Abstract
West Nile virus (WNV) has caused multiple global outbreaks with increased frequency of neuroinvasive disease in recent years. Despite many years of research, there are no licensed therapeutics or vaccines available for human use. One of the major impediments of vaccine development against WNV is the potential enhancement of infection by related flaviviruses in vaccinated subjects through the mechanism of antibody-dependent enhancement of infection (ADE). For instance, the recent finding of enhancement of Zika virus (ZIKV) infection by pre-exposure to WNV further complicates the development of WNV vaccines. Epidemics of WNV and the potential risk of ADE by current vaccine candidates demand the development of effective and safe vaccines. We have previously reported that the domain III (DIII) of the WNV envelope protein can be readily expressed in Nicotiana benthamiana leaves, purified to homogeneity, and promote antigen-specific antibody response in mice. Herein, we further investigated the in vivo potency of a plant-made DIII (plant-DIII) in providing protective immunity against WNV infection. Furthermore, we examined if vaccination with plant-DIII would enhance the risk of a subsequent infection by ZIKV and Dengue virus (DENV). Plant-DIII vaccination evoked antigen-specific cellular immune responses as well as humoral responses. DIII-specific antibodies were neutralizing and the neutralization titers met the threshold correlated with protective immunity by vaccines against multiple flaviviruses. Furthermore, passive administration of anti-plant DIII mouse serum provided full protection against a lethal challenge of WNV infection in mice. Notably, plant DIII-induced antibodies did not enhance ZIKV and DENV infection in Fc gamma receptor-expressing cells, addressing the concern of WNV vaccines in inducing cross-reactive antibodies and sensitizing subjects to subsequent infection by heterologous flavivirus. This study provides the first report of a WNV subunit vaccine that induces protective immunity, while circumventing induction of antibodies with enhancing activity for ZIKV and DENV infection.
Keywords: West Nile virus (WNV), Vaccine, Envelope protein, Domain III (DIII), Antibody-dependent enhancement (ADE), Zika virus (ZIKV), Dengue virus (DENV), Plant-produced vaccine, Plant-made pharmaceuticals
Introduction
West Nile virus (WNV) is a member of the genus Flavivirus in the family Flaviviridae, and shares a high degree of sequence similarity to dengue virus (DENV), Zika virus (ZIKV), tick-borne encephalitis virus (TBEV), and yellow fever virus (YFV) [1]. For example, WNV shares an overall genome structure with these flavivirus and 84%, 66%, 59%, and 52.3% nucleotide sequence identity with TBEV, DENV-2, ZIKV, and YFV, respectively [2, 3]. WNV entered into the Western hemisphere in the United States (US) in 1999, with cases also described in Canada, the Caribbean and Latin American regions [1]. Majority of WNV infection in humans is asymptomatic. Symptomatic WNV infection can cause malaise, fever, and a maculopapular rash, while neuroinvasive disease symptoms include encephalitis, meningitis, and/or possible death [1]. The elderly, individuals who are immunocompromised, or those who carry certain genetic factors are at a higher risk of developing life-threatening neurological diseases [4, 5]. In recent years, outbreaks of WNV have become more frequent and severe with higher instance of patients with neuroinvasive complications [6]. However, currently there is no approved WNV vaccine for human use.
One of the challenges for WNV vaccine development is the increased risk of infection by related flaviviruses in vaccinated subjects due to the phenomenon of antibody-dependent enhancement of infection (ADE). ADE may occur between WNV and related flaviviruses such as DENV and ZIKV due to their high degree of genetic similarity and co-circulation in many parts of the world [7]. As a result, WNV vaccines based on conserved epitopes among related flaviviruses would have the potential to induce cross-reactive antibodies that augment entry and replication of DENV and ZIKV in Fc gamma receptor (FcγR)-expressing cells and lead to DENV or ZIKV infection in vaccinated subjects [8]. Indeed, mutual enhancement between WNV and ZIKV infections has been recently observed [7]. Thus, there is an urgent call for the development of WNV vaccines that are not only effective but also safe with a minimal risk of ADE to combat the threat of WNV infection on a global scale.
WNV Envelope (E) glycoprotein is a major target for the host antibody response and its domain III (DIII) contains the majority of type-specific neutralizing epitopes that elicit a strong host antibody response and/or protective immunity [9]. For approved human vaccines against flaviviruses YFV and TBEV, a neutralizing antibody response has been found to correlate with protection [10, 11]. Neutralizing antibodies have also been demonstrated to play important roles in the protection against infection by other flaviviruses [12]. As a result, DIII has been explored as a promising WNV vaccine candidate and has been expressed in insect and bacterial cell cultures [13, 14]. However, bacterial cell-produced DIII is insoluble and demands a solubilization and refolding process to be effective, which is not only cumbersome but also inconsistent in producing a recombinant DIII protein with native epitopes [14].
In our previous publication, we reported using a plant-based expression system to overcome these challenges, for a robust and scalable production of DIII as a promising WNV vaccine candidate [15]. We demonstrated that DIII was expressed at high levels in Nicotiana benthamiana plants within 4 days post-introduction of the DIII expression cassette. In contrast to E. coli-produced DIII, plant-produced DIII (plant-DIII) was soluble, and can be readily purified to >95% homogeneity without labor-intensive solubilizing and refolding processes [15]. We also demonstrated that immunization of plant-DIII elicited a potent antigen-specific antibody response in mice.
