Abstract
Neurological disorders are a major threat to public health. Stem cell-based regenerative medicine is now a promising experimental paradigm for its treatment, as shown in pre-clinical animal studies. Initial attempts have been on the replacement of neuronal cells only, but glial progenitors (GPs) are now becoming strong alternative cellular therapeutic candidates to replace oligodendrocytes and astrocytes as knowledge accumulates about their important emerging role in various disease processes. There are many examples of successful therapeutic outcomes for transplanted GPs in small animal models, but clinical translation has proved to be challenging due to the 1000-fold larger volume of the human brain compared to mice. Human GPs transplanted into the mouse brain migrate extensively and can induce global cell replacement, but a similar extent of migration in the human brain would only allow for local rather than global cell replacement. We review here the mechanisms that govern cell migration, which could potentially be exploited to enhance the migratory properties of GPs through cell engineering pre-transplantation. We furthermore discuss the (dis)advantages of the various cell delivery routes that are available, with particular emphasis on intra-arterial injection as the most suitable route for achieving global cell distribution in the larger brain. Now that therapeutic success has proven to be feasible in small animal models, future efforts will need to be directed to enhance global cell delivery and migration to make bench-to-bedside translation a reality.
Keywords: Glial progenitors, transplantation, migration, neurological disorder, myelin
Introduction
There is growing awareness about the pivotal role of glia for the function of the central nervous system (CNS). Glial cells provide not only nutritional support for neurons, but also control synapse formation, neurotransmission, cerebral blood flow, and many other processes (Barres 2008). Hence, current neuroscience has become more glial-inclusive rather than being neuron-centric. This emerging shift is also due to the encountered challenges in replacing damaged neurons, while the replacement of glia is much more attainable. As an example for spinal cord injury, attempts to replace neurons have been shifted towards protecting oligodendrocytes that are responsible for remyelination and support axon survival and outgrowth (Almad and Maragakis 2012). Given the current interest in glia replacement therapy, studies on new approaches directed towards optimal delivery and engraftment are warranted.
GP sources
Two decades ago, it was shown that GPs can be derived from multipotent neuroepithelial stem cells, with the A2B5 molecule being a unique identifier (Rao and Mayer-Proschel 1997). Long-term survival and extensive migration of allografted GPs in rat was found to occur exclusively within the white matter environment (Han et al. 2004).
Several studies have reported a successful differentiation of GPs from other sources, e.g., embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) (Fraichard et al. 1995; Wang et al. 2013). ESCs derived from the inner mass of blastocysts are characterized by a high efficiency in generating various cell types, including GPs. (Guillaume and Zhang 2008). A chemically defined culture system has been instrumental in differentiating hESCs into neuroepithelial cells, which, after two to three weeks, became β-III tubulin+ neurons. GFAP+ astrocytes can be generated six to nine weeks after inducing ESC differentiation, and two more weeks are required for O4+ oligodendrocytes to appear. Further modification and optimization of ESC differentiation protocols resulted in a robust method for the in vitro production of MBP(+) oligodendrocytes (Czepiel et al. 2011). iPSC-derived oligodendrocyte precursors were successfully transplanted in hypomyelinated mice (Wang et al. 2013), as well as in a primate model of multiple sclerosis (Thiruvalluvan et al. 2016). The autologous source of GPs is a main benefit of using iPSCs and can potentially overcome immunological barriers associated with allogeneic transplantation. However, both iPSC- and ECS-derived GPs may bear a risk of contamination with undifferentiated, teratoma-forming pluripotent cells, which presence must be excluded prior to clinical application.
GP lineages
Lineage tracing is an area of active current research (Woodworth et al. 2017). The onset of the expression of the transcription factor Sox1 coincides with the induction of the neuroectoderm (Pevny et al. 1998). Subsequent specification towards radial glia is driven by switch of the Sox1 to Pax6 and Pax2/5 (Schwarz et al. 1999; Suter et al. 2009). In turn, Pax6 activates Sox2 expression (Wen et al. 2008) and both transcription factors orchestrate further neurodevelopment (Wen et al. 2008), including the expression of nestin, which appears in mice at E7 (Shimozaki 2014) in rapidly dividing progenitors (Zhang and Jiao 2015) that initially fuel formation of new neurons (Qian et al. 2000). Nestin(+) cells then give rise to NG2 progenitors at E13, when the embryonic brain begins to switch from neurogenesis to gliogenesis (Karram et al. 2005). NG2 cells persist in the brain throughout the entire life-span of animals/humans favoring a fate for glial progeny (Huang et al. 2014); however, they are also capable of neuron generation under a permissive microenvironment (Sypecka et al. 2009). NG2 cells subsequently begin to express A2B5 ganglioside at E13.5 (Staugaitis and Trapp 2009) and rapidly become dividing GPs (Rao and Mayer-Proschel 1997). The appearance of PDGFRα in GPs at E14 commits them to an oligodendroglial lineage, and are then termed oligodendrocyte precursor cells (OPCs) (Hall et al. 1996). Based on in vitro studies, CD44 has long been considered a marker of astrocyte-restricted precursors (ARP) (Liu et al. 2004); however, recent in vivo studies with more advanced lineage-tracing methodology revealed that CD44(+) cells can also yield OPCs (Naruse et al. 2013). The latest studies revealed that the Nkx2.1 transcription factor determines astrocytic fate, but only in the dorsal telencephalon (Minocha et al. 2017). In addition, in vitro conditions can deregulate the fate of progenitor cells, and thus some caution is warranted with the current view of downstream cell differentiation (Dromard et al. 2007). Lineage tracing using increasingly advanced methods may challenge current dogmas. In particular, brain-region specification may occur much earlier and have a more profound effect on progenitor identity than was previously thought. The same factors may determine distinct cell fates in different regions of the CNS and therefore, some reclassification may occur in the near future.
Therapeutic potential of glial progenitors (GPs)
The therapeutic effect elicited by GPs extends beyond maturation toward oligodendrocytes and myelination. In vitro, it has been shown that (GPs) can protect against chronic glutamate toxicity induced by motor neurons (Maragakis et al. 2005). GPs focally allotransplanted into the spinal cord also prolonged survival of rats with ALS, but no cure was achieved (Lepore et al. 2008). In contrast, neonatally transplanted human GPs were capable of rescuing the normal lifespan of dysmyelinated shiverer mice (Wang et al. 2013; Windrem et al. 2008). Human GPs transplanted neonatally displace the host counterparts, even in healthy mice, and can contribute to improved learning (Han et al. 2013; Windrem et al. 2014). These observations further emphasized the important functional role of glia that previously was ascribed only to neurons. Transplanted GPs also rescued some aspects of the disease phenotype in a small animal model of Huntington’s disease (Benraiss et al. 2016), and preserved the electrophysiological function in rats with focal inflammatory spinal cord demyelination (Walczak et al. 2011).
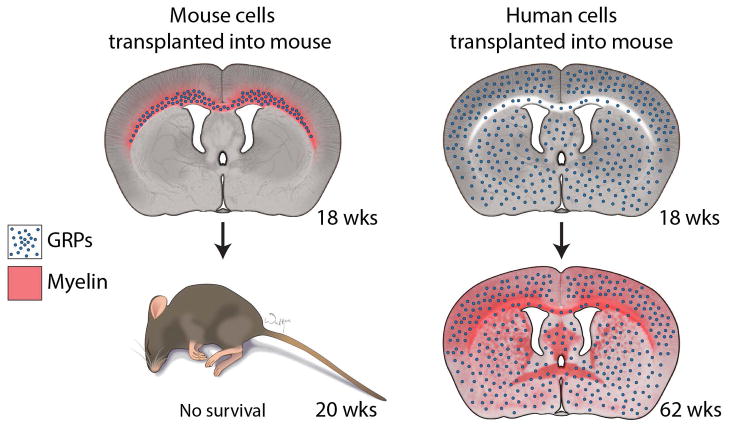
The head-to-head comparison of mouse and human GPs neonatally transplanted into dysmyelinated mice revealed striking species-specific differences in their therapeutic potential (Lyczek et al. 2017). Human GPs extended the life span of dysmyelinated mice, as previously reported, but there was no therapeutic effect after transplantation of their mouse counterparts. The accompanying longitudinal study of brain myelination using magnetic resonance imaging (MRI) revealed even more intriguing information. The typical life span of dysmyelinated (shiverer) mice is up to 200 days, and, at this time point, there was normalization of imaging parameters in the corpus callosum of mice transplanted with mouse GPs, indicating myelination, but there was no prolongation of life span. In contrast, no evidence of myelination was visible at that time in mice transplanted with human GPs, but it prevented the premature death of a majority of dysmyelinated mice. Post-mortem analysis of transplanted mouse GPs revealed very limited migration within the periventricular white matter tracts and early initiation of the production of compact myelin within the area of their distribution. Conversely, human GPs at the same time point had already migrated extensively away from the transplantation site, and were present within the entire brain, including white matter and grey matter areas. No production of compact myelin could be detected by histology, which was confirmed by the MRI findings (Lyczek et al. 2017). This study thus revealed that an extensive migration and distribution of GPs within the entire brain is a pre-requisite to achieve the therapeutic effect (Figure 1). Considering the innate developmental capacity of progenitors derived from a given species and taking into account the fact that mouse GPs were not therapeutic in mice, a similar potential therapeutic effectiveness of human progenitors transplanted in the much larger human brain is an open question, an issue that must be resolved before the clinical application of GPs.
Figure 1. Timeline of migration and differentiation of mouse and human glial progenitors transplanted into dysmyelinated shiverer mice.
