical layers of screening and diagnosis: i) an electronic algorithm identified cases where a chest radiograph was ordered within 12 hours of blood product release ; ii) research nurses reviewed cases of new or worsening hypoxia due to pulmonary edema within 12 hours of transfusion; iii) a pulmonary physician (NHR) triaged cases and ruled out exclusionary diagnoses; and iv) standardized data forms with narrative reports (Appendix 1) were then reviewed by a three-member expert panel of critical care clinicians with expertise in transfusion medicine (DJK, MRL, MAM). Each case was classified as TACO, TRALI, Possible TRALI, TACO/TRALI, or “Other” when an alternative diagnosis was identified (Appendix 2). Screening, record review, data entry, and case adjudication occurred via a centralized study management system.
Clinical diagnosis and imputability of TACO were derived from criteria used in the National Healthcare Safety Network (NHSN) surveillance definition, namely pulmonary edema developing within 6 hours of transfusion characterized by clinical, echocardiographic, or laboratory evidence of left atrial hypertension (Appendices 1 & 2). Controls without hypoxemia, selected from among all transfused patients, were matched to cases by transfusion intensity using a stratified random sampling scheme (1–2, 3–6, and 7 or more blood components transfused within 6 hours) concurrent with case enrollment.
Statistical analysis
For incidence calculations, the total number of transfused components and unique transfused patients during the study period were captured from the hospital transfusion service. For the case-control analysis, the sample size was based upon a priori power calculation (assuming alpha=0.05, power of 0.8, and two sided z-test with pooled variance) from pilot data with 1:2 matching of cases to controls. Data were expressed as medians or proportions and were compared using chi square tests or Wilcoxon rank sum test, as appropriate. Conditional logistic regression model was used to estimate odds ratios (OR) and 95% confidence intervals (CI) for the matched case-control data. Nonsignificant factors (p > 0.05) were eliminated using backward elimination until all remaining factors had a significant association with TACO. A separate logistic regression analysis examined the association of TACO and mortality accounting for comorbidities and other potentially confounding variables. To account for competing risks, length of stay for those discharged alive were modeled using cumulative incidence functions in proportional hazards regression.(30) Gray’s test for equality was performed to compare cumulative incidence of inpatient mortality and length of stay between TACO and control groups. Statistical analysis was performed with SAS/STAT software, Version 9.4, Cary, NC. All reported p-values are two-sided and p-values less than 0.05 were considered to be statistically significant.
RESULTS
A total of 20,845 hospitalized patients were transfused 128,263 blood components at the four hospitals during the study period. The number of inpatient and outpatient blood components transfused annually decreased from 188,017 components in 2011 (prior to the study period) to 140,621 components in 2015 (concurrent with the study period), a 25% decline. The expert panel reviewed 319 cases of new or worsening bilateral pulmonary infiltrates and adjudicated 200 cases of TACO (Appendix 2). The majority (146/200 - 73%) of TACO cases required only two of the three reviewers to make a diagnosis. Using the denominator of unique patients transfused during the study period, we estimated an incidence of 1 TACO case per 100 patients transfused (200/20,845). Using NHSN imputability criteria for TACO, the expert panel classified 55/200 (28%) of TACO cases as Definite, 120/200 (60%) as Probable, and 25/200 (12%) as Possible.
The 200 cases of TACO were matched to 405 transfused controls without evidence of pulmonary edema or hypoxia (Table 1). Cases and controls had a similar distribution of number of blood units transfused due to matching for transfusion intensity. While not specifically matched, cases and controls also had similar proportions of gender, race, and individual and mixed blood components. However, as transfusion intensity increased, plasma transfusion (> 2 components; n=288) and female gender (>4 components; n=141) were more common in TACO compared to controls (83% vs.63%; p<0.001 and 56% vs. 33%; p<0.01, respectively). Table 2 provides a description of comorbid conditions with cases of TACO having a higher prevalence of cardiovascular, renal, and hepatic comorbidities as well as recent surgery compared to controls. Cases of TACO with acute kidney injury were transfused more blood components (3 vs. 2) and were more likely to receive plasma or platelets compared to those with a history of chronic kidney disease (CKD) or CHF (74% vs. 51%; p=0.02).
Table 1.
Case-Control Characteristics
| Patient Characteristics | TACO (N=200) | Controls (N=405) | P-value |
|---|---|---|---|
| Age† | 63 ± 14 | 60 ± 16 | 0.02 |
| Female | 90 (45%) | 186 (46%) | 0.83 |
| Race | |||
| White | 145 (73%) | 274 (68%) | 0.21 |
| Black/African | 16 (8%) | 52 (13%) | |
| Asian | 9 (5%) | 11 (3%) | |
| Other | 6 (3%) | 8 (2%) | |
| Missing/Not reported | 24 (12%) | 55 (14%) | |
| Body mass index | 31 ± 8.7 | 29 ± 7.8 | <0.01 |
| Est. blood volume* | 5.0 ± 0.9 | 4.8 ± 0.9 | 0.03 |
| Number of Components | |||
| 1–2 units | 114 (57%) | 203 (50%) | 0.27 |
| 3–6 units | 54 (27%) | 130 (32%) | |
| 7+ units | 32 (16%) | 72 (18%) | |
| Component Type | |||
| RBC only | 81 (41%) | 175 (43%) | 0.60 |
| Plasma only | 18 (9%) | 27 (7%) | 0.30 |
| Platelets only | 19 (10%) | 47 (12%) | 0.44 |
| Mixed components | 82 (41%) | 156 (39%) | 0.56 |
| Transfusion location | |||
| Intensive care unit | 74 (37%) | 102 (25%) | <0.001 |
| Operating room | 69 (35%) | 99 (24%) | |
| Ward | 48 (24%) | 183 (45%) | |
| Emergency dept. | 7 (3%) | 13 (3%) | |
| Missing | 2 (1%) | 8 (2%) | |
Nadler formula
Table 2.
