Abstract
Background
This retrospective study was performed to determine whether postoperative intravenous ferric carboxymaltose reduces transfusion amounts without influencing clinical outcomes in patients that have undergone hip surgery.
Methods
Between May 2014 and April 2016, the authors adopted a new perioperative blood management protocol involving the administration of intravenous ferric carboxymaltose after hip surgeries. One-to-one matching between the 150 patients treated during this period with 150 patients treated before initiation of the new protocol was performed by propensity scoring for age, sex, diagnosis, and type of hip surgery. Hematologic results and clinical outcomes in these two groups were compared.
Results
Average amounts of perioperative blood loss were not different in the two groups. Ninety-two patients (61%) were transfused in the control group and 70 patients (47%) were transfused in the intravenous ferric carboxymaltose group. The average number of transfused blood units was significantly lower in the intravenous ferric carboxymaltose group (1.7 ± 2.7 units vs. 1.0 ± 1.2 units, p = 0.002). At 6 weeks after surgery, the average hemoglobin concentration recovered to baseline in both groups, but the amount of recovered hemoglobin concentration at 6 weeks was significantly greater in the intravenous ferric carboxymaltose group than in the control group. Clinical outcomes including incidences of postsurgical complications were similar between the two groups.
Conclusions
This study suggests that postoperative intravenous ferric carboxymaltose injection is associated with reduced transfusion amounts and that intravenous ferric carboxymaltose does not influence clinical outcomes after hip surgery.
Keywords: Anemia, Postoperative hemorrhage, Ferric carboxymaltose, Blood transfusion
Orthopedic hip surgery is associated with considerable blood loss and high rates of perioperative transfusion.1) A recent meta-analysis of 19 studies showed that 24% of patients undergoing total hip replacement or total knee replacement and 44% of those with hip fracture had preoperative anemia. It has also been reported that more than 50% of such patients became anemic after surgery due to intraoperative blood loss.2,3) Allogenic red blood cell transfusion is frequently used to treat acute postoperative anemia, and 20%–50% of these patients receive at least one unit of red blood cells.3,4)
However, transfusions prolong the length of hospital stay and increase in-patient costs, the risks of hematogenic infections, and mortality after orthopedic procedures.5,6,7) Furthermore, these complications may involve morbidity or mortality, which makes the development of blood-saving strategies a necessity.8,9) In this respect, the three-pillar concept of patient blood management was developed to reduce the risk of transfusion.10,11)
In addition to perioperative blood loss, iron homeostasis can result in postoperative anemia through a condition called functional iron deficiency, which is caused by the sequestration of iron in macrophages due to the deactivation of ferroportin by inflammatory cytokines.12,13) Accordingly, iron replacement strategies for correcting preoperative anemia have been widely studied.14)
The routine use of oral ferrous sulphate after total hip and knee arthroplasty is not recommended because of its side effects.14) On the other hand, parenteral ferric carboxymaltose is a safe and effective treatment option with the advantages of lower risks of gastrointestinal side effects.15,16,17,18) Although intravenous ferric carboxymaltose (IV-FCM) has been demonstrated to increase hemoglobin levels in preoperative orthopedic surgical patients, no clear role has been established for postoperative iron supplementation.14,19,20)
Hence, we postulated that postoperative IV-FCM might reduce the amount of transfusion after orthopedic hip surgery without adverse influences on clinical outcomes. This study was undertaken to determine whether postoperative IV-FCM would reduce the amount of transfusion without influencing clinical outcomes in patients that have undergone hip surgery.
METHODS
All procedures involving human participants were performed in accordance with the ethical standards of Institutional Review Board of Seoul National University Hospital (IRB No. 1612-008-810) and with the Helsinki Declaration (1964) and its later amendments. Written informed consent was obtained from all patients before surgical treatment and patients also consented to the use of their data in future publications.
