Abstract
The objective of this study was to investigate the expression of the chemokine CXCL10 and its role in joint tissues following articular fracture. We hypothesized that CXCL10 is upregulated following articular fracture and contributes to cartilage degradation associated with post-traumatic arthritis (PTA). To evaluate CXCL10 expression following articular fracture, gene expression was quantified in synovial tissue from knee joints of C57BL/6 mice that develop PTA following articular fracture, and MRL/MpJ mice that are protected from PTA. CXCL10 protein expression was assessed in human cartilage in normal, osteoarthritic (OA), and post-traumatic tissue using immunohistochemistry. The effects of exogenous CXCL10, alone and in combination with IL-1, on porcine cartilage explants were assessed by quantifying the release of catabolic mediators. Synovial tissue gene expression of CXCL10 was upregulated by joint trauma, peaking one day in C57BL/6 mice (25-fold) vs. three days post-fracture in MRL/MpJ mice (15-fold). CXCL10 protein in articular cartilage was most highly expressed following trauma compared with normal and OA tissue. In a dose dependent manner, exogenous CXCL10 significantly reduced total matrix metalloproteinase (MMP) and aggrecanase activity of culture media from cartilage explants. CXCL10 also trended toward a reduction in IL-1α-stimulated total MMP activity (p=0.09) and S-GAG (p=0.09), but not NO release. In conclusion, CXCL10 was upregulated in synovium and chondrocytes following trauma. However, exogenous CXCL10 did not induce a catabolic response in cartilage. CXCL10 may play a role in modulating the chondrocyte response to inflammatory stimuli associated with joint injury and the progression of PTA.
Keywords: cartilage, arthritis, trauma, injury, intra-articular fracture, synovium, inflammation, chemokine
Graphical Abstract
The objective of this study was to investigate the chemokine CXCL10 and its role in joint tissues following articular fracture. We hypothesized that CXCL10 is upregulated following articular fracture and contributes to cartilage degradation associated with post-traumatic arthritis (PTA). CXCL10 was upregulated in synovium and chondrocytes (see legend Figure 2) following trauma, but did not directly induce catabolism of articular cartilage. Findings from this study suggest that CXCL10 may play a role in homeostasis of chondrocytes and modulation of inflammation.
INTRODUCTION
Post-traumatic arthritis (PTA) develops following trauma of weight-bearing joints and accounts for approximately 12% of osteoarthritis (OA) cases of the hip, knee and ankle (1). While the exact etiology of PTA is not yet fully elucidated, it is thought that a major factor involved in the disease progression is inflammation (2). Inflammatory cytokines are elevated following joint injury in humans (3–6) and in animal models (7, 8). Inflammatory cytokines have been shown to upregulate catabolic mediators of cartilage degeneration, such as nitric oxide (NO) and prostaglandin E2, and also extracellular matrix (ECM) degrading enzymes, such as matrix metalloproteases (MMPs) and aggrecanases (9–11). Molecular biomarkers of cartilage degradation products are significantly increased following knee injury in patients (12–15) and are upregulated in animal models of joint injury and in vitro models of injury to articular cartilage (8, 16–18).
Recent studies also suggest that chemokines and their receptors play important roles in rheumatoid arthritis and other forms of inflammatory arthritis. For example, the chemokine CXCL10, or interferon-inducible protein 10 (IP-10), is expressed by synovial cells and fibroblasts (19–21) and upregulated in the serum and plasma of patients with these inflammatory forms of arthritis (22–24). Clinical treatment with anti-rheumatic drugs, including TNF-α blockers, decreased levels of CXCL10 (24, 25). In vitro treatment of synovial cells and human primary fibroblast-like synoviocytes with TNF-α and IL-1β lead to increased levels of CXCL10 (20, 26).
In inflammatory juvenile idiopathic arthritis, CXCL10 plays a role in leukocyte homing to inflamed tissues (27), which may then lead to the perpetuation of chronic inflammation and resulting tissue damage (28). Chemokine receptors including CCR3, the receptor for CXCL10, are present and upregulated in chondrocytes of OA cartilage (29). Additionally, CXCL10 protein secretion is increased by IL-1 and TNF-α in normal and osteoarthritic (OA) human chondrocytes (30). Synovial fluid CXCL10 levels are correlated with osteoarthritis severity in a rat model of diet-induced obesity (31). However, the effects of CXCL10 on cartilage following joint injury, such as articular fracture, and its role in the development of PTA have yet to be determined.
For this study, we hypothesized that CXCL10 is upregulated following intra-articular fracture and contributes to cartilage degradation associated with PTA. The objectives of this study were three-fold: first, to compare synovial gene expression and serum levels of CXCL10 in C57BL/6 mice that develop PTA following articular fracture and MRL/MpJ mice that are protected from PTA; second, to assess CXCL10 protein expression in normal, OA, and post-traumatic human cartilage; and finally, to determine the effects of exogenous CXCL10 on IL-1-mediated catabolism of cartilage explants.
Materials and Methods
CXCL10 Expression following Articular Fracture in the Mouse
All procedures were performed in accordance with an IACUC approved protocol. At 16 weeks of age, C57BL/6 (Charles River Labs) and MRL/MpJ (Jackson Laboratory) male mice were sacrificed to represent the pre-fracture condition (n=6 per strain) or received moderate articular fractures of the left tibial plateau, as previously described (7, 32, 33), and then were sacrificed (n=6 per strain) at 0, 1, 3, 5, and 7 days post-fracture.
At sacrifice, joint capsule tissue was harvested with a 3 mm biopsy punch from 6 animals at each time point, as previously described (34). RNA was isolated using a two-step Trizol protocol. RT-PCR was run on 1μg of RNA in duplicate using SYBR Green Master Mix and the commercially-available RT2 Profiler PCR array for mouse inflammatory cytokines (SABiosciences, Qiagen). Relative mRNA for CXCL10 was first normalized to the geometric mean of three housekeeping genes (GAPDH, HPRT1, and HSP90ab1) in that sample and then compared to mRNA expression at pre-fracture within a mouse strain and also between mouse strains at each time point using the 2-ΔΔCt method (7, 35). In accordance with commercial analysis software provided by the manufacturer, results were considered significant if mRNA expression levels were 3-fold different.