Here, we report a follow-up study of the efficacy of plant-DIII as a promising vaccine against WNV. In this study, we reveal that plant-DIII can also elicit antigen-specific cellular and humoral immune responses, while demonstrating DIII-specific antibodies (anti-plant DIII) neutralized WNV with a neutralization titer threshold that correlated with protective immunity of other known flavivirus vaccines. Importantly, passive transfer of anti-plant DIII serum protected 100% of mice against a lethal WNV challenge. Notably, anti-plant DIII antibodies did not enhance infection of ZIKV and DENV in Fc gamma receptor (FcγR)-expressing cells, offsetting the concern of WNV vaccines in inducing cross-reactive antibodies and sensitizing people to subsequent infection by heterologous flaviviruses. In brief, our plant-DIII based vaccine can effectively prevent WNV infection, along with offering improved safety, and purification efficiency compared to alternative vaccine candidates in development.
Material and methods
Ethics statement and biosafety
All animal experimental procedures were reviewed and approved by the Institutional Animal Care and Use Committees at The University of Southern Mississippi (USM). All the in vitro experiments and animal studies involving live WNV were performed by the certified personnel in biosafety level 3 (BSL3) laboratories following standard biosafety protocols approved by the USM Institutional Biosafety Committees (IBC). Experiments with ZIKV and DENV were conducted with standard biosafety protocols approved by the IBC of Arizona State University by the certified personnel in biosafety level 2 (BSL2) laboratories.
Production of WNV envelope protein DIIII in N. benthamiana plants
Plant expression vectors for WNV E DIII [15] was agroinfiltrated into leaves of N. benthamiana plants as described previously [16–22]. Leaves were harvested 4 days post agroinfiltration (dpi) and DIII was extracted and purified with Ni2+ immobilized metal affinity chromatography (IMAC) as previously described [15]. Details of these methods are provided in Supplementary material.
Mouse immunization
Five-week old female BALB/c mice were divided into 2 groups (n = 6 per group). Group 1 received saline buffer (PBS) with aluminum hydroxide gel (alum, InvivoGen, CA) as mock immunized controls and groups 2 received 25 μg of plant-DIII per dosage. On day 0, each mouse was injected subcutaneously with 100 μl PBS (Group 1) or 100 μl material containing 25 μg purified DIII protein (Group 2) in PBS with alum as adjuvant (DIII protein solution: alum volume ratio = 1:1). Mice were boosted three times (days 21, 42 and 63 post-immunization) with the same dosage and immune protocol as in the 1st immunization. Retro-orbital blood samples were collected on day 0 before the immunization (pre-immune sample) and on days 14 (week 2), 35 (week 5), and 56 (week 8) after the 1st immunization. Mice were humanely euthanized on day 77 (week 11), final blood samples were collected, and the spleens were aseptically removed for in vitro splenocyte cultures.
In vivo passive antibody transfer protection experiments
Serum isolated from PBS or plant DIII-immunized mice at week 11 was heat-inactivated for 30 min at 56°C and stored at −80°C. 5-week old female BALB/c mice were divided into 3 groups (n = 10 per group). Groups 1 and 2 received 50 μl of serum from PBS or plant DIII-immunized mice, respectively. Group 3 received 10 μg of the monoclonal antibody (mAb) E16 [23] as a positive control. Mice anesthetized with 30% isoflurane were passively administered serum or E16 mAb via r.o. injection 1 hr before intraperitoneal (i.p.) inoculation with 102 plaque-forming units (PFU) of WNV (CT2741, kindly provided by Dr. John F. Anderson at the Connecticut Agricultural Experiment Station) in 1% gelatin. Mice were monitored for survival for 25 days post infection. The survival curves were constructed using data from two independent experiments.
Splenocyte culture and cytokine production
Spleens were isolated and mechanically dissociated to prepare single-cell from immunized mice and supernatants from splenocyte cultures were then collected to determine cytokine production as described previously [24]. Details of these methods are provided in Supplementary material.
Plaque reduction neutralization test (PRNT) assay
WNV-specific neutralizing antibodies were measured with a PRNT assay according to our previous report with minor modifications [25–27]. Neutralizing antibody titers were expressed as the reciprocal of the highest dilution of serum that neutralized ≥50% of WNV. Details of the PRNT method are provided in the Supplementary material.
Antibody-dependent enhancement Assay
Sera collected from vaccinated mice at week 11 were pooled and total IgG was isolated using IgG purification kits (GE Healthcare, PA). ADE assay was then performed as described previously [25, 28] with details provided in the Supplementary material.
Statistical analyses
Data analysis was performed using GraphPad Prism software version 6.0 (GraphPad, CA). Comparisons of cytokine levels and neutralization potency between groups was performed using Mann-Whitney test. Comparison of concentrations of cytokines collected at various time points was performed by one-way ANOVA. Survival data from at least two independent experiments (n = 10) were analyzed by a Kaplan-Meier analysis. A p value of < 0.05 indicated statistically significant difference.
Results
Plant-DIII elicited antigen-specific cellular immune responses
BALB/c mice were injected subcutaneously with four doses of plant-DIII with alum as an adjuvant over an 11-week time period (Fig S1). Mice in group 1 received PBS as a negative control and mice in group 2 received 25 μg of plant-DIII. Retro-orbital blood collection from mice was performed on day 0 before the immunization (pre-immune sample), day 14 (week 2), day 35 (week 5), and day 56 (week 8) after the initial dose immunization. On week 11, all mice were euthanized and the spleens were aseptically removed for in vitro splenocyte cultures. Our previous report showed that plant-DIII induced significant titers of antigen-specific IgG (log titers > 3.6 and 4.3 for week 5 and 8, respectively), while no significant DIII-specific IgG titer was detected in sera collected from PBS-injected mice [15]. We have also previously reported that the antibody response generated by plant-DIII, with alum as the adjuvant, included both IgG1 and IgG2 subtypes with higher concentrations of IgG1 detected than IgG2a [15].
