Introduction
Piezo proteins are large membrane proteins which assemble to form mechanically activated Ca2+‐permeable non‐selective cationic channels (Coste et al. 2010, 2012; Murthy et al. 2017; Wu et al. 2017). They serve to regulate membrane potential and Ca2+ signalling coupled to downstream effectors such as calpain in cells of mammals and other classes (Coste et al. 2010, 2012; Li et al. 2014; Murthy et al. 2017; Rode et al. 2017; Wu et al. 2017). They are a distinct type of ion channel subunit which assembles as trimers with a central ion‐conducting pore covered by a single cap and three complex arms reaching out into and curving the membrane (Ge et al. 2015; Guo & MacKinnon, 2017; Saotome et al. 2018; Zhao et al. 2018) (Fig. 1). The last two C‐terminal transmembrane segments (TMs) form the functional pore module (Zhao et al. 2016) while the rest of the protein comprises nine repetitive units of four TMs assembled into a highly curved peripheral blade‐like structure which is critical for mechano‐sensing and transduction (Zhao et al. 2018; Fig. 1). The channels are inherent sensors of membrane tension and increases in this tension seem to be the primary physiological activator (Lewis & Grandl, 2015; Cox et al. 2016; Syeda et al. 2016). Activation or sensitisation to membrane tension occurs in response to a synthetic small molecule called Yoda1, which is a useful pharmacological tool (Syeda et al. 2015).
Figure 1. Structure and topology of the mechanosensitive Piezo1 channel.
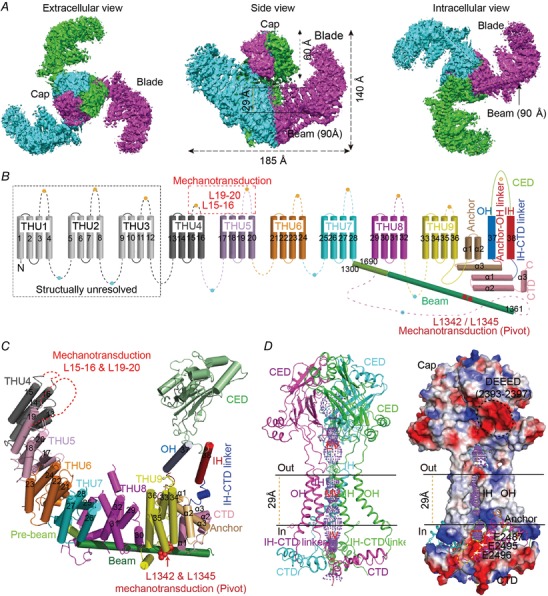
A, the three‐bladed, propeller‐like cryo‐EM structure of the Piezo1 ion channel. B, nine repetitive transmembrane helical units (THUs) and the 38‐TM topology model. C, a cartoon model showing one subunit with featured structural domains labelled. D, the ion‐conducting pore module shown as a ribbon diagram (left) and surface electrostatic potential (right). The functionally identified regions and residues critical for mechanical activation of Piezo1 are indicated in B and C. Figure reproduced with permission from Zhao et al. (2018).
The Piezo proteins are widely expressed and a range of functions is emerging. Piezo1, for example, regulates epithelial cell crowding and division (Gudipaty et al. 2017), is critical for endothelial shear stress‐sensing and vascular development (Li et al. 2014), regulates blood pressure and exercise performance (Rode et al. 2017), and determines neural stem cell lineage (Pathak et al. 2014). Mutations in the human PIEZO1 gene cause anaemia (dehydrated stomatocytosis), consistent with the importance of Piezo1 channels in erythrocyte function (Zarychanski et al. 2012), and generalised lymphatic dysplasia, consistent with functional importance in lymphatic endothelial cells (Fotiou et al. 2015). Piezo2 is important in touch sensation (Woo et al. 2014; Chesler et al. 2016) and airway stretch sensation mediated by sensory neurones (Nonomura et al. 2017). Mutations in the PIEZO2 gene cause distal arthrogryposis and other diseases (Coste et al. 2013; Alper, 2017).
Focus of the review series
Here we present a review series associated with our ‘Piezo channel mechanisms and disease’ symposium at the International Union of Physiological Sciences (IUPS) congress in Rio de Janeiro on 3 August 2017. The series focuses on three key topics from the symposium: Piezo1 channel structure (Wang & Xiao, 2018), Piezo1 in vascular physiology (Beech, 2018) and Piezo1 in genetic disease (Martin‐Almedina et al. 2018). The structure article reviews the breakthrough in determining the tri‐blade propeller‐like arrangement of Piezo1 channels and discusses the hypothesis that the channels comprise discrete mechano‐transduction and ion‐conducting modules which coordinate to fulfil the overall purpose of the channels (Wang & Xiao, 2018). The physiology article reviews current knowledge of the role of Piezo1 channels in the endothelium, discussing the hypothesis that the channels are key sensors of the frictional force of blood flow, leading them to be essential in vascular development and necessary for redistribution of blood flow in exercise and optimal physical performance (Beech, 2018). The disease article reviews PIEZO1 mutations which cause disease, discussing the relationship between stomatocytosis and lymphatic dysplasia and the challenges of understanding the disease consequences of loss‐of‐function and gain‐of‐function mutations (Martin‐Almedina et al. 2018).