Serum was collected at the time of sacrifice. Concentrations of CXCL10 were measured in serum samples using a commercially-available ELISA kit (MCX100; R&D Systems, Minneapolis, MN) and run as directed by the manufacturer. Non-parametric statistical analyses were performed with significance reported at the 95% confidence level. For serum levels of CXCL10, the Mann-Whitney U test was used to compare differences between C57BL/6 and MRL/MpJ strains at each time point, and the Kruskal-Wallis test was used to compare differences with the baseline pre-fracture condition and time post-fracture within a mouse strain. Statistical analysis was performed using Statistica 7 software (StatSoft).
CXCL10 Protein Expression in Human Articular Cartilage
Cartilage was collected from cadavers with no history of joint injury (Normal; n=7), patients with end stage OA undergoing joint replacement (OA; n=11), and from patients with post-traumatic articular fractures (Trauma; n=29). De-identified human surgical waste tissues for the OA and Trauma samples were collected under an IRB approved exemption. Donor and patient demographics are presented in Table 1.
Table 1.
Donor and patient demographics for cartilage samples. Tissue was obtained from donors with no history of joint injury or disease (Normal), patients with end stage osteoarthritis undergoing joint replacement (OA), and patients with articular fracture undergoing surgery (Trauma).
| Age (years) | Sex (M/F) | Joint (n) | |
|---|---|---|---|
| Normal | 40 – 70 | 3 / 3 | Knee (7) |
| OA | 53 – 69 | 5 / 6 | Knee (11) |
| Trauma | 19 – 77 | 18 / 11 | Knee (10), Hip (9), Ankle (7), Elbow (3) |
Immunohistochemistry (IHC) was used to localize CXCL10 protein within the articular cartilage. All tissue samples were formalin-fixed, paraffin-embedded, sectioned at 8 μm, and immunostained for CXCL10. Serial sections were pre-treated with 0.05% proteinase K (Sigma-Aldrich, St. Louis, MO) for 5 minutes at 37°C for antigen retrieval as previously described (36). Endogenous peroxidase was quenched with 3% H2O2 in methanol with 0.1% w/v saponin for membrane permeabilization for 30 minutes. Sections were stained with a polyclonal goat CXCL10 antibody (AF-266-NA, R&D Systems) at 15 μg/mL for 18 hours at 4°C. During every staining protocol, negative controls were run simultaneously by application of 1–2% blocking serum to tissue sections instead of the primary antibody. Chromogenic detection was achieved with DAB substrate (Vectastain, Vector Laboratories, Burlingame, CA), and digital imageswere obtained. Positive CXCL10 staining in each cartilage zone was noted for sections from all samples. The surface, middle, and deep zones were identified by location and morphology of the chondrocytes. Intensity of positive staining for CXCL10 was assessed qualitatively using a discrete four-point scoring system by two blinded graders and reported as a single consensus score (7).
Effects of Exogenous CXCL10 on Cartilage Explants
Six cadaveric porcine knees from 2–3 year old skeletally mature female pigs were obtained from a local abattoir shortly after death. Using sterile technique, four 5 mm diameter biopsy punches of cartilage were taken from the femoral condyles of each joint. Explants were washed for one hour in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen, Carlsbad, CA) with 1,000 units/mL of penicillin/streptomycin/fungizone (Invitrogen). To equilibrate, explants were cultured for 72 hours in DMEM containing 10% heat-inactivated fetal bovine serum (Hyclone, Logan, UT), 0.1 mM nonessential amino acids (Invitrogen), 10 mM HEPES buffer solution (Invitrogen), 100 units/mL of penicillin/streptomycin/fungizone, and 37.5 μg/mL of L-ascorbic acid 2-phosphate (Sigma-Aldrich) at 37°C and 5% CO2. Media were removed, discarded, and replaced with media containing porcine CXCL10 (ab87388, Abcam, Boston, MA) at concentrations of 0, 1, 10, and 100 ng/mL (n=6 per group). After 72 hours, media were collected and frozen, and cartilage explants were weighed.
To assess the effects of CXCL10 on cartilage explants exposed to a pro-inflammatory stimulus, the following additional experiments were performed. Porcine cartilage explants (5 mm diameter from 6 different joints) were harvested, equilibrated for 72 hours, and then incubated with combinations of 0 or 100 ng/ml of porcine CXCL10 (Abcam) and porcine IL-1α (680-PI-010, R&D Systems) at physiologic concentrations of 0, 10, or 100 pg/ml (n=6 per group) (37, 38). IL-1α was selected due to the observation that porcine cartilage catabolism is more sensitive to IL-1α than IL-1β for physiologic concentrations, i.e., less than 1,000 pg/ml (38). After 72 hours, media were collected and frozen, and cartilage explants were weighed.
For both explant studies, media were assessed for total MMP activity, aggrecanase activity, sulfated glycosaminoglycan (S-GAG) release, and nitric oxide (NO) production. Total specific MMP activity in the culture media was measured using a previously described fluorescence-based assay (39). Aggrecanase-1 (ADAMTS-4) activity was measured in the culture media using a commercially available fluorescent peptide (WAAG-3R, AnaSpec, San Jose, CA) as previously described (40). Total S-GAG release was measured in media using the 1,9-dimethylmethylene blue (DMB) assay (41). Total NO release was determined using an established method that measures the concentration of nitrate and nitrite (NOx) in culture media (42). Data were normalized for the cartilage wet weight and expressed as mean ± SEM. One-way ANOVA was performed using linear trend analysis to assess associations of varying doses of exogenous CXCL10 (treated as a categorical variable) with measures of total MMP activity, aggrecanase activity, S-GAG and NO release in media. For the effect of CXCL10 on IL-1-stimulated cartilage explants, statistical analysis was performed using an ANOVA with a Newman-Keuls post-hoc test.