Patient-Specific Risk Factors
| Risk Factor | TACO N=200 |
Controls N=405 |
P-value |
|---|---|---|---|
| Cardiovascular Risk Factors | |||
| History of congestive heart failure | 59 (30%) | 50 (12%) | <0.001 |
| Coronary artery disease | 73 (37%) | 88 (22%) | <0.001 |
| Recent myocardial infarction | 20 (10%) | 21 (5%) | 0.03 |
| Hypertension | 118 (59%) | 193 (48%) | <0.01 |
| Atrial fibrillation | 50 (25%) | 53 (13%) | <0.001 |
| Renal Risk Factors | |||
| Acute kidney injury | 64 (32%) | 71 (18%) | <0.001 |
| Chronic renal failure | 63 (32%) | 84 (21%) | <0.01 |
| Hemodialysis | 32 (16%) | 26 (6%) | <0.001 |
| Gastrointestinal Risk Factors | |||
| Severe liver disease | 54 (27%) | 71 (17%) | <0.01 |
| Upper gastrointestinal bleeding | 24 (12%) | 40 (10%) | 0.42 |
| Lower gastrointestinal bleeding | 19 (10%) | 34 (8%) | 0.65 |
| Hematology/Oncology Risk Factors | |||
| Malignancy/chemotherapy | 38 (19%) | 113 (28%) | 0.02 |
| Immunosuppression | 34 (17%) | 56 (14%) | 0.30 |
| Solid organ transplant | 27 (14%) | 27 (7%) | <0.01 |
| Leukemia/lymphoma | 16 (8%) | 50 (12%) | 0.11 |
| Bone marrow transplant | 3 (2%) | 14 (3%) | 0.17 |
| Infectious Risk Factors | |||
| Pneumonia | 12 (6%) | 19 (5%) | 0.49 |
| Sepsis | 20 (10%) | 39 (10%) | 0.89 |
| COPD* | 29 (15%) | 40 (10%) | 0.09 |
| Perioperative Risk Factors | |||
| Recent surgery | 118 (59%) | 167 (41%) | <0.001 |
| Emergency surgery | 70 (35%) | 65 (16%) | <0.001 |
| Surgery type | |||
| Cardiothoracic | 41 (35%) | 39 (23%) | 0.04 |
| Vascular | 18 (15%) | 16 (10%) | 0.13 |
| Liver | 14 (12%) | 11 (7%) | 0.12 |
| Orthopedic | 9 (8%) | 27 (16%) | 0.03 |
| Spine | 9 (8%) | 29 (17%) | 0.01 |
| Abdominal | 9 (8%) | 27 (16%) | 0.03 |
| Neurosurgery | 5 (4%) | 10 (6%) | 0.52 |
| Genitourinary | 4 (3%) | 3 (2%) | 0.39 |
| Other | 9 (6%) | 5 (3%) | 0.07 |
COPD=Chronic obstructive pulmonary disease
Table 3 shows clinical characteristics and outcomes including transfusion practice and associated pre- and post- transfusion laboratory data. Given stratum-matching for transfusion intensity, there were no differences in the number or total volume of transfused blood components; although, subjects with TACO received a higher number of plasma units. Among those transfused erythrocytes, pre-transfusion hemoglobin thresholds were similar in cases and controls; however, post-transfusion hemoglobin levels were higher in TACO. Despite similar number of RBC units transfused, the change in hemoglobin per RBC unit transfused was higher in TACO compared to controls (0.7 vs. 0.5 g/dL/unit; p= 0.002), and this difference was greater in females with TACO compared to males (0.9 vs. 0.6 g/dL/unit). Pre-transfusion diuretic administration was more common in TACO compared to controls (p<0.001); however, this was not the case in females transfused more than one blood component (20% vs. 16%; p=0.55).
Table 3.
