Abstract
Background
Despite its high prevalence, essential tremor (ET) is among the most poorly understood neurological diseases. The presence and extent of Purkinje cell (PC) loss in ET is the subject of controversy. PCs are a major storehouse of central nervous system gamma-aminobutyric acid (GABA), releasing GABA at the level of the dentate nucleus. It is therefore conceivable that cerebellar dentate GABA concentration could be an in vivo marker of PC number.
Objectives
We used in-vivo 1H magnetic resonance spectroscopy (MRS) to quantify GABA concentrations in two cerebellar volumes of interest, left and right, which included the dentate nucleus, comparing 45 ET cases to 35 age-matched controls.
Methods
1H MRS was performed using a 3.0 Tesla Siemens Tim Trio scanner. The MEGA-PRESS J-editing sequence was used for GABA detection in two cerebellar volumes of interest (left and right) that included the dentate nucleus.
Results
The two groups did not differ with respect to our primary outcome of GABA concentration (given in institutional units). For right dentate: [GABA] in ET cases = 2.01 ± 0.45 and [GABA] in controls = 1.86 ± 0.53, p = 0.17. For left dentate: [GABA] in ET cases = 1.68 ± 0.49 and [GABA] controls = 1.80 ± 0.53, p = 0.33. The controls had similar dentate [GABA] in the right vs. left dentate (p = 0.52); however, in ET cases, the value on the right was considerably higher than on the left (p = 0.001).
Conclusions
We did not detect a reduction in dentate GABA concentration in ET cases vs. controls. One interpretation of the finding is that it does not support the existence of PC loss in ET; however, an alternative interpretation is the observed pattern could be due to the effects of terminal sprouting in ET (i.e., collateral sprouting from surviving PCs making up for the loss of GABA-ergic terminals from PC degeneration). Further research is needed.
Keywords: Essential tremor, magnetic resonance spectroscopy, gamma-aminobutyric acid, dentate nucleus, Purkinje cell, cerebellum, neurodegeneration
Introduction
Despite its extraordinarily high prevalence (1–3), essential tremor (ET) is among the most poorly understood neurological diseases (4, 5). On the most basic biological level, little is known about its underlying pathophysiology and pathological anatomy (4, 5). Recent postmortem studies report a 30 – 40% loss of Purkinje cells (PCs) in ET (6–8), suggesting that on a mechanistic level, this common neurological disease could be neurodegenerative (9–11). However, the presence and extent of such PC loss in ET is the subject of controversy (12–14), and therefore, remains a focus of current scrutiny.
PCs are a major storehouse of the central nervous system inhibitory neurotransmitter gamma-aminobutyric acid (GABA), releasing GABA into the post-synaptic cleft at the level of the cerebellar dentate nucleus (15, 16). Thus, it is conceivable that cerebellar dentate GABA level could be an in vivo marker of PC number (17). In this cross-sectional study, we used in-vivo magnetic resonance spectroscopy (MRS) to quantify GABA concentrations in two cerebellar volumes of interest, left and right, which included the dentate nucleus, comparing ET cases to age-matched controls. The research, by testing the hypothesis that these concentrations will be low in ET patients compared to age-matched controls, could elucidate a critically important question about the underlying pathophysiology of ET. Aside from its scientific value, demonstrating low MRS-assessed GABA concentrations in ET could also have an important clinical implication, that is, such concentrations could serve as an imaging biomarker for ET. No imaging biomarkers for ET currently exist.
Materials and Methods
Subjects and clinical evaluation
ET cases were recruited from several sources (2013 – 2016), including a clinical-epidemiological study of ET (18), one of the author’s (E.D.L.) neurological practices, and study advertisements (17). Inclusion criteria were: (1) a prior diagnosis of ET assigned by a treating neurologist, (2) willingness to undergo a magnetic resonance imaging (MRI) scan, and (3) living within two hours of the recruiting site. Exclusion criteria were: (1) heavy exposure to ethanol (as previously defined)(19), (2) history of a neurodegenerative disease (Parkinson’s disease, Alzheimer’s disease), (3) prior deep brain stimulation or other neurosurgery (e.g., gamma knife, thalamotomy, focused ultrasound) for ET, or (4) a reason to be excluded from MRI scanning (e.g., metal in their bodies). Furthermore, we excluded cases who were taking medications that bind to the GABAA receptor or that enhance GABA tone (e.g., clonazepam, diazepam, lorazepam, gabapentin, phenobarbital, progabide, propofol, tigabine, valproate, vigabatrin); however, a small number of cases (n = 9) who were taking primidone were enrolled specifically for the purposes of a sub-study that proposed to analyze the effects of primidone on MRS results (17). In total, 460 ET cases were excluded for one of the aforementioned reasons.
Normal control subjects were recruited during the same time period and from the same sources as the ET cases with some being spouses of the ET cases. They were matched to ET cases based on age. As cases were more readily available, their recruitment occurred more easily than that of controls, and this contributed to an unequal number of cases and controls. Exclusion criteria included a history of ET or a family history of ET (i.e., a reportedly affected first-degree or second-degree relative) or presence on two hand-drawn screening Archimedes spirals of a tremor rating > 1 (rated by a senior neurologist specializing in movement disorders (E.D.L.) who used the Washington Heights-Inwood Genetic Study of ET (WHIGET) tremor rating scale, as described below) (17, 20).
