Abstract
Background
To evaluate the influence of bone marrow aspirate concentrate (BMAC) on tendon-to-bone healing in a rabbit rotator cuff model and to characterize the composition of growth factors in BMAC.
Methods
In this in vivo study, 40 rabbits were allocated into five groups: control (C), repair + saline (RS), repair + platelet-rich plasma (PRP; RP), repair + BMAC (RB) and repair + PRP + BMAC (RPB). A tear model was created by supraspinatus tendon transection at the footprint. Six weeks after transection, the torn tendon was repaired along with BMAC or PRP administration. Six weeks after repair, shoulder samples were harvested for biomechanical and histological testing. Ten rabbits were used for processing PRP and BMAC, followed by analysis of blood cell composition and the levels of growth factors in vitro.
Results
The ultimate load-to-failure was significantly higher in RPB group compared to RS group (p = 0.025). BMAC-treated groups showed higher values of biomechanical properties than RS group. The histology of BMAC-treated samples showed better collagen fiber continuity and orientation than RS group. BMAC contained significantly higher levels of the several growth factors than PRP.
Conclusions
Locally administered BMAC enhanced tendon-to-bone healing and has potential for clinical applications.
Keywords: Bone marrow, Platelet-rich plasma, Rotator cuff
Rotator cuff tear commonly results in shoulder pain and functional loss, and surgical repair is often required.1) Repair of the rotator cuff typically involves direct reattachment of the torn tendon to the footprint of greater tuberosity. The tendon-to-bone insertion site is the weakest link; a large number of studies have demonstrated a high rate of incomplete healing at the tendon and bone interface.1,2) Previous studies have focused on mechanical augmentation, primarily to improve the fixation strength between tendon and bone, by approaches such as the introduction of different fixation devices, suture pattern, and by improvements in the repair technique and knot configuration; however, numerous studies have indicated that none of these techniques seemed to result in significant improvements in clinical outcomes.3,4)
Animal studies examining tendon-to-bone healing revealed through histology that the rotator cuff insertion site did not regenerate after repair.5,6) It is quite common that rotator cuff healing involves fibrovascular scar tissue formation, which is weaker and fails more easily.5) Recently, there has been growing interest in the biologic augmentation of tissue healing, such as the application of both bone marrow aspirate concentrate (BMAC) and platelet-rich plasma (PRP).7)
BMAC contains a high concentration of platelets, but more importantly, it also contains mesenchymal stem cells (MSCs) and progenitor cells, all of which contribute to tissue regeneration. Furthermore, some in vitro studies have shown that PRP enhances the proliferation of MSCs.8,9) The benefit of this combined with the MSCs-PRP mixture is the ability to obtain source cells and growth factors simultaneously, simply, and cost-effectively. Kim et al.10) obtained encouraging results following local injection of a BMAC-bone morphogenetic protein to improve Achilles tendon-to-bone healing, which was similar to the results of other studies.11,12)
We hypothesized that BMAC administered locally into the repaired site could enhance tendon-to-bone healing following rotator cuff repair in a rabbit model. We also hypothesized that PRP can stimulate the MSCs of BMAC and the PRP-BMAC mixture can improve tissue healing. The purpose of this study was to evaluate the influence of BMAC on tendon-to-bone healing in this chronic rotator cuff tear rabbit model.
METHODS
Ethical Approval
Animal care and all experiments were performed in adherence to Institutional Animal Experiment Committee guidelines and approved by Ethics Committee of Hallym University Kangnam Sacred Heart Hospital (No. IACUC-20150325012). Principles of Laboratory Animal Care were followed, as well as specific national laws where applicable.
In Vivo Study
Sample size estimation
Before the in vivo experiment, a power analysis was conducted with the primary outcome of ultimate load to biomechanical failure. The calculation was based on a previous study that evaluated rotator cuff tendon healing in rabbits.13,14) In that study, the mean difference in ultimate load to failure was 90 N and the standard deviation was 40 N. Using these estimations, an assigned power of 80% would be achieved using eight samples in each group with α = 0.05 and a 25% drop-out rate for biomechanical testing. We tested these assumptions with the Kruskal-Wallis test.
Allocation of rabbits
The rabbits were obtained from a licensed animal center. A total of 44 adult male New Zealand white rabbits, aged 4 to 5 months with a mean bodyweight of 2.9 ± 0.3 kg (range, 2.5 to 3.5 kg), were included in the study. Of the 44 rabbits, four rabbits were utilized to harvest peripheral blood and bone marrow aspirate (BMA); the remaining 40 rabbits were randomly divided into five groups according to the protocol, and each group consists of 16 shoulders from eight rabbits.
Creation of a chronic rotator cuff tear model
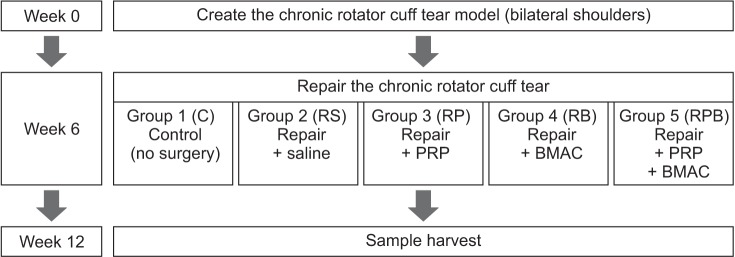
Rubino et al.15) reported that fatty infiltration could appear as early as 6 weeks after surgical detachment; Itoigawa et al.16) reported that fatty infiltration appeared in the muscle at 28 days after creating the tear. Therefore, we believed that 6 weeks, a duration determined in previous studies,14,17) would be an appropriate interval during which to expect the chronic changes and healing of rabbit rotator cuff tendon to occur after repair. The in vivo study was divided into three stages which are illustrated in Fig. 1.
Fig. 1. Flowchart of the in vivo study. PRP: platelet-rich plasma, BMAC: bone marrow aspirate concentrate.