Extensive migration of transplanted human glial progenitors with no signs of myelination at 18 weeks leads to rescue of the normal life-span of shiverer mice. In contrast, no therapeutic effect is seen for transplanted mouse GPs, which was accompanied by a robust production of compact myelin restricted to the corpus callosum due to the limited migration of these cells. Human GPs eventually produce compact myelin with a nearly full reconstitution of myelin by the 62-week time-point, long after the time point of shiverer survival.
While sorting based on the presence of the A2B5 antigen is the current standard method by which to isolate GPs from fetal brain, another study has shown that human fetal cells sorted for the presence of the CD133 marker (referred to as neural stem cells) are also capable of wide migration and myelination in dysmyelinated mice, in a pattern very similar to A2B5-positive human GPs (Uchida et al. 2012). These CD133 populations were transplanted in patients with Pelizeaus-Merzbacher disease, and although it was safe, the benefits were rather modest (Gupta et al. 2012). That clinical trial has been discontinued, however, and the company that sponsored the trial shut down its stem cell operations. While no post-mortem studies from these patients were reported, the MRI data added an interesting clue. A widespread improvement of MRI parameters in the white matter, as found in the preclinical study by Lyczek et al. (Lyczek et al. 2017), was not observed (Gupta et al. 2012). Only a few small hypointensities indicative of potential myelination were observed, which could correspond to the sites of cell injections. Notably, the size of these small regions of cell distribution was comparable to the size of a mouse brain (Gupta et al. 2012). Importantly, the mouse brain is 1000 times smaller than that of a human and thus, migration at a much larger magnitude required to cover the entire human brain appears rather unfeasible. We hypothesize here that the disappointing clinical benefit was likely due to insufficient migration of human CD133-positive cells, which covered only small brain areas around the injection sites.
Factors that enhance the migration of GPs
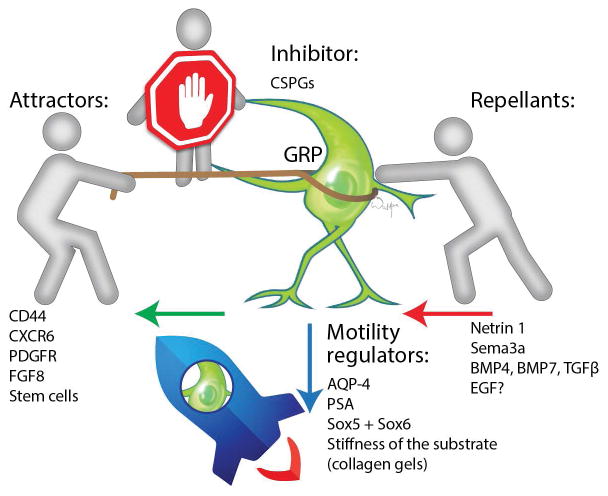
Despite the extensive literature on GPs, attempts to enhance their migratory properties are rare. Paradoxically, both processes of attraction and repulsion are involved in the regulation of GP migration (Figure 3). There are also other physical and biological factors involved that determine the migration of GPs, which could be further employed to fine-tune their motility (i.e., the use of hydrogel scaffolds, or transcription factors) (Table 1). Since some degree of migration of GPs has always been observed, studies on the mechanisms that control their migration were typically based on the use of blocking agents.
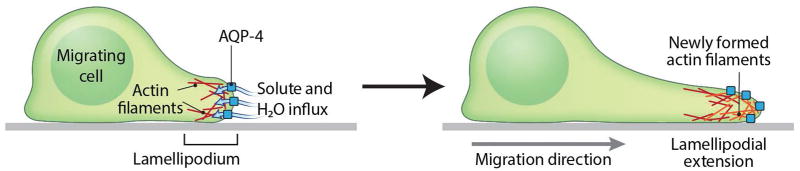
Figure 3. Role of AQP-4 in astrocyte migration.
AQP-4 augments astrocyte migration by accelerating the water influx to the leading edge of the migratory astrocyte, which eventually increases the lamellipodium extension, and ultimately leads to astrocyte migration (adapted from Papadopoulos and Verkman 2013).
Table 1.
Cellular mechanisms that are potentially applicable for genetic engineering of GP migration.
| Mechanism | Molecule/medium | Cell type | Reference |
|---|---|---|---|
| Attraction | CD44 | Astrocyte precursor | Bourguignon et al., 2007 |
| CXCL16/CXCR4 axis | CXCR6-positive GP | Hattermann et al. 2008 | |
| PDGF | Oligodendrocyte precursor | Armstrong et al., 1990 | |
| SDF-1-CXC-4 | Hematopoetic stem cell | Janowski. 2009 | |
| MSC | Nowakowski et al., 2016 | ||
| EPC | Li et al., 2012 | ||
| HGF-c-Met | Tumor cell | Wallace et al., 2013 | |
| IGF-1 | Cardiac stem cell | O’Neill ey al., 2016 | |
| Vascular smooth muscle cell | Beneit et al., 2016 | ||
| Repulsion | CSPGs | GP | Yuan et al., 2016 |
| Nestrin 1 | Small NG2-positive GP | Sugimoto et al., 2001 | |
| Sema3a | Large NG2-positive GP | Sugimoto et al., 2001 | |
| Glioma cell line | Nasarre et al., 2009 | ||
| TGFβ1 | OPC | Choe et al., 2009 | |
| EGF | Newborn glia | Kuhn et al., 2009 | |
| Motility regulation | AQP4 | Astroglia | Ding et al, 2011 |
| NCAM | GP | Wang et al. 1994 | |
| Sox5 and Sox6 | Oligodendroglial cell | Baroti et al., 2016 | |
| ZEB-1 | Epithelial cancer cell | Zhang et al., 2015 | |
| NSC | Kahlert et al., 2015 | ||
| Electric field | Schwann cells, MSCs, and pluripotent stem cells | Iwasa et al. 2017 | |
| OPC | Zhu et al. 2016 |
a) Attractors that affect cell migration
CD44, a transmembrane glycoprotein, was first considered as a key molecule for interactions between cells and hyaluronan, lymphocyte homing, and cell adhesion (Dzwonek and Wilczynski 2015). CD44 is a receptor for hyaluronic acid (HA) and a single-pass, transmembrane glycoprotein that is widely expressed in various physiological and pathological systems responsible for cell-matrix adhesion, cell migration, and signaling (Naor et al. 2002; Saugierveber et al. 1994). CD44 is expressed by lymphocytes, thymocytes, and granulocytes, and was subsequently identified as a human erythrocyte cell surface antigen, a lymphocyte homing receptor. (Dzwonek and Wilczynski 2015). CD44 is now recognized as an important adhesion molecule, involved in several signaling pathways. At first, the role of CD44 in the nervous system was unclear; however, several lines of evidence suggest that CD44 expression occurs in the cerebral white matter, and, more specifically, it in astrocytes and oligodendrocytes (Dzwonek and Wilczynski 2015). Interactions of CD44 and HA were found to be crucial in the regulation of cell migration. It has been shown that a blockade of the CD44 molecule prevents migration of the oligodendrocyte precursor cell line CG4 toward areas of inflammatory demyelinating lesions (Piao et al. 2013). The interaction between CD44 and HA enhances protein kinase N-gamma (PKNγ, a Rac-1 activated serine/threonine kinase) activator, which, in turn, upregulates the phosphorylation of the cytoskeleton protein cortactin. This interaction of CD44/HA and Rac-PKN augments astrocyte migration (Bourguignon et al. 2007). However, it has not been investigated whether overexpression of CD44 is able to enhance the migratory properties of oligodendrocyte progenitors, or whether CD44 is also involved in the migration of transplanted GPs under non-inflammatory conditions, such as dysmyelination and related neurodegenerative diseases.
The CXCR6 molecule is present on the surface of GPs and interacts with CXCL16. CXCL16 is typically released by various cell types in response to injury. It drives the migration of CXCR6-positive GPs toward tissue lesions and facilitates CNS wound healing through astrogliosis (Hattermann et al. 2008). This study hypothesized that stimulation of glial cells by soluble CXCL-16 induces phosphorylation of Akt kinases, activation of the transcription factor AP-1, upregulation of its own receptor, and elevation of cell proliferation and migration. However, CXCR6 is also present in many types of leukocytes (Wilbanks et al. 2001) and thus, uptake of CXCL16 by glial cells may also have anti-inflammatory effects by preventing excessive infiltration of the brain by immune cells. So far, there have beem no attempts to enhance migration of GPs through CXCR6 overexpression.
Platelet-derived growth factor (PDGF) is a potent glial cell mitogen (Maeda et al. 2001). However, it was shown that oligodendrocyte precursors also migrate toward PDGF (Armstrong et al. 1990).
Fibronectin was shown to act synergistically in the pERK1/2-dependent process (Tripathi et al. 2017). In addition, fibroblast growth factor (FGF) plays a very similar pro-migration role both in vitro and in vivo (Cruz-Martinez et al. 2014). Finally, GPs can also be attracted by stem cells, such as mesenchymal stem cells (MSCs), probably through a cocktail of released factors present within exosomes (Jaramillo-Merchan et al. 2013).
b) Repellants that inhibit cell migration
Chondroitin sulfate proteoglycans (CSPGs) consist of a protein core and a chondroitin sulfate chain. They are secreted by various cell types and are involved in various physiological and pathological processes. CSPGs are well known for their presence within the glial scar and for their prominent role in the inhibition of axonal growth (Silver and Miller 2004). It has been recently shown that CSPGs also limit the migration of transplanted GPs, and the induction of chondroitinase expression in surrounding injured tissues facilitates invasion of GPs (Yuan et al. 2016). While such intervention effectively increases the migration of GPs, it can be induced only in the presence of glial scar, which is rather impractical in a scar-free environment, such as dysmyelination and other progressive neurological disorders.
c) Repellants that increase cell migration
Interestingly, the migration of oligodendrocyte precursors in developing optic nerves was rather induced by repulsive cues generated in the optic chiasma. In particular, small NG2-positive GPs were repelled by Netrin 1, while large NG2-negative GPs were directed by repulsion via Sema3a (Sugimoto et al. 2001). It was then found that Sema3a can also serve as a chemorepellent for the migration of glioma cell lines (Nasarre et al. 2009), and Netrin 1 expression within the demyelinating OPC plaques blocks OPC recruitment and remyelination in murine models (Tepavcevic et al. 2014). The repulsive mechanism of glial migration is inspiring, as it could partially explain the radial migration of GPs from the germinal zones toward the cortical surface.
