Abstract
Key points
Although they are unable to utilize muscle glycogen, McArdle mice adapt favourably to an individualized moderate‐intensity endurance exercise training regime. Yet, they fail to reach the performance capacity of healthy mice with normal glycogen availability.
There is a remarkable difference in the protein networks involved in muscle tissue adaptations to endurance exercise training in mice with and without glycogen availability.
Indeed, endurance exercise training promoted the expression of only three proteins common to both McArdle and wild‐type mice: LIMCH1, PARP1 and TIGD4.
In turn, trained McArdle mice presented strong expression of mitogen‐activated protein kinase 12 (MAPK12).
Abstract
McArdle's disease is an inborn disorder of skeletal muscle glycogen metabolism that results in blockade of glycogen breakdown due to mutations in the myophosphorylase gene. We recently developed a mouse model carrying the homozygous p.R50X common human mutation (McArdle mouse), facilitating the study of how glycogen availability affects muscle molecular adaptations to endurance exercise training. Using quantitative differential analysis by liquid chromatography with tandem mass spectrometry, we analysed the quadriceps muscle proteome of 16‐week‐old McArdle (n = 5) and wild‐type (WT) (n = 4) mice previously subjected to 8 weeks’ moderate‐intensity treadmill training or to an equivalent control (no training) period. Protein networks enriched within the differentially expressed proteins with training in WT and McArdle mice were assessed by hypergeometric enrichment analysis. Whereas endurance exercise training improved the estimated maximal aerobic capacity of both WT and McArdle mice as compared with controls, it was ∼50% lower than normal in McArdle mice before and after training. We found a remarkable difference in the protein networks involved in muscle tissue adaptations induced by endurance exercise training with and without glycogen availability, and training induced the expression of only three proteins common to McArdle and WT mice: LIM and calponin homology domains‐containing protein 1 (LIMCH1), poly (ADP‐ribose) polymerase 1 (PARP1 – although the training effect was more marked in McArdle mice), and tigger transposable element derived 4 (TIGD4). Trained McArdle mice presented strong expression of mitogen‐activated protein kinase 12 (MAPK12). Through an in‐depth proteomic analysis, we provide mechanistic insight into how glycogen availability affects muscle protein signalling adaptations to endurance exercise training.
Keywords: Glycogenosis type V, McArdle disease, proteome, signalling networks, training, exercise
Key points
Although they are unable to utilize muscle glycogen, McArdle mice adapt favourably to an individualized moderate‐intensity endurance exercise training regime. Yet, they fail to reach the performance capacity of healthy mice with normal glycogen availability.
There is a remarkable difference in the protein networks involved in muscle tissue adaptations to endurance exercise training in mice with and without glycogen availability.
Indeed, endurance exercise training promoted the expression of only three proteins common to both McArdle and wild‐type mice: LIMCH1, PARP1 and TIGD4.
In turn, trained McArdle mice presented strong expression of mitogen‐activated protein kinase 12 (MAPK12).
Introduction
The importance of endogenous muscle glycogen as a primary fuel source during exertion (particularly for intense endurance bouts) has been a fundamental concept in exercise physiology for half a century (Bergstrom et al. 1967; Pernow & Saltin, 1971). Accordingly, carbohydrate‐rich diets have been traditionally recommended for athletes to ensure the replenishment of muscle glycogen stores to meet the metabolic demands of intense training exercise sessions and competitions (Bartlett et al. 2015). However, training with low muscle glycogen availability might induce some beneficial metabolic adaptations in the muscle tissue, including activation of key cell signalling kinases (e.g. protein kinase, AMP‐activated, catalytic subunit α 1 and p38 mitogen‐activated protein kinase (p38MAPK)), transcription factors (e.g. protein 53 (p53), peroxisome proliferator‐activated receptor delta (PPARδ)) and transcriptional co‐activators (e.g. peroxisome proliferator‐activated receptor‐1α (PGC‐1α)) (Bartlett et al. 2015), increased fat oxidation (Lane et al. 2015), or delayed liver glycogenolysis (Webster et al. 2016). Elucidating the muscle metabolic adaptations to endurance exercise training as a function of glycogen availability is of interest as it may help to gain insight into the mechanisms that mediate the muscle adaptations to this type of training. This issue can be solved effectively by studying McArdle's disease because it allows the investigation of the effects of total unavailability of muscle glycogen on muscle adaptations to endurance exercise training without the need for dietary (e.g. extreme restrictions in carbohydrate intake) or pharmacological manipulations.