We adopted a new perioperative blood management protocol for patients that undergo orthopedic hip surgery in May 2014 that involves postoperative screening by blood testing immediately after surgery. Patients meeting the following criteria were administered 1 g of ferric carboxymaltose intravenously (2 vials of Ferinject; Vifor France SA, Puteaux, France). The criteria used were (1) an immediate postoperative hemoglobin concentration of < 10 g/dL or (2) a hemoglobin concentration fall of > 3 g/dL versus the preoperative value. According to this protocol, 150 of 846 patients were intravenously injected with ferric carboxymaltose postoperatively between May 2014 and April 2016, and these 150 patients were enrolled in the present study. In addition to this IV-FCM protocol, we adopted a strict transfusion protocol, which triggered transfusion when hemoglobin concentrations fell below 8 g/dL or symptoms of acute anemia (e.g., dizziness, chest pain, tachycardia, and persistent hypotension) occurred.
A biostatistician performed one-to-one matching with patients that underwent hip surgery before the introduction of the new protocol using the propensity scoring method. Variables included for the propensity score matching were sex, age, initial diagnosis, and type of hip surgery. After propensity score matching, all variables were successfully matched (Table 1). Finally, the data of 300 patients (IV-FCM group, n = 150; control group, n = 150) were included in the analysis. In both groups, there were 43 men and 107 women with ages ranging from 20 to 91 years. The most common diagnosis in both groups was osteonecrosis of the femoral head (39%), followed by femur neck fracture (15%), degenerative arthritis (13%), intertrochanteric fracture (11%) and dysplastic hip (7%) (Table 1).
Table 1. Baseline Characteristics of the Study Subjects.
| Variable | Control group (n = 150) | IV-FCM group (n = 150) |
|---|---|---|
| Sex (male:female) | 43:107 | 43:107 |
| Age (yr) | 63.6 ± 15.7 | 63.4 ± 15.9 |
| Diagnosis (no. of hips) | ||
| Osteonecrosis of femoral head | 58 (39) | |
| Femur neck fracture | 22 (15) | |
| Degenerative arthritis | 20 (13) | |
| Intertrochanteric fracture | 17 (11) | |
| Dysplasia | 11 (7) | |
| Miscellaneous (bearing surface wear, periprosthetic joint infection) | 22 (15) | |
| Surgery | ||
| THA | 95 (47) | |
| Bipolar hemiarthroplasty | 21 (18) | |
| Intramedullary nailing | 15 (16) | |
| Revisional THA | 12 (14) | |
| Open reduction and internal fixation | 7 (5) | |
Values are presented as mean ± standard deviation or number (%).
IV-FCM: intravenous ferric carboxymaltose, THA: total hip arthroplasty.
Intermittent pneumatic compression devices and low-dose aspirin (100 mg qd) were used for venous thromboembolism prophylaxis and negative pressure blood drainage was applied for 2–3 days in all patients after surgery.
Clinical data were collected retrospectively from medical records. In particular, medical and drug histories were checked for items that might affect bleeding or thromboembolic tendency, such as histories of cerebral infarction, coronary artery disease, or valvular heart disease and the use of antiplatelet or anticoagulant medication. Perioperative blood losses were evaluated, and we checked whether transfusion was performed postoperatively. Perioperative blood loss was defined as the sum of estimated blood loss during surgery and postoperative drainage amount through the negative pressure drainage system. Postoperative transfusion amounts were also evaluated. Blood hemoglobin concentrations were measured at 1 and 6 weeks after surgery and compared to values obtained preoperatively and immediately after surgery. Hospital stay (defined as the number of days spent in hospital from the day of surgery until discharge) and postsurgical complications were noted, as were periprosthetic joint infection, urinary tract infections, respiratory infections, and venous thromboembolism during admission. Infection was defined as a positive bacterial culture and the need for antibiotics. Adverse effects of IV-FCM associated drug reactions, such as hypersensitivity and infusion site reactions, were noted by nurses administering IV-FCM. Patients were monitored during admission and any cardiovascular event, infection, or other adverse drug reaction requiring intervention was evaluated.