It has also been shown that bone morphogenetic protein-4 (BMP4), BMP7, and transforming growth factor beta1 (TGF-β1) produced by the meninges and pericytes repelled ventral OPCs into the cortex at the mouse embryonic stage. Thus, the data suggest that the mesenchymal TGF-β family proteins promote migration of ventral OPCs into the cortex during corticogenesis in a repulsive fashion (Choe et al. 2014).
Epidermal growth factor (EGF) is a well-known mitogen. It has been shown that intraventricular infusion of EGF increased the number of newborn glia in the subventricular zone (SVZ), and induced their migration toward the olfactory bulb and striatum (Kuhn et al. 1997). However, it is difficult to conclude whether EGF-induced gliogenesis and cell migration was just a consequence or whether EGF supported both proliferation and repulsion to send out glia from the SVZ toward the striatum and olfactory bulb.
d) Motility regulators
Aquaporin-4 (AQP-4), a member of the water-selective channel proteins and the most abundant aquaporin in the brain found in primates and rodents, plays an important role in water and ion homeostasis in the CNS. AQP-4 mediates the movement of water into and out of the brain, and it has been suggested that AQP-4 enhances the migration of astroglia by facilitating the water influx across the leading edge of the migrating astrocyte as a result of increased water permeability of the plasma membrane (Ding et al. 2011). The transmembrane fluxes that occur during cell movement are driven by the osmotic gradient created due to actin depolymerization. The increased hydrostatic pressure causes then a lamellipodial extension. This plasma membrane protrusion is soon followed by actin re-polymerization to stabilize the expansion of the membrane (Papadopoulos and Verkman 2013) (Figure 4). Of 13 aquaporins discovered thus far, only AQP-4 has been identified to participate in neurological diseases, especially in brain edema (Ding et al. 2011). It has been shown that inhibition of AQP-4 expression negatively affects the migration of astroglia (Kong et al. 2008; Saadoun et al. 2005). During the process of oligodendrocyte progenitor migration, there is an increase of cell volume in an AQP-4-dependent fashion (Happel et al. 2013). In normal cerebral activity, it is important to maintain and stabilize the internal osmotic environment (Simard and Nedergaard 2004).
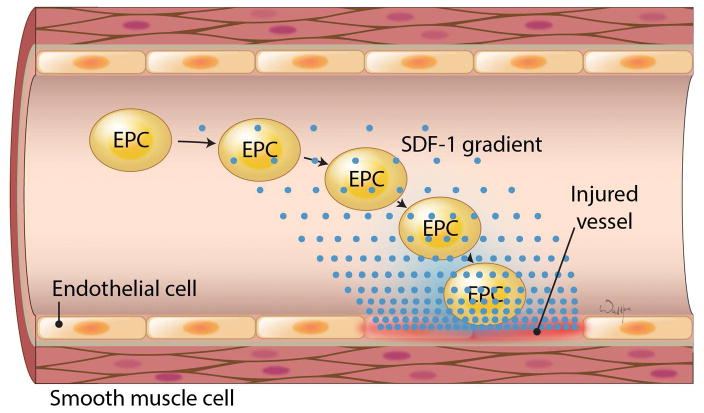
Figure 4. SDF-1 mediated migration of EPCs to the injury site.
(adapted from Li et al. 2012).
The neural cell adhesion molecule (NCAM) is predominantly expressed in neurons, and the polysialated version of this molecule (PSA-NCAM) facilitates cell migration, and is considered to serve as a marker of neuroblasts. Interestingly, the polysialic acid (Psachoulia et al.) is also present on the surface of GPs, as its enzymatic cleavage blocks their migration. (Wang et al. 1994). The transcription factors SOX5 and SOX6 are very attractive targets of genetic engineering to facilitate global replacement of glia in humans. These two factors jointly increase migration through the induction of PDGFR expression (Baroti et al. 2016), while, at the same time, serve as repressors of their differentiation (Stolt et al. 2006). Thus, a conditional overexpression of both transcription factors could facilitate the maintenance of a migratory phenotype of GPs while avoiding initiation of the differentiation process, and, once adequate distribution is achieved, their expression could be switched off to finalize the maturation of transplanted cells.
The migration of GPs is dependent not only on chemical and/or biological factors, but also on the physicochemical properties of the substrate, such as its stiffness. Stiff collagen gels promote the migration of GPs, in the fashion of a “paved highway”, while soft collagen gels had a rather suppressive effect, which may be compared to the effect of “muddy roads (Mori et al. 2013).
Other major migratory pathways that could be potentially exploited to manipulate the migratory properties of glia
A number of migratory pathways shown to promote the migration of MSCs or neural stem/progenitor cells could be considered and used to enhance the migration of GPs. The stromal-derived factor 1 (SDF-1) – CXC receptor 4 (CXCR-4) axis is a known master regulator responsible for the management of the hematopoietic stem cell (HSC) population in bone marrow (Janowski 2009). This axis is also involved in stroke-induced neurogenesis (Merino et al. 2015). There is also increasing evidence indicating that SDF-1 and its cellular receptor, CXCR-4, are also involved in guiding the migration of MSCs (Nowakowski et al. 2016) and endothelial progenitor cells (EPCs) in response to injury (Figure 5) (Li et al. 2012). The SDF-1–CXCR4 axis is also a “villain” in tumor metastasis (Weidle et al. 2016). Interestingly, the routing of CXCR4 receptors between the nucleus and the cell surface can depend on cell culture conditions, which can also affect migratory properties (Janowski et al. 2011).
Hepatocyte growth factor-c-mesenchymal-epithelial transition (HGF–c-Met) is another axis that regulates various migratory streams. It draws the neuroblasts from the SVZ toward the olfactory bulb (Garzotto et al. 2008), and also enhances the migration of the ovarian surface epithelium in order to replenish the area damaged due to expulsion of the ovum (Wallace et al. 2013). This signaling pathway is also frequently used to determine the invasiveness and motility of tumors (Wallace et al. 2013). The insulin like growth factor 1 (IGF-1) molecule is another regulator various functions, including the frequently reported effect on cell migration for cardiac stem cells (O’Neill et al. 2016), as well as vascular smooth muscle cells (Beneit et al. 2016). Similar to some other factors described above, IGF-1 is extensively involved in tumor invasion (Le Coz et al. 2016).
While most of the factors that contribute to cell motility belong to the surface-protein family, cell migration can also be regulated more centrally on the level of transcription factors. Zinc finger E-box binding homeobox 1 (ZEB-1) is a transcription factor that was first characterized as an inducer of tumor invasion and metastasis of several tumors, including kidney tumors, by promoting epithelial-mesenchymal transition (EMT). EMT is a process that is prominent in embryonic development, but its aberrant induction is linked to invasiveness of epithelial cancer cells via promoting their migration, invasion, and dissemination (Zhang et al. 2015). ZEB-1 increases EMT by suppression of CDH1 (encoding E cadherin, an epithelial marker) and microRNA 200 (Gu et al. 2016). This leads to the activation of the TGF-β1 signaling pathway and elicits unregulated proliferation of cancer cells and their invasion (Gu et al. 2016). In addition, ZEB-1 has been implicated in animal organ development, cartilage development, and regulation of MSC proliferation, as well as the establishment of a motile and drug-resistance tumor phenotype (Gu et al. 2016). However, there is little reference to the concrete role of ZEB-1 in the migration of stem cells. It has been found that inhibition of ZEB-1 expression in human fetal neural stem/progenitor cells impaired the migration of human neural stem cells (Kahlert et al. 2015). These authors suggest that ZEB-1 might play an important role in immature, non-neoplastic cell migration during brain development. They stained three fetal human brains and observed moderate to strong expression in the cells nearest to the ventricles, as well as moderate expression in the germinal matrix. Their results strongly support the notion that, during fetal brain development, a maximum number of stem cells gather around the ventricle with a dense germinal matrix and then migrate outward to generate neurons and astrocytes. All the above-mentioned axes and molecules can potentially be exploited to engineer GPs, with the goal of inducing their migratory properties.
Electric field (EF) modulation as a novel method for manipulating glial cell migration
The generation of an electric field may be a novel method that can be used for regulating the migration of different cell types including Schwann cells, human mesenchymal cells, and human embryonic and pluripotent stem cells (Iwasa et al. 2017). Almost all types of cells generate a membrane potential specific for particular tissue, ranging from 3 mV/mm to 1000 mV/mm, generating a specific electric field (Funk et al. 2009). EF plays an important role in axon guidance, nerve growth, and neurogenesis, and, applied at a physiological magnitude, could serve as a guiding cue for glial cell migration (Borgens 1988). It has been recently shown that EF regulates the directed migration of OPC in a β-1 integrin-dependent fashion (Zhu et al. 2016). However, demonstration of its efficacy in large animal models is warranted, as the delivery of a homogenous EF over an extended range could be challenging in larger brains.