Upon enrollment, a trained research assistant conducted an in-person evaluation of all ET cases and controls, administering demographic and medical history questionnaires, which included collection of data on medications and daily use of ethanol, which was coded ordinally (Table 1) (17). The Montreal Cognitive Assessment (MoCA) was administered to briefly assess global cognitive function (21). During this assessment, a videotaped neurological examination was also performed on all ET cases and controls, which included one test for postural tremor and five for kinetic tremor (including pouring, drinking, using a spoon, finger-nose-finger maneuver, and drawing an Archimedes spiral, 12 tests total). A senior neurologist specializing in movement disorders (E.D.L.) used a reliable and valid clinical rating scale, the WHIGET tremor rating scale, to rate postural and kinetic tremor during each test: 0 (none), 1 (mild), 2 (moderate), 3 (severe), resulting in a total tremor score (range = 0 – 36) (17, 20). Diagnoses of ET were re-confirmed by E.D.L. using the videotaped neurological examination and WHIGET diagnostic criteria (moderate or greater amplitude kinetic tremor [tremor rating ≥ 2] during at least three tests or a head tremor, in the absence of Parkinson’s disease, dystonia or another cause) (22).
Table 1.
Demographic and Clinical Comparison of ET Cases and Controls
| Variable | ET Cases | Controls | Significance |
|---|---|---|---|
| N | 45 | 35 | |
| Age in years | 74.98 ± 6.16 | 73.26 ± 6.06 | 0.22a |
| Male gender | 26 (57.8) | 10 (28.6) | 0.009b |
| Right handed | 38 (84.4) | 31 (88.6) | 0.85c |
| White race | 43 (95.6) | 32 (91.4) | 0.65c |
| Married | 28 (62.2) | 22 (62.9) | 0.95b |
| Education (years) | 17.9 ± 3.7 [18.0] | 18.2 ± 4.8 [17.5] | 0.87d |
| Current smoker | 1 (2.2) | 1 (2.9) | 1.00c |
| MoCA score | 27.5 ± 2.3 [28.0] | 28.2 ± 1.7 [28.0] | 0.22d |
| Number of prescription medications | 3.5 ± 2.5 [3.0] | 3.2 ± 2.9 [3.0] | 0.40d |
| Ethanol use <1 drink/week <1 drink/day 1 drink/day > 1 drink/day |
21 (46.7) 14 (31.1) 3 (6.7) 7 (15.6) |
20 (57.1) 6 (17.1) 5 (14.3) 4 (11.4) |
0.34b |
| Total tremor score | 21.5 ± 4.1 [22.0] | 5.6 ± 2.3 [5.0] | <0.001d |
| Neck or jaw tremor | 26 (57.8) | NA | NA |
| Age of tremor onset in years | 41.5 ± 20.4 [47.5] | NA | NA |
| Tremor duration in years | 34.0 ± 19.6 [30.0] | NA | NA |
| Prescribed medication for tremor at some point | 29 (64.4) | NA | NA |
| Left dentate volume (c.c.) | 1.37 ± 0.22 | 1.40 ± 0.21 | 0.42a |
| Right dentate volume (c.c.) | 1.47 ± 0.24 | 1.49 ± 0.21 | 0.69a |
| Left dentate GABA concentration (i.u.) | 1.68 ± 0.49 | 1.80 ± 0.53 | 0.69a |
| Right dentate GABA concentration (i.u.) | 2.01 ± 0.45 | 1.86 ± 0.53 | 0.53a |
All values represent mean ± standard deviation or number (percentage).
c.c. = cubic centimeters, i.u. = institutional units, MoCA = Montreal Cognitive Assessment.
Student’s t test
Chi-square test
Fisher’s exact test
Mann-Whitney test
The study protocol was approved by the Human Subjects Institutional Review Board at Yale University, Purdue University and Weill Cornell Medical College. Written informed consent was obtained from each subject upon enrollment in the study.
In vivo MRI/MRS measurements
MRI and 1H MRS exams were performed on a 3.0 Tesla Siemens Tim Trio scanner (Siemens Healthcare, Erlangen, Germany), equipped with a 32-channel head coil (17). All scans were performed at Weill Cornell Medical College. Fast T2-weighted images were acquired in all three orientations to ensure exact localization of the MRS volumes of interest (VOIs) (17). GABA-edited MRS data was acquired from two VOIs containing the left and right cerebellar dentate nucleus, respectively (both 25 mm × 25 mm × 25 mm, 128 averages) (Figure 1). Both dentate nuclei were clearly identified on the T2-weighted images on both axial and coronal planes. Each GABA VOI was placed on the axial plane such that the entire dentate nucleus was included, given the fact that this is the level at which the Purkinje cells release their GABA into the synaptic cleft (15), while minimizing contributions from vascular and CSF compartments. The VOI was then confirmed to be completely within the cerebellum on the coronal plane. The MEGA-PRESS J-editing sequence was used for GABA detection (TR/TE = 1500/68 ms) (23).
Figure 1.
