We read with interest the recent work by Uhde et al,1 which demonstrated that physically asymptomatic non-coeliac gluten/wheat sensitivity involves compromised intestinal epithelium barrier dysfunction in conjunction with systemic immune activation events. We also read with interest the recent work by Marchesi et al,2 which comprehensively reviewed the role of microbiota in physical disorders of the gut and extra-gut organs.
But common to these Gut papers was the lack of accounting for anxiety and depression, comorbidities often experienced in gastroenterology clinics. Patients who are otherwise physically asymptomatic often do not explicitly divulge these mental disorders, or the disorders are unintentionally overlooked, yet they report ‘diminished quality of life’.
In response to this gap, we explored roles of dysbiosis and gut barrier integrity in individuals physically asymptomatic for gastrointestinal distress, yet nonetheless experiencing mental distress. We hypothesised that anxiety and depressive disorders are linked to human gut dysbiosis with microbiota that secrete lipopolysaccharide (LPS) endotoxin into plasma, which in conjunction with compromised gut barrier integrity has systemic manifestations including the brain. We further hypothesised that this correlates with altered intestinal epithelium paracellular integrity molecules discharged into blood as biomarkers, namely zonulin and intestinal fatty acid-binding protein-2 (FABP2). Zonulin is the master regulator of endothelial and epithelium tight junctions, modulating both gut and blood–brain barriers.3
We analysed stool faecal microbiota and plasma in 50 subjects who self-reported to be asymptomatic for gastrointestinal physical disorders; 22 volunteers met Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition criteria for a depressive disorder or an anxiety disorder (DEP/ANX), and 28 more were control reference subjects (NODEPANX). Subjects refrained from probiotics and antibiotics during 30 days prior to providing samples (see online supplementary methods).
LEfSe analyses (online supplementary figures S1 and S2) revealed gut dysbiosis in the DEP/ANX subjects, in contrast to microbiome of control subjects. PICRUSt analyses (figure 1) predicted KEGG orthologue gene differences consistent with a pathophysiological gut metagenomic signature in DEP/ANX versus NODEPANX microbiome. Notable in figure 1 were over-represented LPS biosynthesis genes in the gut microbiome of DEP/ANX subjects. Over-represented KEGG genes of DEP/ANX microbiome (figure 1) also reflected deleterious metabolism of mood neurotransmitter pathways and host intestinal protective glycosaminoglycan mucins, in contrast to beneficial counterparts in controls.
Figure 1.
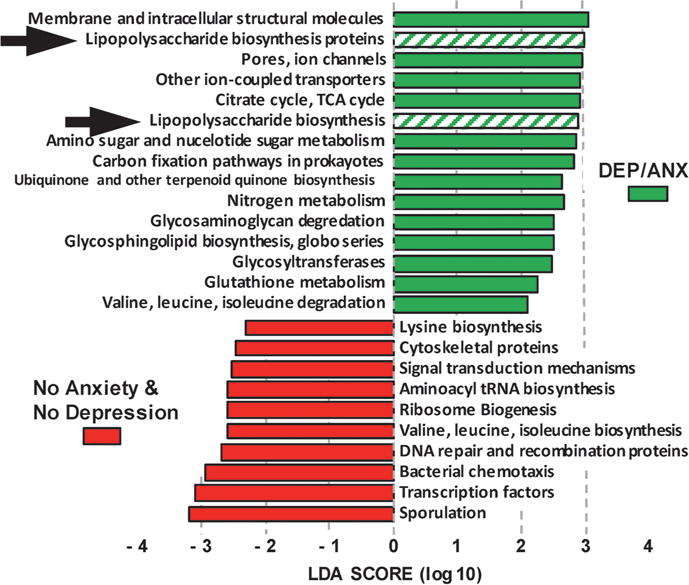
PICRUSt metagenome KEGG orthologue gene distribution in microbiomes of depressive disorder or an anxiety disorder (DEP/ANX) versus control subjects (see online supplementary material). LDA, linear discriminate analysis; TCA, tricarboxylic acid; tRNA, transfer RNA.
Figure 2 shows that plasma levels of LPS, zonulin and FABP2 were each significantly elevated in DEP/ANX versus NODEPANX controls. The enhanced plasma LPS in DEP/ANX likely derived from the skewed distribution of gram-negative taxa of this cohort (see online supplementary figures S1 and S2) with their attending over-represented LPS biosynthesis genes (figure 1). FABP2 and zonulin are biomarkers of gut epithelium tight junction barrier integrity, reportedly upregulated and released by the presence of dysbiotic microbiota.3,4 Uhde et al1 assayed FABP2, but not zonulin nor LPS.
Figure 2.