Safety concerns related to the manipulation of GP migration
PDGF is involved not only in migration, but also in supporting the proliferation of GPs. An increase of PDGF expression in the SVZ through transduction can lead to the formation of glioma (Assanah et al. 2009). Likewise, EGF is also a potent mitogen and overexpression of EGF receptors in OPC resulted in white matter hyperplasia (Ivkovic et al. 2008). While TGF-β is neuroprotective after injury, it is also known as a suppressor and promoter of tumorigenesis and is involved in the formation of brain tumors (Aigner and Bogdahn 2008; Golestaneh and Mishra 2005). High-grade human gliomas activate latent TGF-β by secreting thrombospondin-1 (Sasaki et al. 2001). It essentially changes the anti-proliferative effect of TGF-β into an oncogene, which can then lead to tumor development (Aigner and Bogdahn 2008). The link between TGF-β and malignant transformation is believed to result from an acquired resistance to its growth inhibitory effects (Canoll and Goldman 2008). Therefore, any engineering approaches toward the enhancement of GP migration should be pursued cautiously, and further extensive preclinical testing is needed.
Cell delivery routes optimal for wide distribution of GPs
Direct injection of GPs to the brain parenchyma results in a highly restricted biodistribution. Extensive cell migration could theoretically be achieved via cell engineering to express/inhibit certain signaling pathways, as described above, however, that approach would require extensive testing to ensure safety and to tune migration efficiency to clinical needs. An alternative approach is to focus on improving the initial biodistribution of the cell transplant. This could be achieved by adapting and fine-tuning the cell delivery technique. Multiple injections into the brain parenchyma are one such option to widely distribute transplanted cells, but this approach is highly impractical due to the multiple burr hole placement required, as well as the risk of major cerebral bleeding, which is 1% per stereotactic introduction of the needle of electrode to the brain (DeLong et al. 2014). Other routes of stem cell delivery, including placement of cells within the cerebrospinal fluid spaces or within various vascular compartments, are other alternative methods with the potential to successfully target glial cell grafts into vast brain territories.
a) Intracerebroventricular/intrathecal route
The advantage of an intracerebroventricular (ICV) route is that it gives the cells access to a very large surface area of the brain throughout the entire neuroaxis. The challenge is, however, that the cells still are required to migrate relatively long distances across the ependyma and into the brain parenchyma. This route has been successfully used to deliver MSCs for neuroprotection in animal studies and in preterm infants with intraventricular hemorrhage (Ahn et al. 2014; Park et al. 2015). An alternative is to inject in the intrathecal (IT) space, where superparamagnetic iron oxide (SPIO)-labeled MSCs were found to reach the occipital horns of the ventricles as shown on MRI, indicating the possible migration into the meninges, subarachnoid space, and spinal cord in patients with multiple sclerosis (MS) and amyotrophic lateral sclerosis (ALS) (Karussis et al. 2010). The ICV route was previously shown to be an effective way to distribute transplanted human GPs within the rodent CNS (Learish et al. 1999). Building on the success of preclinical studies, this approach has been used clinically in patients for ICV delivery of human umbilical cord blood-derived neural stem cells. In that study, MRI cell tracking using SPIO-labeled cells allowed non-invasive assessment of cell distribution and no label was found in the brain parenchyma, but all the transplanted cells located within the occipital horn, probably due to gravitational forces (Jozwiak et al. 2010). Long-term follow-up revealed the gradual disappearance of the signal (Janowski et al. 2014). However, no reliable conclusion can be drawn from this study about the migratory properties of transplanted cells, as long-term MRI tracking is compromised by cell proliferation or excretion of iron oxide from transplanted neural stem cells, prior to migration from the ventricles, throughout the brain parenchyma (Cromer Berman et al. 2013; Walczak et al. 2007). To date, all studies on the migration of GPs have been performed in the rodent brain, and, while this model is suitable for initial screening, the small size of the brain is a major limitation and detracts from clinical relevance.
b) Intra-arterial route
With the constant urge for more accurate methods of cell delivery and for far more precise transplantation, the focus has now shifted toward intra-arterial (IA) delivery (Lu et al. 2001; Yavagal et al. 2014). The vascular tree seems to be an ideal route by which to distribute cells widely within the desired brain territory, and the use of arteries bypasses the peripheral filtering organs. It has been already shown that intra-arterial infusion of GPs at a proper velocity is safe (Janowski et al. 2013). In addition, the recently introduced IA injection under high-speed, real-time MRI guidance adds to the predictability and precision of the procedure of GP delivery (Janowski et al. 2016). The same method can be used for blood brain barrier opening, which can additionally facilitate homing of stem cells (Janowski et al. 2016). However, safe and precise delivery is insufficient, as the injected cells still need to traverse the vessel wall. It has been shown that genetic engineering of GRPs to express VLA-4 allows for their docking to the LPS-induced inflamed endothelium (Gorelik et al. 2012), and effective extravasation of GPs in an animal model of stroke (Jablonska et al. 2017). The IA route has already been validated in a large animal model and transplantation of even large MSCs was safe and therapeutic in a canine ischemia model (Chung et al. 2009). There were also no adverse events as a result of IA delivery of bone marrow mononuclear cells in subacute ischemic stroke patients (Ghali et al. 2016). However, the effective diapedesis in diseases without or with little neuroinflammatory component, such as dysmyelination and related neurodegenerative disorders, is still unresolved and thus, this route of delivery also may have its limitations.
c) Intranasal route
The intranasal route has been recently considered as an effective route for stem cell delivery to the mouse brain (Yu et al. 2017). The growing popularity of this route is attributable to the non-invasive nature of administration, excellent safety, as well as its selectivity reducing cell distribution in peripheral organs (Li et al. 2015; Reitz M 2012). Another advantage is the bypass of the blood-brain barrier, with direct cell migration from the nasal mucosa (Li et al. 2015; Reitz M 2012). There are two intracranial routes through which intranasally transplanted cells reach the brain: (1) through the olfactory bulb and subsequent migration throughout the brain; and (2) through the CSF with subsequent migration along the surface of the cortex, followed by invasion into the brain parenchyma (Danielyan et al. 2009). Because of rapid migration of stem cells to the injury site, this method can be employed at an early stage of disease. It can also evade problems of a low cell survival rate and the inconvenience of frequent invasive surgical administration (Danielyan et al. 2014). However, as of to date, only MSCs and NSCs have been introduced to the brain using the intranasal route (Oppliger et al. 2016; van Velthoven CT 2010; Wu et al. 2013). GPs are a population of delicate cells and it remains to be proven that they can survive in the nasal cavity sufficiently long and can sense chemotaxis toward the brain. In addition, the intranasal route has so far only been used in rodents where the distance of cell migration is just a few millimeters, while the clinical scenario would require cell movements over several centimeters.
Local distribution of cells for neuroregeneration
While global glia replacement could be a versatile therapeutic strategy for a wide array of neurological disorders, the local control of cell migration may be applied to increase the precision of GP delivery in focal diseases. It has been shown that NG2(+) progenitors penetrate the glial scar and facilitate axonal growth after spinal cord injury (Vadivelu et al. 2015). GPs have also been shown to be therapeutic in a rat model of transverse myelitis (Walczak et al. 2011), which led to a FDA approval of the first-in-man clinical trial for this disease (www.qthera.com). Local administration of GPs can also be applied to remyelinate MS lesions at an early disease stage (Harlow et al. 2015). In stroke patients, myelin content is the only independent variable predicting motor function in the upper extremities, providing another rationale for local GP delivery (Lakhani et al. 2017).
The importance of MRI cell tracking to evaluate GP migration in humans
There is a vast diversity of direct and indirect labeling methods to follow personalized stem cell-based therapy in vivo (Janowski et al. 2012). Non-invasive monitoring of stem cell delivery and migration by means of MRI cell tracking will be essential to thoroughly evaluate the above proposed improvements in patients (Bulte 2009). As an example, in the discontinued trial by Gupta et al., (Gupta et al. 2012) no information on the dispersion of transplanted human NSCs was obtained, and such MRI cell tracking information may provide clues as to why limited therapeutic effects were observed. Future efforts toward clinical translation of GP-based therapy should ideally include MRI-based cell tracking studies in a comprehensive manner.
Large animal models to address the gap between mice and men
Most animal studies have been conducted in rodents, given the relative low cost and high speed of experimental reproduction. Often, this has led to achieving sufficient statistical power to draw unambiguous conclusions about therapeutic outcome. Nevertheless, the ability of rodent experiments to predict the overall effectiveness of stem cell-based therapy in larger mammals remains controversial and is subject to frequent failures (Cibelli et al. 2013). The differences in organ size and physiology greatly affect the outcome of cell replacement approaches, as it is more difficult to repopulate large tissue territories. Studies in rabbits, dogs, pigs, sheep, goats, and non-human primates have improved the capability to predict clinical effectiveness, compared to studies in mice alone (Cibelli et al. 2013). It is critical to select the most appropriate animal species when studying a specific disease. The use of swine, both minipigs and full-size breeds, appears to be one of the best animal models to study several diseases that require genetic engineering, such as Alzheimer’s disease, Huntington disease and cystic fibrosis, but also diseases without genetic modification including ophthalmological diseases, diabetes, cardiovascular disease, and some cancers (Cibelli et al. 2013). Humanized pigs are particularly compelling as donors for transplantation into non-human primates prior to advancing clinical xenotransplantation (Suzuki et al. 2012). Large animals are also more appropriate for studying accuracy and efficacy of stem cell transplantations due to more clinically relevant anatomical features and follow-up time (Cibelli et al. 2013). Two studies assessing the survival of human pluripotent and embryo-derived dopamine neurons in monkeys are good examples to this extent (Daadi et al. 2012; Kriks et al. 2011). However, the use of large animals is not without its own challenges, such as the limited availability of specific antibodies and growth factors, as well as the more cumbersome production of pluripotent stem cells.