Clinical Characteristics & Treatments
| Characteristic | TACO N=200 |
Controls N=405 |
P value |
|---|---|---|---|
| Indication for Transfusion | |||
| Hemorrhage | 65 (33%) | 124 (31%) | 0.64 |
| Transfusion Factors | |||
| # of Units TX in 6 hours | 2 (1–4) | 2 (1–4) | 0.99 |
| TX volume in 6 hours (liters) | 0.6 (0.4–1.2) | 0.7 (0.3–1.2) | 0.73 |
| # of RBC units - 6 hours | 1 (1–2) | 1 (1–2) | 0.84 |
| # of Plt units - 6 hours | 0 (0–1) | 0 (0–1) | 0.78 |
| # of Plasma units - 6 hours | 0 (0–2) | 0 (0–2) | 0.04 |
| Laboratory Variables^ | |||
| Pre-TX Hemoglobin level | 7.6 (6.9–8.6) | 7.5 (6.9–8.6) | 0.87 |
| Post-TX Hemoglobin level | 9.1 (8.3–9.9) | 8.6 (7.8–9.8) | 0.001 |
| Pre-TX Platelet count | 52 (24–95) | 50 (20–150) | 0.59 |
| Post-TX Platelet count | 75 (43–124) | 94 (57–153) | 0.07 |
| Pre-TX INR | 1.8 (1.5–2.6) | 1.7 (1.3–2.3) | 0.27 |
| Post-TX INR | 1.6 (1.3–1.9) | 1.4 (1.3–1.8) | 0.13 |
| Respiratory | |||
| Mechanical ventilation pre-TX | 40 (20%) | 88 (22%) | 0.62 |
| PaO2/FiO2 ratio pre-TX | 334 (246–413) | 359 (269–454) | 0.23 |
| PaO2/FiO2 ratio post-TX | 155 (120–208) | 344 (265–429) | <0.001 |
| Cardiovascular | |||
| Electrocardiogram performed* | 181 (91%) | 302 (75%) | <0.001 |
| Abnormal EKG | 166 (83%) | 236 (58%) | <0.001 |
| Echocardiogram performed* | 156 (78%) | 184 (45%) | <0.001 |
| Abnormal echo features | 142 (71%) | 133 (33%) | <0.001 |
| Cardiomegaly# | 68 (34%) | 46 (11%) | <0.001 |
| Vasopressor use pre-TX | 58 (29%) | 86 (21%) | 0.04 |
| Antihypertensive Med Pre-TX | 96 (48%) | 156 (39%) | 0.03 |
| Antihypertensive Med Post-TX | 115 (58%) | 169 (42%) | <0.001 |
| Elevated blood pressure at TX | 75 (38%) | 114 (28%) | 0.02 |
| Renal | |||
| Fluid balance prior 6 hours (liters) | 1 (0.4–2.2) | 0.6 (0.1–1.5) | <.0.001 |
| Fluid balance hospital LOS (liters) | 2 (0.3–6.0) | 1.7 (0.1–4.9) | 0.10 |
| Receipt of Albumin | 60 (30%) | 99 (24%) | 0.14 |
| Creatinine level (mg/dL) | 1.4 (0.9–2.4) | 1.0 (0.7–1.6) | <0.001 |
| Diuretics pre-TX† | 63 (32%) | 61 (15%) | <0.001 |
| Diuretics post-transfusion | 130 (65%) | 75 (19%) | <0.001 |
| Negative fluid balance at 24 hrs | 72 (36%) | 78 (19%) | <0.001 |
| APACHE II score pre-TX‡ | 17.3 ± 6.0 | 17.9 ± 6.0 | 0.23 |
TX=Transfusion/transfused; RBC=Red blood cells; Plt=platelet; LOS=length of stay; INR=international normalized ratio; PaO2/FiO2=partial pressure of oxygen in arterial blood/fraction of inspired oxygen; EKG=electrocardiogram; echo=echocardiogram; Med=medication; BP=blood pressure
Hemoglobin levels in g/dL, platelet counts in K/ul, and PT/INR in cases and controls who were transfused red blood cells (n=463), platelets (n=242), and plasma (n=220), respectively.
Median pre-TX hemoglobin levels and number of RBC units transfused were 7.2 g/dl (IQR-6.8–8) and 1 unit for medical cases and 7.8 g/dL (IQR 7.2–9) and 2 units for surgical cases, respectively.
Pre- or Post- Transfusion
Cardiomegaly as reported on chest radiograph studies
Pre-TX diuretic dose; 40 mg (IQR 20–80) in cases and 40 mg (IQR 20–60) in controls; p=0.29
APACHE=Acute physiology and chronic health evaluation
After multivariable modeling, several cardiovascular and renal conditions were associated with TACO after adjusting for other covariates, including a history of congestive heart failure (CHF), elevated blood pressure (systolic or diastolic blood pressure > 140 or 90 mmHg, respectively), cardiomegaly on baseline chest radiography, increasing fluid balance, pre-transfusion diuretic use, and acute kidney injury (Table 4). Additional risk factors for TACO included liver failure, emergency surgery, and number of plasma units transfused. Given a significant interaction between plasma transfusion and gender, separate OR were calculated for males and females. This analysis showed a stronger effect size for the number of plasma units transfused and TACO in females (OR 1.6; 95% CI 1.2.–2.0) and a borderline result in males (OR=1.2; 95% CI 1.0–1.3). In an adjusted analysis of patients transfused more than three blood components, acute kidney injury, female gender, and the number of RBC units remained independently associated with TACO (Appendix 5).
Table 4.