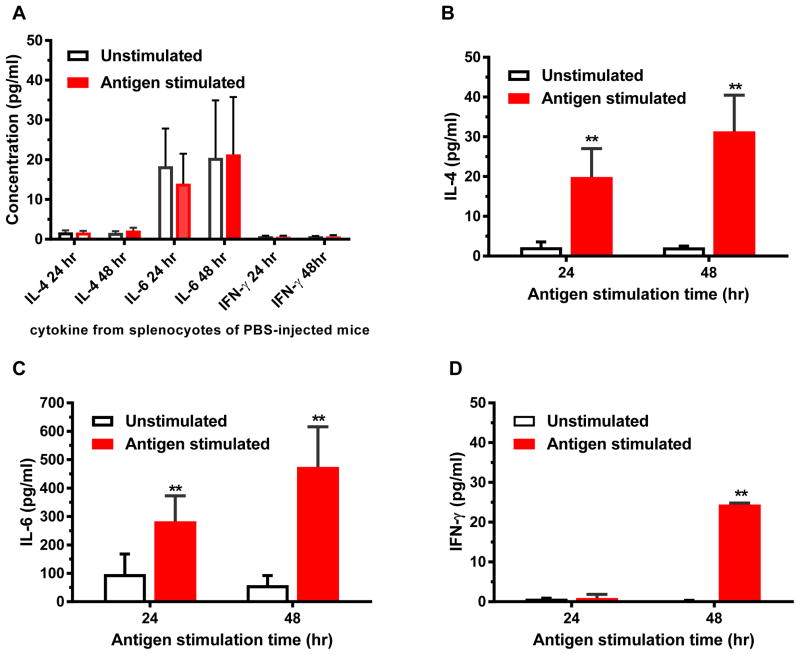
To determine if plant-DIII induced an antigen-specific cellular immune response in mice, the production of cytokines by splenocytes from immunized mice was measured after in vitro antigen stimulation for 24 and 48 hr. As expected, splenocytes of mice in the group that received PBS did not produce significant titers of cytokines after in vitro stimulation with plant-DIII (Fig 1A). In contrast, splenocytes from plant DIII-inoculated mice secreted significant levels of IL-4 (Fig 1B), IL-6 (Fig 1C), and IFN-γ (Fig 1D). The mean concentrations of IL-6 and IL-4 are higher than that of IFN-γ after both 24 and 48 hr stimulation (p = 0.0009 and 0.0008 for 24 hr and 48 hr samples, respectively). In addition, the competency of splenocytes in producing cytokines was demonstrated by the detection of high levels of IFN-γ upon stimulation with the positive control, ConA (Fig S2). These results demonstrated that plant-DIII evoked both Th1 and Th2-type antigen-specific cellular immune responses.
Figure 1. Splenocyte cytokine production from plant-DIII immunized mice.
Spleen cells from mice injected with PBS (A) or 25 μg plant-DIII (B–D) were stimulated in vitro with DIII for 24 to 48 hr. The production of IL-4 (A and B), IL-6 (A and C), and IFN-γ (A and D) was quantitated by a multiplex mouse cytokine kit. Mean concentration (pg/ml) and standard deviation (SD) from at least two independent experiments with technical triplicates for each sample are presented. Compared with unstimulated samples, significant induction of cytokines by antigen stimulation are observed (p = 0.002 for IL-4 and IL6 both 24hr and 48 hr; and p =0.002 for IFN-γ 48 hr). Significant difference in cytokine levels are also observed between splenocytes from plant-DIII vaccinated mice and control mice receiving PBS (p = 0.002 for IL-4 and IL-6 both 24hr and 48 hr; and p < 0.002 for IFN-γ 48 hr).
Serum from plant-DIII immunized mice exhibited potent neutralizing activities against WNV
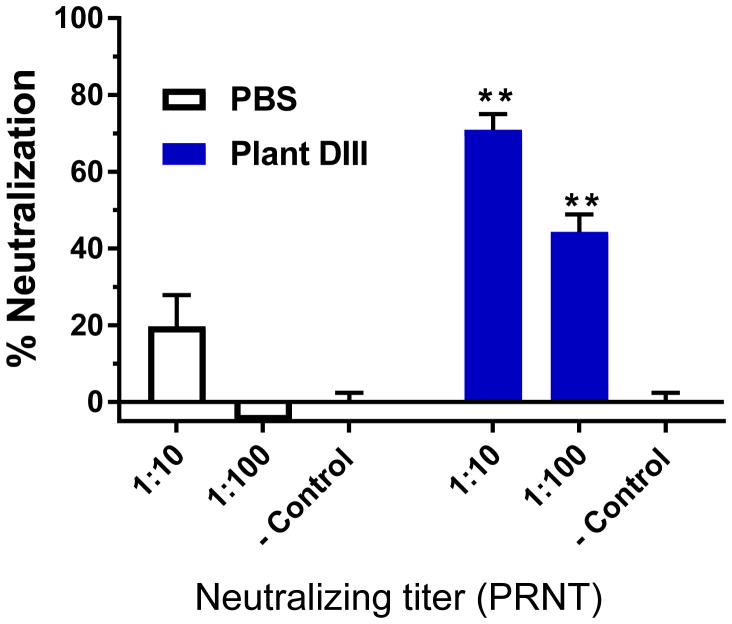
The ability of induced antibodies in response to plant DIII immunization to confer neutralization against WNV was examined by a PRNT assay with serum samples collected 2 weeks after the 2nd immunization (week 5 serum). No significant reduction of WNV infection was detected for sera from mice injected with PBS (Fig 2). In contrast, sera from mice that received plant-DIII vaccination exhibited potent neutralizing activities against WNV infection as early as week 5 (p <0.005 comparing anti-plant DIII sera versus PBS sera) (Fig 2). Specifically, greater than 44% and 70% of WNV infection was reduced by sera from plant DIII-vaccinated mice that have been diluted by 100 and 10 folds, respectively (Fig 2). Neutralization titers that correlate with protection in humans and animal models have been established for several flaviviruses including YFV, TBEV, and lately ZIKV [29–32]. These studies revealed that antibody responses with neutralization titers >10 are sufficient to provide protective immunity against these viral infections [29–32]. Our results indicate that plant-DIII also induced a neutralization titer that is > 10, meeting the established protective threshold.