Results
CXCL10 Gene and Protein Expression is Upregulated in Mouse Articular Fracture Model
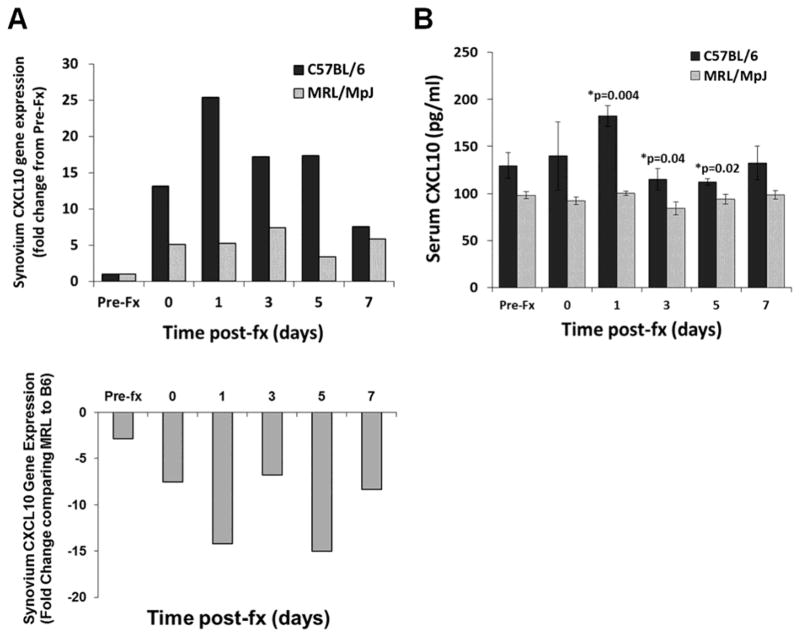
Comparing pre- to post-fracture, CXCL10 gene expression was increased in C57BL/6 mice: 13-fold increase within 4 hours of fracture (day 0), a maximum 25-fold increase at day 1, 17-fold increase at days 3 and 5, and 8-fold increase at day 7. In contrast, CXCL10 gene expression was also increased in MRL/MpJ mice, although to a lesser extent: a 5-fold increase on days 0 and 1, a maximum 7-fold increase on day 3, and a 5-fold increase at day 7 (Figure 1A). Prior to fracture, CXCL10 expression was similar between mouse strains. However, at all post-fracture time points, CXCL10 gene expression in MRL/MpJ mice was lower (range 7 to 15-fold) compared with C57BL/6 mice (Figure 1A).
Figure 1.
Gene expression and serum levels of CXCL10 in C57BL/6 and MRL/MpJ mice following fracture. (A) RT-PCR data showing relative change of CXCL10 gene expression in synovial tissue from C57BL/6 mice (black bars) and MRL/MpJ mice (light bars) (n=6 for each strain) following fracture (fx). Top shows fold change from Pre-Fracture (Pre-Fx) for each strain and bottom shows fold change in MRL/MpJ (MRL) strain compared to C57BL/6 (B6) strain. Greater than 3-fold change in expression was considered significant. (B) Serum concentrations of CXCL10 from C57BL/6 (black bars) and MRL/MpJ (light bars) mice following fracture (fx), data presented as mean ± SEM (Mann-U Whitney, *p < 0.05).
Similar to relative gene expression between mouse strains, serum concentrations of CXCL10 were significantly greater in C57BL/6 mice at 1, 3 and 5 days post-fracture compared to MRL/MpJ mice (Figure 1B). Serum concentrations of CXCL10 varied over time in C57BL/6 mice and trended towards an increase one day post-fracture (Kruskal Wallis, p=0.06). CXCL10 serum concentrations were not significantly different by time post-fracture in MRL/MpJ mice (Kruskal Wallis, p=0.25).
CXCL10 Protein Expression is Upregulated in Human Articular Cartilage following Trauma
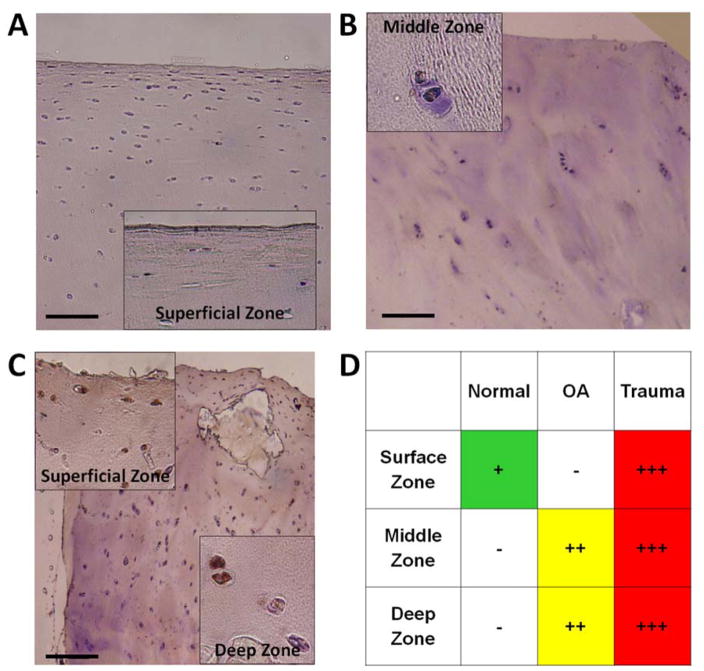
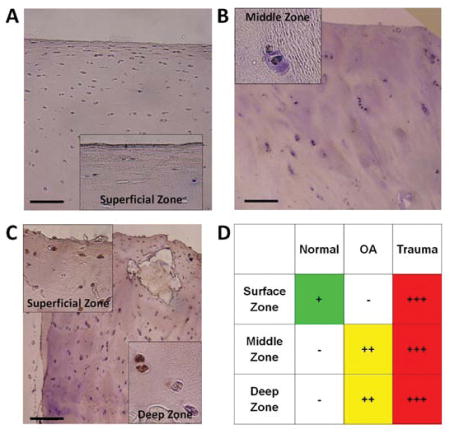
By immunostaining, CXCL10 was detected in chondrocytes in all human cartilage samples. There was minimal CXCL10 in normal cartilage with positive staining only in chondrocytes within the superficial cartilage zone (Figure 2A). There was slightly more CXCL10 in OA cartilage with localization to the middle and deep cartilage zones (Figure 3B). CXCL10 was most abundant in trauma cartilage and detected in all cartilage layers (Figure 2C). Additionally, CXCL10 increased with patient age for both OA and trauma cartilage. Overall, CXCL10 protein expression was highest in cartilage following joint trauma (Figure 2D).