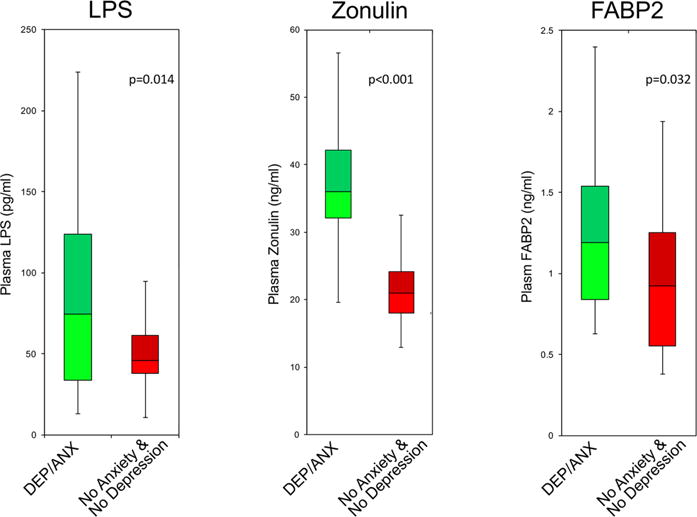
Plasma LPS endotoxin, zonulin and fatty acid-binding protein-2 (FABP2) blood markers of gut dysbiosis and gut permeability pathophysiological epithelium integrity.3,4 Analysis of variance yielded significant mean differences (p<0.05) (see online supplementary material).
These results support our hypothesis that anxiety and depressive disorders are associated with gut dysbiosis linked with discharge of intestinal epithelial integrity molecules into blood of subjects asymptomatic for gastrointestinal physical distress. They provide evidence substantiating prior human and preclinical studies of mental illness models that predicted attending gut dysbiosis and altered intestinal barrier integrity.5–7
In conclusion, these data represent the first correlation of human gut dysbiosis with the tripartite consensus clustering of zonulin, FABP2 and LPS plasma biomarkers of increased gut permeability in individuals with DEP/ANX disorders versus NODEPANX controls. These findings highlight that gut can be considered a novel target for DEP/ANX management, notably in individuals physically asymptomatic for gastrointestinal distress.
Supplementary Material
Acknowledgments
We thank Sara Croft for technical assistance.
Funding University of Florida Clinical and Translational Science Institute grant (BRS, CJP) from the National Center For Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR001427; National Institute of Health (NIH) grants HL33610, HL56921 (MKR, CJP); UM1 HL087366 (CJP); Gatorade Trust through funds distributed by the University of Florida, Department of Medicine (CJP); PCORI-OneFlorida Clinical Research Consortium CDRN-1501-26692 (CJP); and internal funds from the University of Florida Department of Physiology and Functional Genomics (BRS, MKR).
Footnotes
Correction notice This article has been corrected since it was published Online First. Figure 2 has been replaced.
Contributors BRS and MKR had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: BRS, MKW, CJP. Acquisition, analysis, interpretation of data: BRS, MKR, KS, EMR, RG, RCH. Drafting of the manuscript: BRS, MKR, CJP. Critical revision of the manuscript: BRS, MKW, CJP, KS, EMR, RCH. Statistical analysis: BRS, EMR. Obtained funding: BRS, MKR, CJP. Administrative, technical or material support: EMR, KS, CJP. Study supervision: BRS.
Disclaimer The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Competing interests None declared.
Patient consent Detail has been removed from this case description/these case descriptions to ensure anonymity. The editors and reviewers have seen the detailed information available and are satisfied that the information backs up the case the authors are making.
Ethics approval The University of Florida Institutional Review Board approved the study.
Provenance and peer review Not commissioned; internally peer reviewed.
Data sharing statement Unpublished data may be accessed from the authors.
► Additional material is published online only. To view please visit the journal online (http://dx.doi.org/10.1136/gutjnl-2017-314759).
References
- 1.Uhde M, Ajamian M, Caio G, et al. Intestinal cell damage and systemic immune activation in individuals reporting sensitivity to wheat in the absence of coeliac disease. Gut. 2016;65:1930–7. doi: 10.1136/gutjnl-2016-311964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marchesi JR, Adams DH, Fava F, et al. The gut microbiota and host health: a new clinical frontier. Gut. 2016;65:330–9. doi: 10.1136/gutjnl-2015-309990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sturgeon C, Fasano A, Zonulin FA. Zonulin, a regulator of epithelial and endothelial barrier functions, and its involvement in chronic inflammatory diseases. Tissue Barriers. 2016;4:e1251384. doi: 10.1080/21688370.2016.1251384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guerrant RL, Leite AM, Pinkerton R, et al. Biomarkers of environmental enteropathy, inflammation, stunting, and impaired growth in children in northeast Brazil. PLoS One. 2016;11:e0158772. doi: 10.1371/journal.pone.0158772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vuong HE, Yano JM, Fung TC, et al. The microbiome and host behavior. Annu Rev Neurosci. 2017 doi: 10.1146/annurev-neuro-072116-031347. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lasselin J, Elsenbruch S, Lekander M, et al. Mood disturbance during experimental endotoxemia: predictors of state anxiety as a psychological component of sickness behavior. Brain Behav Immun. 2016;57:30–7. doi: 10.1016/j.bbi.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 7.Grigoleit JS, Kullmann JS, Wolf OT, et al. Dose-dependent effects of endotoxin on neurobehavioral functions in humans. PLoS One. 2011;6:e28330. doi: 10.1371/journal.pone.0028330. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