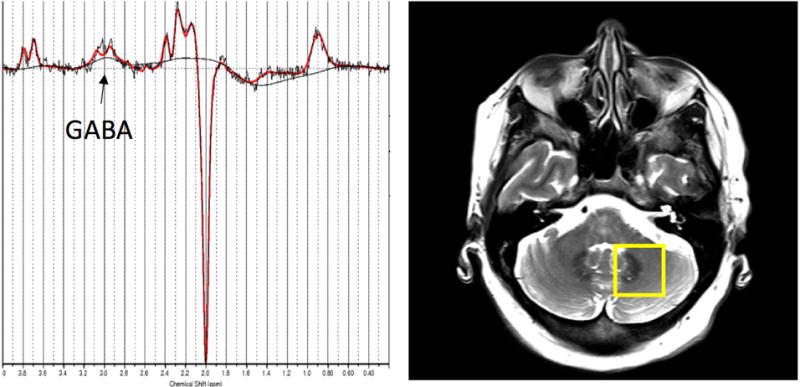
Left: Representative GABA-edited spectrum from the dentate VOI showing the raw data (black) and the LCModel fit (red). Right: Placement of the GABA VOI, containing the left cerebellar dentate.
196 averages were acquired with the spectrally selective editing pulse centered at 1.9 ppm (edit-on) and 196 averages with the pulse centered at 7.5 ppm (edit-off) in an interleaved fashion (17). The resulting difference spectrum contains a GABA peak at 3.0 ppm, which also includes contributions from co-edited macromolecules and homocarnosine, a dipeptide consisting of GABA and histidine (17). Therefore, the signal will be referred to as GABA+. For both VOIs, a reference spectrum was acquired without water suppression. These reference spectra were then used for phase and frequency correction of the corresponding water-suppressed spectra. FASTESTMAP shimming (IPR#577; Siemens Healthcare) was performed before each voxel measurement to achieve water line widths of < 20 Hz (24). In order to determine voxel tissue composition, high-resolution MPRAGE images were acquired (TR/TE/TI = 2300/2.91/900 ms, flip angle = 9°, bandwidth: 240 Hz/pixel, voxel size: 1.0 mm × 1.0 mm × 1.2 mm, GRAPPA = 2). Every effort was made to ensure the subjects were as comfortable as possible without moving in the scanner (17).
Data processing and analysis
MRS data processing and quantification were performed with LCModel 6.3–0L (25), fitting each spectrum as a weighted linear combination of basis spectra from individual metabolites (17). For fitting the MEGA-PRESS spectra, basis sets were generated from density matrix simulations of the sequence using published values for chemical shifts and J-couplings from Kaiser et al. (26), with an exact treatment of metabolite evolution during the two frequency-selective MEGA inversion pulses (17). LCModel fitting %SD values were lower than 20% for GABA+ in all spectra. GABA+ concentrations were derived from raw GABA+ output values from LCModel, multiplied with a water calibration factor provided by LCModel (FCALIB factor). Due to scaling uncertainties, GABA+ concentration values are given in institutional units, but are proportional to and in the range of the true GABA+ concentrations in mM (17).
The reproducibility of GABA-edited MRS in the cerebellum was determined by Long et al. (27) who showed that the intra-individual test-retest coefficient of variance (CV) in an elderly population only ranged between 4 and 13.8%. However, an elderly population shows a rather high inter-individual CV, ranging from 13 – 24 %, compared to younger populations with typical inter-individual CVs of 4 – 15% (28–30).
To determine and correct for the tissue composition of the MRS voxels, MPRAGE images were segmented into grey matter (GM), white matter (WM) and cerebrospinal fluid (CSF) using statistical parametric mapping (SPM12, Wellcome Department of Imaging Neuroscience, London, UK) (17). The MRS voxels were then registered to the tissue maps using an in-house MATLAB 2013a (MathWorks Inc., Natick, MA, USA) code to calculate the proportion of each type of tissue within each voxel. GABA levels corrected for CSF were obtained using the method described by Chowdhury et al (31).
The volume of the left and right dentate nucleus, in c.c., was estimated from the high resolution 3D T1-Weighted MRI sequence using SPM12 and the SUIT toolbox (32). This refers to the Spatially Unbiased Infra-tentorial Template of the cerebellum and brainstem (33). The cerebellum was isolated and normalized or deformed into the SUIT atlas template. This procedure aligns individual fissures and reduces their spatial spread as well as improves on alignment of the deep cerebellar nuclei resulting in an estimate of 34 cerebellar lobules including the dentate.
Sample size calculation
Given the 30 – 40% loss of PCs in our postmortem studies of ET (6, 34), at the start of the study, we had hypothesized a similar reduction in cerebellar dentate GABA level in ET cases. Given an average (right and left) dentate GABA concentration = 1.83 ± 0.53 in controls, and assuming alpha = 0.05, our sample had 85.7% power to detect as little as a 20% reduction in average GABA concentration in ET cases.
Statistical analyses
All analyses were performed in SPSS (version 24.0). We compared demographic and clinical features of ET cases and controls using Student’s t tests, chi-square tests, and Fisher’s exact tests (for normally distributed variables) and Mann Whitney tests (for variables that were not normally distributed) (Table 1). We also compared dentate GABA concentration by group (e.g., men vs. women, ET case vs. controls) using Student’s t tests and within subjects (right vs. left) using a paired sample t test. We performed a series of secondary analyses in which we (1) excluded ET cases taking primidone, (2) compared ET cases with head and jaw tremor to controls. We correlated dentate GABA concentration with total tremor score, tremor duration and daily use of ethanol (ordinally distributed), using either Pearson’s or Spearman’s r, depending on the distribution of the variables.