Figure 2.
Immunohistochemistry for CXCL10 in human articular cartilage. Representative images from CXCL10 staining observed in articular cartilage from (A) Normal (n=7), (B) OA (n=11), and (C) Trauma tissue (n=29). Scale bars are 100 μm, with 20x magnification on insert, brown = positive IHC stain, and blue = hematoxylin counterstain. (D) Positive CXCL10 staining was qualitatively graded in the surface, middle and deep zones of the articular cartilage from all samples. Staining was discretely graded on a four-point qualitative scale, (−) to (+++), with (−) indicating minimal staining and (+++) indicating maximal staining in the cartilage zones.
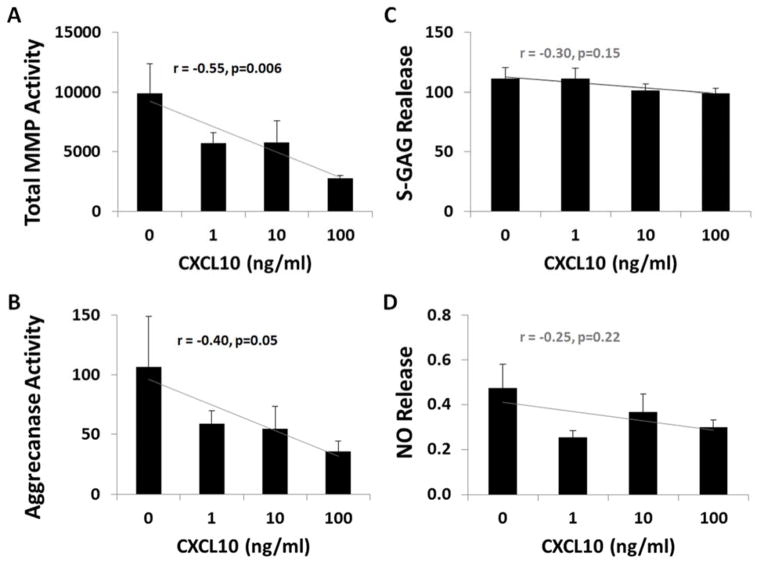
Figure 3.
Effect of exogenous CXCL10 on cartilage explants. All outcomes measured in media from cartilage explants cultured in different concentrations of CXCL10 (0, 1, 10, 100 ng/ml). Data normalized to wet weight of cartilage and reported as mean ± SEM with statistical analysis using one-way ANOVA with trend analysis. (A) MMP activity in fluorescent units/mg of cartilage (r = −0.55, p = 0.006). (B) Aggrecanase-1 (ADAMTS-4) activity in fluorescent units/ mg of cartilage (r = −0.40, p = 0.05). (C) S-GAG release in μg/mg of cartilage (r = −0.30, p = 0.15). (D) NO release in μM/mg of cartilage (r = −0.25, p = 0.22).
Anti-Inflammatory Effects of Exogenous CXCL10 on Cartilage Explants
Exogenous CXCL10 at physiologic concentrations (19, 22, 24) significantly reduced total MMP activity in culture media from cartilage explants in a dose dependent manner (linear trend r = −0.55, p=0.006). CXCL10 also reduced aggrecanase activity in a dose dependent manner (linear trend r = −0.40, p=0.05). Other catabolic measures, including S-GAG release (Figure 3C: linear trend r = −0.30, p=0.15) and NO release (Figure 3D: linear trend r = −0.25, p=0.22), were not significantly altered by CXCL10.
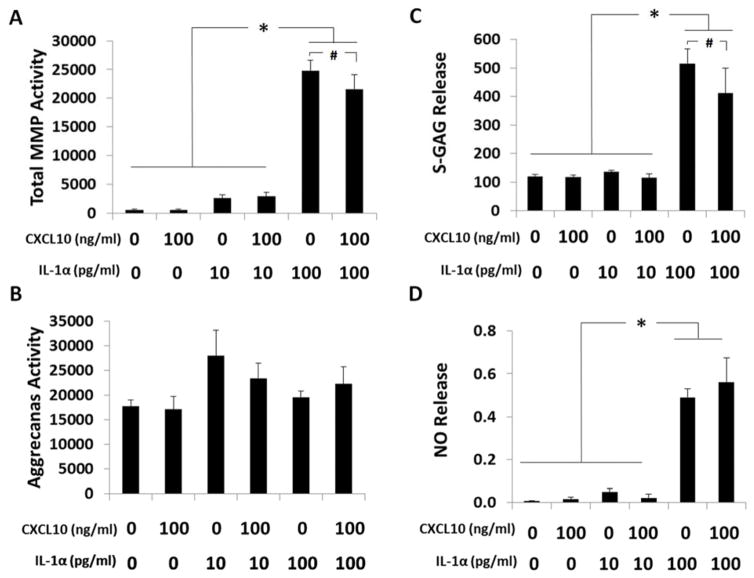
Effect of Exogenous CXCL10 on IL-1α-Stimulated Cartilage Explants
To examine if the observed anti-inflammatory response of CXCL10 could overcome a pro-inflammatory challenge, cartilage explants were cultured with exogenous CXCL10 in combination with physiologic concentrations of IL-1α (38). IL-1α (100 pg/mL) significantly upregulated total MMP activity, S-GAG and NO release (all p<0.001), but not aggrecanase activity (p=0.17) in media from cartilage explants (Figure 4). CXCL10 reduced IL-1α stimulated total MMP activity (p=0.09) and S-GAG release (p=0.09) but not NO release (p=0.34).