Conclusion and perspective
Piezo channels are relatively newly discovered – perhaps one of the last major ion channel families which had to be identified. What has been particularly striking has been the unanimous agreement from independent investigators across the world that these channels are indeed bona fide sensors of membrane tension in mammalian and other cell types and that their primary biological purpose is likely to be as sensors of mechanical force and transducers of this force into biological effect. Because of the importance of mechanical force sensation in biology, the implications are substantial. The symposium reviews provide important insight into this new field but should naturally be seen alongside the wider field and literature. As we are at the beginning of the Piezo era of biological discovery, we can expect and hope for many more original research articles on this topic as well as more symposia – small and large – and review articles as this field achieves its true potential and perhaps position alongside other great fields such as those focused on other membrane proteins such as the ionotropic glutamate receptors and tyrosine kinase receptors. We encourage you to read and enjoy the review articles and join the Piezo field.
Additional information
Competing interests
None of the authors has any conflicts of interests.
Acknowledgements
We thank The Physiological Society and IUPS for the opportunity and conference framework and The Journal of Physiology for sponsoring the symposium. Our research is supported by research grants from the Wellcome Trust (110044/Z/15/Z), British Heart Foundation (RG/17/11/33042) and Medical Research Council (MR/L019051/1) (D.J.B.) and the National Key R&D Program of China (2016YFA0500402, 2015CB910102) and the National Natural Science Foundation of China (31630090) (B.X.).
Edited by: Ole Petersen
References
- Alper SL (2017). Genetic diseases of PIEZO1 and PIEZO2 dysfunction. Curr Top Membr 79, 97–134. [DOI] [PubMed] [Google Scholar]
- Beech DJ (2018). Endothelial Piezo1 channels as sensors of exercise. J Physiol 596, 979–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesler AT, Szczot M, Bharucha‐Goebel D, Ceko M, Donkervoort S, Laubacher C, Hayes LH, Alter K, Zampieri C, Stanley C, Innes AM, Mah JK, Grosmann CM, Bradley N, Nguyen D, Foley AR, Le Pichon CE & Bonnemann CG (2016). The role of PIEZO2 in human mechanosensation. N Engl J Med 375, 1355–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste B, Houge G, Murray MF, Stitziel N, Bandell M, Giovanni MA, Philippakis A, Hoischen A, Riemer G, Steen U, Steen VM, Mathur J, Cox J, Lebo M, Rehm H, Weiss ST, Wood JN, Maas RL, Sunyaev SR & Patapoutian A (2013). Gain‐of‐function mutations in the mechanically activated ion channel PIEZO2 cause a subtype of Distal Arthrogryposis. Proc Natl Acad Sci USA 110, 4667–4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste B, Mathur J, Schmidt M, Earley TJ, Ranade S, Petrus MJ, Dubin AE & Patapoutian A (2010). Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 330, 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coste B, Xiao B, Santos JS, Syeda R, Grandl J, Spencer KS, Kim SE, Schmidt M, Mathur J, Dubin AE, Montal M & Patapoutian A (2012). Piezo proteins are pore‐forming subunits of mechanically activated channels. Nature 483, 176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox CD, Bae C, Ziegler L, Hartley S, Nikolova‐Krstevski V, Rohde PR, Ng CA, Sachs F, Gottlieb PA & Martinac B (2016). Removal of the mechanoprotective influence of the cytoskeleton reveals PIEZO1 is gated by bilayer tension. Nat Commun 7, 10366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotiou E, Martin‐Almedina S, Simpson MA, Lin S, Gordon K, Brice G, Atton G, Jeffery I, Rees DC, Mignot C, Vogt J, Homfray T, Snyder MP, Rockson SG, Jeffery S, Mortimer PS, Mansour S & Ostergaard P (2015). Novel mutations in PIEZO1 cause an autosomal recessive generalized lymphatic dysplasia with non‐immune hydrops fetalis. Nat Commun 6, 8085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge J, Li W, Zhao Q, Li N, Chen M, Zhi P, Li R, Gao N, Xiao B & Yang M (2015). Architecture of the mammalian mechanosensitive Piezo1 channel. Nature 527, 64–69. [DOI] [PubMed] [Google Scholar]