The primary study variables were perioperative blood loss, number of blood units transfused, and changes in hemoglobin concentrations at 1 week and 6 weeks after surgery. The secondary variables were duration of hospital stay and the prevalence of IV-FCM-associated postsurgical complications and adverse drug reactions. Statistical analysis was performed using the Student t-test in SPSS ver. 23.0 (IBM Corp., Armonk, NY, USA). Statistical significance was accepted for p-values of < 0.05.
RESULTS
Average amounts of perioperative blood loss in the two groups were not significantly different (p = 0.143). Ninety-two patients (61%) were transfused in the control group and 70 patients (47%) were transfused in the IV-FCM group. Furthermore, the average number of transfused blood units was significantly lower in the IV-FCM group (1.7 ± 2.7 vs. 1.0 ± 1.2 units, p = 0.002) (Table 2).
Table 2. Postoperative Clinical Outcomes in the Two Study Groups.
| Variable | Control group | IV-FCM group | p-value |
|---|---|---|---|
| Perioperative blood loss (mL) | 815 ± 666 | 915 ± 508 | 0.143 |
| No. of patients transfused | 92 (61) | 70 (47) | NA |
| No. of units transfused (pack) | 1.7 ± 2.7 | 1.0 ± 1.2 | 0.002* |
| Length of hospital stay (day) | 11.8 ± 10.3 | 7.6 ± 3.3 | 0* |
| Follow-up period (mo) | 33.3 ± 23.4 | 20.7 ± 12.0 | 0* |
| Postsurgical complication | 6 (3) | 7 (4) | NA |
| Periprosthetic joint infection | 1 | 1 | NA |
| Urinary tract and upper respiratory infections | 5 | 4 | NA |
| Venous thromboembolism | 0 | 2 | NA |
Values are presented as mean ± standard deviation or number (%).
IV-FCM: intravenous ferric carboxymaltose, NA: not significant.
*p-value < 0.05.
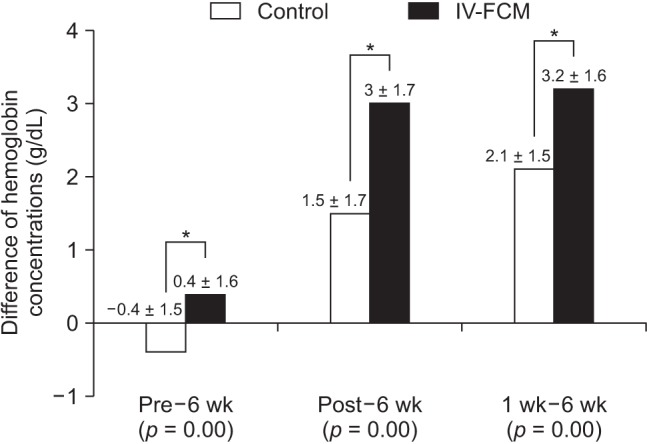
Preoperative hemoglobin concentrations were similar in the two groups. In both groups, average postoperative hemoglobin concentrations were significantly lower than preoperative concentrations. Moreover, the average hemoglobin concentration was significantly lower in the IV-FCM group immediately after surgery and 1 week after surgery than the average concentration before surgery (baseline). At 6 weeks after surgery, average hemoglobin concentrations recovered to baseline in both groups. However, at 6 weeks after surgery, the average hemoglobin concentration was significantly higher in the IV-FCM group than in the control group (12.5 vs. 12.1 g/dL) (Fig. 1). When average hemoglobin concentrations at 6 weeks after surgery were compared with those measured preoperatively, immediate after surgery, and at 1 week after surgery, the amount of recovered hemoglobin concentration at 6 weeks was significantly greater in the IV-FCM group than in the control group at all intervals (Fig. 2).