To see whether the arm with the most severe tremor corresponded to the side of the dentate with the lowest GABA concentration, two indices were created. The first was the tremor score on the right minus the tremor score on the left, which we called tremor asymmetry. The second was right dentate GABA concentration minus left dentate GABA concentration, which we called GABA asymmetry. We then correlated the two using a Pearson’s correlation coefficient.
Results
The ET cases and controls were similar in age, race and a variety of additional demographic factors (marital status, education, smoking) and clinical variables (handedness, MoCA score, number of prescription medications, ethanol use) (Table 1). They differed by gender; however, gender was not associated with dentate GABA concentration (for right dentate GABA concentration in men [1.95 ± 0.48] vs. women [1.95 ± 0.50], t = 0.01, p = 0.99, and for left dentate GABA concentration in men [1.69 ± 0.48] vs. women [1.77 ± 0.54], t = 0.61, p = 0.54). The age of ET onset was ≤ 60 in 39 (86.7%) of 45 ET cases and 64.4% had been prescribed medication at some point for their tremor. The median tremor duration was 30 years. None of the cases or controls had been exposed to chemotherapy. None had been heavy ethanol users and few of the cases or controls drank more than one drink per day (Table 1).
In our main analysis, we compared dentate GABA concentration in ET cases and controls. The two groups did not differ: for right dentate GABA concentration, ET cases = 2.01 ± 0.45 and controls = 1.86 ± 0.53, p = 0.17; for left dentate GABA concentration, ET cases = 1.68 ± 0.49 and controls = 1.80 ± 0.53, p = 0.33. The respective volumes of the dentate for each group is also given in Table 1. When we compared dentate GABA concentration/dentate volume in cases vs. controls, there were no differences on the right (1.42 ± 0.45 vs. 1.29 ± 0.42, p = 0.18) or the left (1.28 ± 0.47 vs. 1.31 ± 0.47, p = 0.75).
In the majority of ET cases (34/45 = 75.6%) and the majority of controls (22/35 = 62.9%), the dentate GABA concentration was higher in the right than the left dentate. In ET cases as a whole, the mean GABA concentration was significantly higher in the right than left dentate nucleus (2.01 ± 0.45 vs. 1.68 ± 0.49, p = 0.001, Table 1, Figure 2A); by contrast, the mean dentate GABA concentration was similar on the right and left among controls (1.86 ± 0.53 vs. 1.80 ± 0.53, p = 0.52, Table 1, Figure 2B). Restricting the sample to the 38 right handed cases and 31 right handed controls yielded similar results: in ET cases as a whole, the mean GABA concentration was significantly higher in the right than left dentate nucleus (1.98 ± 0.40 vs. 1.69 ± 0.51, p = 0.004); by contrast, the mean dentate GABA concentration was similar on the right and left among controls (1.84 ± 0.55 vs. 1.77 ± 0.55, p = 0.55). The mean value of GABA asymmetry (right dentate GABA concentration minus left dentate GABA concentration) was 0.34 ± 0.62 in ET cases and 0.07 ± 0.62 in controls (p = 0.05) (Figure 3).
Figure 2A.
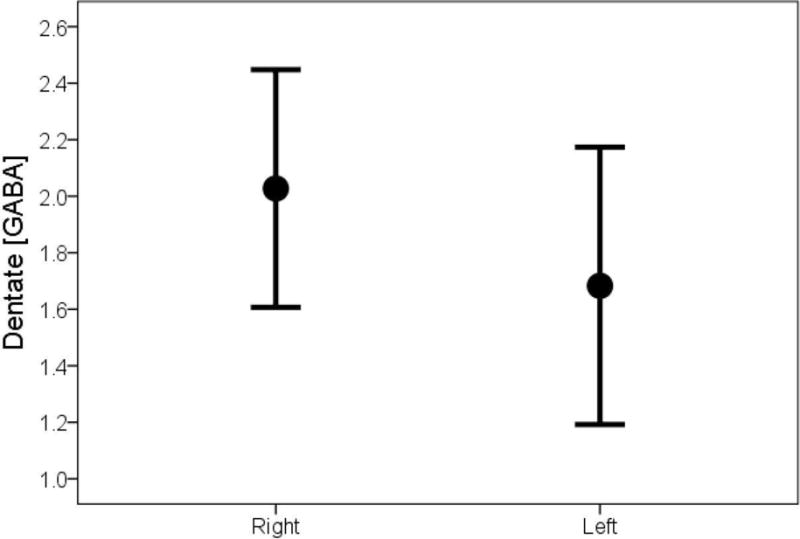
Dentate GABA concentration in ET cases, comparing right to left side
Figure 2B.