Multivariable analysis evaluating risk factors for TACO versus control status
| Characteristic | Odds Ratio | 95% CI | P-value |
|---|---|---|---|
| History of congestive heart failure | 2.0 | 1.2–3.5 | 0.01 |
| History of coronary artery disease | 1.7 | 1.1–2.8 | 0.02 |
| Acute kidney injury | 1.9 | 1.1–3.1 | 0.02 |
| Hemodialysis | 2.2 | 1.1–4.3 | 0.03 |
| Liver failure | 2.1 | 1.3–3.6 | <0.01 |
| Emergency surgery | 2.2 | 1.4–3.7 | 0.001 |
| Fluid balance in the 6 hours prior to TX (per liter) | 1.5 | 1.3–1.7 | <0.001 |
| # of plasma units in 6 hours – in females (per unit)† | 1.6 | 1.2–2.0 | <0.001 |
| # of plasma units in 6 hours – in males (per unit)† | 1.2 | 1.0–1.3 | 0.04 |
| Abnormal electrocardiogram prior to TX | 2.2 | 1.4–3.6 | <0.001 |
| Use of diuretics pre-TX | 2.4 | 1.4–4.2 | 0.001 |
| Elevated blood pressure at the time of TX* | 1.9 | 1.2–2.9 | <0.01 |
| Cardiomegaly on chest radiograph prior to TX | 1.9 | 1.1–3.5 | 0.03 |
TX=Transfusion
Separate odds ratios were calculated given a significant interaction between gender and the number of plasma units transfused in 6 hours
Systolic blood pressure greater than 140 mmHg or diastolic blood pressure > 90 mmHg
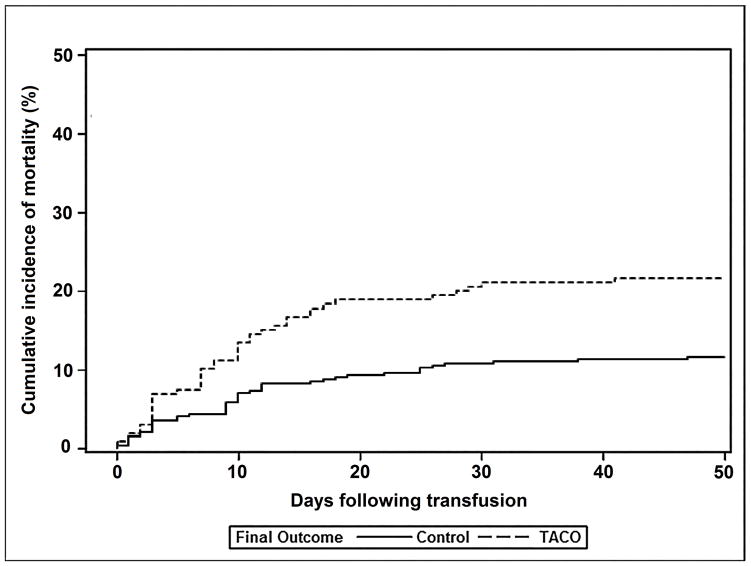
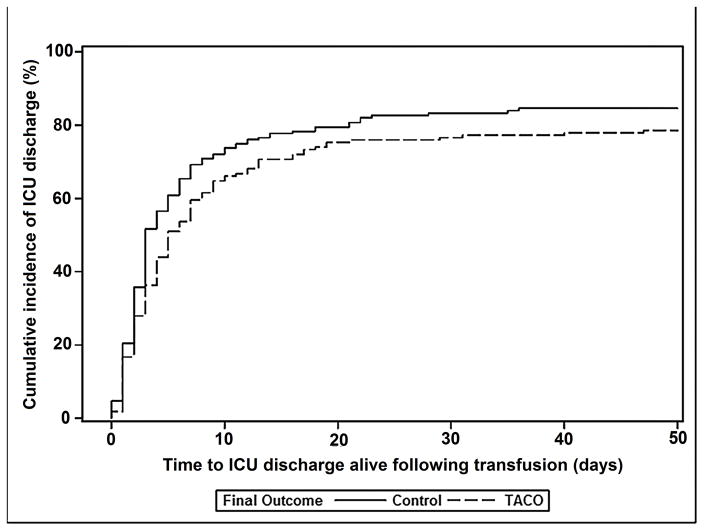
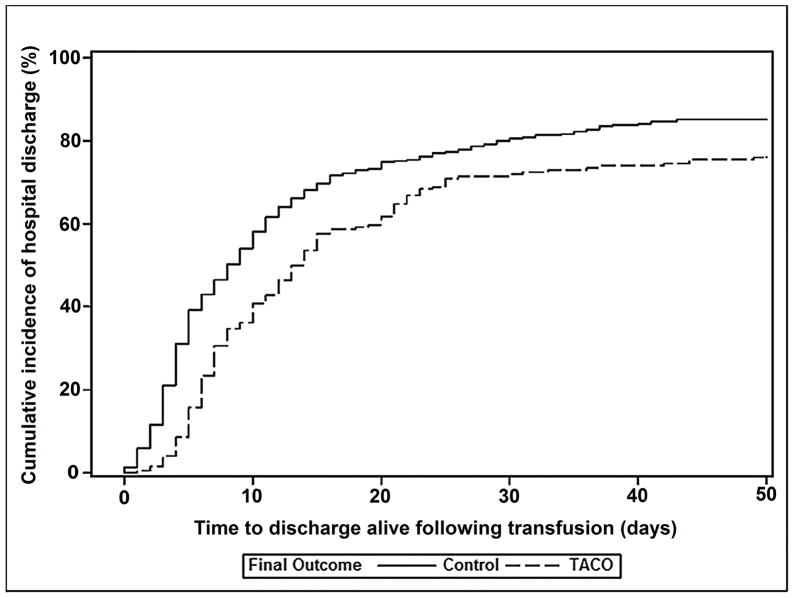
There were no significant differences in the proportion of cases and controls receiving mechanical ventilation prior to transfusion (Table 3). However, patients who developed TACO were more likely to require mechanical ventilation compared with controls (71% versus 49%; p<0.001). Fully 82% of TACO cases (164/200) required ICU admission and 71% of cases transfused outside of the ICU (90/126) subsequently required ICU level of care. There were 41/200 (21%) in-hospital deaths in cases of TACO and 44/405 (11%) deaths within the control group. Cumulative incidence of in-hospital mortality was significantly higher for TACO patients compared to controls (p=0.02; Figure 1a), and TACO remained associated with mortality after adjusting for other potentially confounding variables (OR 2.4; 95% CI 1.4–4.0; p=0.001) (Appendix 6). Amongst subjects discharged alive, cumulative incidence of time to discharge following transfusion from the intensive care unit (ICU) and hospital was increased in TACO compared with controls (p<0.001 & p<0.04, respectively; Figures 1b & 1c).