Figure 2. Neutralization of WNV by anti-plant DIII serum.
Pooled sera from week 5 of vaccinated mice receiving PBS or plant-DIII were diluted 10 and 100 folds, respectively and incubated with 102 PFU of WNV prior to infection in Vero cells. WNV-specific neutralizing antibodies in the sera were assessed with a PRNT assay. PBS buffer was used as the negative control (− Control) in the PRNT assay. Mean % neutralization and SD from two independent experiments with technical triplicates for each sample are presented. ** indicates p values < 0.005 of DIII-immunized serum compared to that from PBS-inoculated control mice.
Plant-DIII induced protective immunity that protects mice against lethal WNV infection
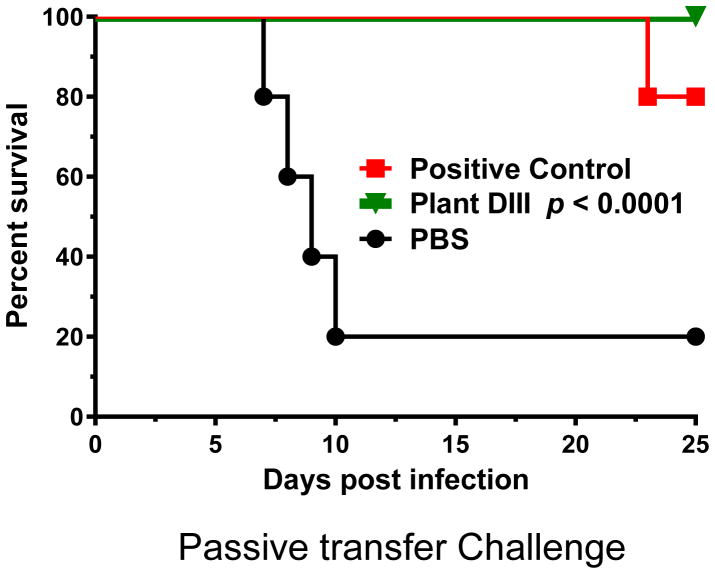
Although the PRNT results suggested the protective nature of plant-DIII evoked immune responses, it was essential to demonstrate this activity in vivo. Challenge studies were performed in 5-week-old wild-type BALB/c mice (n = 10 per group) to examine if sera from plant DIII-vaccinated mice protected against WNV infection in recipient mice. Mice were first passively administered pooled heat-inactivated sera collected at week 11 from plant DIII-inoculated or PBS-injected mice (negative control) via an r.o. route and then infected with 102 PFU of WNV, which causes a baseline mortality of 80–90% [23]. A protective E16 mAb [23] was also r.o. injected in parallel as a positive control. Indeed, 80% of mice in the negative control group that received PBS-injected serum succumbed to infection and died within 10 days of WNV inoculation (Fig 3). In contrast, 80% of mice that received the positive control E16 mAb were protected (Fig 3). Notably, 100% of mice receiving plant DIII-vaccinated serum (with anti-DIII log titer > 4.4 and neutralization titer >10) were completely protected from the lethal infection (p < 0.0001) (Fig 3). These results indicate that plant-DIII elicited an antibody response that can protect mice against WNV infection.
Figure 3. Mice protected from WNV infection by passive administration of serum collected from plant-DIII immunized mice.
Serum was collected from mice that were immunized with plant-DIII (Plant DIII) or PBS. After heat-inactivation, BALB/c mice were passively administered (r.o.) 50 μl of serum or 10 μg of E16 mAb (Positive Control) before infection with 102 PFU of WNV. The p-value is indicated for the plant DIII curve. Data reflect two independent experiments with n = 10 mice per treatment.