Figure 4.
Effect of exogenous CXCL10 on IL-1α stimulated cartilage explants. All outcomes measured in media from cartilage explants cultured with or without CXCL10 (0, 100 ng/ml) and different physiologic concentrations of IL-1α (0, 10, 100 pg/ml). Data normalized to wet weight of cartilage and reported as mean ± SEM with statistical analysis using ANOVA with Newman-Keuls post-hoc test. (A) Total MMP activity in fluorescent units/mg of cartilage (*p<0.0002, #p=0.09). (B) Aggrecanase-1 (ADAMTS-4) activity in fluorescent units/mg of cartilage (p=0.17). (C) S-GAG release in μg/mg of cartilage (*p<0.0004, #p=0.09). (D) NO release in μM/mg of cartilage (*p<0.0002).
Discussion
An association of local and systemic CXCL10 has been reported clinically (19, 22, 24, 25) and in animal models of inflammatory arthritis (24, 43, 44). Based on these findings, serum CXCL10 might serve as a clinical marker of disease activity in some forms of arthritis, including PTA. However, the role of chemokines like CXCL10 on cartilage and disease progression following injury is unknown. For this reason, we investigated the endogenous expression of CXCL10 in joint tissues in the context of injury and OA and the impact of exogenous CXCL10 on cartilage inflammation and degradative pathways.
Our results confirm that CXCL10 was endogenously upregulated, most especially by trauma, at both a transcriptional (gene expression) and post-transcriptional (protein by immunohistochemistry observed in all layers of cartilage after fracture and serum analyses by ELISA) level. Although elevated in mouse synovium following articular fracture and in serum of the PTA-prone C57BL/6 mice, CXCL10 was upregulated to a lesser extent in the MRL/MpJ mice that are less susceptible to PTA. These data are consistent with CXCL10 transcriptional regulation by inflammatory cytokines (21, 45). The greater CXCL10 upregulation in the synovium and serum of C57BL/6 mice following articular fracture compared to MRL/MpJ mice may contribute to the increased macrophage infiltration reported in the C57BL/6 mice (7), as CXCL10 and other chemokines have been implicated in immune cell chemotaxis (27, 28). This association of increased CXCL10 expression in synovium and serum with increased synovial inflammation and arthritic changes in the joint is similar to findings for rheumatoid arthritis (19, 20, 22, 24, 46). However, the direct effect of this chemokine on cartilage, or role in cartilage metabolism, has not been established.
Other research has shown that CXCL10 gene expression is upregulated in normal and OA chondrocytes stimulated with IL-1 or TNF-α, and that in response to incubation with exogenous IL-1β, human chondrocytes had increased gene expression of various other chemokines (47). However, the effect of exogenous CXCL10 on cartilage catabolism alone or in combination with pro-inflammatory cytokines has not been reported. In this study, CXCL10 alone or in combination with IL-1α did not induce NO production in cartilage, and consistently and most notably, reduced total MMP and aggrecanase activity. This suggests that CXCL10 may play a role in modulating inflammation following injury or an inflammatory stimulus. A homeostatic role for CXCL10 has already been suggested based on its upregulation in response to in vitro exposure of late-stage OA human chondrocytes to the damage-associated molecular pattern (DAMPs) molecule, soluble high mobility groups box-1 (HMGB1) (48).
The acute upregulation of CXCL10 following articular fracture may be part of the natural healing process. CXCL10 has been identified as playing a role in skin fibrosis, wound healing and tissue remodeling by significantly inducing type 1 collagen and hyaluronan production in human dermal fibroblasts (49). It has also been reported that osteochondral healing may be enhanced by CXCL10 through recruitment of subchondral progenitor cells (50). Human osteoclasts have been shown to express high levels of CXCL10 (51), and CXCL10 plays an important role in osteoblast activity through upregulation of enzymes involved in the bone remodeling process. CXCL10 has also been shown to effectively recruit human annulus fibrosus cells in vitro, which suggests that CXCL10 is involved in the spontaneous repair of annulus tears in the spine (52). Based on these reports, CXCL10 may enhance healing following an articular fracture by recruiting cells to the site of the osteochondral injury. Articular fracture induces an inflammatory response in the synovium including infiltration of immune cells and increased cytokine production (6, 53). Results from this study demonstrate articular fracture increases CXCL10 levels. To our surprise, exogenous CXCL10 did not have a catabolic effect on articular cartilage. The complexity of the intra-articular environment following a joint injury, including blood, immune cells, inflammation and altered articulation, makes identification of the etiology of PTA a challenging scientific question. A complex cascade of biomolecular pathways are likely involved, which may include chemokines like CXCL10. More studies are needed to determine the exact role of CXCL10 in the whole joint following injury and in the progression of PTA.
These data in combination suggest that although CXCL10 in joint tissues is upregulated following trauma, CXCL10 does not directly induce catabolism of articular cartilage but may play a role in homeostasis of chondrocytes and modulation of inflammation. The complex signaling that occurs between cartilage, subchondral bone, and synovium is likely to have an important role in maintaining cartilage homeostasis. Identifying key chemokines, like CXCL10, that are upregulated following joint injury may provide new insight into the interaction of joint tissues and opens possibilities of regulating signaling to promote healing and prevent the development of PTA.