Figure 1.
Cumulative incidence of in-hospital mortality (Figure 1a), being discharged alive from the intensive care unit (Figure 1b), and being discharged alive from the hospital (Figure 1c) for TACO cases (dotted line) and transfused controls without pulmonary edema (solid line). Analyses counted from the time of transfusion until death or discharge at 50 days.
DISCUSSION
Our results provide contemporary data in both academic and community hospital settings on the incidence and risk factors for TACO following a decline in blood utilization. In the largest multicenter study to date, we found a lower incidence of TACO in both medical and surgical patients than previously reported.(14, 17, 31, 32) In addition to chronic comorbidities such as CHF and CKD, multivariable analysis identified plasma transfusion, emergency surgery, acute kidney injury, pre-transfusion diuretic use, elevated blood pressure, and cardiomegaly on chest radiograph as risk factors for TACO. Despite restrictive transfusion practice, TACO remained associated with increased need for mechanical ventilation, hospital and ICU length of stay, and mortality - even after adjustment for relevant covariates.(14, 17, 26, 33)
It is well known that pulmonary transfusion reactions are under-appreciated given the resources required to identify and characterize them.(34) Active surveillance methodologies using electronic health records provide more uniform reporting of characteristics and outcomes of TACO and TRALI.(26, 35) However, the impact of decreased blood utilization on pulmonary transfusion reactions has not been studied. The incidence of TACO in both community and academic hospital settings is lower than that reported prior to the advent of patient blood management.
We found that the number of transfused blood components per case of TACO (2 units) was lower than previously published studies (3–6 units) conducted prior to widespread adoption of restrictive transfusion strategies. (14, 17, 33) In addition, pre-transfusion hemoglobin levels for TACO in the medical ICU were lower (7.1 g/dL (IQR 6.6–7.6)) than those previously reported (8.6 g/dL (IQR 7.3–9.6)).(14, 36) In contrast, the prevalence of cardiovascular and renal comorbidities in cases of TACO, including CHF and chronic kidney disease, was similar to those in earlier studies. Reduced blood utilization was also accompanied by other relevant changes in clinical practice including lower fluid balances and a higher use of diuretics prior to and following transfusion.(14, 26, 37)
Despite advances in clinical and transfusion practice, measures of severity of illness and outcomes related to TACO were unchanged compared to prior publications. The proportion of patients requiring mechanical ventilation and PaO2/FiO2 ratios were similar to what has been previously reported.(26, 35) In addition, hospital and ICU length of stay after the development of pulmonary edema and mortality were increased compared to controls even after adjustment for potentially confounding factors. The lack of improvement of these measures despite more restrictive transfusion practice suggests that recipient characteristics or transfusion factors beyond transfusion intensity are more relevant to outcomes related to TACO. We and others have demonstrated that plasma transfusion is an independent risk factor for TACO, especially in females, after controlling for volumes of transfused blood components and intravenous fluids and estimated circulating blood volumes.(16) Whether the pathogenesis of TACO is explained simply by elevated pulmonary venous pressures leading to transudation of fluid into the alveoli remains unclear. Additional translational research examining clinical and biomarker predictors of TACO will hopefully improve our understanding of its pathophysiology.
Our findings also suggest that additional quality improvement efforts in clinical and transfusion practice are achievable. We found higher post-transfusion hemoglobin levels in cases of TACO compared to controls despite similar pre-transfusion levels and number of RBC units transfused. Post-transfusion hemoglobin levels and the change in hemoglobin level per RBC unit transfused were significantly higher in TACO, even after accounting for estimated circulating blood volumes. These differences are likely multifactorial and related to hemodilution from non-erythrocyte blood products and intravenous fluids as well as more liberal RBC transfusion in female and surgical patients. These findings raise the question of whether more restrictive transfusion or other modifications to transfusion practice could further reduce TACO incidence and morbidity. Future studies could examine the impact of single unit RBC orders, specific blood infusion rates, timing and dosing of pre-transfusion diuretics, and use of coagulation factor concentrates in place of plasma to reverse coagulopathy in high risk patients.
While risk factors for TACO have been more clearly defined in medical and surgical populations, relevant comorbidities are not always identified or documented prior to transfusion. Acute kidney injury was independently associated with TACO, and we found a greater number of blood components transfused to patients with acute kidney injury compared to those with CKD or CHF. Acute changes in kidney function and the associated risk for TACO may not be recognized when transfusion decisions are made. We also found that relevant echocardiographic abnormalities occurred more frequently than clinical documentation of CHF, and inclusion of echocardiographic data in our multivariable regression analysis abrogated the significance of other cardiovascular risk factors.