Antibody-dependent enhancement activities of IgGs from plant DIII-vaccinated mice
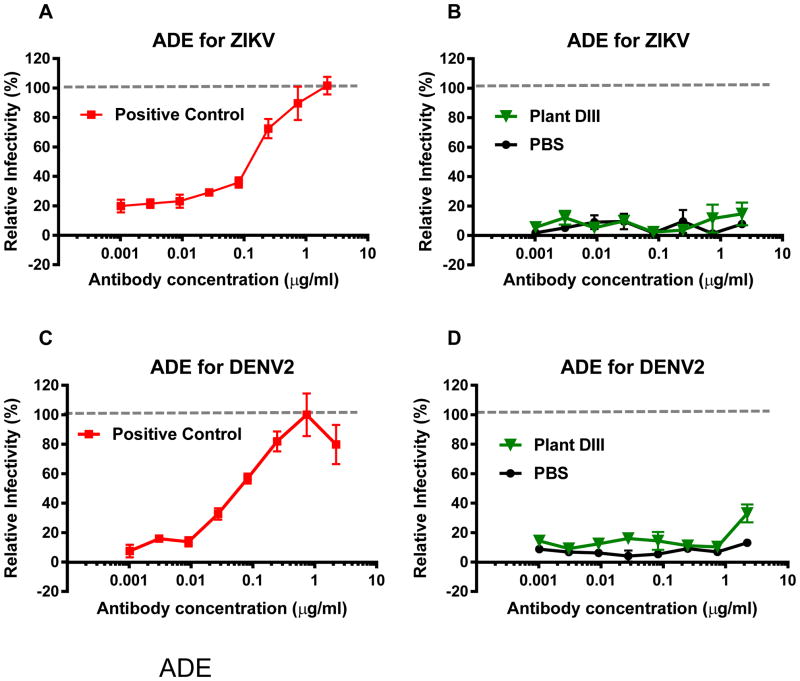
One of the major challenges of vaccine development for WNV and other flaviviruses is the risk of ADE of heterologous flavivirus (e.g. ZIKV and DENV) infection. As such, we investigated if plant-DIII vaccine would induce antibodies that enhance the infection of ZIKV or DENV. As previously demonstrated [33], 4G2, an anti-DENV E mAb that cross-reacts with E of other flaviviruses, efficiently caused ADE of ZIKV (Fig 4A) and DENV-2 (Fig 4C) infection in K562 cells that express the human FcγR. In contrast, IgGs from the negative control mice (week 11) that received PBS did not promote ADE for ZIKV (Fig 4B) or DENV-2 (Fig 4D). Likewise, IgGs from plant-DIII vaccinated mice displayed no significant ADE activity for ZIKV (Fig 4B) or DENV-2 (Fig 4D). PRNT analysis revealed that high concentrations of plant-DIII evoked IgGs used in the ADE assay have neutralizing activity (data not shown), confirming the lack of ADE is not due to insufficient amount anti-DIII IgGs in the assay.
Figure 4. Lack of enhancement of ZIKV and DENV infection by antibodies in anti-plant DIII serum.
Serum collected at week 11 from PBS or plant-DIII vaccinated mice were pooled and IgGs were isolated from the pool sera. Serial dilutions of IgGs were mixed with ZIKV (A and B) or DENV-2 (C and D) and incubated with FcγR expressing K562 cells. After 48 hr of incubation, cells were fixed, permeabilized and analyzed by flow cytometry for viral infection. Anti-flavivirus E mAb 4G2 was used as an ADE positive control (A and C). Enhancement by IgGs from sera is expressed as a % relative to that of 4G2 (B and D).
Discussion
The continuous global outbreaks of WNV and its clinical effects on the central nervous system and long-term morbidity call for the development of vaccines especially in the absence of an effective treatment. Current WNV vaccine candidates include inactivated WNV, live-chimeric virus based on canarypox virus or YFV, DNA-based vaccines, and protein subunit vaccines based on the WNV E protein [34–36]. Studies with these candidates demonstrated that neutralizing antibody responses are essential in providing protection against WNV infection [34–37]. Among these vaccine candidates, the protein subunit-based vaccines with WNV E are projected to be the safest due to lack of risks associated with incomplete inactivation of a live virus, unfavorable host responses to viral vectors, and oncogenesis due to the potential insertion of DNA vaccine fragment into the host genome. However, attempts to produce a WNV E protein or its sub-domains in traditional expression systems such as E. coli or insect cell cultures are challenged by several obstacles including purification and epitope preservation [14, 38]. Safety concerns of WNV vaccines in worsening symptoms of secondary ZIKV and DENV infections via ADE further ignite an urgent need for safer WNV vaccines.
Neutralizing antibody responses with neutralization titers > 10 against E protein has been found to correlate with protection of various flavivirus vaccines and vaccine candidates including those against YFV, TBEV, ZIKV, and WNV [10, 11, 29–32]. We have shown that some anti-plant DIII antibodies compete with a known protective mouse antibody [15, 39–41], suggesting potentially protective antibodies can be produced by plant-DIII immunization. Consistent with this hypothesis, our current study demonstrated that anti-plant DIII antibodies have potent neutralizing activities that meets an established threshold (neutralization titer >10) required for protective immunity by various flavivirus vaccines in a mouse model. This was later confirmed by our in vivo study in which 100% of mice that received mouse plant-DIII sera were protected from a lethal challenge of WNV infection (p < 0.0001 compared to mice receiving PBS-injected mouse sera). Collectively, these results demonstrated that plant-DIII can elicit a neutralizing antibody response that provides protective immunity against WNV infection.
Our current study also demonstrated that plant-DIII also evoked antigen-specific cellular immune responses. Consistent with our previous finding that plant-DIII elicited both IgG1 and IgG2a responses [15], our cytokine profiling also indicated the induction of both Th1 and Th2-type cytokines with mean concentrations of Th-2 type cytokines higher than that of the Th1-type. This is not totally unexpected as comparative studies with flavivirus antigens showed that the adjuvant alum tends to skew the response toward the Th2-type [42, 43]. In this study, alum was used as the adjuvant because it has been successfully employed in licensed human vaccines against other flaviviruses [44]. While a Th1-type response is generally more preferable than a Th2-type for preventing and treating viral infection, studies have shown that a Th1/Th2 mixed response, one that was induced by plant-DIII with alum, can be just effective [45]. The background levels of IL-6 observed secreted by splenocytes of PBS-injected mice were higher than that of the other cytokines. Further experiment is required to investigate the reasons behind this observation. The induction of both humoral and cellular responses by plant-DIII indicates its potential in clearing WNV infection, as well as in providing sterilizing immunity against subsequent WNV challenge.