Concluding remarks
The pivotal role of glia in neurological disorders has been increasingly recognized. Human glial progenitors have been shown to be highly therapeutic in a vast array of small animal models of neurological disorders. However, no robust therapeutic effects have been observed in patients with Pelizeaus-Merzbacher disease using highly myelinogenic progenitors, and further attempts have been discontinued. It has been shown recently in a small animal model that the therapeutic efficacy of transplanted glial progenitors depends on the extent of their migration. Human glial progenitors widely migrated and were highly therapeutic despite the lack of compact myelin at the time critical for a rescue of animals. In contrast, mouse glial progenitors migrated very sparsely and early differentiated and started formation of compact myelin within the corpus callosum surrounding the cerebral ventricles, which did not translate to any therapeutic benefit. Therefore, the translation of very promising preclinical findings into effective therapies requires that the migration of glial progenitors must be within the desired brain territories, which in some circumstances such as global dysmyelination needs to be the entire brain. Future studies on grafting glial progenitors should consider including cell engineering methods to enhance cell migration.
Figure 2. Mechanisms governing the migration of glial progenitors.
Four major factors direct the migration of glial progenitors: Inhibitors, attractors, repellants, and motility regulators.
Main points.
Global glia replacement following cell transplantation is needed for clinical translation.
Clinical promise has not lived up to its success in mice, due to the 1000-fold larger volume of the human brain.
Transplanted cell migration may be improved through cell engineering and optimizing delivery.
Acknowledgments
Our studies are supported by R01 NS091100, R01 NS091110, R56 NS098520, NMSS RG 4994-A-3, and ALSA 16-IIP-252. We thank Mary McAllister for editorial assistance and I-Hsun Wu for preparation of figures.
Footnotes
Conflict of interest:
The authors declare no conflict of interest.
References
- Ahn SY, Chang YS, Park WS. Mesenchymal stem cells transplantation for neuroprotection in preterm infants with severe intraventricular hemorrhage. Korean J Pediatr. 2014;57:251–6. doi: 10.3345/kjp.2014.57.6.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aigner L, Bogdahn U. TGF-beta in neural stem cells and in tumors of the central nervous system. Cell and Tissue Research. 2008;331:225–241. doi: 10.1007/s00441-007-0466-7. [DOI] [PubMed] [Google Scholar]
- Almad AA, Maragakis NJ. Glia: an emerging target for neurological disease therapy. Stem Cell Research & Therapy. 2012;3:37. doi: 10.1186/scrt128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong RC, Harvath L, Dubois-Dalcq ME. Type 1 astrocytes and oligodendrocyte-type 2 astrocyte glial progenitors migrate toward distinct molecules. J Neurosci Res. 1990;27:400–7. doi: 10.1002/jnr.490270319. [DOI] [PubMed] [Google Scholar]
- Assanah MC, Bruce JN, Suzuki SO, Chen A, Goldman JE, Canoll P. PDGF Stimulates the Massive Expansion of Glial Progenitors in the Neonatal Forebrain. Glia. 2009;57:1835–1847. doi: 10.1002/glia.20895. [DOI] [PubMed] [Google Scholar]
- Baroti T, Zimmermann Y, Schillinger A, Liu L, Lommes P, Wegner M, Stolt CC. Transcription factors Sox5 and Sox6 exert direct and indirect influences on oligodendroglial migration in spinal cord and forebrain. Glia. 2016;64:122–38. doi: 10.1002/glia.22919. [DOI] [PubMed] [Google Scholar]
- Barres BA. The Mystery and Magic of Glia: A Perspective on Their Roles in Health and Disease. Neuron. 2008;60:430–440. doi: 10.1016/j.neuron.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Beneit N, Fernandez-Garcia CE, Martin-Ventura JL, Perdomo L, Escribano O, Michel JB, Garcia-Gomez G, Fernandez S, Diaz-Castroverde S, Egido J, et al. Expression of insulin receptor (IR) A and B isoforms, IGF-IR, and IR/IGF-IR hybrid receptors in vascular smooth muscle cells and their role in cell migration in atherosclerosis. Cardiovasc Diabetol. 2016;15:161. doi: 10.1186/s12933-016-0477-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benraiss A, Wang S, Herrlinger S, Li X, Chandler-Militello D, Mauceri J, Burm HB, Toner M, Osipovitch M, Jim Xu Q, et al. Human glia can both induce and rescue aspects of disease phenotype in Huntington disease. Nat Commun. 2016;7:11758. doi: 10.1038/ncomms11758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgens RB. Stimulation of neuronal regeneration and development by steady electrical fields. Adv Neurol. 1988;47:547–64. [PubMed] [Google Scholar]
- Bourguignon LY, Peyrollier K, Gilad E, Brightman A. Hyaluronan-CD44 interaction with neural Wiskott-Aldrich syndrome protein (N-WASP) promotes actin polymerization and ErbB2 activation leading to beta-catenin nuclear translocation, transcriptional up-regulation, and cell migration in ovarian tumor cells. J Biol Chem. 2007;282:1265–80. doi: 10.1074/jbc.M604672200. [DOI] [PubMed] [Google Scholar]
- Bulte JW. In vivo MRI cell tracking: clinical studies. AJR Am J Roentgenol. 2009;193:314–25. doi: 10.2214/AJR.09.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canoll P, Goldman JE. The interface between glial progenitors and gliomas. Acta Neuropathologica. 2008;116:465–477. doi: 10.1007/s00401-008-0432-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choe Y, Huynh T, Pleasure SJ. Migration of oligodendrocyte progenitor cells is controlled by transforming growth factor beta family proteins during corticogenesis. J Neurosci. 2014;34:14973–83. doi: 10.1523/JNEUROSCI.1156-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung DJ, Choi CB, Lee SH, Kang EH, Lee JH, Hwang SH, Han H, Choe BY, Lee SY, Kim HY. Intraarterially delivered human umbilical cord blood-derived mesenchymal stem cells in canine cerebral ischemia. J Neurosci Res. 2009;87:3554–67. doi: 10.1002/jnr.22162. [DOI] [PubMed] [Google Scholar]
- Cibelli J, Emborg ME, Prockop DJ, Roberts M, Schatten G, Rao M, Harding J, Mirochnitchenko O. Strategies for Improving Animal Models for Regenerative Medicine. Cell Stem Cell. 2013;12:271–274. doi: 10.1016/j.stem.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromer Berman SM, Kshitiz, Wang CJ, Orukari I, Levchenko A, Bulte JW, Walczak P. Cell motility of neural stem cells is reduced after SPIO-labeling, which is mitigated after exocytosis. Magn Reson Med. 2013;69:255–62. doi: 10.1002/mrm.24216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Martinez P, Martinez-Ferre A, Jaramillo-Merchan J, Estirado A, Martinez S, Jones J. FGF8 activates proliferation and migration in mouse post-natal oligodendrocyte progenitor cells. PLoS One. 2014;9:e108241. doi: 10.1371/journal.pone.0108241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czepiel M, Balasubramaniyan V, Schaafsma W, Stancic M, Mikkers H, Huisman C, Boddeke E, Copray S. Differentiation of Induced Pluripotent Stem Cells Into Functional Oligodendrocytes. Glia. 2011;59:882–892. doi: 10.1002/glia.21159. [DOI] [PubMed] [Google Scholar]
- Daadi MM, Grueter BA, Malenka RC, Redmond DE, Steinberg GK. Dopaminergic Neurons from Midbrain-Specified Human Embryonic Stem Cell-Derived Neural Stem Cells Engrafted in a Monkey Model of Parkinson’s Disease. Plos One. 2012:7. doi: 10.1371/journal.pone.0041120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielyan L, Beer-Hammer S, Stolzing A, Schafer R, Siegel G, Fabian C, Kahle P, Biedermann T, Lourhmati A, Buadze M, et al. Intranasal delivery of bone marrow-derived mesenchymal stem cells, macrophages, and microglia to the brain in mouse models of Alzheimer’s and Parkinson’s disease. Cell Transplant. 2014;23(Suppl 1):S123–39. doi: 10.3727/096368914X684970. [DOI] [PubMed] [Google Scholar]
- Danielyan L, Schafer R, von Ameln-Mayerhofer A, Buadze M, Geisler J, Klopfer T, Burkhardt U, Proksch B, Verleysdonk S, Ayturan M, et al. Intranasal delivery of cells to the brain. Eur J Cell Biol. 2009;88:315–24. doi: 10.1016/j.ejcb.2009.02.001. [DOI] [PubMed] [Google Scholar]
- DeLong MR, Huang KT, Gallis J, Lokhnygina Y, Parente B, Hickey P, Turner DA, Lad SP. Effect of advancing age on outcomes of deep brain stimulation for Parkinson disease. JAMA Neurol. 2014;71:1290–5. doi: 10.1001/jamaneurol.2014.1272. [DOI] [PubMed] [Google Scholar]
- Ding T, Ma Y, Li W, Liu X, Ying G, Fu L, Gu F. Role of aquaporin-4 in the regulation of migration and invasion of human glioma cells. Int J Oncol. 2011;38:1521–31. doi: 10.3892/ijo.2011.983. [DOI] [PubMed] [Google Scholar]