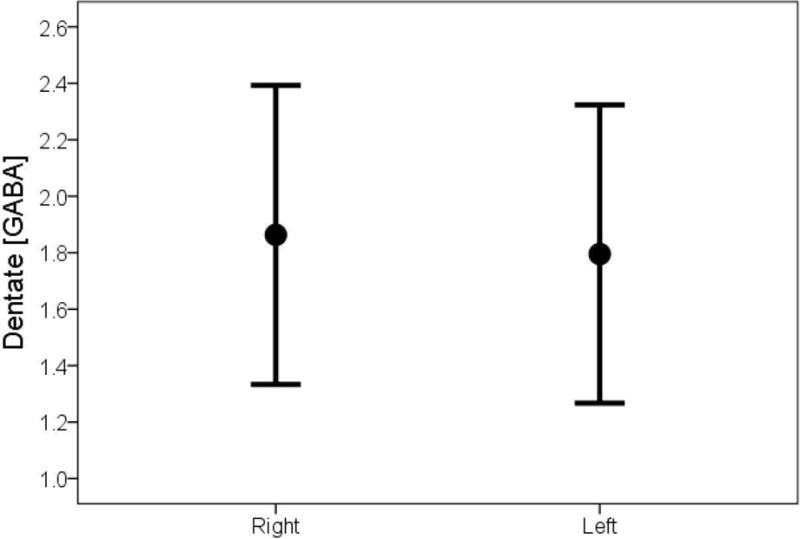
Dentate GABA concentration in controls, comparing right to left side
Figure 3.
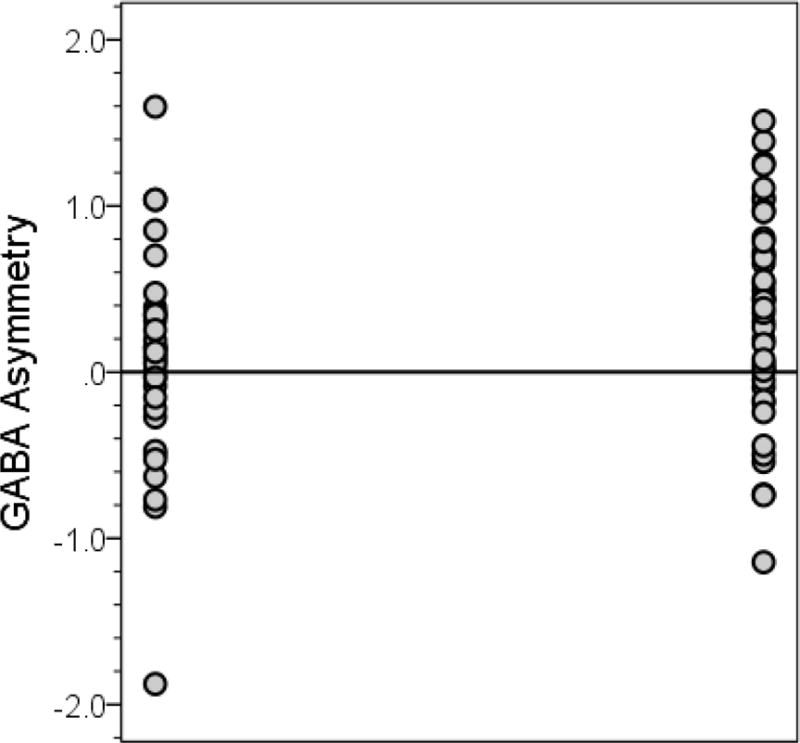
Dentate GABA asymmetry in controls (left) and ET cases (right)
The mean value of GABA asymmetry (right dentate GABA concentration minus left dentate GABA concentration) was 0.34 ± 0.62 in ET cases (values on right) and 0.07 ± 0.62 in controls (values on left) (for case-control comparison, p = 0.05). The horizontal line at zero indicates a point in which the GABA concentration in the left and right dentate are equal. Values above the line indicate that the right dentate GABA concentration is greater than the left; values below the line indicate that the left dentate GABA concentration is greater than the right.
Nine ET cases were taking primidone; in a prior analysis, we did not find a difference in dentate GABA concentrations between 6 ET patients taking daily primidone and 26 ET patients not taking primidone (17). Nonetheless, in a secondary analysis, we excluded these nine cases, comparing dentate GABA concentration in the remaining 36 ET cases and 35 controls. The two groups did not differ: for right dentate GABA concentration, ET cases = 2.01 ± 0.46 and controls = 1.86 ± 0.52, p = 0.21; for left dentate GABA concentration, ET cases = 1.69 ± 0.52 and controls = 1.80 ± 0.53, p = 0.39.
In another secondary analysis, we compared 25 ET cases with head or jaw tremor to the 35 controls. In these analyses, ET cases with head or jaw tremor had higher dentate GABA concentration rather than lower dentate GABA concentration than controls on the right. Thus, for right dentate GABA concentration, ET cases with head or jaw tremor = 2.14 ± 0.38 and controls = 1.86 ± 0.53, p = 0.03; for left dentate GABA concentration, ET cases with head or jaw tremor = 1.70 ± 0.53 and controls = 1.80 ± 0.53, p = 0.48.
In ET cases, neither total tremor score nor tremor duration was correlated with dentate GABA concentration: total tremor score and right dentate GABA concentration Pearson’s r = −0.05, p = 0.73, total tremor score and left dentate GABA concentration Pearson’s r = −0.20, p = 0.20, tremor duration and right dentate GABA concentration Pearson’s r = −0.05, p = 0.73, and tremor duration and left dentate GABA concentration Pearson’s r = 0.14, p = 0.38. Daily use of ethanol, coded ordinally (Table 1), was not correlated with right dentate GABA concentration in ET cases (Spearman’s r = 0.07, p = 0.66) or left dentate GABA concentration in ET cases (Spearman’s r = −0.17, p = 0.28).
Finally, no correlation was found between tremor asymmetry and GABA asymmetry in ET cases (r = 0.15, p = 0.34).