Vaccine development for some flaviviruses, such as DENV, has been impeded by the issue of ADE. For example, previous infection or vaccination against one serotype of DENV may predispose these individuals to develop the more severe dengue hemorrhagic fever/dengue shock syndrome (DHF/DSS) through ADE if they are exposed to another serotype of DENV subsequently [46]. The recent outbreaks of ZIKV further complicate vaccine development for flaviviruses as antibodies against DENV, WNV, and ZIKV have been shown to cross-react and enhance the replication of each other both in cell culture and in mice infection studies, suggesting ADE can occur between these related viruses, which are co-circulating in many parts of the world [7, 47–49]. Therefore, minimizing ADE with heterologous flavivirus infection must be an important consideration for WNV vaccine development.
Structural studies have indicated that domain I and II (DI and DII) of the E protein are highly conserved among flaviviruses [50]. Likewise, the majority of flavivirus cross-reactive but subneutralizing antibodies in human humoral response to flavivirus E protein are targeted to epitopes on DII or DI [49, 51, 52]. In contrast, antibodies against DIII epitopes are overall virus-specific, have potent neutralizing activity, and many are protective against flavivirus challenge in mice [12, 49, 53]. For example, antibodies against ZIKV DIII did not enhance DENV infection, while ZIKV DI and DII-specific antibodies exhibited strong ADE activity both in vitro and in vivo [49]. Of note, our results provided direct evidence that antibodies induced by plant-DIII did not enhance ZIKA or DENV infection in vitro, warranting further testing in animal models. In contrast, all other current WNV vaccine candidates based on inactivated, chimeric virus, or DNA, have the potential to elicit DI/DII-cross reactive, subneutralizing antibodies that can enhance ZIKV or/and DENV infection in vaccinated subjects. Therefore, our plant-DIII based vaccine may have a safety advantage over the current WNV vaccine candidates in development.
In summary, we have demonstrated potent efficacy of a plant-produced DIII vaccine that protects against WNV infection both in vitro and in vivo. More importantly, we also further demonstrated the lack of ADE activity for ZIKV and DENV infection in vitro with our plant-DIII vaccine candidate. To our knowledge, this is the first report of a WNV subunit vaccine that induces protective immunity, while circumventing the induction of cross-reactive antibodies that may enhance ZIKV and DENV infection via ADE. Altogether, our study has provided a proof-of-principle for further development of an effective and potentially safer recombinant protein-based subunit vaccine against WNV infection.
Supplementary Material
Acknowledgments
The authors thank Dr. J. Anderson at the Connecticut Agricultural Experiment Station for providing WNV-CT2741. We also thank E. Cheng for providing the graphic of a mouse in the figure. This work was supported in part by grants from National Institute of Allergy and Infectious Diseases (NIAID) # U01 AI075549 and # R33AI101329 to QC.
Footnotes
Conflicts of Interest
The authors declare that there is no conflict of interests regarding the publication of this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Petersen LR, Brault AC, Nasci RS. West Nile virus: review of the literature. Jama. 2013;310:308–15. doi: 10.1001/jama.2013.8042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ye Q, Liu Z-Y, Han J-F, Jiang T, Li X-F, Qin C-F. Genomic characterization and phylogenetic analysis of Zika virus circulating in the Americas. Infection, Genetics and Evolution. 2016;43:43–9. doi: 10.1016/j.meegid.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Chang H-H, Huber RG, Bond PJ, Grad YH, Camerini D, Maurer-Stroh S, et al. Systematic analysis of protein identity between Zika virus and other arthropod-borne viruses. Bulletin of the World Health Organization. 2017;95:517–25I. doi: 10.2471/BLT.16.182105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bode AV, Sejvar JJ, Pape WJ, Campbell GL, Marfin AA. West Nile virus disease: a descriptive study of 228 patients hospitalized in a 4-county region of Colorado in 2003. Clinical Infect Dis. 2006;42:1234–40. doi: 10.1086/503038. [DOI] [PubMed] [Google Scholar]
- 5.Diamond MS, Klein RS. A genetic basis for human susceptibility to West Nile virus. Trends Microbiol. 2006;14:287–9. doi: 10.1016/j.tim.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Hart J, Tillman G, Kraut MA, Chiang H-S, Strain JF, Li Y, et al. West Nile virus neuroinvasive disease: neurological manifestations and prospective longitudinal outcomes. BMC Infectious Diseases. 2014;14:248. doi: 10.1186/1471-2334-14-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bardina SV, Bunduc P, Tripathi S, Duehr J, Frere JJ, Brown JA, et al. Enhancement of Zika virus pathogenesis by preexisting antiflavivirus immunity. Science. 2017;356:175–80. doi: 10.1126/science.aal4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morens DM. Antibody-dependent of enhancement of infection and the pathogenesis of viral disease. Clin Inf Dis. 1994;19:500–12. doi: 10.1093/clinids/19.3.500. [DOI] [PubMed] [Google Scholar]
- 9.Oliphant T, Engle M, Nybakken G, Doane C, Johnson S, Huang L, et al. Development of a humanized monoclonal antibody with therapeutic potential against West Nile virus. Nature Medicine. 2005;11:522–30. doi: 10.1038/nm1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Belmusto-Worn VE, Sanchez JL, McCarthy K, Nichols R, Bautista CT, Magill AJ, et al. Randomized, double-blind, phase III. Pivotal field trial of the comparative immunogenicity, safety, and tolerability of two yellow fever 17D vaccines (ARILVAX™ and YF-VAX®) in healthy infants and children in Peru. American Journal of Tropical Medicine and Hygiene. 2005;72:189–97. [PubMed] [Google Scholar]