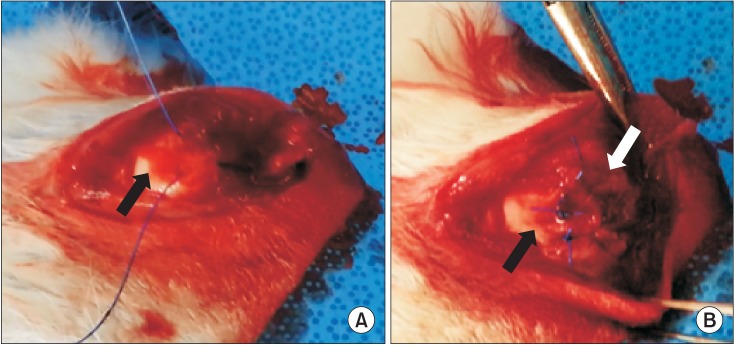
All rabbits except the control group accepted the surgery. All experimental procedures were performed in both shoulders and performed under general anesthesia by a single surgeon (KCN). Anesthesia was carried out with intramuscular injection of xylazine HCl (2 mL/kg; Fangzheng Animal Pharmaceutics, Changchun, China) combined with 0.5% lidocaine hydrochloride (Hualu Pharmaceutical, Liaocheng, China) injected into the skin incision to increase the anesthetic effect and reduce post-operative pain. The rabbits were secured on the operating table in a supine position; bilateral shoulders were shaved. Under aseptic conditions, a 3.0 cm-anterolateral skin incision was made to expose the supraspinatus tendon, which was then precisely cut from its insertion site, and wrapped with a 10 mm (length) by 8 mm (diameter) silicone Penrose drain (Yushin Corp., Bucheon, Korea) to inhibit adhesion to the surrounding soft tissue for 6 weeks (Fig. 2). According to location of the tear site, a midsubstance tear indicates strong tendon-to-bone healing whereas an insertion tear suggests relatively weak tendon-to-bone healing. This method of making chronic rotator cuff model was previously reported.13) The incision was sutured in layers. Postoperatively, the rabbits were provided free access to water, food, and activity without limb immobilization in their respective cages.
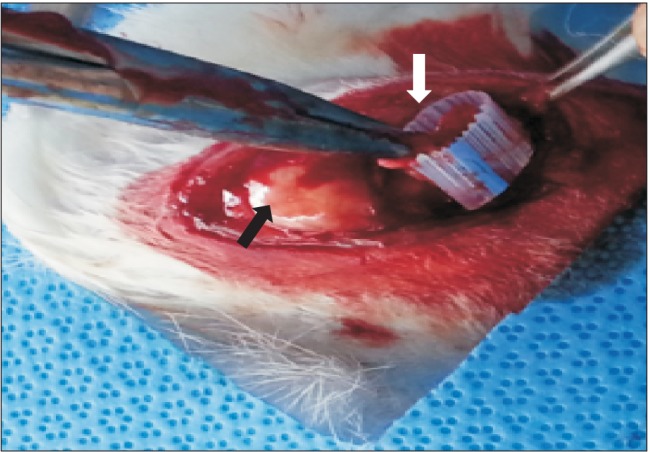
Fig. 2. Creation of a chronic rotator cuff tear model. The supraspinatus tendon is completely detached at the insertion site and wrapped with a Penrose drain to inhibit adhesion to the surrounding tissue. The black arrow indicates the greater tuberosity and the white arrow indicates the supraspinatus tendon.
PRP and BMAC preparation
Since all rabbits were from an inbred strain, they are considered syngeneic and most antigens of the erythrocytes were removed during preparation. Therefore, transplantation of blood within these rabbits is similar to autograft transplantation with a low-risk of graft rejection. According to the study design, four rabbits, under the same anesthesia conditions as above, were used to harvest bone morrow aspirate from bilateral femurs, tibias, and humeri, under sterile conditions using an 18 G spinal needle into 20 mL syringes prefilled with 3.5 mL of citrate dextrose (acid citrate dextrose [ACD]-A) to prevent clotting. Next, whole blood was harvested by cardiac puncture into 20 mL syringes containing ACD-A. Blood samples were centrifuged at 350 g for 5 minutes. After centrifugation, the PRP and BMAC were separated with aid of autologous conditioned plasma (ACP; Arthrex, Naples, FL, USA) preparation kit. The total amount of PRP and BMAC collected from the four rabbits was approximately 25 mL and 16 mL, respectively. A volume of 2 mL processed PRP and BMAC was used for the analysis of complete blood cell count. Approximately 0.5 mL aliquots of each processed PRP were prepared for the present study. The preparation of PRP and BMAC as well as the counting of platelets and leukocytes were performed by a specialist who was blinded to the study, and the activator of PRP was not used.
Repair with PRP and BMAC treatment
After 6 weeks, the torn supraspinatus tendon was repaired as follows: under the same anesthesia and approach, the Penrose drains (Yushin Corp.) were removed and the greater tuberosity was prepared with a scalpel blade. Two interosseous bone tunnels were created at the anterior and posterior aspects of the footprint and 5 mm distal to the articular surface. Size 2-0 Ti-Cron (Medtronic, Minneapolis, MN, USA) nonabsorbable sutures were passed through the tunnels for reattaching the supraspinatus tendon to the footprint and then tied over the lateral humeral cortex (Fig. 3). The control group did not receive any surgery. In the repair + PRP (RP) group, we injected 0.5 mL of the prepared PRP without dilution into the supraspinatus muscle adjacent to the repair site. In the repair + BMAC (RB) group, we injected 0.5 mL of BMAC. The repair + PRP + BMAC (RPB) group was injected with 0.25 mL PRP + 0.25 mL of BMAC. In the repair + saline (RS) group, we injected 0.5 mL of saline. Every injection was administered in the repair site, footprint of the supraspinatus tendon, after the repair and the overall volume of injection were the same between the repair groups. The incision was closed in the same manner as described above, and the rabbits were allowed free cage activity without limb immobilization.
Fig. 3. (A) Repair of the torn supraspinatus tendon to the greater tuberosity in an open transosseous manner. The black arrow indicates the greater tuberosity. (B) Each suture end is then tied over the lateral humeral cortex, reattaching the supraspinatus tendon to the footprint. The white arrow indicates the repaired supraspinatus tendon.