Fig. 1. Graph showing changes in perioperative hemoglobin concentrations in the control and intravenous ferric carboxymaltose (IV-FCM) groups. Values are presented as mean ± standard deviation. Pre: preoperative, Post: immediately after surgery, 1 wk: 1 week after surgery, 6 wk: 6 weeks after surgery. *p < 0.05.
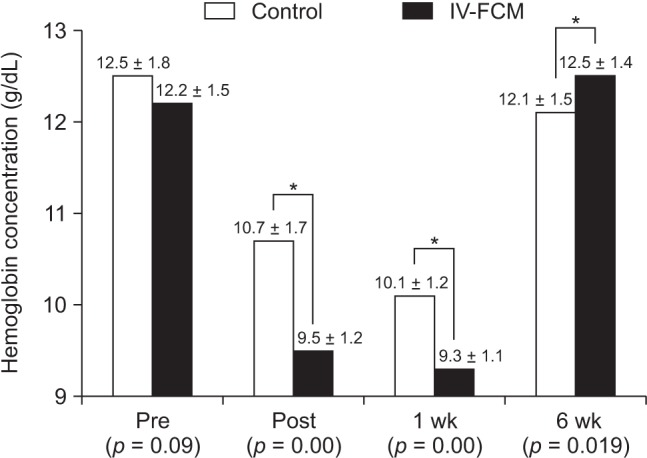
Fig. 2. Graph showing differences in hemoglobin concentrations at 6 weeks after surgery compared to those at the preoperative assessment (Pre–6 wk), immediately after surgery (Post–6 wk), and at 1 week after surgery (1 wk–6 wk). The amount of recovered hemoglobin concentration at 6 weeks was significantly greater in the IV-FCM group than in the control group at all intervals. *p < 0.05.
The average hospital stay was significantly shorter in the IV-FCM group than in the control group (7.6 days vs. 11.8 days). However, the prevalence of postsurgical complications, including periprosthetic joint infections, urinary tract and upper respiratory infections, and venous thromboembolism were similar in the two groups (Table 2). No adverse drug reaction previously reported to be a possible side effect of FCM, such as hypersensitivity, cardiovascular event or infusion site reaction, was observed.21,22,23)
DISCUSSION
This study was undertaken to determine whether IV-FCM administered immediately after surgery could reduce transfusion frequency and amounts and significantly increase hemoglobin levels after hip surgery. The IV-FCM group was found to have transfusions less frequently and to have been transfused with smaller amounts than the control group (61% vs. 47% and 1.7 ± 2.7 vs. 1.0 ± 1.2 units, respectively). Furthermore, hemoglobin concentration recovery was greater in the IV-FCM group at 6 weeks postoperatively. Finally, IV-FCM was not found to affect surgery-associated complications or adverse drug reactions.
Blood loss is not the only factor that can influence iron status. Inflammatory cytokines, such as interleukins and tumor necrosis factor-alpha (TNF-α), that are released after hip surgery affect iron homeostasis, as these cytokines lead to iron uptake by activated macrophages. Accordingly, surgery per se may cause changes in iron metabolism, that is, a decrease in serum levels of iron and transferrin and an increase in serum ferritin concentration.12) In this respect, IV-FCM may confer benefits by directly supplementing hemoglobin levels and inhibiting the formation of TNF-α.13)
The average amount of perioperative blood loss was not different in our two study groups (p = 0.143), but IV-FCM reduced transfusion frequency and amounts. This result may have been due to the adoption of a strict transfusion protocol. In particular, although IV-FCM is not a substitute for blood products, IV-FCM was administered instead of routine blood transfusion, and thus, indiscriminate transfusions were probably reduced.
Average hemoglobin concentrations were significantly lower in the IV-FCM group immediately after and 1 week after surgery, which was probably associated with the indications for IV-FCM used. Nevertheless, the amount of recovered hemoglobin concentration at 6 weeks was significantly greater in the IV-FCM group than in the control group at every time interval.