- Gudipaty SA, Lindblom J, Loftus PD, Redd MJ, Edes K, Davey CF, Krishnegowda V & Rosenblatt J (2017). Mechanical stretch triggers rapid epithelial cell division through Piezo1. Nature 543, 118–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo YR & MacKinnon R (2017). Structure‐based membrane dome mechanism for Piezo mechanosensitivity. Elife 6, e33660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis AH & Grandl J (2015). Mechanical sensitivity of Piezo1 ion channels can be tuned by cellular membrane tension. Elife 4, e12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Hou B, Tumova S, Muraki K, Bruns A, Ludlow MJ, Sedo A, Hyman AJ, McKeown L, Young RS, Yuldasheva NY, Majeed Y, Wilson LA, Rode B, Bailey MA, Kim HR, Fu Z, Carter DA, Bilton J, Imrie H, Ajuh P, Dear TN, Cubbon RM, Kearney MT, Prasad RK, Evans PC, Ainscough JF & Beech DJ (2014). Piezo1 integration of vascular architecture with physiological force. Nature 515, 279–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin‐Almedina S, Mansour S & Ostergaard P (2018). Human phenotypes caused by PIEZO1 mutations; one gene, two overlapping phenotypes? J Physiol 596, 985–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy SE, Dubin AE & Patapoutian A (2017). Piezos thrive under pressure: mechanically activated ion channels in health and disease. Nat Rev Mol Cell Biol 18, 771–783. [DOI] [PubMed] [Google Scholar]
- Nonomura K, Woo SH, Chang RB, Gillich A, Qiu Z, Francisco AG, Ranade SS, Liberles SD & Patapoutian A (2017). Piezo2 senses airway stretch and mediates lung inflation‐induced apnoea. Nature 541, 176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak MM, Nourse JL, Tran T, Hwe J, Arulmoli J, Le DT, Bernardis E, Flanagan LA & Tombola F (2014). Stretch‐activated ion channel Piezo1 directs lineage choice in human neural stem cells. Proc Natl Acad Sci USA 111, 16148–16153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rode B, Shi J, Endesh N, Drinkhill MJ, Webster PJ, Lotteau SJ, Bailey MA, Yuldasheva NY, Ludlow MJ, Cubbon RM, Li J, Futers TS, Morley L, Gaunt HJ, Marszalek K, Viswambharan H, Cuthbertson K, Baxter PD, Foster R, Sukumar P, Weightman A, Calaghan SC, Wheatcroft SB, Kearney MT & Beech DJ (2017). Piezo1 channels sense whole body physical activity to reset cardiovascular homeostasis and enhance performance. Nat Commun 8, 350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saotome K, Murthy SE, Kefauver JM, Whitwam T, Patapoutian A & Ward AB (2018). Structure of the mechanically activated ion channel Piezo1. Nature 554, 481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syeda R, Florendo MN, Cox CD, Kefauver JM, Santos JS, Martinac B & Patapoutian A (2016). Piezo1 channels are inherently mechanosensitive. Cell Rep 17, 1739–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syeda R, Xu J, Dubin AE, Coste B, Mathur J, Huynh T, Matzen J, Lao J, Tully DC, Engels IH, Petrassi HM, Schumacher AM, Montal M, Bandell M & Patapoutian A (2015). Chemical activation of the mechanotransduction channel Piezo1. Elife 4, e07369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y & Xiao B (2018). The mechanosensitive Piezo1 channel: structural features and molecular bases underlying its ion permeation and mechanotransduction. J Physiol 596, 969–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo SH, Ranade S, Weyer AD, Dubin AE, Baba Y, Qiu Z, Petrus M, Miyamoto T, Reddy K, Lumpkin EA, Stucky CL & Patapoutian A (2014). Piezo2 is required for Merkel‐cell mechanotransduction. Nature 509, 622–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Lewis AH & Grandl J (2017). Touch, tension, and transduction – the function and regulation of Piezo ion channels. Trends Biochem Sci 42, 57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarychanski R, Schulz VP, Houston BL, Maksimova Y, Houston DS, Smith B, Rinehart J & Gallagher PG (2012). Mutations in the mechanotransduction protein PIEZO1 are associated with hereditary xerocytosis. Blood 120, 1908–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Wu K, Geng J, Chi S, Wang Y, Zhi P, Zhang M & Xiao B (2016). Ion permeation and mechanotransduction mechanisms of mechanosensitive piezo channels. Neuron 89, 1248–1263. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Zhou H, Chi S, Wang Y, Wang J, Geng J, Wu K, Liu W, Zhang T, Dong M‐Q, Wang J, Li X & Xiao B (2018). Structure and mechanogating mechanism of the Piezo1 channel. Nature 554, 487–492. [DOI] [PubMed] [Google Scholar]