McArdle's disease (glycogen storage disease type V, OMIM® 232600) is the most prevalent disorder of muscle glycogen metabolism. This autosomal recessive disease is caused by pathogenic mutations (the most common of which is the stop codon mutation p.R50X) in both alleles of the PYGM gene (MIM#608455) encoding the skeletal‐muscle isoform of glycogen phosphorylase, ‘myophosphorylase’, which leads to total deficiency of the enzyme (Santalla et al. 2014). Because myophosphorylase catalyses the breakdown of glycogen into glucose 1‐phosphate in skeletal muscle fibres, patients are unable to obtain energy from their muscle glycogen stores (Santalla et al. 2014). This disorder provokes ‘exercise intolerance’ in virtually all affected individuals, which typically manifests in the form of acute crises of undue fatigue and muscle pain and stiffness since childhood (Lucia et al. 2012). Paradoxically, patients who are physically active are less severely affected than their inactive peers (Lucia et al. 2012). Prior non‐controlled studies have reported benefits of supervised, moderate‐intensity ‘aerobic’ exercise interventions (60–70% of maximum heart rate) for patients with McArdle's disease, in the form of increased peak oxygen uptake (Haller et al. 2006; Mate‐Munoz et al. 2007) associated with improvements in the muscle levels of two key aerobic enzymes, citrate synthase and β‐hydroxyacyl coenzyme A dehydrogenase (Haller et al. 2006). No other molecular data are, however, available on muscle tissue adaptations to training in these patients.
We recently generated a knock‐in mouse model of McArdle's disease (mice homozygous for the pygm p.R50X mutation; Nogales‐Gadea et al. 2012; Brull et al. 2015). Because this model closely mimics the phenotypes observed in patients (Nogales‐Gadea et al. 2012), it can serve as a useful tool to assess the effects of potential treatment interventions for McArdle's disease, including endurance exercise training. It was therefore the aim of our study to identify key proteins and pathways involved in the endurance exercise training adaptations at the muscle tissue level in McArdle's disease. To do this, we examined, using a controlled design, the effects of submaximal endurance training on the skeletal muscle proteome of wild‐type (wt/wt) and McArdle (p.R50x/p.R50x) mice.
Methods
Ethical approval
The study received ethical institutional (Centre of Energy, Environment and Technical Research, CIEMAT) review board approval (reference number 179/15). Experiments were carried out according to the guidelines laid down by the institution's welfare committee, and conformed to the principles and regulations as described by Grundy (2015). All procedures were carried out according to European and Spanish legislative and regulatory guidelines (European convention ETS 123, on the use and protection of vertebrate mammals in experimentation and for other scientific purposes, and Spanish Law 32/2007, and R.D. 1201/2005 on the protection and use of animals in scientific research). The investigators of the present study understand the ethical principles under which The Journal of Physiology operates and our work complies with its animal ethics checklist. Whenever possible, efforts were made to minimize animal discomfort (see ‘Endurance exercise training intervention in McArdle and wild‐type mice’).
Animals
Founder p.R50X/p.R50X knock‐in mice of mixed genetic background (Nogales‐Gadea et al. 2012) were backcrossed onto the wild‐type CB7Bl/6J background for 10 generations. All the animals were genotyped with LoxP‐F and LoxP‐R as previously reported (Nogales‐Gadea et al. 2012).
A total of 36 male mice (age: 8 weeks; wild‐type: n = 18; McArdle: n = 18) were housed in Eurostandard type IIL microisolator cages (five mice maximum in each) under controlled conditions of temperature and humidity (20 ± 2°C and 55 ± 10%, respectively) at the animal facility of the CIEMAT (registration no. ES280790000183, Madrid, Spain). The cages were lit (fluorescent lighting) from 07.00 to 19.00 h, and food (Harlan Teklad Global Diets 2914) and water (50 μm filtered and UV irradiated) were provided ad libitum.