Discussion
In this cross-sectional study, we used in-vivo magnetic resonance spectroscopy (MRS) to quantify GABA concentrations in two cerebellar volumes of interest, left and right, which included the dentate nucleus, comparing ET cases to age-matched controls. To our knowledge, there are no prior studies of dentate GABA concentration in ET cases and controls. We did not find a case-control difference. In ET but not controls, there was a difference in mean right vs. left dentate nucleus concentration.
The research tested the hypothesis that low dentate GABA concentration in ET cases would be a marker for PC loss in ET. One interpretation of the null finding (i.e., absence of a case-control difference) is that it does not support the existence of PC loss in ET; however, there are other possibilities. A second interpretation, albeit speculative, is that the lack of an observed case-control difference in dentate GABA concentration could be due to the compensatory effects of terminal sprouting. Indeed, it is well established in animal models with PC loss, that when PCs are injured and/or transected, functional plasticity of the remaining PC synapses in deep nuclei results in a compensatory sprouting of their terminal boutons (35, 36). Indeed, PCs are endowed with considerable capability for terminal arbor growth and remodeling, in which injured PCs may develop a new terminal GABA-ergic arbor (35, 36). Studies of ET patients have indeed shown considerable remodeling of injured PC axons in the cerebellar cortex, although similar studies in the dentate nuclei have not been undertaken (37). In weaver mice, in which there is a considerable degeneration of PCs with resultant ataxia and tremor, and terminal sprouting is extensive, GABA-ergic innervation in mutants (i.e., mean density of GABA positive terminals in deep nuclei) is identical to or even greater than that of wild type mice. In other words, collateral sprouting from surviving PCs makes up for (or even over-compensates for) the loss of GABA-ergic terminals from PC degeneration (36). Our second observation, that the normal situation (i.e., the equivalence of right and left dentate GABA concentrations seen in our controls) is altered in ET, suggests that the PC-dentate-GABA system is disturbed in ET. The observed side-side difference in dentate GABA in ET could be the result of side-side differences in regenerative biology in ET. In other words, greater collateral sprouting in the right than left dentate nucleus in ET could lead both to a higher dentate GABA concentration on the right (2.01 ± 0.45) than left (1.68 ± 0.49) in ET and to an equivalence (or even slightly higher) right dentate concentration in ET cases (2.01 ± 0.45) than controls (1.86 ± 0.53) (Table 1). This interpretation of our data is speculative at the moment. Although postmortem studies in humans have demonstrated some changes in dentate GABA receptors (38), additional postmortem studies of PC sprouting in ET are needed.
A third interpretation of our data is that the readout we have chosen is not a good marker of PC loss. Indeed, there are several methodological as well as biological issues, which could explain our null finding. First, MRS measures the overall concentration of GABA and does not provide information about the concentrations of GABA within different compartments (intracellular or extracellular at the synaptic or extrasynaptic levels) (39). The GABA concentration we were primarily interested in was PC synaptic GABA. There is some evidence to suggest that MRS-GABA concentrations may better reflect the extrasynaptic GABA tone (39–41). Second, our VOI was large compared to the size of the dentate nucleus. The large VOI size was necessary in order to obtain a GABA-edited spectrum with sufficient signal to noise ratio for adequate quantification. Thus, it is conceivable that our sensitivity to detect very small changes in dentate GABA may have been insufficient, especially if the GABA concentration of the surrounding cerebellar tissue, which was co-measured, did not change. Third, the biological signal we are attempting to measure could be more subtle than we had hypothesized and the technology may not allow us to detect such a signal. Fourth, while the major postmortem brain changes observed in ET are in the cerebellum, in 10–15% of ET cases, there are brainstem Lewy bodies with less involvement of the cerebellum (6). The clinical features that differentiate these two groupings of ET cases are not clear. Inclusion of both groupings would have lowered our study power. Fifth, in addition to the GABA present in PC nerve terminals in the dentate nucleus, the deep cerebellar nuclei, at least in mice, contain intrinsic neuronal populations that also express GABA (42). The presence of such GABA-ergic neurons could have made it more difficult for us to detect changes specific to the PC-associated GABA pool across our study groups. Finally, we measured static GABA concentrations rather than dynamic concentrations (i.e., change over time), and future studies would benefit from a longitudinal approach to this question.
While it is conceivable that a larger dentate volume in ET cases could have masked a reduction in GABA concentration in our ET cases, we do not think this was the case. First, there is no conceivable biological reason why dentate volume would be larger in ET cases than similarly aged controls. Second, our estimate of right and left dentate volumes was similar in ET cases and controls (Table 1).
In summary, we did not detect a reduction in dentate GABA concentration in ET cases vs. controls. One interpretation of the finding is that it does not support the existence of PC loss in ET; however, an alternative interpretation is the observed pattern could be due to the effects of terminal sprouting in ET (i.e., collateral sprouting from surviving PCs making up for the loss of GABA-ergic terminals from PC degeneration).
Acknowledgments
This work was supported by NINDS R01 NS085136 from the National Institutes of Health.
Footnotes
Conflicts of Interest: None of the authors has any conflicts of interest.