Another byproduct of our multivariable analysis was the confirmation of risk factors of TACO that are clinically recognized. For example, cardiovascular factors such as cardiomegaly on chest radiograph, elevated blood creatinine level, pre-transfusion use of diuretics, and elevated blood pressure at the time of transfusion were all associated with TACO; to our knowledge, this is the first time this has been demonstrated in a case-control study. Indeed, some of these characteristics have been under consideration by the International Society of Blood Transfusion (ISBT) Working Party on Haemovigilance in their revised criteria for TACO.(38)
Further systematic assessment of these risk factors may be relevant to the prevention of TACO. While pre-transfusion diuretic administration was more common in TACO than controls, their use could be further increased, especially in patients with risk factors and females transfused multiple units. Furthermore, the timing and dosing of diuretics prior to transfusion may not have been adequate to prevent TACO or may have mitigated its severity. Future implementation science research focused on the development of real-time predictive algorithms embedded into the electronic health record could alert treating clinicians to patients at increased risk of TACO.(39) For example, clinical decision support systems incorporating blood pressure parameters or creatinine clearance could identify patients at risk for systolic or diastolic dysfunction and trigger recommendations for pre-transfusion echocardiography or concomitant diuretic administration. Better ways of identifying at risk individuals and further minimizing transfusion and overall fluid balance may decrease associated morbidity.
Our study has both strengths and limitations. Strengths include the use of active surveillance in a multicenter study population composed of medical and surgical patients, the use of an electronic screening algorithm, collection of granular clinical data, and expert panel review for outcome adjudication. Although efforts were made to audit our screening methodology, cases of TACO in which chest radiography was not ordered within 12 hours of transfusion may have been missed. Although efforts were made to risk-adjust mortality using comorbidities and severity of illness, potential for unmeasured confounding effects clearly remains. Additional studies will address questions regarding the role of BNP in classifying cases independent of known or unrecognized pre-transfusion risk factors such as diastolic dysfunction.
In conclusion, our study of TACO found a lower incidence and fewer transfused blood components per case than that reported in studies prior to patient blood management. Elevated blood pressure, increased creatinine, or cardiomegaly on chest radiograph may be useful in identifying patients at risk for TACO, especially when echocardiography is not available. Despite more restrictive transfusion practice, TACO continues to be associated with increased need for mechanical ventilation, length of stay, and hospital mortality. Additional research is needed to examine the benefit of single unit RBC transfusions, diuretic dosing and timing, and alternatives to plasma transfusion in the prevention of TACO.
Supplementary Material
Acknowledgments
Author contributions: NHR and ELM designed and supervised the study, reviewed the data and drafted the manuscript. DJK, MRL and MAM adjudicated cases. DC and DB organized the data collection and statistical analyses. JEH, DJT and JLG collected data. All authors contributed to the final version of the manuscript.
The authors have no conflicts of interest to disclose relevant to this manuscript.
Funding/Support: Study: The authors were supported by research contracts from the National Heart, Lung, and Blood Institute (NHLBI Contracts HHSN2682011000002I, HHSN2682011000003I, HHSN2682011000004I, HHSN2682011000005I, and HHSN268201100006I for the Recipient Epidemiology and Donor Evaluation Study-III (REDS-III). The funding source designated an investigator-led steering committee, which independently oversaw the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript; and decision to submit the manuscript for publication.
The NHLBI Recipient Epidemiology Donor Evaluation Study - III (REDS-III), domestic component, is the responsibility of the following persons:
-
Hubs:
A.E. Mast and J.L. Gottschall, BloodCenter of Wisconsin (BCW), Milwaukee, WI
D.J. Triulzi and J.E. Kiss, The Institute For Transfusion Medicine (ITXM), Pittsburgh, PA
E.L. Murphy and E. St. Lezin, University of California, San Francisco (UCSF), San Francisco, CA
E.L. Snyder, Yale University School of Medicine, New Haven, CT and R.G Cable, American Red Cross Blood Services, Farmington CT
-
Data coordinating center:
D. J. Brambilla and M. T. Sullivan, RTI International, Rockville, MD
-
Publication Committee Chairman:
R. Y. Dodd, American Red Cross, Holland Laboratory, Rockville, MD
-
Steering Committee Chairman:
S. H. Kleinman, University of British Columbia, Victoria, BC, Canada
-
National Heart, Lung, and Blood Institute, National Institutes of Health:
S. A. Glynn and K. Malkin
Footnotes
Copyright form disclosure: Drs. Roubinian, Hendrickson, Triulzi, Gottschall, Michalkiewicz, Brambilla, Kor, Looney, Murphy, Matthay, and Kleinman received support for article research from the National Institutes of Health (NIH). Drs. Roubinian, Hendrickson, and Looney’s institutions received funding from the NIH. Drs. Brambilla, Kor, and Murphy’s institutions received funding from the National Heart, Lung, and Blood Institute (NHLBI). Dr. Brambilla disclosed work for hire. Dr. Kor received funding from NHLBI and UptoDate (royalties). Dr. Matthay’s institution received funding from a GlaxoSmithKline grant and an Amgen grant, and he received funding from consulting for Bayer, Cerus Therapeutics, Boehringer-Ingelheim, Thesan Pharmaceuticals, and aTyr Pharmaceuticals. Dr. Chowdhury disclosed that she does not have any potential conflicts of interest.