Furthermore, the average hospital stay was significantly shorter in the IV-FCM group than in the control group (7.6 vs. 11.8 days), and it is not reasonable to ascribe this to IV-FCM. During recent years, we have promoted early ambulation and rehabilitation programs, and thus, have shortened hospital stays after hip surgeries. Accordingly, we believe this result was not due to IV-FCM but due to postoperative care protocol changes. Nevertheless, the prevalence of postsurgical complications, such as periprosthetic joint infections, urinary tract and upper respiratory infections and venous thromboembolism were not different in the two groups.
IV iron supplementation raises several concerns. The first is life-threatening hypersensitivity;23) others include cardiovascular events and infections.8,21,22) In the present study, we encountered no adverse drug reaction, such as hypersensitivity, a cardiovascular event, or an infusion site reaction. These results concur with the previous findings of a systematic review of 10,390 patients that participated in 103 trials, in which it was concluded intravenous iron supplementation is not associated with increased risks of adverse drug reactions or infections.24)
Few studies have investigated the effects of postoperative intravenous iron supplementation on blood transfusion requirements or on the recoveries of hemoglobin concentrations.25,26,27) Nevertheless, our findings concur with these previous findings regarding significant reduction in blood transfusion frequency and more rapid recovery of postoperative hemoglobin concentrations after IV-FCM. The results of the present study suggest that postoperative IV-FCM administration confers benefits after diverse surgical procedures including general orthopedic hip surgery procedures and arthroplasty.
However, our study has several limitations. First, we matched patients using four criteria, that is sex, age, diagnosis, and type of hip surgery, and it is believed that other criteria can also affect clinical outcomes, such as comorbidities and medication history. We checked whether patients had a history of cerebral infarction, coronary artery disease, or valvular heart disease and whether they use antiplatelets, including aspirin, or anticoagulants, such as clopidogrel or warfarin. Nine of the 150 patients in the IV-FCM group and 19 of 150 patients in the control group (6% vs 12.7%) were found to have such a history. However, we believe comorbidities and medications were not powerful confounding factors because the average perioperative blood loss was not significantly different in the two groups. Second, IV-FCM effectively reduced transfusion and enhanced postoperative hemoglobin recovery rates. However, IV-FCM does not directly increase hemoglobin concentration like transfusion, but indirectly increases its concentration by promoting hemoglobin synthesis. Iron supplementation could improve hemoglobin synthesis and facilitate the correction of anemia, and thus, variables like serum iron, transferrin, and ferritin levels should have been considered when evaluating the iron status. Third, we evaluated the effects of FCM which is more expensive than other forms of iron supplementation like ferric hydroxide sucrose. Although ferric hydroxide sucrose (Venoferrum) is cheaper than Ferinject, it is difficult to fulfill insurance guidelines on the use of ferric hydroxide sucrose. Finally, there is a concern of administration of IV-FCM in geriatric patients, taking into account their poor hematopoietic functions compared to younger patients. However, our study concurs with the previous report showing that administration of FCM in geriatric patients is an effective treatment option for the treatment of functional iron deficiency.28)
Our retrospective results show that postoperative IV-FCM reduces transfusion amounts without influencing clinical outcomes in patients that had undergone hip surgery. A blood management strategy based on postoperative IV-FCM may be beneficial for such patients. Randomized trials are required to determine whether the described blood management program is worthwhile.
ACKNOWLEDGEMENTS
This study was supported by a grant from the Seoul National University Hospital Research Fund (No. 06-03-0630), Seoul, Korea.