References
- 1.Louis ED, Ferreira JJ. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov Disord. 2010;25(5):534–41. doi: 10.1002/mds.22838. [DOI] [PubMed] [Google Scholar]
- 2.Benito-Leon J, Bermejo-Pareja F, Morales JM, Vega S, Molina JA. Prevalence of essential tremor in three elderly populations of central Spain. Mov Disord. 2003;18(4):389–94. doi: 10.1002/mds.10376. [DOI] [PubMed] [Google Scholar]
- 3.Seijo-Martinez M, Del Rio MC, Alvarez JR, Prado RS, Salgado ET, Esquete JP, et al. Prevalence of Essential Tremor on Arosa Island, Spain: a Community-based, Door-to-Door Survey. Tremor Other Hyperkinet Mov (N Y) 2013;3 doi: 10.7916/D89P30BB. pii: tre-03-192-4299-1. eCollection 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Louis ED. Understanding essential tremor: progress on the biological front. Curr Neurol Neurosci Rep. 2014;14(6):450. doi: 10.1007/s11910-014-0450-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Louis ED. Essential tremor: from bedside to bench and back to bedside. Curr Opin Neurol. 2014;27(4):461–7. doi: 10.1097/WCO.0000000000000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Louis ED, Faust PL, Vonsattel JP, Honig LS, Rajput A, Robinson CA, et al. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain. 2007;130(Pt 12):3297–307. doi: 10.1093/brain/awm266. [DOI] [PubMed] [Google Scholar]
- 7.Choe M, Cortes E, Vonsattel JG, Kuo SH, Faust PL, Louis ED. Purkinje cell loss in essential tremor: Random sampling quantification and nearest neighbor analysis. Mov Disord. 2016;31(3):393–401. doi: 10.1002/mds.26490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Louis ED, Babij R, Lee M, Cortes E, Vonsattel JP. Quantification of cerebellar hemispheric purkinje cell linear density: 32 ET cases versus 16 controls. Mov Disord. 2013;28(13):1854–9. doi: 10.1002/mds.25629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grimaldi G, Manto M. Is essential tremor a Purkinjopathy? The role of the cerebellar cortex in its pathogenesis. Mov Disord. 2013;28(13):1759–61. doi: 10.1002/mds.25645. [DOI] [PubMed] [Google Scholar]
- 10.Bonuccelli U. Essential tremor is a neurodegenerative disease. J Neural Transm. 2012;119(11):1383–7. doi: 10.1007/s00702-012-0878-8. [DOI] [PubMed] [Google Scholar]
- 11.Benito-Leon J. Essential tremor: a neurodegenerative disease? Tremor Other Hyperkinet Mov (N Y) 2014;4:252. doi: 10.7916/D8765CG0. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rajput AH, Robinson CA, Rajput ML, Robinson SL, Rajput A. Essential tremor is not dependent upon cerebellar Purkinje cell loss. Parkinsonism Relat Disord. 2012;18(5):626–8. doi: 10.1016/j.parkreldis.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 13.Symanski C, Shill HA, Dugger B, Hentz JG, Adler CH, Jacobson SA, et al. Essential tremor is not associated with cerebellar Purkinje cell loss. Mov Disord. 2014;29(4):496–500. doi: 10.1002/mds.25845. [DOI] [PubMed] [Google Scholar]
- 14.Louis ED, Faust PL, Vonsattel JP. Purkinje cell loss is a characteristic of essential tremor: Towards a more mature understanding of pathogenesis. Parkinsonism Relat Disord. 2012;18(8):1003–4. doi: 10.1016/j.parkreldis.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 15.Gambarana C, Loria CJ, Siegel RE. GABAA receptor messenger RNA expression in the deep cerebellar nuclei of Purkinje cell degeneration mutants is maintained following the loss of innervating Purkinje neurons. Neuroscience. 1993;52(1):63–71. doi: 10.1016/0306-4522(93)90182-f. [DOI] [PubMed] [Google Scholar]
- 16.Linnemann C, Sultan F, Pedroarena CM, Schwarz C, Thier P. Lurcher mice exhibit potentiation of GABA(A)-receptor-mediated conductance in cerebellar nuclei neurons in close temporal relationship to Purkinje cell death. J Neurophysiol. 2004;91(2):1102–7. doi: 10.1152/jn.00163.2003. [DOI] [PubMed] [Google Scholar]
- 17.Louis ED, Hernandez N, Dyke JP, Ma R, Dydak U. Effect of Primidone on Dentate Nucleus gamma-Aminobutyric Acid Concentration in Patients With Essential Tremor. Clin Neuropharmacol. 2016;39(1):24–8. doi: 10.1097/WNF.0000000000000127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michalec M, Hernandez N, Clark LN, Louis ED. The spiral axis as a clinical tool to distinguish essential tremor from dystonia cases. Parkinsonism Relat Disord. 2014;20(5):541–4. doi: 10.1016/j.parkreldis.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harasymiw JW, Bean P. Identification of heavy drinkers by using the early detection of alcohol consumption score. Alcohol Clin Exp Res. 2001;25(2):228–35. [PubMed] [Google Scholar]
- 20.Louis ED. Utility of the hand-drawn spiral as a tool in clinical-epidemiological research on essential tremor: data from four essential tremor cohorts. Neuroepidemiology. 2015;44(1):45–50. doi: 10.1159/000371850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–9. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 22.Louis ED, Ottman R, Ford B, Pullman S, Martinez M, Fahn S, et al. The Washington Heights-Inwood Genetic Study of Essential Tremor: methodologic issues in essential-tremor research. Neuroepidemiology. 1997;16(3):124–33. doi: 10.1159/000109681. [DOI] [PubMed] [Google Scholar]