Study design and endurance exercise training intervention
Pre‐training phase
Mice were allowed to adapt to the treadmill (Harvard Apparatus; Panlab, Barcelona, Spain) in three sessions (on three separate days) as described (Fiuza‐Luces et al. 2013); adaptation involved a gradual increase in running time, treadmill velocity and inclination, starting with placement of the mouse on the treadmill with movement at a very low speed during the first day (0% inclination and 0–5 cm s−1 speed for 1 min, with 0.1 mA electrical stimulation) and ending with a 20 min period at low running intensity on the third day (15% and 12 cm s−1, electrical stimulation 0.1 mA, 1 Hz, 200 ms). A total of four treadmills were used and each mouse was consistently trained and tested on the same treadmill.
Maximal endurance exercise performance test
Once the mice had adapted to the treadmill, they were subjected to a gradual test until exhaustion to determine total running distance as a proxy of their maximal aerobic capacity (Hoydal et al. 2007). The test was performed after a warm‐up period of 20 min at a speed of 12 cm s−1 (with 15% inclination), and followed a previous protocol from our group with slight modifications in workload increases (Fiuza‐Luces et al. 2013). Thus, the initial velocity was 5 cm s−1, and this was followed by workload increases of 3 cm s−1 every 2 min until exhaustion, while treadmill inclination was kept constant at 15% during the whole test and use of electrical stimulation (0.1 mA, 1 Hz, 200 ms). Mice were defined as exhausted when they spent more than 5 s continuously on the electric grid and were unable to continue running at the next speed (Ayala et al. 2009).
Group assignment
McArdle mice were paired‐matched based on the total running distance they reached during the aforementioned test, and each pair was randomly assigned to an exercise (n = 9, subjected to an 8‐week exercise training programme) or a control (‘sedentary’) group (n = 9, allowed to freely move in the cage, but did not perform the programme). The same method of group (exercise or control) assignment and number of animals per group was applied for wild‐type mice.
Endurance exercise training intervention in McArdle and wild‐type mice
Training load over a period of time is the result of the combination of frequency, duration and intensity of the different sessions. Thus, for the training loads of the two McArdle and wild‐type exercise groups during the 8‐week period to be comparable despite their different fitness level at baseline, we ensured that the weekly frequency of training sessions and the duration and relative intensity (the latter expressed as a percentage of the velocity reached at the end of the tests, V max) of each session was the same for the two groups. Thus, the intervention in both exercise groups included five weekly sessions (from Monday to Friday; session duration, 30–50 min), which was performed between 08.00 and 12.00 h. The duration and relative intensity of each session were also the same and increased gradually in the same manner in the two groups, beginning at low workloads in the first session (30 min at 50% of V max and 0% gradient on the first day) and ending with 50 min at 70–75% of V max and 15% gradient at the end of the programme (Fiuza‐Luces et al. 2013). All the sessions included a warm‐up period (15 min at 40% (start of the programme) to 50% of V max (end)) and were followed by a cool‐down period (5 min at 35% of V max), both at the same treadmill slope used for the core part.
Whenever possible, efforts were made to minimize animal discomfort. Thus, only gentle tail touching was used to prompt the mice to run and no electrical stimulation was applied during the training sessions. In addition, because carbohydrate ingestion 30–40 min prior to exercise attenuates the risk of muscle damage in McArdle's disease (Lucia et al. 2008), for ethical reasons, during the hour before each session the McArdle mice were fed one Fruit CrunchiesTM pellet (weight, 190 mg; 52% energy from carbohydrate, 20.2% protein, 11.5% fibre, 6.3% fat, 5.1% ash and <10% moisture) (Bio‐ServTM, LBS Serving Biotechnology, UK). We verified that all exercise sessions started after the mice had eaten the pellet (which consistently took ≤60 min). Ingestion of pellets was chosen instead of parenteral administration of glucose to minimize animal discomfort. To ensure similar conditions in all groups, all the study mice also consumed the aforementioned pellet at the same time of the day.
Post‐training phase
At the end of the endurance exercise training programme, all animals repeated the aforementioned performance test. Finally, 48 h after the last test, mice were killed by intraperitoneal injection with a lethal dose of Avertin (0.2%, 0.15 ml g−1). We dissected the quadriceps muscles, trimmed of connective tissue, which were immediately snap‐frozen in liquid nitrogen before storage at −80°C for proteomics analysis. We chose the quadriceps instead of other limb muscles (tibialis anterior, extensor digitorum longus, or soleus) based on its high glycolytic phenotype, for technical reasons (i.e. large size, allowing sufficient amount of sample for present and future investigations), and also for its use in recent research from our group aiming at identifying molecular markers of important cell functions (energy‐sensing pathways, oxidative phosphorylation and autophagy/proteasome systems, oxidative damage, and sarcoplasmic reticulum Ca2+ handling) in McArdle mice (Fiuza‐Luces et al. 2016).