Gross examination and biomechanical testing
Six weeks after repair, all rabbits were anesthetized and euthanized with carbon dioxide asphyxiation, followed by harvesting of the bilateral complexes of supraspinatus, and humeral head of each rabbit. After harvest, we examined the status of the repaired supraspinatus tendon. We checked the limb integrity, as well as other conditions (e.g., inflammation).
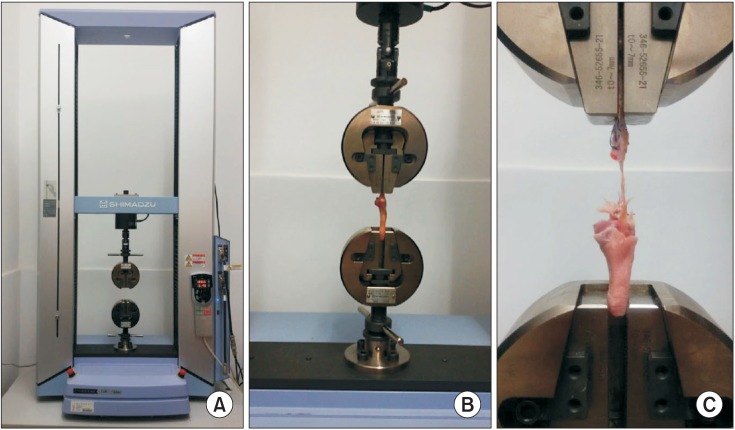
The right shoulder of each rabbit was used for the biomechanical tests. The specimens intended for biomechanical testing were wrapped with saline-moistened gauze and stored in a tube at −80℃ in liquid nitrogen. The samples were thawed at room temperature and rested overnight prior to the test. The sample was sprayed with saline solution to retain moisture during testing. All mechanical testing was performed in a blinded fashion by allocating the randomized number to each rabbit before test. The supraspinatus tendon was transferred to the material testing machine (AGS-X; Shimadzu, Kyoto, Japan) and placed along its length (Fig. 4). The tendon was fixed in a screw grip by sandpaper; the proximal humerus was placed in a block made with dental base acrylic resin powder to prevent fracture through the humeral physis. The biomechanical testing protocol was based on a previous study.13) Briefly, after a preload of 5 N for 5 seconds, cyclic loading from 5 to 50 N at a loading rate of 15 N/sec for five cycles was performed, followed by pull to failure at a rate of 1 mm/sec until the tendon was pulled apart from the bone or ruptured at the tendon midsubstance. Data on ultimate load-to-failure were automatically collected with a data acquisition system on a personal computer by analyzing the strength-distance curve (Trapezium, Shimadzu), and the failure site was recorded. Stiffness was determined by the slope of the linear portion of stress-distance curve. Young's modulus was defined from the steepest preyield slope of the stress-distance curve; the yield load was defined as the load for which the stiffness greatly decreases due to yielding on the structure or part of the material.
Fig. 4. (A) AGS-X materials testing machine (Shimadzu, Japan). (B) Fixture clamping system. (C) Tensile testing of the repaired supraspinatus tendon.
Histological testing
The left shoulder of each rabbit was dissected to yield a specimen consisting only of the humerus with the attached supraspinatus tendon and muscle. Samples were fixed with neutral buffered 10% formalin (pH 7.4), further decalcified with acetic acid for 2 weeks, and then embedded in paraffin blocks. Specimens were sectioned in the coronal plane in line with the repaired supraspinatus tendon at 4 µm in thickness, followed by staining with hematoxylin and eosin (H&E) and Masson trichrome. An independent, blinded pathologist experienced in musculoskeletal pathology, semi-quantitatively assessed the vascularity, cellularity, collagen fiber continuity, and proportion of fibers oriented parallel to the tendon-to-bone interface based on the methods described by Chung et al.18) Parameters were evaluated in four grades. The collagen fiber continuity and collagen fibers oriented parallel were graded as present with < 25% of proportion (grade 0), 25%–50% of proportion (grade 1), 50%–75% of proportion (grade 2), and > 75% of proportion (grade 3). Other parameters were graded as absent or minimally present (grade 0), mildly present (grade 1), moderately present (grade 2), and severe or markedly present (grade 3) (Table 1).
Table 1. Results of Histological Test*.
| Variable | RS group (n = 7) | RP group (n = 7) | RB group (n = 8) | RPB group (n = 8) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G0 | G1 | G2 | G3 | G0 | G1 | G2 | G3 | G0 | G1 | G2 | G3 | G0 | G1 | G2 | G3 | |
| Vascularity | 2 | 4 | 1 | 0 | 1 | 4 | 2 | 0 | 2 | 3 | 2 | 1 | 1 | 1 | 5 | 1 |
| Cellularity | 1 | 4 | 2 | 0 | 0 | 4 | 3 | 0 | 0 | 5 | 2 | 1 | 0 | 3 | 2 | 3 |
| Collagen fiber continuity | 2 | 3 | 2 | 0 | 2 | 2 | 3 | 0 | 1 | 3 | 2 | 2 | 0 | 2 | 4 | 2 |
| Collagen fiber parallel arrangement | 1 | 4 | 2 | 0 | 1 | 4 | 2 | 0 | 1 | 1 | 4 | 2 | 0 | 2 | 3 | 3 |
| Inflammation | 5 | 2 | 0 | 0 | 4 | 2 | 1 | 0 | 7 | 1 | 0 | 0 | 6 | 2 | 0 | 0 |
| Absorption rate | 4 | 2 | 1 | 0 | 4 | 3 | 0 | 0 | 5 | 1 | 2 | 0 | 6 | 2 | 0 | 0 |
RS: repair + saline, RP: repair + platelet-rich plasma, RB: repair + bone marrow aspirate concentrate, RPB: repair + platelet-rich plasma + bone marrow aspirate concentrate.
*The histological grades were as follows: G0, absent, minimal or < 25%; G1, mild degree or 25%–50%; G2, moderate degree or 50%–75%; and G3, severe (marked) degree or > 75%.