Footnotes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Liodakis E, Antoniou J, Zukor DJ, Huk OL, Epure LM, Bergeron SG. Major complications and transfusion rates after hemiarthroplasty and total hip arthroplasty for femoral neck fractures. J Arthroplasty. 2016;31(9):2008–2012. doi: 10.1016/j.arth.2016.02.019. [DOI] [PubMed] [Google Scholar]
- 2.Spahn DR. Anemia and patient blood management in hip and knee surgery: a systematic review of the literature. Anesthesiology. 2010;113(2):482–495. doi: 10.1097/ALN.0b013e3181e08e97. [DOI] [PubMed] [Google Scholar]
- 3.Sehat KR, Evans RL, Newman JH. Hidden blood loss following hip and knee arthroplasty: correct management of blood loss should take hidden loss into account. J Bone Joint Surg Br. 2004;86(4):561–565. [PubMed] [Google Scholar]
- 4.Rosencher N, Kerkkamp HE, Macheras G, et al. Orthopedic surgery transfusion hemoglobin European overview (OSTHEO) study: blood management in elective knee and hip arthroplasty in Europe. Transfusion. 2003;43(4):459–469. doi: 10.1046/j.1537-2995.2003.00348.x. [DOI] [PubMed] [Google Scholar]
- 5.Ponnusamy KE, Kim TJ, Khanuja HS. Perioperative blood transfusions in orthopaedic surgery. J Bone Joint Surg Am. 2014;96(21):1836–1844. doi: 10.2106/JBJS.N.00128. [DOI] [PubMed] [Google Scholar]
- 6.Browne JA, Adib F, Brown TE, Novicoff WM. Transfusion rates are increasing following total hip arthroplasty: risk factors and outcomes. J Arthroplasty. 2013;28(8 Suppl):34–37. doi: 10.1016/j.arth.2013.03.035. [DOI] [PubMed] [Google Scholar]
- 7.Saleh A, Small T, Chandran Pillai AL, Schiltz NK, Klika AK, Barsoum WK. Allogenic blood transfusion following total hip arthroplasty: results from the nationwide inpatient sample, 2000 to 2009. J Bone Joint Surg Am. 2014;96(18):e155. doi: 10.2106/JBJS.M.00825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hill GE, Frawley WH, Griffith KE, Forestner JE, Minei JP. Allogeneic blood transfusion increases the risk of postoperative bacterial infection: a meta-analysis. J Trauma. 2003;54(5):908–914. doi: 10.1097/01.TA.0000022460.21283.53. [DOI] [PubMed] [Google Scholar]
- 9.Carson JL, Duff A, Berlin JA, et al. Perioperative blood transfusion and postoperative mortality. JAMA. 1998;279(3):199–205. doi: 10.1001/jama.279.3.199. [DOI] [PubMed] [Google Scholar]
- 10.Kotze A, Carter LA, Scally AJ. Effect of a patient blood management programme on preoperative anaemia, transfusion rate, and outcome after primary hip or knee arthroplasty: a quality improvement cycle. Br J Anaesth. 2012;108(6):943–952. doi: 10.1093/bja/aes135. [DOI] [PubMed] [Google Scholar]
- 11.Shander A, Van Aken H, Colomina MJ, et al. Patient blood management in Europe. Br J Anaesth. 2012;109(1):55–68. doi: 10.1093/bja/aes139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Iperen CE, Kraaijenhagen RJ, Biesma DH, Beguin Y, Marx JJ, van de Wiel A. Iron metabolism and erythropoiesis after surgery. Br J Surg. 1998;85(1):41–45. doi: 10.1046/j.1365-2168.1998.00571.x. [DOI] [PubMed] [Google Scholar]
- 13.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352(10):1011–1023. doi: 10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 14.Mundy GM, Birtwistle SJ, Power RA. The effect of iron supplementation on the level of haemoglobin after lower limb arthroplasty. J Bone Joint Surg Br. 2005;87(2):213–217. doi: 10.1302/0301-620x.87b2.15122. [DOI] [PubMed] [Google Scholar]