Paired comparisons were made between the maximal distance measured before and after the training period in the exercise and control group, in both McArdle and wild‐type mice (Wilcoxon test). Analyses were performed with Stata statistical software (version 13, Stata Corp; College Station, TX, USA) for Mac. The statistical significance level was set at P < 0.05.
Proteomic analysis
Proteomic analysis was performed in 10 McArdle (5 per group) and 8 wild‐type (4 per group) mice.
Sample preparation
Muscle samples were extracted in lysis buffer (2% SDS, 10 mm Tris(2‐carboxyethyl) phosphine hydrochloride (TCEP) and 50 mm Tris–HCl, pH 7.5) by homogenizing the tissue with 3 cycles at 6500 rpm for 60 s each using a MagNA Lyser Instrument (Roche; Mannheim, Germany). Thereafter, samples were boiled for 5 min and incubated for 30 min at room temperature with agitation. Samples were centrifuged at 16,000 g for15 min, and protein concentration in the supernatant was determined with a Direct Detect IR spectrometer (Millipore Ibérica; Madrid, Spain).
Protein digestion and isobaric labelling
For the quantitative differential analysis by liquid chromatography with tandem mass spectrometry (LC‐MS/MS) using isobaric tags (TMT 10‐plex), ∼100 μg of total proteins was digested using the filter‐aided protocol as previously described with minor modifications (Cardona et al. 2015). Proteins were diluted in 7 m urea and 0.1 mm Tris–HCl (pH 8.5) (UA) and loaded onto 10 kDa centrifugal filter devices (NanoSep 10k Omega, Pall Life Sciences; Port Washington, NY, USA). The buffer was replaced by washing filters with UA, and proteins were then alkylated using 50 mm iodoacetamide (IAA) in UA for 30 min in the dark. The excess of alkylating reagent was eliminated by washing three times with UA and three additional times with 50 mm ammonium bicarbonate. Proteins were digested overnight at 37°C with modified trypsin (Promega Biotech Ibérica; Alcobendas, Madrid, Spain) in 50 mm ammonium bicarbonate at 30:1 protein:trypsin (w/w) ratio. The resulting peptides were eluted by centrifugation with 50 mm ammonium bicarbonate (twice) and 0.5 m sodium chloride. Trifluoroacetic acid (TFA) was added to a final concentration of 1% and the peptides were desalted onto C18 Oasis‐HLB cartridges (Waters; Milford, MA, USA) and dried‐down for further analysis.
For stable isobaric labelling, the resulting tryptic peptides were dissolved in 100 mm tri‐ethyl‐ammonium bicarbonate (TEAB) buffer, and the peptide concentration was determined by measuring amide bonds with the Direct Detect system (Millipore Ibérica). Equal amounts of each peptide sample were labelled using 10‐plex TMT Reagents (Thermo Fisher Scientific; Waltham, MA) according to the manufacturer's protocol. Peptides were labelled with TMT reagents previously reconstituted with 70 μl of acetonitrile; after incubation at room temperature for 2 h, the reaction was stopped with 0.5% TFA, incubated for 30 min, and peptides were combined. Samples were concentrated in a Speed Vac, desalted onto C18 Oasis‐HLB cartridges and dried‐down for further analysis. To increase proteome coverage, TMT‐labelled samples were fractionated by high‐pH reverse phase chromatography (High pH Reversed‐Phase Peptide Fractionation Kit, Pierce; Rockford, IL, USA) and concentrated as before.