In Vitro Study
PRP and BMAC preparation
For the in vitro study, 10 mature male New Zealand white rabbits weighing between 2.5 kg and 3 kg with a mean bodyweight of 2.8 ± 0.3 kg and a mean age of 4.4 ± 0.6 months were used; anesthesia was conducted as described above in the in vivo study. Rabbits of the in vitro and the in vivo test were sacrificed, respectively. Whole blood samples were obtained via cardiac puncture using a 20-mL syringe which contained 3.5 mL of ACD-A. Bone morrow aspirate was collected by stabbing the bilateral femurs and humeri with an 18 G spinal needle into 20 mL syringes prefilled with 3.5 mL of ACD-A. PRP and BMAC were processed using the ACP preparation kit, following the manufacturer's instructions. Approximately 4 mL of PRP or BMAC was produced per rabbit, of which 2 mL was collected for the analysis of blood cell count, and the remaining 2 mL was used for the growth factor analysis.
Hematological analysis
Ten serial samples of whole blood, PRP, BMA, and BMAC were analyzed immediately after collection using an automated blood cell counter (Advia 2120i; Siemens Healthcare Diagnostics Inc., Erlangen, Germany). The number of platelets and leukocytes were evaluated using the same instrument.
Quantification of growth factors
Ten serial samples of whole blood, PRP, BMA and BMAC were used for evaluating growth factor levels. Samples for each were snap frozen (−20℃) to preserve growth factor integrity, and thawed on ice prior to the enzyme-linked immunosorbent assay (ELISA). Samples were agitated thoroughly, centrifuged at 1,000 g for 15 minutes and the supernatants were aspirated for further testing. The following growth factors were chosen for evaluation as they are known to have a specific role in tissue-healing and regeneration: platelet-derived growth factor-AB (PDGF-AB), insulin-like growth factor 1 (IGF-1), transforming growth factor beta 1 (TGF-β1), and vascular endothelial growth factor (VEGF).19) Using ELISA, levels of each growth factor were determined according to the manufacturer's instructions (Quantikine; R&D Systems, Minneapolis, MN, USA).
Statistical Analysis
Data were presented as the mean ± standard deviation. Nonparametric statistical methods were used for all analyses. The Kruskal-Wallis test, followed by post-hoc Bonferroni corrections, and the Mann-Whitney U-test, were used to evaluate differences between groups and samples. All statistical analyses were performed using IBM SPSS ver. 22.0 (IBM Corp., Armonk, NY, USA), and a p-value of < 0.05 was considered statistically significant.
RESULTS
Gross Examination
No rabbits died during surgery or postoperatively. Two rabbits (one in group RS and one in group RP) showed a deep infection in the left shoulder at the time of harvest and were excluded from the final histological analysis. No infection was found in any other rabbits. In addition, no rabbits experienced a retear, as the supraspinatus tendon was attached to the footprint of the greater tuberosity in all groups, and healing was observed in all rabbits.
Biomechanical Evaluation
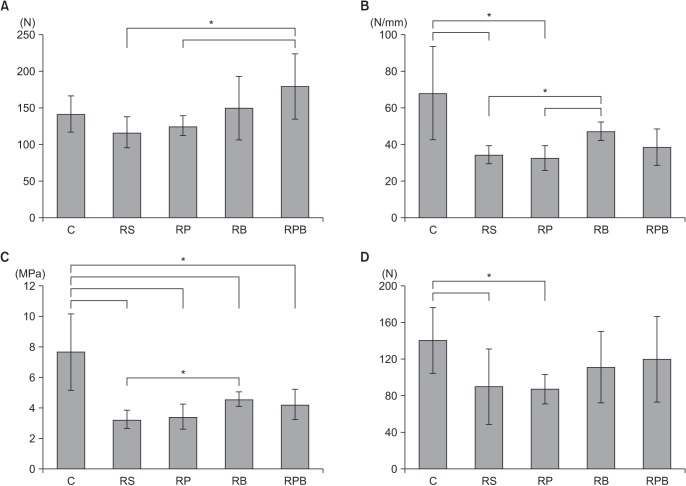
In the failure mode analysis, the ratio of insertion tear to the midsubstance tear was 3 to 5 in the control group, but other groups failed at the insertion site. Results of the biomechanical evaluation are summarized in Fig. 5. Comparing all groups, the Kruskal-Wallis test showed statistically significant differences of all parameters (ultimate load-to-failure, stiffness, Young's modulus, and yield strength: p = 0.042, p = 0.002, p = 0.000, and p = 0.047, respectively).
Fig. 5. Biomechanical tests among the groups. (A) The ultimate load-to-failure is significantly higher in the repair + PRP + BMAC (RPB) group than the repair + saline (RS) group. (B) Stiffness is significantly higher in the repair + BMAC (RB) group than both the RS and repair + PRP (RP) groups. (C) The Young's modulus is significantly lower in all experimental groups than the control group. (D) Yield strength of the RB and RPB groups showed no statistical differences from the control group. Asterisks and error bars show the statistical difference between the two groups (*p < 0.05). C: control, PRP: platelet-rich plasma, BMAC: bone marrow aspirate concentrate.
The ultimate load-to-failure was significantly higher in the RPB group compared to the RS group (p = 0.025) and RP group (p = 0.038) in post-hoc test and also higher than the control group (p = 0.101). The RB group showed higher ultimate load-to-failure than both the RS and RP groups (p = 0.610 and p = 0.534, respectively).
The RB group showed a significantly higher stiffness than both the RS and RP groups (p = 0.002 and p = 0.006, respectively). Stiffness of the RB and RPB groups showed no statistical difference compared to the control group (p = 0.165 and p = 0.053, respectively).
Although Young's modulus of the four repair groups showed significant differences from the control group (p = 0.001 for RS group, p = 0.006 for RP group, p = 0.001 for RB group, and p = 0.001 for RPB group), the RB group showed a higher Young's modulus than both the RS and RP groups (p = 0.002 and p = 0.053, respectively). The RPB group also showed a higher Young's modulus than both the RS and RP groups (p = 0.937 and p = 0.172, respectively).