- Dromard C, Bartolami S, Deleyrolle L, Takebayashi H, Ripoll C, Simonneau L, Prome S, Puech S, Tran VB, Duperray C, et al. NG2 and Olig2 expression provides evidence for phenotypic deregulation of cultured central nervous system and peripheral nervous system neural precursor cells. Stem Cells. 2007;25:340–53. doi: 10.1634/stemcells.2005-0556. [DOI] [PubMed] [Google Scholar]
- Dzwonek J, Wilczynski GM. CD44: molecular interactions, signaling and functions in the nervous system. Frontiers in Cellular Neuroscience. 2015:9. doi: 10.3389/fncel.2015.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraichard A, Chassande O, Bilbaut G, Dehay C, Savatier P, Samarut J. In-Vitro Differentiation of Embryonic Stem-Cells into Glial-Cells and Functional-Neurons. Journal of Cell Science. 1995;108:3181–3188. doi: 10.1242/jcs.108.10.3181. [DOI] [PubMed] [Google Scholar]
- Funk RHW, Monsees T, Ozkucur N. Electromagnetic effects - From cell biology to medicine. Progress in Histochemistry and Cytochemistry. 2009;43:177–264. doi: 10.1016/j.proghi.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Garzotto D, Giacobini P, Crepaldi T, Fasolo A, De Marchis S. Hepatocyte growth factor regulates migration of olfactory interneuron precursors in the rostral migratory stream through Met-Grb2 coupling. J Neurosci. 2008;28:5901–9. doi: 10.1523/JNEUROSCI.1083-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghali AA, Yousef MK, Ragab OA, ElZamarany EA. Intra-arterial Infusion of Autologous Bone Marrow Mononuclear Stem Cells in Subacute Ischemic Stroke Patients. Front Neurol. 2016;7:228. doi: 10.3389/fneur.2016.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golestaneh N, Mishra B. TGF-beta, neuronal stem cells and glioblastoma. Oncogene. 2005;24:5722–5730. doi: 10.1038/sj.onc.1208925. [DOI] [PubMed] [Google Scholar]
- Gorelik M, Orukari I, Wang J, Galpoththawela S, Kim H, Levy M, Gilad AA, Bar-Shir A, Kerr DA, Levchenko A, et al. Use of MR cell tracking to evaluate targeting of glial precursor cells to inflammatory tissue by exploiting the very late antigen-4 docking receptor. Radiology. 2012;265:175–85. doi: 10.1148/radiol.12112212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu YP, Zhao Y, Zhou YR, Xie YJ, Ju P, Long YS, Liu JN, Ni DS, Cao F, Lyu ZS, et al. Zeb1 Is a Potential Regulator of Six2 in the Proliferation, Apoptosis and Migration of Metanephric Mesenchyme Cells. International Journal of Molecular Sciences. 2016:17. doi: 10.3390/ijms17081283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillaume DJ, Zhang SC. Human embryonic stem cells: a potential source of transplantable neural progenitor cells. Neurosurg Focus. 2008;24:E3. doi: 10.3171/FOC/2008/24/3-4/E2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N, Henry RG, Strober J, Kang SM, Lim DA, Bucci M, Caverzasi E, Gaetano L, Mandelli ML, Ryan T, et al. Neural stem cell engraftment and myelination in the human brain. Sci Transl Med. 2012;4:155ra137. doi: 10.1126/scitranslmed.3004373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A, Giese NA, Richardson WD. Spinal cord oligodendrocytes develop from ventrally derived progenitor cells that express PDGF alpha-receptors. Development. 1996;122:4085–94. doi: 10.1242/dev.122.12.4085. [DOI] [PubMed] [Google Scholar]
- Han SS, Liu Y, Tyler-Polsz C, Rao MS, Fischer I. Transplantation of glial-restricted precursor cells into the adult spinal cord: survival, glial-specific differentiation, and preferential migration in white matter. Glia. 2004;45:1–16. doi: 10.1002/glia.10282. [DOI] [PubMed] [Google Scholar]
- Han X, Chen M, Wang F, Windrem M, Wang S, Shanz S, Xu Q, Oberheim NA, Bekar L, Betstadt S, et al. Forebrain engraftment by human glial progenitor cells enhances synaptic plasticity and learning in adult mice. Cell Stem Cell. 2013;12:342–53. doi: 10.1016/j.stem.2012.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happel P, Moller K, Schwering NK, Dietzel ID. Migrating oligodendrocyte progenitor cells swell prior to soma dislocation. Sci Rep. 2013;3:1806. doi: 10.1038/srep01806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow DE, Honce JM, Miravalle AA. Remyelination Therapy in Multiple Sclerosis. Front Neurol. 2015;6:257. doi: 10.3389/fneur.2015.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattermann K, Ludwig A, Gieselmann V, Held-Feindt J, Mentlein R. The chemokine CXCL16 induces migration and invasion of glial precursor cells via its receptor CXCR6. Mol Cell Neurosci. 2008;39:133–41. doi: 10.1016/j.mcn.2008.03.009. [DOI] [PubMed] [Google Scholar]
- Huang W, Zhao N, Bai X, Karram K, Trotter J, Goebbels S, Scheller A, Kirchhoff F. Novel NG2-CreERT2 knock-in mice demonstrate heterogeneous differentiation potential of NG2 glia during development. Glia. 2014;62:896–913. doi: 10.1002/glia.22648. [DOI] [PubMed] [Google Scholar]
- Ivkovic S, Canoll P, Goldman JE. Constitutive EGFR signaling in oligodendrocyte progenitors leads to diffuse hyperplasia in postnatal white matter. Journal of Neuroscience. 2008;28:914–922. doi: 10.1523/JNEUROSCI.4327-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasa SN, Babona-Pilipos R, Morshead CM. Environmental Factors That Influence Stem Cell Migration: An “Electric Field”. Stem Cells International. 2017 doi: 10.1155/2017/4276927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowski M. Functional diversity of SDF-1 splicing variants. Cell Adh Migr. 2009;3:243–9. doi: 10.4161/cam.3.3.8260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowski M, Bulte JW, Walczak P. Personalized nanomedicine advancements for stem cell tracking. Adv Drug Deliv Rev. 2012;64:1488–507. doi: 10.1016/j.addr.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowski M, Lukomska B, Domanska-Janik K. Migratory capabilities of human umbilical cord blood-derived neural stem cells (HUCB-NSC) in vitro. Acta Neurobiol Exp (Wars) 2011;71:24–35. doi: 10.55782/ane-2011-1820. [DOI] [PubMed] [Google Scholar]
- Janowski M, Lyczek A, Engels C, Xu J, Lukomska B, Bulte JW, Walczak P. Cell size and velocity of injection are major determinants of the safety of intracarotid stem cell transplantation. J Cereb Blood Flow Metab. 2013;33:921–7. doi: 10.1038/jcbfm.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowski M, Walczak P, Kropiwnicki T, Jurkiewicz E, Domanska-Janik K, Bulte JW, Lukomska B, Roszkowski M. Long-term MRI cell tracking after intraventricular delivery in a patient with global cerebral ischemia and prospects for magnetic navigation of stem cells within the CSF. PLoS One. 2014;9:e97631. doi: 10.1371/journal.pone.0097631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowski M, Walczak P, Pearl MS. Predicting and optimizing the territory of blood-brain barrier opening by superselective intra-arterial cerebral infusion under dynamic susceptibility contrast MRI guidance. J Cereb Blood Flow Metab. 2016;36:569–75. doi: 10.1177/0271678X15615875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaramillo-Merchan J, Jones J, Ivorra JL, Pastor D, Viso-Leon MC, Armengol JA, Molto MD, Geijo-Barrientos E, Martinez S. Mesenchymal stromal-cell transplants induce oligodendrocyte progenitor migration and remyelination in a chronic demyelination model. Cell Death Dis. 2013;4:e779. doi: 10.1038/cddis.2013.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jozwiak S, Habich A, Kotulska K, Sarnowska A, Kropiwnicki T, Janowski M, Jurkiewicz E, Lukomska B, Kmiec T, Walecki J, et al. Intracerebroventricular Transplantation of Cord Blood-Derived Neural Progenitors in a Child With Severe Global Brain Ischemic Injury. Cell Med. 2010;1:71–80. doi: 10.3727/215517910X536618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahlert UD, Suwala AK, Raabe EH, Siebzehnrubl FA, Suarez MJ, Orr BA, Bar EE, Maciaczyk J, Eberhart CG. ZEB1 Promotes Invasion in Human Fetal Neural Stem Cells and Hypoxic Glioma Neurospheres. Brain Pathol. 2015;25:724–32. doi: 10.1111/bpa.12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karram K, Chatterjee N, Trotter J. NG2-expressing cells in the nervous system: role of the proteoglycan in migration and glial-neuron interaction. J Anat. 2005;207:735–44. doi: 10.1111/j.1469-7580.2005.00461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karussis D, Karageorgiou C, Vaknin-Dembinsky A, Gowda-Kurkalli B, Gomori JM, Kassis I, Bulte JW, Petrou P, Ben-Hur T, Abramsky O, et al. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol. 2010;67:1187–94. doi: 10.1001/archneurol.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong H, Fan Y, Xie J, Ding J, Sha L, Shi X, Sun X, Hu G. AQP4 knockout impairs proliferation, migration and neuronal differentiation of adult neural stem cells. J Cell Sci. 2008;121:4029–36. doi: 10.1242/jcs.035758. [DOI] [PubMed] [Google Scholar]