Protein identification and quantification
Labelled peptides were analysed by LC‐MS/MS using a C‐18 reversed phase nano‐column (75 μm I.D. × 50 cm, 2 μm particle size, Acclaim PepMap RSLC 100 C18; Thermo Fisher Scientific, Waltham, MA, USA) in a continuous acetonitrile gradient consisting of 0–30% B in 360 min and 50–90% B in 3 min (A = 0.1% formic acid; B = 90% acetonitrile, 0.1% formic acid). A flow rate of 200 nl min−1 was used to elute peptides from the nano‐column to an emitter nanospray needle for real time ionization and peptide fragmentation on an Orbitrap Fusion mass spectrometer (Thermo Fisher Scientific). An enhanced FT‐resolution spectrum (resolution = 70,000) followed by the MS/MS spectra from the Nth most intense parent ions were analysed along the chromatographic run. Dynamic exclusion was set at 40 s.
For peptide identification, all spectra were analysed with Proteome Discoverer (version 2.1.0.81, Thermo Fisher Scientific) using SEQUEST‐HT (Thermo Fisher Scientific). For database searching at the Uniprot database containing all sequences from mouse and contaminants (27April 2016; 48,644 entries), the parameters were selected as follows: trypsin digestion with two maximum missed cleavage sites, precursor and fragment mass tolerances of 2 Da and 0.02 Da, respectively, carbamidomethyl cysteine and TMT modifications at N‐terminal and Lys residues as fixed modifications, and methionine oxidation as dynamic modification. Peptide identification was performed using the probability ratio method (Martinez‐Bartolome et al. 2008) and false discovery rate (FDR) was calculated using inverted databases and the refined method (Navarro & Vazquez, 2009) with an additional filtering for precursor mass tolerance of 15 ppm (Bonzon‐Kulichenko et al. 2015).
Identified peptides with an FDR equal to or lower than 1% FDR were used to quantify the relative abundance of each protein from reporter ion intensities, and statistical analysis of quantitative data was performed using the WSPP statistical model previously described (Navarro et al. 2014). In this model, protein log2‐ratios are expressed as standardized variables; that is, in units of standard deviation (SD) according to their estimated variances (Z q values).
Functional protein analysis
Functional protein analysis of the whole set of quantified proteins was performed using a novel algorithm system biology triangle (SBT), developed specifically for the analysis of coordinated protein responses in high‐throughput quantitative proteomics experiments (Garcia‐Marques et al. 2016). This algorithm correlates the performance of a group of proteins inside a category (biological process) in terms of their quantitative behaviour (relative abundance); thus, changes can be detected in functional biological processes far beyond individual protein responses. Variations in the abundance of annotated functional categories were visualized by comparing the cumulative frequency (sigmoid) plots of the standardized variable with that of the normal distribution, as in previous research (Isern et al. 2013). Individual protein changes were also considered for further analysis.
Determination of the differentially expressed proteins
The Kolmogorov–Smirnov test was applied to test if the data followed a normal distribution. Student's t test or the Wilcoxon rank‐sum test was applied to identify differentially expressed proteins (i.e. ‘proteins of interest’) between exercise and control groups in both McArdle and wild‐type mice. P‐values were adjusted for multiple comparisons with FDR correction.
Biocomputational analysis of proteomics data
Processing of protein expression data
Murine proteins were converted into the corresponding human equivalent UniProt reviewed protein according to the following steps: (i) UniProt ID automatic crossing of the murine proteins with human proteome with corresponding databases (InParanoid (Sonnhammer & Ostlund, 2015) and the Mouse Genome Database (MGD; Blake et al. 2017)), (ii) gene name automatic crossing of the murine proteins with human proteome with corresponding databases (InParanoid; Turk et al. 1990) and (iii) Manual Blast (Altschul et al. 1990), selecting the best reviewed match presenting at least an identity value ≥ 70% and E‐value ≤ 10−6.
Molecular characterization of adaptation to endurance exercise training
Adaptation to endurance exercise training was characterized at the molecular level via manual curation of the literature. This characterization was performed in two steps: in the first step, we identified the main pathophysiological processes related to ‘manifestative’ adaptations in the skeletal muscle in response to endurance exercise training (‘manifestative’ signatures), which were further characterized at the protein level to provide a final list of ‘condition effector’ proteins (hereafter termed ‘effectors’) (Appendix, Table A1). Hence, effectors are those proteins that, according to the existing literature, have previously been reported to play a critical role in the process of interest for a given study viz., skeletal muscle adaptations to endurance exercise training (databases: PubMed, ScienceDirect and Scopus; key words (and combinations thereof): protein, muscle, physical adaptations, sports, exercise, McArdle's disease and McArdle).