- 15.Breymann C, Gliga F, Bejenariu C, Strizhova N. Comparative efficacy and safety of intravenous ferric carboxymaltose in the treatment of postpartum iron deficiency anemia. Int J Gynaecol Obstet. 2008;101(1):67–73. doi: 10.1016/j.ijgo.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Bisbe E, Molto L, Arroyo R, Muniesa JM, Tejero M. Randomized trial comparing ferric carboxymaltose vs oral ferrous glycine sulphate for postoperative anaemia after total knee arthroplasty. Br J Anaesth. 2014;113(3):402–409. doi: 10.1093/bja/aeu092. [DOI] [PubMed] [Google Scholar]
- 17.Suh YS, Nho JH, Choi HS, Ha YC, Park JS, Koo KH. A protocol avoiding allogeneic transfusion in joint arthroplasties. Arch Orthop Trauma Surg. 2016;136(9):1213–1226. doi: 10.1007/s00402-016-2516-7. [DOI] [PubMed] [Google Scholar]
- 18.Song JH, Park JW, Lee YK, et al. Management of blood loss in hip arthroplasty: Korean Hip Society current consensus. Hip Pelvis. 2017;29(2):81–90. doi: 10.5371/hp.2017.29.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khalafallah A, Al-Barzan AM, Chan J, et al. A prospective randomized controlled trial to assess the effect of intravenous versus oral iron therapy in the treatment of preoperative anaemia. J Blood Disord Transfus. 2012;3(4):127. [Google Scholar]
- 20.Beris P, Munoz M, Garcia-Erce JA, Thomas D, Maniatis A, Van der. Perioperative anaemia management: consensus statement on the role of intravenous iron. Br J Anaesth. 2008;100(5):599–604. doi: 10.1093/bja/aen054. [DOI] [PubMed] [Google Scholar]
- 21.Fishbane S. Review of issues relating to iron and infection. Am J Kidney Dis. 1999;34(4 Suppl 2):S47–S52. doi: 10.1053/AJKD034s00047. [DOI] [PubMed] [Google Scholar]
- 22.Zager RA, Johnson AC, Hanson SY, Wasse H. Parenteral iron formulations: a comparative toxicologic analysis and mechanisms of cell injury. Am J Kidney Dis. 2002;40(1):90–103. doi: 10.1053/ajkd.2002.33917. [DOI] [PubMed] [Google Scholar]
- 23.Walters BA, Van Wyck DB. Benchmarking iron dextran sensitivity: reactions requiring resuscitative medication in incident and prevalent patients. Nephrol Dial Transplant. 2005;20(7):1438–1442. doi: 10.1093/ndt/gfh811. [DOI] [PubMed] [Google Scholar]
- 24.Avni T, Bieber A, Grossman A, Green H, Leibovici L, Gafter-Gvili A. The safety of intravenous iron preparations: systematic review and meta-analysis. Mayo Clin Proc. 2015;90(1):12–23. doi: 10.1016/j.mayocp.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 25.Munoz M, Naveira E, Seara J, Cordero J. Effects of postoperative intravenous iron on transfusion requirements after lower limb arthroplasty. Br J Anaesth. 2012;108(3):532–534. doi: 10.1093/bja/aes012. [DOI] [PubMed] [Google Scholar]
- 26.Munoz M, Gomez-Ramirez S, Cuenca J, et al. Very-short-term perioperative intravenous iron administration and postoperative outcome in major orthopedic surgery: a pooled analysis of observational data from 2547 patients. Transfusion. 2014;54(2):289–299. doi: 10.1111/trf.12195. [DOI] [PubMed] [Google Scholar]
- 27.Khalafallah AA, Yan C, Al-Badri R, et al. Intravenous ferric carboxymaltose versus standard care in the management of postoperative anaemia: a prospective, open-label, randomised controlled trial. Lancet Haematol. 2016;3(9):e415–e425. doi: 10.1016/S2352-3026(16)30078-3. [DOI] [PubMed] [Google Scholar]
- 28.Rohrig G, Steinmetz T, Stein J, et al. Efficacy and tolerability of ferric carboxymaltose in geriatric patients with anemia: data from three non-interventional studies. MMW Fortschr Med. 2014;156(Suppl 2):48–53. [PubMed] [Google Scholar]