- Kriks S, Shim JW, Piao JH, Ganat YM, Wakeman DR, Xie Z, Carrillo-Reid L, Auyeung G, Antonacci C, Buch A, et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature. 2011;480:547–U177. doi: 10.1038/nature10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn HG, Winkler J, Kempermann G, Thal LJ, Gage FH. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J Neurosci. 1997;17:5820–9. doi: 10.1523/JNEUROSCI.17-15-05820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhani B, Hayward KS, Boyd LA. Hemispheric asymmetry in myelin after stroke is related to motor impairment and function. Neuroimage Clin. 2017;14:344–353. doi: 10.1016/j.nicl.2017.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Coz V, Zhu C, Devocelle A, Vazquez A, Boucheix C, Azzi S, Gallerne C, Eid P, Lecourt S, Giron-Michel J. IGF-1 contributes to the expansion of melanoma-initiating cells through an epithelial-mesenchymal transition process. Oncotarget. 2016;7:82511–82527. doi: 10.18632/oncotarget.12733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Learish RD, Brustle O, Zhang SC, Duncan ID. Intraventricular transplantation of oligodendrocyte progenitors into a fetal myelin mutant results in widespread formation of myelin. Ann Neurol. 1999;46:716–22. [PubMed] [Google Scholar]
- Lepore AC, Rauck B, Dejea C, Pardo AC, Rao MS, Rothstein JD, Maragakis NJ. Focal transplantation-based astrocyte replacement is neuroprotective in a model of motor neuron disease. Nat Neurosci. 2008;11:1294–301. doi: 10.1038/nn.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DW, Liu ZQ, Wei J, Liu Y, Hu LS. Contribution of endothelial progenitor cells to neovascularization (Review) Int J Mol Med. 2012;30:1000–6. doi: 10.3892/ijmm.2012.1108. [DOI] [PubMed] [Google Scholar]
- Li YH, Feng L, Zhang GX, Ma CG. Intranasal delivery of stem cells as therapy for central nervous system disease. Experimental and Molecular Pathology. 2015;98:145–151. doi: 10.1016/j.yexmp.2015.01.016. [DOI] [PubMed] [Google Scholar]
- Liu Y, Han SS, Wu Y, Tuohy TM, Xue H, Cai J, Back SA, Sherman LS, Fischer I, Rao MS. CD44 expression identifies astrocyte-restricted precursor cells. Dev Biol. 2004;276:31–46. doi: 10.1016/j.ydbio.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Lu D, Li Y, Wang L, Chen J, Mahmood A, Chopp M. Intraarterial administration of marrow stromal cells in a rat model of traumatic brain injury. J Neurotrauma. 2001;18:813–9. doi: 10.1089/089771501316919175. [DOI] [PubMed] [Google Scholar]
- Lyczek A, Arnold A, Zhang J, Campanelli JT, Janowski M, Bulte JW, Walczak P. Transplanted human glial-restricted progenitors can rescue the survival of dysmyelinated mice independent of the production of mature, compact myelin. Exp Neurol. 2017;291:74–86. doi: 10.1016/j.expneurol.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda Y, Solanky M, Menonna J, Chapin J, Li W, Dowling P. Platelet-derived growth factor-alpha receptor-positive oligodendroglia are frequent in multiple sclerosis lesions. Ann Neurol. 2001;49:776–85. doi: 10.1002/ana.1015. [DOI] [PubMed] [Google Scholar]
- Maragakis NJ, Rao MS, Llado J, Wong V, Xue H, Pardo A, Herring J, Kerr D, Coccia C, Rothstein JD. Glial restricted precursors protect against chronic glutamate neurotoxicity of motor neurons in vitro. Glia. 2005;50:145–59. doi: 10.1002/glia.20161. [DOI] [PubMed] [Google Scholar]
- Merino JJ, Bellver-Landete V, Oset-Gasque MJ, Cubelos B. CXCR4/CXCR7 molecular involvement in neuronal and neural progenitor migration: focus in CNS repair. J Cell Physiol. 2015;230:27–42. doi: 10.1002/jcp.24695. [DOI] [PubMed] [Google Scholar]
- Minocha S, Valloton D, Arsenijevic Y, Cardinaux JR, Guidi R, Hornung JP, Lebrand C. Nkx2.1 regulates the generation of telencephalic astrocytes during embryonic development. Sci Rep. 2017;7:43093. doi: 10.1038/srep43093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori H, Takahashi A, Horimoto A, Hara M. Migration of glial cells differentiated from neurosphere-forming neural stem/progenitor cells depends on the stiffness of the chemically cross-linked collagen gel substrate. Neurosci Lett. 2013;555:1–6. doi: 10.1016/j.neulet.2013.09.012. [DOI] [PubMed] [Google Scholar]
- Naor D, Nedvetzki S, Golan I, Melnik L, Faitelson Y. CD44 in cancer. Crit Rev Clin Lab Sci. 2002;39:527–79. doi: 10.1080/10408360290795574. [DOI] [PubMed] [Google Scholar]
- Naruse M, Shibasaki K, Yokoyama S, Kurachi M, Ishizaki Y. Dynamic changes of CD44 expression from progenitors to subpopulations of astrocytes and neurons in developing cerebellum. PLoS One. 2013;8:e53109. doi: 10.1371/journal.pone.0053109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasarre C, Koncina E, Labourdette G, Cremel G, Roussel G, Aunis D, Bagnard D. Neuropilin-2 acts as a modulator of Sema3A-dependent glioma cell migration. Cell Adh Migr. 2009;3:383–9. doi: 10.4161/cam.3.4.9934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowakowski A, Walczak P, Lukomska B, Janowski M. Genetic Engineering of Mesenchymal Stem Cells to Induce Their Migration and Survival. Stem Cells International. 2016 doi: 10.1155/2016/4956063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill HS, O’Sullivan J, Porteous N, Ruiz Hernandez E, Kelly HM, O’Brien FJ, Duffy GP. A Collagen Cardiac Patch Incorporating Alginate Microparticles Permits the Controlled Release of HGF and IGF-1 to Enhance Cardiac Stem Cell Migration and Proliferation. J Tissue Eng Regen Med. 2016 doi: 10.1002/term.2392. [DOI] [PubMed] [Google Scholar]
- Oppliger B, Joerger-Messerli M, Mueller M, Reinhart U, Schneider P, Surbek DV, Schoeberlein A. Intranasal Delivery of Umbilical Cord-Derived Mesenchymal Stem Cells Preserves Myelination in Perinatal Brain Damage. Stem Cells Dev. 2016;25:1234–42. doi: 10.1089/scd.2016.0027. [DOI] [PubMed] [Google Scholar]
- Papadopoulos MC, Verkman AS. Aquaporin water channels in the nervous system. Nat Rev Neurosci. 2013;14:265–77. doi: 10.1038/nrn3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park WS, Sung SI, Ahn SY, Yoo HS, Sung DK, Im GH, Choi SJ, Chang YS. Hypothermia augments neuroprotective activity of mesenchymal stem cells for neonatal hypoxic-ischemic encephalopathy. PLoS One. 2015;10:e0120893. doi: 10.1371/journal.pone.0120893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pevny LH, Sockanathan S, Placzek M, Lovell-Badge R. A role for SOX1 in neural determination. Development. 1998;125:1967–78. doi: 10.1242/dev.125.10.1967. [DOI] [PubMed] [Google Scholar]
- Piao JH, Wang Y, Duncan ID. CD44 is required for the migration of transplanted oligodendrocyte progenitor cells to focal inflammatory demyelinating lesions in the spinal cord. Glia. 2013;61:361–7. doi: 10.1002/glia.22438. [DOI] [PubMed] [Google Scholar]
- Psachoulia K, Jamen F, Young KM, Richardson WD. Cell cycle dynamics of NG2 cells in the postnatal and ageing brain. Neuron Glia Biology. 2009;5:57–67. doi: 10.1017/S1740925X09990354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X, Shen Q, Goderie SK, He W, Capela A, Davis AA, Temple S. Timing of CNS cell generation: a programmed sequence of neuron and glial cell production from isolated murine cortical stem cells. Neuron. 2000;28:69–80. doi: 10.1016/s0896-6273(00)00086-6. [DOI] [PubMed] [Google Scholar]
- Rao MS, Mayer-Proschel M. Glial-restricted precursors are derived from multipotent neuroepithelial stem cells. Dev Biol. 1997;188:48–63. doi: 10.1006/dbio.1997.8597. [DOI] [PubMed] [Google Scholar]
- Reitz MDM, Sedlacik J, Meissner H, Fiehler J, Kim SU, Westphal M, Schmidt NO. Intranasal delivery of neural stem/progenitor cells:a noninvasive passage to target intracerebralglioma. Stem Cells Transl Med. 2012 doi: 10.5966/sctm.2012-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadoun S, Papadopoulos MC, Watanabe H, Yan D, Manley GT, Verkman AS. Involvement of aquaporin-4 in astroglial cell migration and glial scar formation. J Cell Sci. 2005;118:5691–8. doi: 10.1242/jcs.02680. [DOI] [PubMed] [Google Scholar]
- Sasaki A, Naganuma H, Satoh E, Kawataki T, Amagasaki K, Nukui H. Participation of thrombospondin-1 in the activation of latent transforming growth factor-beta in malignant glioma cells. Neurologia Medico-Chirurgica. 2001;41:253–258. doi: 10.2176/nmc.41.253. [DOI] [PubMed] [Google Scholar]
- Saugierveber P, Munnich A, Bonneau D, Rozet JM, Lemerrer M, Gil R, Boespflugtanguy O. X-Linked Spastic Paraplegia and Pelizaeus-Merzbacher Disease Are Allelic Disorders at the Proteolipid Protein Locus. Nature Genetics. 1994;6:257–262. doi: 10.1038/ng0394-257. [DOI] [PubMed] [Google Scholar]
- Schwarz M, Alvarez-Bolado G, Dressler G, Urbanek P, Busslinger M, Gruss P. Pax2/5 and Pax6 subdivide the early neural tube into three domains. Mech Dev. 1999;82:29–39. doi: 10.1016/s0925-4773(99)00005-2. [DOI] [PubMed] [Google Scholar]