Contextualization of the differentially expressed proteins within ‘adaptation to exercise training’ protein network
Effector proteins were used to focus the analysis on the biological condition of interest in the human biological network. The direct interactions (physical interactions or functional relationships) among the differentially expressed proteins (in exercise vs. control groups), as well as the interactions between the differentially expressed proteins and the effectors of skeletal muscle adaptations to endurance exercise training, were assessed. Different publicly available databases were consulted for the human protein network generation (e.g. Reactome, Molecular INTeraction database (MINT) and BioGrid) (Herrando‐Grabulosa et al. 2016; Iborra‐Egea et al. 2017).
Mechanistic evaluation of the differentially expressed proteins in relation to endurance exercise training: artificial neuronal networks analysis
The possible molecular relationship between the differentially expressed proteins and skeletal muscle adaptations to endurance exercise training was evaluated by means of artificial neuronal networks (ANNs), following TPMS technology protocols (Herrando‐Grabulosa et al. 2016; Iborra‐Egea et al. 2017). This approach involves the generation of mathematical models of the biological processes through the use of artificial intelligence techniques. Then, mathematical models were solved by ANNs, which are supervised algorithms that identify relationships between the different nodes in the network. ANN analysis yields a score for each differential protein based on the validations of the prediction capacity of the mathematical models towards known drugs and diseases, as described in databases. The higher the score, the stronger is the predicted mechanistic relationship between the evaluated protein and the biological process. Each score is associated with a P‐value that describes the probability of the result being a true positive one. Aiming to facilitate the understanding of the results, the obtained scores were divided into three categories: >76, strong (P < 0.05); 40–76, medium‐strong (P = 0.05–0.25); and <40, weak (P > 0.25).
Proteins presenting 200+ interactions (‘sticky proteins’), or proteins that do not have reported interactions, were not included in the topology as they may disrupt the correct assessment of existing relationships (Pache et al. 2008). Relationships between the differentially expressed proteins and skeletal muscle adaptations to endurance exercise training were assessed both for individual proteins and for combinations of two proteins; thus, from all the reported protein interactions, the most interesting are those presenting a synergic effect. The synergy criteria were applied according to the approach described by Berenbaum (1989), in which a significant synergic effect is considered when the overall effect is >20% of the sum of the individual effects of the two proteins.
Visualization of the protein network
Cytoscape 3.5.1. software was used to study the representation of all the reported interactions (both from the significantly associated proteins and non‐significantly associated proteins) according to the ANN score.
Enrichment analysis
The pathways and biological processes enriched within the differentially expressed proteins in the exercise and control groups within both McArdle and wild‐type mice were assessed using a hypergeometric enrichment analysis approach (Rivals et al. 2007). Specifically, the enrichment was run over several sets of proteins, including: Gene Ontology (GO) terms (Biological Process, Cellular Component, Molecular Function) according to European Molecular Biology Laboratory (EMBL)–European Bioinformatics Institute (EBI)/UniProt‐GO (The UniProt Consortium, 2017); pathways from the Kyoto Encyclopedia of Genes and Genomes (KEGG; Kanehisa et al. 2014), the Pharmacogenomics Knowledgebase (PharmGKB; Whirl‐Carrillo et al. 2012) and the Small Molecule Pathway Database (SMPDB; Frolkis et al. 2010); pathological conditions, signatures and pathways from the Biological Effectors Database (BED; Iborra‐Egea et al. 2017) and the regulatory molecular mechanisms included in Transcriptional Regulatory Relationships Unraveled by Sentence‐based Text‐mining (TRRUST) database (Han et al. 2015). Only those pathways that showed a statistically significant presence were presented (FDR P‐value < 0.05).