Acknowledgments
Funding Sources: This study was supported by the Arthritis Foundation, NIH grants AG028716, AR55434, AR50245, and AR48852, a VA Rehabilitation Research Service Award, and research grant from DePuy/Synthes.
Walter Chad Hembree, MD and Adi Kanlic for procuring human cartilage and immunostaining; Stephen Johnson for his technical support; Duke University School of Medicine Anatomical Gifts Program; Duke University URS Independent Study Grant; DePuy/Synthes research support; NIH AG028716, AR55434, AR50245, and AR48852, and a VA Rehabilitation Research Service Award.
Footnotes
All authors have read and approved the final submitted manuscript. ALM, VBK, FG, and SAO contributed to the research design, interpretation of data, drafting and revising of the manuscript; BDF, CLK and JLK contributed to data acquisition, data analysis, data interpretation, drafting and revising of the manuscript.
References
- 1.Brown TD, Johnston RC, Saltzman CL, Marsh JL, Buckwalter JA. Posttraumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. Journal of orthopaedic trauma. 2006;20(10):739–44. doi: 10.1097/01.bot.0000246468.80635.ef. [DOI] [PubMed] [Google Scholar]
- 2.Guilak F, Fermor B, Keefe FJ, Kraus VB, Olson SA, Pisetsky DS, et al. The role of biomechanics and inflammation in cartilage injury and repair. Clin Orthop Relat Res. 2004;(423):17–26. doi: 10.1097/01.blo.0000131233.83640.91. [DOI] [PubMed] [Google Scholar]
- 3.Elsaid KA, Fleming BC, Oksendahl HL, Machan JT, Fadale PD, Hulstyn MJ, et al. Decreased lubricin concentrations and markers of joint inflammation in the synovial fluid of patients with anterior cruciate ligament injury. Arthritis and rheumatism. 2008;58(6):1707–15. doi: 10.1002/art.23495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scanzello CR, McKeon B, Swaim BH, DiCarlo E, Asomugha EU, Kanda V, et al. Synovial inflammation in patients undergoing arthroscopic meniscectomy: molecular characterization and relationship to symptoms. Arthritis and rheumatism. 2011;63(2):391–400. doi: 10.1002/art.30137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brophy RH, Rai MF, Zhang Z, Torgomyan A, Sandell LJ. Molecular analysis of age and sex-related gene expression in meniscal tears with and without a concomitant anterior cruciate ligament tear. The Journal of bone and joint surgery American volume. 2012;94(5):385–93. doi: 10.2106/JBJS.K.00919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furman BD, Kimmerling KA, Zura RD, Reilly RM, Zlowodzki MP, Huebner JL, et al. Articular Ankle Fracture Results in Increased Synovitis, Synovial Macrophage Infiltration, and Synovial Fluid Inflammatory Cytokines and Chemokines. Arthritis Rheumatol. 2015 doi: 10.1002/art.39064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewis JS, Jr, Furman BD, Zeitler E, Huebner JL, Kraus VB, Guilak F, et al. Genetic and cellular evidence of decreased inflammation associated with reduced incidence of posttraumatic arthritis in MRL/MpJ mice. Arthritis and rheumatism. 2013;65(3):660–70. doi: 10.1002/art.37796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei L, Fleming BC, Sun X, Teeple E, Wu W, Jay GD, et al. Comparison of differential biomarkers of osteoarthritis with and without posttraumatic injury in the Hartley guinea pig model. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2010;28(7):900–6. doi: 10.1002/jor.21093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amin AR, Di Cesare PE, Vyas P, Attur M, Tzeng E, Billiar TR, et al. The expression and regulation of nitric oxide synthase in human osteoarthritis-affected chondrocytes: evidence for up-regulated neuronal nitric oxide synthase. The Journal of experimental medicine. 1995;182(6):2097–102. doi: 10.1084/jem.182.6.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Attur MG, Patel IR, Patel RN, Abramson SB, Amin AR. Autocrine production of IL-1 beta by human osteoarthritis-affected cartilage and differential regulation of endogenous nitric oxide, IL-6, prostaglandin E2, and IL-8. Proceedings of the Association of American Physicians. 1998;110(1):65–72. [PubMed] [Google Scholar]
- 11.Kobayashi M, Squires GR, Mousa A, Tanzer M, Zukor DJ, Antoniou J, et al. Role of interleukin-1 and tumor necrosis factor alpha in matrix degradation of human osteoarthritic cartilage. Arthritis and rheumatism. 2005;52(1):128–35. doi: 10.1002/art.20776. [DOI] [PubMed] [Google Scholar]
- 12.Catterall JB, Stabler TV, Flannery CR, Kraus VB. Changes in serum and synovial fluid biomarkers after acute injury ( NCT00332254) Arthritis research & therapy. 2010;12(6):R229. doi: 10.1186/ar3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindhorst E, Vail TP, Guilak F, Wang H, Setton LA, Vilim V, et al. Longitudinal characterization of synovial fluid biomarkers in the canine meniscectomy model of osteoarthritis. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2000;18(2):269–80. doi: 10.1002/jor.1100180216. [DOI] [PubMed] [Google Scholar]
- 14.Hazell PK, Dent C, Fairclough JA, Bayliss MT, Hardingham TE. Changes in glycosaminoglycan epitope levels in knee joint fluid following injury. Arthritis and rheumatism. 1995;38(7):953–9. doi: 10.1002/art.1780380711. [DOI] [PubMed] [Google Scholar]
- 15.Lohmander LS, Dahlberg L, Ryd L, Heinegard D. Increased levels of proteoglycan fragments in knee joint fluid after injury. Arthritis and rheumatism. 1989;32(11):1434–42. doi: 10.1002/anr.1780321113. [DOI] [PubMed] [Google Scholar]