Yield strength of the RB and RPB groups showed no statistical differences from the control group (p = 0.259 and p = 0.234, respectively), and they showed higher values than the RS and RP groups though they were not significantly different.
Histological Evaluation
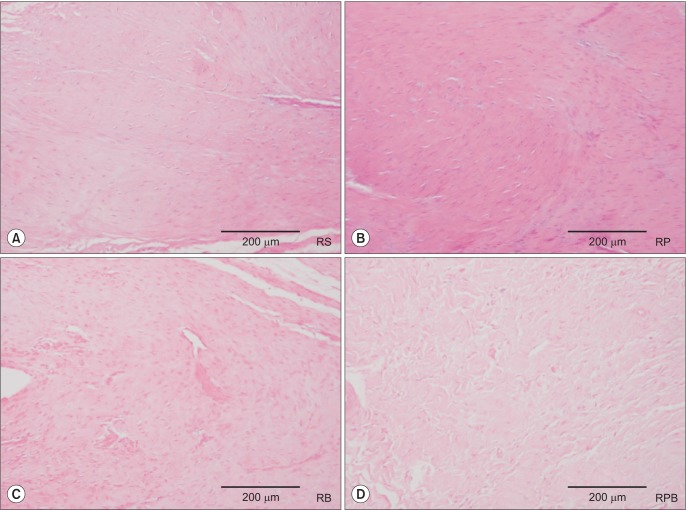
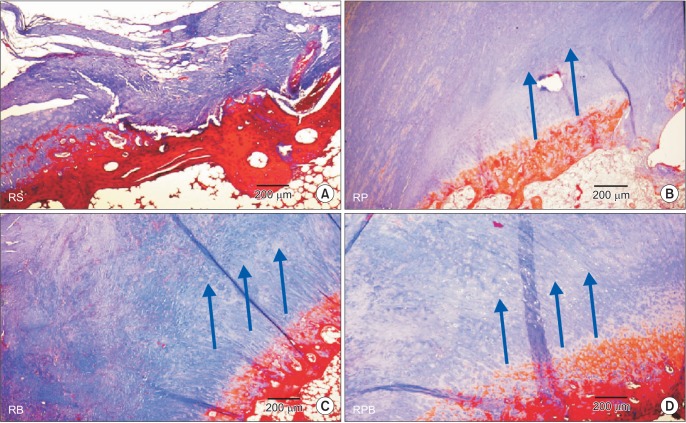
Increased amounts of cellular and vascular fibrous tissues appeared at the tendon-to-bone interface in all repair groups (Fig. 6). However, compared to the RS (Fig. 7A) and RP (Fig. 7B) groups, the BMAC-treated groups (RB group and RPB group, Fig. 7C and 7D, respectively) showed better collagen, as collagen fibers in the interface were more perpendicular to the bone. However, there was no obvious difference in tendon-to-bone healing between the RB and RPB groups. The RPB group showed relatively higher vascularity, cellularity, and collagen fiber continuity and parallel arrangement than the other groups.
Fig. 6. Tendon-to-bone interface H&E staining shows increased cellular and vascular fibrous tissues in the four groups: (A) repair + saline (RS) group, (B) repair + platelet-rich plasma (RP) group, (C) repair + bone marrow aspirate concentrate (RB) group, and (D) repair + platelet-rich plasma + bone marrow aspirate concentrate (RPB) group.
Fig. 7. Masson-trichrome staining demonstrates better collagen fiber continuity and arrangement at tendon-to-bone attachment sites in the bone marrow aspirate concentrate (BMAC)-treated groups: (A) repair + saline (RS) group, (B) repair + platelet-rich plasma (RP) group, (C) repair + BMAC (RB) group, and (D) repair + platelet-rich plasma + BMAC (RPB) group. The arrows indicate the improved collagen fiber continuity and arrangement of the RB and RPB groups compared to the control and RS groups. Collagen fibers run parallel along the tendon-to-bone attachment sites (in this figure, they run diagonally). However, there are few collagen fibers in the control and RS groups.
Hematological Analysis
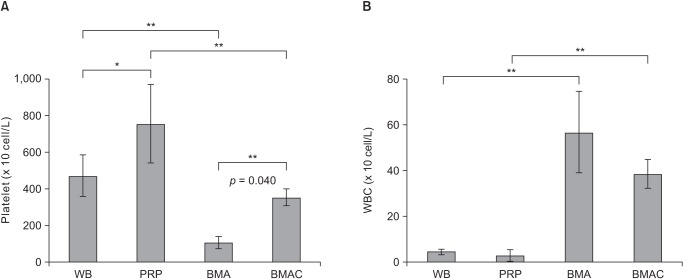
The average platelet counts were 471.09 ± 113.61 × 103/µL in whole blood, 754.32 ± 214.30 × 103/µL in PRP, 106.52 ± 33.12 × 103/µL in BMA, and 353.39 ± 45.68 × 103/µL in BMAC. With respect to the platelet counts in both BMA and the whole blood series, all processed samples showed a significant increase in platelet number as compared to each native source by 3.3- and 1.6-fold, respectively. In PRP, the platelet level was significantly higher than that in BMAC (p = 0.004) (Fig. 8A). In addition, the mean number of leukocytes was 4.39 ± 1.14 × 103/µL in whole blood, 2.83 ± 2.48 × 103/µL in PRP, 56.77 ± 17.87 × 103/µL in BMA, and 38.59 ± 6.22 × 103/µL in BMAC. In terms of the white blood cell concentration, BMAC was significantly higher than PRP (p = 0.001) (Fig. 8B).
Fig. 8. The platelet counts (A) and WBC counts (B) of BMA and peripheral blood series. Asterisks and error bars show the statistical difference between the two groups (*p < 0.05, **p < 0.01). WB: whole blood, PRP: platelet-rich plasma, BMA: bone marrow aspirate, BMAC: bone marrow aspirate concentrate, WBC: white blood cell.