- 11.Heinz FX, Holzmann H, Essl A, Kundi M. Field effectiveness of vaccination against tick-borne encephalitis. Vaccine. 2007;25:7559–67. doi: 10.1016/j.vaccine.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 12.Zhao H, Fernandez E, Dowd Kimberly A, Speer Scott D, Platt Derek J, Gorman Matthew J, et al. Structural Basis of Zika Virus-Specific Antibody Protection. Cell. 2016;166:1016–27. doi: 10.1016/j.cell.2016.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alonso-Padilla J, Jiménez de Oya N, Blázquez A-B, Escribano-Romero E, Escribano JM, Saiz J-C. Recombinant West Nile virus envelope protein E and domain III expressed in insect larvae protects mice against West Nile disease. Vaccine. 2011;29:1830–5. doi: 10.1016/j.vaccine.2010.12.081. [DOI] [PubMed] [Google Scholar]
- 14.Chu JJ, Rajamanonmani R, Li J, Bhuvanakantham R, Lescar J, Ng ML. Inhibition of West Nile virus entry by using a recombinant domain III from the envelope glycoprotein. J Gen Virol. 2005;86:405–12. doi: 10.1099/vir.0.80411-0. [DOI] [PubMed] [Google Scholar]
- 15.He J, Peng L, Lai H, Hurtado J, Stahnke J, Chen Q. A Plant-Produced Antigen Elicits Potent Immune Responses against West Nile Virus in Mice. Biomed Res Int. 2014;2014:10. doi: 10.1155/2014/952865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leuzinger K, Dent M, Hurtado J, Stahnke J, Lai H, Zhou X, et al. Efficient Agroinfiltration of Plants for High-level Transient Expression of Recombinant Proteins. Journal of Visualized Experiments. 2013 doi: 10.3791/50521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Q, Dent M, Hurtado J, Stahnke J, McNulty A, Leuzinger K, et al. Transient Protein Expression by Agroinfiltration in Lettuce. Methods in molecular biology (Clifton, NJ) 2016;1385:55–67. doi: 10.1007/978-1-4939-3289-4_4. [DOI] [PubMed] [Google Scholar]
- 18.Chen Q, Davis K. The potential of plants as a system for the development and production of human biologics. F1000Research. 2016;5 doi: 10.12688/f1000research.8010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Q. Expression and Purification of Pharmaceutical Proteins in Plants. Biological Engineering. 2008;1:291–321. [Google Scholar]
- 20.Tuse D, Tu T, McDonald K. Manufacturing Economics of Plant-Made Biologics: Case Studies in Therapeutic and Industrial Enzymes. Biomed Res Int. 2014;2014:10. doi: 10.1155/2014/256135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Q, Lai H. Gene delivery into plant cells for recombinant protein production. Biomed Res Int. 2014;2014:10. doi: 10.1155/2015/932161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen Q. Advances in Botanical Research. Academic Press; 2018. Recombinant Therapeutic Molecules Produced in Plants. [Google Scholar]
- 23.Lai H, Engle M, Fuchs A, Keller T, Johnson S, Gorlatov S, et al. Monoclonal antibody produced in plants efficiently treats West Nile virus infection in mice. Proc Natl Acad Sci U S A. 2010;107:2419–24. doi: 10.1073/pnas.0914503107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang M, Dent M, Lai H, Sun H, Chen Q. Immunization of Zika virus envelope protein domain III induces specific and neutralizing immune responses against Zika virus. Vacccine. 2017;35:4287–94. doi: 10.1016/j.vaccine.2017.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dent M, Hurtado J, Paul AM, Sun H, Lai H, Yang M, et al. Plant-produced anti-dengue virus monoclonal antibodies exhibit reduced antibody-dependent enhancement of infection activity. J Gen Virol. 2016;97:3280–90. doi: 10.1099/jgv.0.000635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paul AM, Shi Y, Acharya D, Douglas JR, Cooley A, Anderson JF, et al. Delivery of antiviral small interfering RNA with gold nanoparticles inhibits dengue virus infection in vitro. J Gen Virol. 2014;95:1712–22. doi: 10.1099/vir.0.066084-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang M, Sun H, Lai H, Hurtado J, Chen Q. Plant-produced Zika virus envelope protein elicits neutralizing immune responses that correlate with protective immunity against Zika virus in mice. Plant Biotechnology Journal. 2017 doi: 10.1111/pbi.12796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang M, Lai H, Sun H, Chen Q. Virus-like particles that display Zika virus envelope protein domain III induce potent neutralizing immune responses in mice. Scientific reports. 2017;7:7679. doi: 10.1038/s41598-017-08247-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hombach J, Solomon T, Kurane I, Jacobson J, Wood D. Report on a WHO consultation on immunological endpoints for evaluation of new Japanese encephalitis vaccines, WHO, Geneva, 2–3 September, 2004. Vaccine. 2005;23:5205–11. doi: 10.1016/j.vaccine.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 30.Kreil TR, Burger I, Bachmann M, Fraiss S, Eibl MM. Antibodies protect mice against challenge with tick-borne encephalitis virus (TBEV)-infected macrophages. Clinical & Experimental Immunology. 1997;110:358–61. doi: 10.1046/j.1365-2249.1997.4311446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mason RA, Tauraso NM, Spertzel RO, Ginn RK. Yellow fever vaccine: direct challenge of monkeys given graded doses of 17D vaccine. Appl Microbiol. 1973;25:539–44. doi: 10.1128/am.25.4.539-544.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Larocca RA, Abbink P, Peron JPS, de A, Zanotto PM, Iampietro MJ, Badamchi-Zadeh A, et al. Vaccine protection against Zika virus from Brazil. Nature. 