- 23.Mullins PG, McGonigle DJ, O’Gorman RL, Puts NA, Vidyasagar R, Evans CJ, et al. Current practice in the use of MEGA-PRESS spectroscopy for the detection of GABA. Neuroimage. 2014;86:43–52. doi: 10.1016/j.neuroimage.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gruetter R, Tkac I. Field mapping without reference scan using asymmetric echo-planar techniques. Magn Reson Med. 2000;43(2):319–23. doi: 10.1002/(sici)1522-2594(200002)43:2<319::aid-mrm22>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 25.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30(6):672–9. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 26.Kaiser LG, Young K, Meyerhoff DJ, Mueller SG, Matson GB. A detailed analysis of localized J-difference GABA editing: theoretical and experimental study at 4 T. NMR Biomed. 2008;21(1):22–32. doi: 10.1002/nbm.1150. [DOI] [PubMed] [Google Scholar]
- 27.Long Z, Dyke JP, Ma R, Huang CC, Louis ED, Dydak U. Reproducibility and effect of tissue composition on cerebellar gamma-aminobutyric acid (GABA) MRS in an elderly population. NMR Biomed. 2015;28(10):1315–23. doi: 10.1002/nbm.3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Near J, Ho YC, Sandberg K, Kumaragamage C, Blicher JU. Long-term reproducibility of GABA magnetic resonance spectroscopy. Neuroimage. 2014;99:191–6. doi: 10.1016/j.neuroimage.2014.05.059. [DOI] [PubMed] [Google Scholar]
- 29.Bogner W, Gruber S, Doelken M, Stadlbauer A, Ganslandt O, Boettcher U, et al. In vivo quantification of intracerebral GABA by single-voxel (1)H-MRS-How reproducible are the results? Eur J Radiol. 2010;73(3):526–31. doi: 10.1016/j.ejrad.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 30.O’Gorman RL, Michels L, Edden RA, Murdoch JB, Martin E. In vivo detection of GABA and glutamate with MEGA-PRESS: reproducibility and gender effects. J Magn Reson Imaging. 2011;33(5):1262–7. doi: 10.1002/jmri.22520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chowdhury FA, O’Gorman RL, Nashef L, Elwes RD, Edden RA, Murdoch JB, et al. Investigation of glutamine and GABA levels in patients with idiopathic generalized epilepsy using MEGAPRESS. J Magn Reson Imaging. 2015;41(3):694–9. doi: 10.1002/jmri.24611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ashburner J, Friston KJ. Voxel-based morphometry–the methods. Neuroimage. 2000;11(6 Pt 1):805–21. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 33.Diedrichsen J, Zotow E. Surface-Based Display of Volume-Averaged Cerebellar Imaging Data. PLoS One. 2015;10(7):e0133402. doi: 10.1371/journal.pone.0133402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Axelrad JE, Louis ED, Honig LS, Flores I, Ross GW, Pahwa R, et al. Reduced purkinje cell number in essential tremor: a postmortem study. Arch Neurol. 2008;65(1):101–7. doi: 10.1001/archneurol.2007.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gianola S, Rossi F. Long-term injured purkinje cells are competent for terminal arbor growth, but remain unable to sustain stem axon regeneration. Exp Neurol. 2002;176(1):25–40. doi: 10.1006/exnr.2002.7924. [DOI] [PubMed] [Google Scholar]
- 36.Grusser-Cornehls U, Baurle J. Mutant mice as a model for cerebellar ataxia. Prog Neurobiol. 2001;63(5):489–540. doi: 10.1016/s0301-0082(00)00024-1. [DOI] [PubMed] [Google Scholar]
- 37.Babij R, Lee M, Cortes E, Vonsattel JP, Faust PL, Louis ED. Purkinje cell axonal anatomy: quantifying morphometric changes in essential tremor versus control brains. Brain. 2013;136(Pt 10):3051–61. doi: 10.1093/brain/awt238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paris-Robidas S, Brochu E, Sintes M, Emond V, Bousquet M, Vandal M, et al. Defective dentate nucleus GABA receptors in essential tremor. Brain. 2012;135:105–16. doi: 10.1093/brain/awr301. [DOI] [PubMed] [Google Scholar]
- 39.Marjanska M, Lehericy S, Valabregue R, Popa T, Worbe Y, Russo M, et al. Brain dynamic neurochemical changes in dystonic patients: A magnetic resonance spectroscopy study. Mov Disord. 2013;28(2):201–9. doi: 10.1002/mds.25279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stagg CJ, Bestmann S, Constantinescu AO, Moreno LM, Allman C, Mekle R, et al. Relationship between physiological measures of excitability and levels of glutamate and GABA in the human motor cortex. J Physiol. 2011;589(Pt 23):5845–55. doi: 10.1113/jphysiol.2011.216978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Myers JF, Evans CJ, Kalk NJ, Edden RA, Lingford-Hughes AR. Measurement of GABA using J-difference edited 1H-MRS following modulation of synaptic GABA concentration with tiagabine. Synapse. 2014;68(8):355–62. doi: 10.1002/syn.21747. [DOI] [PubMed] [Google Scholar]
- 42.Uusisaari M, Knopfel T. Functional classification of neurons in the mouse lateral cerebellar nuclei. Cerebellum. 2011;10(4):637–46. doi: 10.1007/s12311-010-0240-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