Quantification of Growth Factors
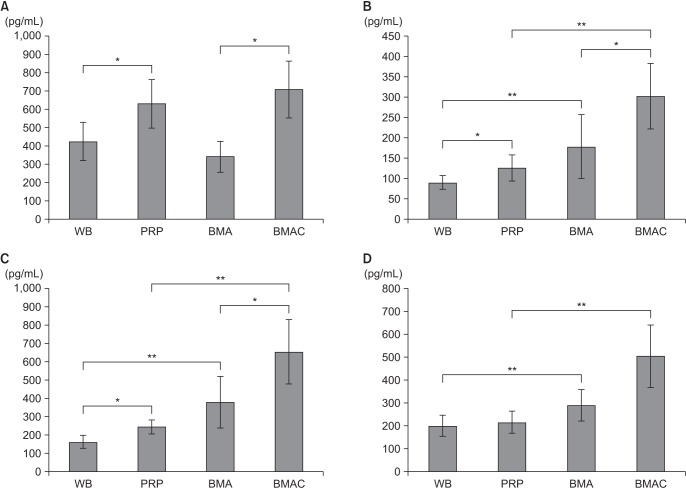
Results of the growth factor evaluation are summarized in Fig. 9. The PDGF-AB levels in BMAC and PRP were not significantly different (p = 0.672), but were significantly higher than in both BMA and whole blood (p = 0.037 and p = 0.008, respectively). The IGF-1 and TGF-β1 levels were significantly higher in BMAC than in the other groups, with BMAC having the highest concentration and whole blood the lowest concentration (p < 0.05). The IGF-1 and TGF-β1 levels of PRP were significantly higher than whole blood (p = 0.021 and p = 0.046, respectively). The VEGF level in BMAC was the highest, but the difference in BMA was not significant (p = 0.073).
Fig. 9. Growth factor levels in BMA and peripheral blood. (A) Platelet-derived growth factor-AB level. (B) Insulin-like growth factor 1 level. (C) Transforming growth factor beta 1 level. (D) Vascular endothelial growth factor level. Asterisks and error bars show the statistical difference between the two groups (*p < 0.05, **p < 0.01). WB: whole blood, PRP: platelet-rich plasma, BMA: bone marrow aspirate, BMAC: bone marrow aspirate concentrate.
DISCUSSION
This study showed that the local application of BMAC after rotator cuff repair enhanced the mechanical strength of the tendon-to-bone junction. Improved biological healing was also observed histologically in BMAC-treated groups.
BMAC has emerged as an important biological tool in orthopedics; it contains platelets, and more importantly, MSCs and progenitor cells, all of which facilitate tissue healing.11) Our in vitro data demonstrated that BMAC released significantly higher levels of growth factors IGF, TGF-β1, and VEGF than PRP, although we did not perform a nucleated cell count analysis. However, a recent study showed that the number of nucleated cells in BMAC was three times higher than that in PRP.20) Furthermore, nucleated cells were simple to obtain from BMA through a modification of the PRP processing protocol. Our in vivo data indicated that the BMAC-treated groups (RB and RPB groups) had increased ultimate load-to-failure, stiffness, Young's modulus, and yield load. The above parameters represent critical mechanical features of the repaired tendon, although some of the differences were not statistically significant compared to the RS and RP groups. In addition, the BMAC-treated groups showed better collagen fiber continuity and more regularly arranged collagen fibers than the RS and RP groups, whereas there was little benefit to PRP treatment compared to saline. An earlier study reported that local administration of MSCs after rotator cuff repair in a rat model did not show any benefits in terms of tendon structure and strength, despite confirmed biological activity at the repair site.21)
Therefore, we speculated that isolated MSCs or PRP alone were not sufficient to support tendon-to-bone healing in a rotator cuff model during the tissue repair and regenerative process, and that a combination of pluripotent cells with a local environment that encourages the differentiation of transplanted cells might improve healing. It is possible that the combination of growth factors and nucleated cells played a significant role in our model, and the dominant healing mechanism may have resulted from biosynthetic effects of the transplanted cells and growth factors, which then recruited host tenogenic progenitor cells by chemotaxis. Nonetheless, further research is required to better understand the underlying mechanisms of repair.
In the current study, we demonstrated a consistent increase in platelet counts in the BMAC and PRP samples by 3.3- and 1.6-fold, respectively, relative to each baseline, BMA and whole blood. Thus, we believe that BMAC was processed efficiently, and the level of platelets was within the working range. However, the platelet concentration of the PRP did not reach the therapeutic range, even though the baseline platelet count was relatively higher (over 400 × 103/µL). We also did not activate the PRP before the injection. We intended to induce the spontaneous activation following exposure to natural collagen in connective tissue. However, there are several methods for activating PRP, and PRP activation could cause the release of growth factors and inflammatory mediators. In these respects, it appears that the effect was reduced in the RP group compared with a similar study of Chung et al.18)
A number of animal studies6,22,23,24) not only support the use of BMAC to augment tendon repair by improving enthesis regeneration but also highlight the function of BMAC to improve repair quality. In the current study, the RB group showed a higher mean ultimate load-to-failure, stiffness, and Young's modulus than the RS group. Although the difference between these two groups did not reach statistical significance, these findings were in accordance with gross observations of the specimens, in which more vascularity, cellularity, collagen fiber continuity and parallel arrangement were observed in the tendon-to-bone junction site in the BMAC-treated group. The use of BMAC is particularly attractive because BMA can be processed within 30 minutes; therefore, prolonged, culture-based expansion is not necessary. We believe that BMAC, which was produced by simultaneous concentration of platelets and bone marrow cells, is a more practical and cost-effective approach for surgical applications than cultured MSCs.