2016;536:474–8. doi: 10.1038/nature18952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crill WD, Chang GJ. Localization and characterization of flavivirus envelope glycoprotein cross-reactive epitopes. J Virol. 2004;78:13975–86. doi: 10.1128/JVI.78.24.13975-13986.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pinto AK. A Hydrogen Peroxide-Inactivated Virus Vaccine Elicits Humoral and Cellular Immunity and Protects against Lethal West Nile Virus Infection in Aged Mice. J Virol. 2013;87:1926. doi: 10.1128/JVI.02903-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Minke JM, Siger L, Karaca K, Austgen L, Gordy P, Bowen R, et al. Recombinant canarypoxvirus vaccine carrying the prM/E genes of West Nile virus protects horses against a West Nile virus-mosquito challenge. Arch Virol Suppl. 2004:221–30. doi: 10.1007/978-3-7091-0572-6_20. [DOI] [PubMed] [Google Scholar]
- 36.Monath TP, Liu J, Kanesa-Thasan N, Myers GA, Nichols R, Deary A, et al. A live, attenuated recombinant West Nile virus vaccine. Proc Natl Acad Sci U S A. 2006 doi: 10.1073/pnas.0601932103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun H, Chen Q, Lai H. Development of Antibody Therapeutics against Flaviviruses. International Journal of Molecular Sciences. 2018;19:54. doi: 10.3390/ijms19010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martina BE, Koraka P, van den Doel P, van Amerongen G, Rimmelzwaan GF, Osterhaus ADME. Immunization with West Nile virus envelope domain III protects mice against lethal infection with homologous and heterologous virus. Vaccine. 2008;26:153–7. doi: 10.1016/j.vaccine.2007.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.He J, Lai H, Engle M, Gorlatov S, Gruber C, Steinkellner H, et al. Generation and Analysis of Novel Plant-Derived Antibody-Based Therapeutic Molecules against West Nile Virus. PLoS ONE. 2014;9:e93541. doi: 10.1371/journal.pone.0093541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lai H, He J, Hurtado J, Stahnke J, Fuchs A, Mehlhop E, et al. Structural and functional characterization of an anti-West Nile virus monoclonal antibody and its single-chain variant produced in glycoengineered plants. Plant Biotechnology Journal. 2014;12:1098–107. doi: 10.1111/pbi.12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vogt MR, Moesker B, Goudsmit J, Jongeneelen M, Austin SK, Oliphant T, et al. Human monoclonal antibodies against West Nile virus induced by natural infection neutralize at a postattachment step. J Virol. 2009;83:6494–507. doi: 10.1128/JVI.00286-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Demento SL, Bonafe N, Cui W, Kaech SM, Caplan MJ, Fikrig E, et al. TLR9-Targeted Biodegradable Nanoparticles as Immunization Vectors Protect against West Nile Encephalitis. The Journal of Immunology. 2010;185:2989–97. doi: 10.4049/jimmunol.1000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lindblad EB. Aluminium compounds for use in vaccines. Immunol Cell Biol. 2004;82:497–505. doi: 10.1111/j.0818-9641.2004.01286.x. [DOI] [PubMed] [Google Scholar]
- 44.Firbas C, Jilma B. Product review on the JE vaccine IXIARO. Human Vaccines & Immunotherapeutics. 2015;11:411–20. doi: 10.4161/21645515.2014.983412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Phoolcharoen W, Dye JM, Kilbourne J, Piensook K, Pratt WD, Arntzen CJ, et al. A nonreplicating subunit vaccine protects mice against lethal Ebola virus challenge. Proc Natl Acad Sci U S A. 2011;108:20695–700. doi: 10.1073/pnas.1117715108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Halstead SB. Dengue Antibody-Dependent Enhancement: Knowns and Unknowns. Microbiology spectrum. 2014:2. doi: 10.1128/microbiolspec.AID-0022-2014. [DOI] [PubMed] [Google Scholar]
- 47.Dejnirattisai W, Supasa P, Wongwiwat W, Rouvinski A, Barba-Spaeth G, Duangchinda T, et al. Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus. Nat Immunol. 2016 doi: 10.1038/ni.3515. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barba-Spaeth G, Dejnirattisai W, Rouvinski A, Vaney M-C, Medits I, Sharma A, et al. Structural basis of potent Zika–dengue virus antibody cross-neutralization. Nature. 2016;536:48–53. doi: 10.1038/nature18938. [DOI] [PubMed] [Google Scholar]
- 49.Stettler K, Beltramello M, Espinosa DA, Graham V, Cassotta A, Bianchi S, et al. Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection. Science. 2016;353:823. doi: 10.1126/science.aaf8505. [DOI] [PubMed] [Google Scholar]
- 50.Sirohi D, Chen Z, Sun L, Klose T, Pierson TC, Rossmann MG, et al. The 3.8 Å resolution cryo-EM structure of Zika virus. Science. 2016;352:467–70. doi: 10.1126/science.aaf5316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beltramello M, Williams KL, Simmons CP, Macagno A, Simonelli L, Quyen Nguyen Than H, et al. The Human Immune Response to Dengue Virus Is Dominated by Highly Cross-Reactive Antibodies Endowed with Neutralizing and Enhancing Activity. Cell host & microbe. 2010;8:271–83. doi: 10.1016/j.chom.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin HE, Tsai WY, Liu IJ, Li PC, Liao MY, Tsai JJ, et al. Analysis of epitopes on dengue virus envelope protein recognized by monoclonal antibodies and polyclonal human sera by a high throughput assay. PLoS Negl Trop Dis. 2012;6:e1447. doi: 10.1371/journal.pntd.0001447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oliphant T, Nybakken GE, Austin SK, Xu Q, Bramson J, Loeb M, et al. Induction of Epitope-Specific Neutralizing Antibodies against West Nile Virus. J Virol. 2007;81:11828–39. doi: 10.1128/JVI.00643-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.