References
- 1.Waters JH, Ness PM. Patient blood management: a growing challenge and opportunity. Transfusion. 2011;51(5):902–903. doi: 10.1111/j.1537-2995.2011.03122.x. [DOI] [PubMed] [Google Scholar]
- 2.Goodnough LT, Shah N. The next chapter in patient blood management: real-time clinical decision support. Am J Clin Pathol. 2014;142(6):741–747. doi: 10.1309/AJCP4W5CCFOZUJFU. [DOI] [PubMed] [Google Scholar]
- 3.Oliver JC, Griffin RL, Hannon T, et al. The success of our patient blood management program depended on an institution-wide change in transfusion practices. Transfusion. 2014;54(10 Pt 2):2617–2624. doi: 10.1111/trf.12536. [DOI] [PubMed] [Google Scholar]
- 4.Collins RA, Wisniewski MK, Waters JH, et al. Effectiveness of multiple initiatives to reduce blood component wastage. Am J Clin Pathol. 2015;143(3):329–335. doi: 10.1309/AJCP42WMHSSTPHXI. [DOI] [PubMed] [Google Scholar]
- 5.Roubinian NH, Escobar GJ, Liu V, et al. Decreased red blood cell use and mortality in hospitalized patients. JAMA Intern Med. 2014;174(8):1405–1407. doi: 10.1001/jamainternmed.2014.2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roubinian NH, Escobar GJ, Liu V, et al. Trends in red blood cell transfusion and 30-day mortality among hospitalized patients. Transfusion. 2014;54(10 Pt 2):2678–2686. doi: 10.1111/trf.12825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thakkar RN, Lee KH, Ness PM, et al. Relative impact of a patient blood management program on utilization of all three major blood components. Transfusion. 2016;56(9):2212–2220. doi: 10.1111/trf.13718. [DOI] [PubMed] [Google Scholar]
- 8.Mehra T, Seifert B, Bravo-Reiter S, et al. Implementation of a patient blood management monitoring and feedback program significantly reduces transfusions and costs. Transfusion. 2015;55(12):2807–2815. doi: 10.1111/trf.13260. [DOI] [PubMed] [Google Scholar]
- 9.Shehata N, Forster A, Lawrence N, et al. Changing trends in blood transfusion: an analysis of 244,013 hospitalizations. Transfusion. 2014;54(10 Pt 2):2631–2639. doi: 10.1111/trf.12644. [DOI] [PubMed] [Google Scholar]
- 10.Yerrabothala S, Desrosiers KP, Szczepiorkowski ZM, et al. Significant reduction in red blood cell transfusions in a general hospital after successful implementation of a restrictive transfusion policy supported by prospective computerized order auditing. Transfusion. 2014;54(10 Pt 2):2640–2645. doi: 10.1111/trf.12627. [DOI] [PubMed] [Google Scholar]
- 11.Whitaker B, Rajbhandary S, Kleinman S, et al. Trends in United States blood collection and transfusion: results from the 2013 AABB Blood Collection, Utilization, and Patient Blood Management Survey. Transfusion. 2016;56(9):2173–2183. doi: 10.1111/trf.13676. [DOI] [PubMed] [Google Scholar]
- 12.Hendrickson JE, Hillyer CD. Noninfectious serious hazards of transfusion. Anesth Analg. 2009;108(3):759–769. doi: 10.1213/ane.0b013e3181930a6e. [DOI] [PubMed] [Google Scholar]
- 13.Alam A, Lin Y, Lima A, et al. The prevention of transfusion-associated circulatory overload. Transfus Med Rev. 2013;27(2):105–112. doi: 10.1016/j.tmrv.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Li G, Rachmale S, Kojicic M, et al. Incidence and transfusion risk factors for transfusion-associated circulatory overload among medical intensive care unit patients. Transfusion. 2011;51(2):338–343. doi: 10.1111/j.1537-2995.2010.02816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toy P, Gajic O, Bacchetti P, et al. Transfusion-related acute lung injury: incidence and risk factors. Blood. 2012;119(7):1757–1767. doi: 10.1182/blood-2011-08-370932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clifford L, Jia Q, Subramanian A, et al. Risk Factors and Clinical Outcomes Associated with Perioperative Transfusion-associated Circulatory Overload. Anesthesiology. 2017;126(3):409–418. doi: 10.1097/ALN.0000000000001506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy EL, Kwaan N, Looney MR, et al. Risk factors and outcomes in transfusion-associated circulatory overload. Am J Med. 2013;126(4):357.e329–338. doi: 10.1016/j.amjmed.2012.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toy P, Bacchetti P, Grimes B, et al. Recipient clinical risk factors predominate in possible transfusion-related acute lung injury. Transfusion. 2015;55(5):947–952. doi: 10.1111/trf.12954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arinsburg SA, Skerrett DL, Karp JK, et al. Conversion to low transfusion-related acute lung injury (TRALI)-risk plasma significantly reduces TRALI. Transfusion. 2012;52(5):946–952. doi: 10.1111/j.1537-2995.2011.03403.x. [DOI] [PubMed] [Google Scholar]
- 20.Lin Y, Saw CL, Hannach B, et al. Transfusion-related acute lung injury prevention measures and their impact at Canadian Blood Services. Transfusion. 2012;52(3):567–574. doi: 10.1111/j.1537-2995.2011.03330.x. [DOI] [PubMed] [Google Scholar]