Several early basic science studies that investigated the effect of PRP on tendon healing using animal models reported significant improvements in early tendon healing;25,26,27) however, most of these studies examined the acute stage of tendon injury. Differences between acute tendon injury and chronic tears should be kept in mind when considering the effect of PRP on tendon repair, since differences in clinical stages may be related to different biological paths for tendon healing and remodeling. In our study, PRP showed little benefit over saline solution; however, the combination of PRP and BMAC (RPB group) showed significantly increased ultimate load-to-failure compared to the RS group, and demonstrated a slight increase over the RP and RB groups, but without statistical significance. We are unaware of the exact reason why the combination of PRP and BMAC showed a greater benefit than BMAC alone in this study, since comparative studies on the capabilities of BMAC and PRP in tendon regeneration are rare. Previous studies28) have reported that PRP administration resulted in the preferential upregulation of tenocyte-related genes including collagen type I alpha 1 chain (COL1), collagen type III alpha 1 chain (COL3), and tenascin C (TNC), and promoted the differentiation of tendon stem cells into active tenocytes. However, the possible effects of increased proliferation require further investigation. Variations among PRP preparations, which result in different concentrations of growth factors in various PRP products is the most common problem in PRP therapy.29) In addition, the biology of a number of PRP-related factors remain unknown, such as the optimal dosage, best time, effect of multiple injections, release kinetics of growth factors and the effects of local tissue pH. Understanding these factors may determine the best approach for a given PRP formulation.19)
To our knowledge, this is the first study comparing the effects of BMAC and PRP in a rabbit model for chronic rotator cuff tear repair. Our main objective was to evaluate the cellular composition and growth factor levels of BMAC in a rabbit model. Prior to this study, we were concerned that platelets in the bone marrow are premature and would not provide growth factors. However, we found that BMAC is superior to PRP, and the growth factors (IGF-1, TGF-β1, and VEGF) were significantly higher in BMAC than PRP. These differences in cellular and cytokine composition between PRP and BMAC should be considered for therapeutic applications. We believe that BMAC provides advantages over PRP, as it contains “working cells.” In support of this, Nishimoto et al.30) found that BMAC accelerated wound healing on an ischemic limb, whereas PRP had no effect.
The present study has several limitations. First, this was an animal study, the results of which may differ when applied to humans. We also used an inbred rabbit strain to create allogeneic PRP and to standardize the PRP preparation. This allogeneic PRP may have influenced the degree of inflammatory responses in the host rabbit. On the other hand, the standardized PRP decreased the technical bias. In our rabbit model, we were unable to immobilize the repaired limbs, which may have led to a higher or unforeseen stress on the repaired tendon postoperatively. A sample size calculation for histologic test was not performed in this study. Safranin O staining for histologic test, which is better in evaluation of bone tendon interface was not performed in this study. We also used only single observation time point (6 weeks after repair) which is the middle phase of the healing process, and we could not compare overall regeneration process. Finally, we did not confirm the existence of MSCs by stem cell marker, nor were we able to determine whether PRP remained in the desired site after incision closure. However, we believe that these cells persist in vivo for several weeks and then gradually disappear over time.
The major findings of our study are (1) locally administered BMAC enhanced tendon-to-bone healing, (2) BMAC is enriched with growth factors compared to PRP, and (3) this approach shows potential for human clinical applications.
ACKNOWLEDGEMENTS
This research was supported by Hallym University Research Fund. The authors would like to thank Ms. Miseon Son for her invaluable technical assistance and contribution to statistical analysis and graphic design.
Footnotes
CONFLICT OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Seida JC, LeBlanc C, Schouten JR, et al. Systematic review: nonoperative and operative treatments for rotator cuff tears. Ann Intern Med. 2010;153(4):246–255. doi: 10.7326/0003-4819-153-4-201008170-00263. [DOI] [PubMed] [Google Scholar]
- 2.Harryman DT, 2nd, Mack LA, Wang KY, Jackins SE, Richardson ML, Matsen FA., 3rd Repairs of the rotator cuff: correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991;73(7):982–989. [PubMed] [Google Scholar]
- 3.Lorbach O, Pape D, Raber F, Kohn D, Kieb M. Arthroscopic rotator cuff repair using a single-row of triple-loaded suture anchors with a modified suture configuration. Arch Orthop Trauma Surg. 2011;131(8):1073–1076. doi: 10.1007/s00402-011-1283-8. [DOI] [PubMed] [Google Scholar]
- 4.Livermore RW, Chong AC, Prohaska DJ, Cooke FW, Jones TL. Knot security, loop security, and elongation of braided polyblend sutures used for arthroscopic knots. Am J Orthop (Belle Mead NJ) 2010;39(12):569–576. [PubMed] [Google Scholar]
- 5.Gerber C, Schneeberger AG, Perren SM, Nyffeler RW. Experimental rotator cuff repair: a preliminary study. J Bone Joint Surg Am. 1999;81(9):1281–1290. doi: 10.2106/00004623-199909000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Kovacevic D, Rodeo SA. Biological augmentation of rotator cuff tendon repair. Clin Orthop Relat Res. 2008;466(3):622–633. doi: 10.1007/s11999-007-0112-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheung EV, Silverio L, Sperling JW. Strategies in biologic augmentation of rotator cuff repair: a review. Clin Orthop Relat Res. 2010;468(6):1476–1484. doi: 10.1007/s11999-010-1323-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murphy MB, Blashki D, Buchanan RM, et al. Adult and umbilical cord blood-derived platelet-rich plasma for mesenchymal stem cell proliferation, chemotaxis, and cryopreservation. Biomaterials. 2012;33(21):5308–5316. doi: 10.1016/j.biomaterials.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 9.Xie X, Wang Y, Zhao C, et al. Comparative evaluation of MSCs from bone marrow and adipose tissue seeded in PRP-derived scaffold for cartilage regeneration. Biomaterials. 2012;33(29):7008–7018. doi: 10.1016/j.biomaterials.2012.06.058. [DOI] [PubMed] [Google Scholar]