- Shimozaki K. Sox2 transcription network acts as a molecular switch to regulate properties of neural stem cells. World J Stem Cells. 2014;6:485–90. doi: 10.4252/wjsc.v6.i4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–56. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Simard M, Nedergaard M. The neurobiology of glia in the context of water and ion homeostasis. Neuroscience. 2004;129:877–96. doi: 10.1016/j.neuroscience.2004.09.053. [DOI] [PubMed] [Google Scholar]
- Staugaitis SM, Trapp BD. NG2-positive glia in the human central nervous system. Neuron Glia Biol. 2009;5:35–44. doi: 10.1017/S1740925X09990342. [DOI] [PubMed] [Google Scholar]
- Stolt CC, Schlierf A, Lommes P, Hillgartner S, Werner T, Kosian T, Sock E, Kessaris N, Richardson WD, Lefebvre V, et al. SoxD proteins influence multiple stages of oligodendrocyte development and modulate SoxE protein function. Dev Cell. 2006;11:697–709. doi: 10.1016/j.devcel.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y, Taniguchi M, Yagi T, Akagi Y, Nojyo Y, Tamamaki N. Guidance of glial precursor cell migration by secreted cues in the developing optic nerve. Development. 2001;128:3321–30. doi: 10.1242/dev.128.17.3321. [DOI] [PubMed] [Google Scholar]
- Suter DM, Tirefort D, Julien S, Krause KH. A Sox1 to Pax6 switch drives neuroectoderm to radial glia progression during differentiation of mouse embryonic stem cells. Stem Cells. 2009;27:49–58. doi: 10.1634/stemcells.2008-0319. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Iwamoto M, Saito Y, Fuchimoto D, Sembon S, Suzuki M, Mikawa S, Hashimoto M, Aoki Y, Najima Y, et al. Il2rg Gene-Targeted Severe Combined Immunodeficiency Pigs. Cell Stem Cell. 2012;10:753–758. doi: 10.1016/j.stem.2012.04.021. [DOI] [PubMed] [Google Scholar]
- Sypecka J, Sarnowska A, Domanska-Janik K. Crucial role of the local micro-environment in fate decision of neonatal rat NG2 progenitors. Cell Prolif. 2009;42:661–71. doi: 10.1111/j.1365-2184.2009.00618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepavcevic V, Kerninon C, Aigrot MS, Meppiel E, Mozafari S, Arnould-Laurent R, Ravassard P, Kennedy TE, Nait-Oumesmar B, Lubetzki C. Early netrin-1 expression impairs central nervous system remyelination. Ann Neurol. 2014;76:252–68. doi: 10.1002/ana.24201. [DOI] [PubMed] [Google Scholar]
- Thiruvalluvan A, Czepiel M, Kap YA, Mantingh-Otter I, Vainchtein I, Kuipers J, Bijlard M, Baron W, Giepmans B, Bruck W, et al. Survival and Functionality of Human Induced Pluripotent Stem Cell-Derived Oligodendrocytes in a Nonhuman Primate Model for Multiple Sclerosis. Stem Cells Transl Med. 2016;5:1550–1561. doi: 10.5966/sctm.2016-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi A, Parikh ZS, Vora P, Frost EE, Pillai PP. pERK1/2 Peripheral Recruitment and Filopodia Protrusion Augment Oligodendrocyte Progenitor Cell Migration: Combined Effects of PDGF-A and Fibronectin. Cell Mol Neurobiol. 2017;37:183–194. doi: 10.1007/s10571-016-0359-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida N, Chen K, Dohse M, Hansen KD, Dean J, Buser JR, Riddle A, Beardsley DJ, Wan Y, Gong X, et al. Human neural stem cells induce functional myelination in mice with severe dysmyelination. Sci Transl Med. 2012;4:155ra136. doi: 10.1126/scitranslmed.3004371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadivelu S, Stewart TJ, Qu Y, Horn K, Liu S, Li Q, Silver J, McDonald JW. NG2+ progenitors derived from embryonic stem cells penetrate glial scar and promote axonal outgrowth into white matter after spinal cord injury. Stem Cells Transl Med. 2015;4:401–11. doi: 10.5966/sctm.2014-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Velthoven CTKA, van Bel F, Heijnen CJ. Nasal administration of stem cells:a promising novel route to treat neonatal ischemic braindamage. Pediatr Res. 2010;68:419–22. doi: 10.1203/PDR.0b013e3181f1c289. [DOI] [PubMed] [Google Scholar]
- Walczak P, All AH, Rumpal N, Gorelik M, Kim H, Maybhate A, Agrawal G, Campanelli JT, Gilad AA, Kerr DA, et al. Human glial-restricted progenitors survive, proliferate, and preserve electrophysiological function in rats with focal inflammatory spinal cord demyelination. Glia. 2011;59:499–510. doi: 10.1002/glia.21119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walczak P, Kedziorek DA, Gilad AA, Barnett BP, Bulte JW. Applicability and limitations of MR tracking of neural stem cells with asymmetric cell division and rapid turnover: the case of the shiverer dysmyelinated mouse brain. Magn Reson Med. 2007;58:261–9. doi: 10.1002/mrm.21280. [DOI] [PubMed] [Google Scholar]
- Wallace GCt, Dixon-Mah YN, Vandergrift WA, 3rd, Ray SK, Haar CP, Mittendorf AM, Patel SJ, Banik NL, Giglio P, Das A. Targeting oncogenic ALK and MET: a promising therapeutic strategy for glioblastoma. Metab Brain Dis. 2013;28:355–66. doi: 10.1007/s11011-013-9401-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Rougon G, Kiss JZ. Requirement of polysialic acid for the migration of the O-2A glial progenitor cell from neurohypophyseal explants. J Neurosci. 1994;14:4446–57. doi: 10.1523/JNEUROSCI.14-07-04446.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Bates J, Li X, Schanz S, Chandler-Militello D, Levine C, Maherali N, Studer L, Hochedlinger K, Windrem M, et al. Human iPSC-derived oligodendrocyte progenitor cells can myelinate and rescue a mouse model of congenital hypomyelination. Cell Stem Cell. 2013;12:252–64. doi: 10.1016/j.stem.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidle UH, Birzele F, Kollmorgen G, Ruger R. Molecular Mechanisms of Bone Metastasis. Cancer Genomics Proteomics. 2016;13:1–12. [PubMed] [Google Scholar]
- Wen J, Hu Q, Li M, Wang S, Zhang L, Chen Y, Li L. Pax6 directly modulate Sox2 expression in the neural progenitor cells. Neuroreport. 2008;19:413–7. doi: 10.1097/WNR.0b013e3282f64377. [DOI] [PubMed] [Google Scholar]
- Wilbanks A, Zondlo SC, Murphy K, Mak S, Soler D, Langdon P, Andrew DP, Wu L, Briskin M. Expression Cloning of the STRL33/BONZO/TYMSTR Ligand Reveals Elements of CC, CXC, and CX3C Chemokines. The Journal of Immunology. 2001;166:5145–5154. doi: 10.4049/jimmunol.166.8.5145. [DOI] [PubMed] [Google Scholar]
- Windrem MS, Schanz SJ, Guo M, Tian GF, Washco V, Stanwood N, Rasband M, Roy NS, Nedergaard M, Havton LA, et al. Neonatal chimerization with human glial progenitor cells can both remyelinate and rescue the otherwise lethally hypomyelinated shiverer mouse. Cell Stem Cell. 2008;2:553–65. doi: 10.1016/j.stem.2008.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windrem MS, Schanz SJ, Morrow C, Munir J, Chandler-Militello D, Wang S, Goldman SA. A competitive advantage by neonatally engrafted human glial progenitors yields mice whose brains are chimeric for human glia. J Neurosci. 2014;34:16153–61. doi: 10.1523/JNEUROSCI.1510-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodworth MB, Girskis KM, Walsh CA. Building a lineage from single cells: genetic techniques for cell lineage tracking. Nat Rev Genet. 2017;18:230–244. doi: 10.1038/nrg.2016.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Li K, Yan Y, Gran B, Han Y, Zhou F, Guan YT, Rostami A, Zhang GX. Intranasal Delivery of Neural Stem Cells: A CNS-specific, Non-invasive Cell-based Therapy for Experimental Autoimmune Encephalomyelitis. J Clin Cell Immunol. 2013:4. doi: 10.4172/2155-9899.1000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yavagal DR, Lin B, Raval AP, Garza PS, Dong C, Zhao W, Rangel EB, McNiece I, Rundek T, Sacco RL, et al. Efficacy and dose-dependent safety of intra-arterial delivery of mesenchymal stem cells in a rodent stroke model. PLoS One. 2014;9:e93735. doi: 10.1371/journal.pone.0093735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Li G, Lesniak MS, Balyasnikova IV. Intranasal Delivery of Therapeutic Stem Cells to Glioblastoma in a Mouse Model. J Vis Exp. 2017 doi: 10.3791/55845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan XB, Jin Y, Haas C, Yao L, Hayakawa K, Wang Y, Wang C, Fischer I. Guiding migration of transplanted glial progenitor cells in the injured spinal cord. Sci Rep. 2016;6:22576. doi: 10.1038/srep22576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Jiao J. Molecular Biomarkers for Embryonic and Adult Neural Stem Cell and Neurogenesis. Biomed Res Int. 2015;2015:727542. doi: 10.1155/2015/727542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang PJ, Sun YT, Ma L. ZEB1: At the crossroads of epithelial-mesenchymal transition, metastasis and therapy resistance. Cell Cycle. 2015;14:481–487. doi: 10.1080/15384101.2015.1006048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu BF, Nicholls M, Gu Y, Zhang GF, Zhao C, Franklin RJM, Song B. Electric Signals Regulate the Directional Migration of Oligodendrocyte Progenitor Cells (OPCs) via beta 1 Integrin. International Journal of Molecular Sciences. 2016:17. doi: 10.3390/ijms17111948. [DOI] [PMC free article] [PubMed] [Google Scholar]