- 21.Wiersum-Osselton JC, Middelburg RA, Beckers EA, et al. Male-only fresh-frozen plasma for transfusion-related acute lung injury prevention: before-and-after comparative cohort study. Transfusion. 2011;51(6):1278–1283. doi: 10.1111/j.1537-2995.2010.02969.x. [DOI] [PubMed] [Google Scholar]
- 22.Eder AF, Herron RM, Strupp A, et al. Effective reduction of transfusion-related acute lung injury risk with male-predominant plasma strategy in the American Red Cross (2006–2008) Transfusion. 2010;50(8):1732–1742. doi: 10.1111/j.1537-2995.2010.02652.x. [DOI] [PubMed] [Google Scholar]
- 23.Bolton-Maggs PH, Cohen H. Serious Hazards of Transfusion (SHOT) haemovigilance and progress is improving transfusion safety. Br J Haematol. 2013;163(3):303–314. doi: 10.1111/bjh.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bolton-Maggs PH. Conference report: the 2015 SHOT symposium and report--what’s new? Transfus Med. 2015;25(5):295–298. doi: 10.1111/tme.12257. [DOI] [PubMed] [Google Scholar]
- 25. [last accessed 8/22/2017];Fatalities Reported to FDA Following Blood Collection and Transfusion. 2014 [Google Scholar]
- 26.Clifford L, Singh A, Wilson GA, et al. Electronic health record surveillance algorithms facilitate the detection of transfusion-related pulmonary complications. Transfusion. 2013;53(6):1205–1216. doi: 10.1111/j.1537-2995.2012.03886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roubinian NH, Hendrickson JE, Triulzi DJ, et al. Incidence and clinical characteristics of transfusion-associated circulatory overload using an active surveillance algorithm. Vox Sang. 2017;112(1):56–63. doi: 10.1111/vox.12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kleinman S, Busch MP, Murphy EL, et al. The National Heart, Lung, and Blood Institute Recipient Epidemiology and Donor Evaluation Study (REDS-III): a research program striving to improve blood donor and transfusion recipient outcomes. Transfusion. 2014;54(3 Pt 2):942–955. doi: 10.1111/trf.12468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Epidemiology. 2007;18(6):805–835. doi: 10.1097/EDE.0b013e3181577511. [DOI] [PubMed] [Google Scholar]
- 30.Austin PC, Lee DS, Fine JP. Introduction to the Analysis of Survival Data in the Presence of Competing Risks. Circulation. 2016;133(6):601–609. doi: 10.1161/CIRCULATIONAHA.115.017719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Narick C, Triulzi DJ, Yazer MH. Transfusion-associated circulatory overload after plasma transfusion. Transfusion. 2012;52(1):160–165. doi: 10.1111/j.1537-2995.2011.03247.x. [DOI] [PubMed] [Google Scholar]
- 32.Clifford L, Jia Q, Yadav H, et al. Characterizing the epidemiology of perioperative transfusion-associated circulatory overload. Anesthesiology. 2015;122(1):21–28. doi: 10.1097/ALN.0000000000000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rana R, Fernández-Pérez ER, Khan SA, et al. Transfusion-related acute lung injury and pulmonary edema in critically ill patients: a retrospective study. Transfusion. 2006;46(9):1478–1483. doi: 10.1111/j.1537-2995.2006.00930.x. [DOI] [PubMed] [Google Scholar]
- 34.Raval JS, Mazepa MA, Russell SL, et al. Passive reporting greatly underestimates the rate of transfusion-associated circulatory overload after platelet transfusion. Vox Sang. 2015;108(4):387–392. doi: 10.1111/vox.12234. [DOI] [PubMed] [Google Scholar]
- 35.Roubinian NH, Looney MR, Kor DJ, et al. Cytokines and clinical predictors in distinguishing pulmonary transfusion reactions. Transfusion. 2015;55(8):1838–1846. doi: 10.1111/trf.13021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li G, Daniels CE, Kojicic M, et al. The accuracy of natriuretic peptides (brain natriuretic peptide and N-terminal pro-brain natriuretic) in the differentiation between transfusion-related acute lung injury and transfusion-related circulatory overload in the critically ill. Transfusion. 2009;49(1):13–20. doi: 10.1111/j.1537-2995.2008.01941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lieberman L, Maskens C, Cserti-Gazdewich C, et al. A retrospective review of patient factors, transfusion practices, and outcomes in patients with transfusion-associated circulatory overload. Transfus Med Rev. 2013;27(4):206–212. doi: 10.1016/j.tmrv.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 38.Transfusion-associated circulatory overload (TACO) Draft revised reporting criteria - International Society of Blood Transfusion Working Party on Haemovigilance in collaboration with The International Haemovigilance Network - November 2016. In.
- 39.Escobar GJ, Turk BJ, Ragins A, et al. Piloting electronic medical record-based early detection of inpatient deterioration in community hospitals. J Hosp Med. 2016;11(Suppl 1):S18–S24. doi: 10.1002/jhm.2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.