- 16.Sui Y, Lee JH, DiMicco MA, Vanderploeg EJ, Blake SM, Hung HH, et al. Mechanical injury potentiates proteoglycan catabolism induced by interleukin-6 with soluble interleukin-6 receptor and tumor necrosis factor alpha in immature bovine and adult human articular cartilage. Arthritis and rheumatism. 2009;60(10):2985–96. doi: 10.1002/art.24857. [DOI] [PubMed] [Google Scholar]
- 17.Lee JH, Fitzgerald JB, Dimicco MA, Grodzinsky AJ. Mechanical injury of cartilage explants causes specific time-dependent changes in chondrocyte gene expression. Arthritis and rheumatism. 2005;52(8):2386–95. doi: 10.1002/art.21215. [DOI] [PubMed] [Google Scholar]
- 18.Furman BD, Mangiapani DS, Zeitler E, Bailey KN, Horne PH, Huebner JL, et al. Targeting pro-inflammatory cytokines following joint injury: acute intra-articular inhibition of interleukin-1 following knee injury prevents post-traumatic arthritis. Arthritis research & therapy. 2014;16(3):R134. doi: 10.1186/ar4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Proost P, Vynckier AK, Mahieu F, Put W, Grillet B, Struyf S, et al. Microbial Toll-like receptor ligands differentially regulate CXCL10/IP-10 expression in fibroblasts and mononuclear leukocytes in synergy with IFN-gamma and provide a mechanism for enhanced synovial chemokine levels in septic arthritis. European journal of immunology. 2003;33(11):3146–53. doi: 10.1002/eji.200324136. [DOI] [PubMed] [Google Scholar]
- 20.Ruschpler P, Lorenz P, Eichler W, Koczan D, Hanel C, Scholz R, et al. High CXCR3 expression in synovial mast cells associated with CXCL9 and CXCL10 expression in inflammatory synovial tissues of patients with rheumatoid arthritis. Arthritis research & therapy. 2003;5(5):R241–52. doi: 10.1186/ar783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Broeren MG, de Vries M, Bennink MB, Arntz OJ, van Lent PL, van der Kraan PM, et al. Suppression of the inflammatory response by disease-inducible interleukin-10 gene therapy in a three-dimensional micromass model of the human synovial membrane. Arthritis research & therapy. 2016;18:186. doi: 10.1186/s13075-016-1083-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanaoka R, Kasama T, Muramatsu M, Yajima N, Shiozawa F, Miwa Y, et al. A novel mechanism for the regulation of IFN-gamma inducible protein-10 expression in rheumatoid arthritis. Arthritis research & therapy. 2003;5(2):R74–81. doi: 10.1186/ar616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Jager W, Hoppenreijs EP, Wulffraat NM, Wedderburn LR, Kuis W, Prakken BJ. Blood and synovial fluid cytokine signatures in patients with juvenile idiopathic arthritis: a cross-sectional study. Annals of the rheumatic diseases. 2007;66(5):589–98. doi: 10.1136/ard.2006.061853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuan WP, Tam LS, Wong CK, Ko FW, Li T, Zhu T, et al. CXCL 9 and CXCL 10 as Sensitive markers of disease activity in patients with rheumatoid arthritis. The Journal of rheumatology. 2010;37(2):257–64. doi: 10.3899/jrheum.090769. [DOI] [PubMed] [Google Scholar]
- 25.Ichikawa T, Kageyama Y, Kobayashi H, Kato N, Tsujimura K, Koide Y. Etanercept treatment reduces the serum levels of interleukin-15 and interferon-gamma inducible protein-10 in patients with rheumatoid arthritis. Rheumatology international. 2010;30(6):725–30. doi: 10.1007/s00296-009-1356-y. [DOI] [PubMed] [Google Scholar]
- 26.Wong CK, Chen da P, Tam LS, Li EK, Yin YB, Lam CW. Effects of inflammatory cytokine IL-27 on the activation of fibroblast-like synoviocytes in rheumatoid arthritis. Arthritis research & therapy. 2010;12(4):R129. doi: 10.1186/ar3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martini G, Zulian F, Calabrese F, Bortoli M, Facco M, Cabrelle A, et al. CXCR3/CXCL10 expression in the synovium of children with juvenile idiopathic arthritis. Arthritis research & therapy. 2005;7(2):R241–9. doi: 10.1186/ar1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee EY, Lee ZH, Song YW. The interaction between CXCL10 and cytokines in chronic inflammatory arthritis. Autoimmunity reviews. 2013;12(5):554–7. doi: 10.1016/j.autrev.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 29.Borzi RM, Mazzetti I, Cattini L, Uguccioni M, Baggiolini M, Facchini A. Human chondrocytes express functional chemokine receptors and release matrix-degrading enzymes in response to C-X-C and C-C chemokines. Arthritis and rheumatism. 2000;43(8):1734–41. doi: 10.1002/1529-0131(200008)43:8<1734::AID-ANR9>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 30.De Ceuninck F, Dassencourt L, Anract P. The inflammatory side of human chondrocytes unveiled by antibody microarrays. Biochem Biophys Res Commun. 2004;323(3):960–9. doi: 10.1016/j.bbrc.2004.08.184. [DOI] [PubMed] [Google Scholar]
- 31.Collins KH, Reimer RA, Seerattan RA, Leonard TR, Herzog W. Using diet-induced obesity to understand a metabolic subtype of osteoarthritis in rats. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2015;23(6):957–65. doi: 10.1016/j.joca.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 32.Furman BD, Strand J, Hembree WC, Ward BD, Guilak F, Olson SA. Joint degeneration following closed intraarticular fracture in the mouse knee: a model of posttraumatic arthritis. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2007;25(5):578–92. doi: 10.1002/jor.20331. [DOI] [PubMed] [Google Scholar]
- 33.Ward BD, Furman BD, Huebner JL, Kraus VB, Guilak F, Olson SA. Absence of posttraumatic arthritis following intraarticular fracture in the MRL/MpJ mouse. Arthritis and rheumatism. 2008;58(3):744–53. doi: 10.1002/art.23288. [DOI] [PubMed] [Google Scholar]