- 10.Kim HJ, Nam HW, Hur CY, et al. The effect of platelet rich plasma from bone marrow aspirate with added bone morphogenetic protein-2 on the Achilles tendon-bone junction in rabbits. Clin Orthop Surg. 2011;3(4):325–331. doi: 10.4055/cios.2011.3.4.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishimoto S, Fukuda K, Kawai K, et al. Supplementation of bone marrow aspirate-derived platelet-rich plasma for treating radiation-induced ulcer after cardiac fluoroscopic procedures: a preliminary report. Indian J Plast Surg. 2012;45(1):109–114. doi: 10.4103/0970-0358.96599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yun JH, Han SH, Choi SH, et al. Effects of bone marrow-derived mesenchymal stem cells and platelet-rich plasma on bone regeneration for osseointegration of dental implants: preliminary study in canine three-wall intrabony defects. J Biomed Mater Res B Appl Biomater. 2014;102(5):1021–1030. doi: 10.1002/jbm.b.33084. [DOI] [PubMed] [Google Scholar]
- 13.Oh JH, Chung SW, Kim SH, Chung JY, Kim JY. effect of the adipose-derived stem cell for the improvement of fatty degeneration and rotator cuff healing in rabbit model. J Shoulder Elbow Surg. 2014;23(4):445–455. doi: 10.1016/j.jse.2013.07.054. [DOI] [PubMed] [Google Scholar]
- 14.Uhthoff HK, Seki M, Backman DS, Trudel G, Himori K, Sano H. Tensile strength of the supraspinatus after reimplantation into a bony trough: an experimental study in rabbits. J Shoulder Elbow Surg. 2002;11(5):504–509. doi: 10.1067/mse.2002.126760. [DOI] [PubMed] [Google Scholar]
- 15.Rubino LJ, Stills HF, Jr, Sprott DC, Crosby LA. Fatty infiltration of the torn rotator cuff worsens over time in a rabbit model. Arthroscopy. 2007;23(7):717–722. doi: 10.1016/j.arthro.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 16.Itoigawa Y, Kishimoto KN, Sano H, Kaneko K, Itoi E. Molecular mechanism of fatty degeneration in rotator cuff muscle with tendon rupture. J Orthop Res. 2011;29(6):861–866. doi: 10.1002/jor.21317. [DOI] [PubMed] [Google Scholar]
- 17.Hirose K, Kondo S, Choi HR, Mishima S, Iwata H, Ishiguro N. Spontaneous healing process of a supraspinatus tendon tear in rabbits. Arch Orthop Trauma Surg. 2004;124(6):374–377. doi: 10.1007/s00402-004-0663-8. [DOI] [PubMed] [Google Scholar]
- 18.Chung SW, Song BW, Kim YH, Park KU, Oh JH. Effect of platelet-rich plasma and porcine dermal collagen graft augmentation for rotator cuff healing in a rabbit model. Am J Sports Med. 2013;41(12):2909–2918. doi: 10.1177/0363546513503810. [DOI] [PubMed] [Google Scholar]
- 19.Foster TE, Puskas BL, Mandelbaum BR, Gerhardt MB, Rodeo SA. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med. 2009;37(11):2259–2272. doi: 10.1177/0363546509349921. [DOI] [PubMed] [Google Scholar]
- 20.Zhong W, Sumita Y, Ohba S, et al. In vivo comparison of the bone regeneration capability of human bone marrow concentrates vs. platelet-rich plasma. PLoS One. 2012;7(7):e40833. doi: 10.1371/journal.pone.0040833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gulotta LV, Kovacevic D, Ehteshami JR, Dagher E, Packer JD, Rodeo SA. Application of bone marrow-derived mesenchymal stem cells in a rotator cuff repair model. Am J Sports Med. 2009;37(11):2126–2133. doi: 10.1177/0363546509339582. [DOI] [PubMed] [Google Scholar]
- 22.Geuze RE, Kruyt MC, Verbout AJ, Alblas J, Dhert WJ. Comparing various off-the-shelf methods for bone tissue engineering in a large-animal ectopic implantation model: bone marrow, allogeneic bone marrow stromal cells, and platelet gel. Tissue Eng Part A. 2008;14(8):1435–1443. doi: 10.1089/ten.tea.2007.0210. [DOI] [PubMed] [Google Scholar]
- 23.Hoffmann A, Gross G. Tendon and ligament engineering: from cell biology to in vivo application. Regen Med. 2006;1(4):563–574. doi: 10.2217/17460751.1.4.563. [DOI] [PubMed] [Google Scholar]
- 24.Okamoto N, Kushida T, Oe K, Umeda M, Ikehara S, Iida H. Treating Achilles tendon rupture in rats with bone-marrow-cell transplantation therapy. J Bone Joint Surg Am. 2010;92(17):2776–2784. doi: 10.2106/JBJS.I.01325. [DOI] [PubMed] [Google Scholar]
- 25.Lyras DN, Kazakos K, Verettas D, et al. The effect of platelet-rich plasma gel in the early phase of patellar tendon healing. Arch Orthop Trauma Surg. 2009;129(11):1577–1582. doi: 10.1007/s00402-009-0935-4. [DOI] [PubMed] [Google Scholar]
- 26.Aspenberg P, Virchenko O. Platelet concentrate injection improves Achilles tendon repair in rats. Acta Orthop Scand. 2004;75(1):93–99. doi: 10.1080/00016470410001708190. [DOI] [PubMed] [Google Scholar]
- 27.Bosch G, van Schie HT, de Groot MW, et al. Effects of platelet-rich plasma on the quality of repair of mechanically induced core lesions in equine superficial digital flexor tendons: a placebo-controlled experimental study. J Orthop Res. 2010;28(2):211–217. doi: 10.1002/jor.20980. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J, Wang JH. Platelet-rich plasma releasate promotes differentiation of tendon stem cells into active tenocytes. Am J Sports Med. 2010;38(12):2477–2486. doi: 10.1177/0363546510376750. [DOI] [PubMed] [Google Scholar]
- 29.Mazzocca AD, McCarthy MB, Chowaniec DM, et al. The positive effects of different platelet-rich plasma methods on human muscle, bone, and tendon cells. Am J Sports Med. 2012;40(8):1742–1749. doi: 10.1177/0363546512452713. [DOI] [PubMed] [Google Scholar]
- 30.Nishimoto S, Kawai K, Tsumano T, Fukuda K, Fujiwara T, Kakibuchi M. Impacts of bone marrow aspirate and peripheral blood derived platelet-rich plasma on the wound healing in chronic ischaemic limb. J Plast Surg Hand Surg. 2013;47(3):169–174. doi: 10.3109/2000656X.2012.752739. [DOI] [PubMed] [Google Scholar]