- 34.Van Meurs JB, Van Lent PL, Joosten LA, Van der Kraan PM, Van den Berg WB. Quantification of mRNA levels in joint capsule and articular cartilage of the murine knee joint by RT-PCR: kinetics of stromelysin and IL-1 mRNA levels during arthritis. Rheumatology international. 1997;16(5):197–205. doi: 10.1007/BF01330296. [DOI] [PubMed] [Google Scholar]
- 35.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25(4):402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 36.Zwerina J, Redlich K, Polzer K, Joosten L, Kronke G, Distler J, et al. TNF-induced structural joint damage is mediated by IL-1. PNAS. 2007;104(28):11742–7. doi: 10.1073/pnas.0610812104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vangsness CT, Jr, Burke WS, Narvy SJ, MacPhee RD, Fedenko AN. Human knee synovial fluid cytokines correlated with grade of knee osteoarthritis--a pilot study. Bulletin of the NYU hospital for joint diseases. 2011;69(2):122–7. [PubMed] [Google Scholar]
- 38.McNulty AL, Rothfusz NE, Leddy HA, Guilak F. Synovial fluid concentrations and relative potency of interleukin-1 alpha and beta in cartilage and meniscus degradation. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2013;31(7):1039–45. doi: 10.1002/jor.22334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilusz RE, Weinberg JB, Guilak F, McNulty AL. Inhibition of integrative repair of the meniscus following acute exposure to interleukin-1 in vitro. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2008;26(4):504–12. doi: 10.1002/jor.20538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Backus JD, Furman BD, Swimmer T, Kent CL, McNulty AL, Defrate LE, et al. Cartilage viability and catabolism in the intact porcine knee following transarticular impact loading with and without articular fracture. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2011;29(4):501–10. doi: 10.1002/jor.21270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochimica et biophysica acta. 1986;883(2):173–7. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 42.Granger DL, Anstey NM, Miller WC, Weinberg JB. Measuring nitric oxide production in human clinical studies. Methods in enzymology. 1999;301:49–61. doi: 10.1016/s0076-6879(99)01068-x. [DOI] [PubMed] [Google Scholar]
- 43.Clutter SD, Wilson DC, Marinov AD, Hirsch R. Follistatin-like protein 1 promotes arthritis by up-regulating IFN-gamma. J Immunol. 2009;182(1):234–9. doi: 10.4049/jimmunol.182.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kwak HB, Ha H, Kim HN, Lee JH, Kim HS, Lee S, et al. Reciprocal cross-talk between RANKL and interferon-gamma-inducible protein 10 is responsible for bone-erosive experimental arthritis. Arthritis and rheumatism. 2008;58(5):1332–42. doi: 10.1002/art.23372. [DOI] [PubMed] [Google Scholar]
- 45.Burke SJ, Goff MR, Lu D, Proud D, Karlstad MD, Collier JJ. Synergistic expression of the CXCL10 gene in response to IL-1beta and IFN-gamma involves NF-kappaB, phosphorylation of STAT1 at Tyr701, and acetylation of histones H3 and H4. J Immunol. 2013;191(1):323–36. doi: 10.4049/jimmunol.1300344. [DOI] [PubMed] [Google Scholar]
- 46.Benigni G, Dimitrova P, Antonangeli F, Sanseviero E, Milanova V, Blom A, et al. CXCR3/CXCL10 Axis Regulates Neutrophil-NK Cell Cross-Talk Determining the Severity of Experimental Osteoarthritis. J Immunol. 2017;198(5):2115–24. doi: 10.4049/jimmunol.1601359. [DOI] [PubMed] [Google Scholar]
- 47.Sandell LJ, Xing X, Franz C, Davies S, Chang LW, Patra D. Exuberant expression of chemokine genes by adult human articular chondrocytes in response to IL-1beta. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2008;16(12):1560–71. doi: 10.1016/j.joca.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Amin AR, Islam AB. Genomic analysis and differential expression of HMG and S100A family in human arthritis: upregulated expression of chemokines, IL-8 and nitric oxide by HMGB1. DNA and cell biology. 2014;33(8):550–65. doi: 10.1089/dna.2013.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim MS, Song HJ, Lee SH, Lee CK. Comparative study of various growth factors and cytokines on type I collagen and hyaluronan production in human dermal fibroblasts. Journal of cosmetic dermatology. 2014;13(1):44–51. doi: 10.1111/jocd.12073. [DOI] [PubMed] [Google Scholar]
- 50.Endres M, Andreas K, Kalwitz G, Freymann U, Neumann K, Ringe J, et al. Chemokine profile of synovial fluid from normal, osteoarthritis and rheumatoid arthritis patients: CCL25, CXCL10 and XCL1 recruit human subchondral mesenchymal progenitor cells. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2010;18(11):1458–66. doi: 10.1016/j.joca.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 51.Grassi F, Piacentini A, Cristino S, Toneguzzi S, Cavallo C, Facchini A, et al. Human osteoclasts express different CXC chemokines depending on cell culture substrate: molecular and immunocytochemical evidence of high levels of CXCL10 and CXCL12. Histochemistry and cell biology. 2003;120(5):391–400. doi: 10.1007/s00418-003-0587-3. [DOI] [PubMed] [Google Scholar]
- 52.Hegewald AA, Neumann K, Kalwitz G, Freymann U, Endres M, Schmieder K, et al. The chemokines CXCL10 and XCL1 recruit human annulus fibrosus cells. Spine. 2012;37(2):101–7. doi: 10.1097/BRS.0b013e318210ed55. [DOI] [PubMed] [Google Scholar]
- 53.Lewis JS, Hembree WC, Furman BD, Tippets L, Cattel D, Huebner JL, et al. Acute joint pathology and synovial inflammation is associated with increased intra-articular fracture severity in the mouse knee. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2011;19(7):864–73. doi: 10.1016/j.joca.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]