Results
The training loads were common to both McArdle and wild‐type exercise groups over the 8‐week period and are shown in detail in Appendix, Table A2. The endurance exercise training programme was successful in inducing a significant improvement in the total running distance of both McArdle (141 (31 SD) m (pre‐training) vs. 167 (48 SD) m (post‐training); Wilcoxon test P = 0.035) and wild‐type (275 (30 SD) vs. 353 (146 SD) m, P = 0.041) mice, whereas no significant change was found during the same time period in their sedentary controls (140 (41 SD) vs. 116 (48 SD) m, P = 0.129 for McArdle and 302 (85 SD) vs. 296 (103 SD) m, P = 0.726 for wild‐type mice). Although the relative improvement in total running distance did not differ between McArdle and wild‐type mice (Mann–Whitney test, P = 0.111), the total running distance of McArdle mice was ∼50% lower than that of wild‐type mice both before and after the endurance exercise intervention. Individual data (total running distance) of the mice used for proteomic analyses are shown in Fig. 1. When expressed as V max, the test results were as follows: McArdle, exercise group: 26 (3) cm s−1 (pre‐training) vs. 29 (4) cm s−1 (post‐training), Wilcoxon test P = 0.054; wild‐type, exercise group: 36 (6) (pre‐training) vs. 41 (8) cm s−1 (post‐training), P = 0.028; McArdle, sedentary control group: 26 (4) vs. 24 (5) cm s−1, P = 0.123; wild‐type, sedentary control group: 38 (5) vs. 37 (7) cm s−1, P = 0.180.
Figure 1. Individual responses during the intervention period in maximal distance during a gradual treadmill running test until exhaustion in mice used for proteomic analysis.
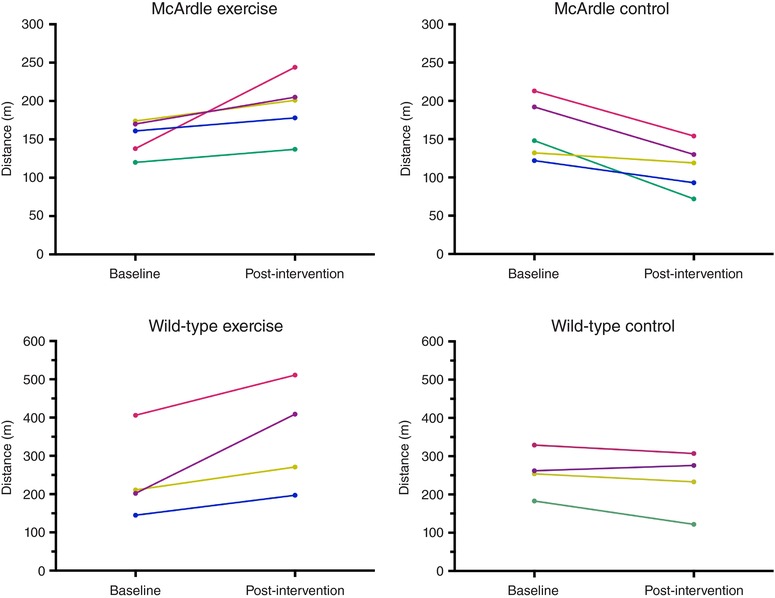
The body mass of the mice showed an increasing trend over time irrespective of genotype or intervention (exercise or control): (i) McArdle, exercise group: 20.8 (1.4) g (pre) and 22.0 (1.3) g (post), Wilcoxon test P = 0.997; (ii) McArdle, control group: 20.8 (1.3) g (pre) and 22.1 (0.81) g (post), P = 0.031; wild‐type, exercise group: 18.7 (2.0) g (pre) and 21.3 (1.0) g (post), P = 0.083; wild‐type, control group: 19.0 (1.9) g (pre) and 21.7 (2.1) g (post), P = 0.805.
Proteomics
Proteins of interest
Data provided by whole proteomic analysis regarding differential proteins within the sedentary and the trained groups of McArdle and wild‐type mice, respectively, were analysed by applying Student's t test and the Wilcoxon rank‐sum test. A total of 74 differentially expressed proteins between the trained and sedentary wild‐type mice and 123 differentially expressed proteins between the trained and sedentary McArdle mice were found (Appendix, Table A3). Of these differentially expressed proteins in response to endurance exercise training, only three were common to wild‐type and McArdle mice: LIM and calponin homology domains‐containing protein 1 (LIMCH1); poly (ADP‐ribose) polymerase 1 (PARP‐1, also known as NAD+ ADP‐ribosyltransferase 1 or poly (ADP‐ribose) synthase 1); and tigger transposable element derived 4 (TIGD4). Expression of all three proteins was higher in endurance exercise training than in untrained conditions, in both wild‐type and McArdle mice; however, statistical significance was not reached for any after FDR adjustment.
Contextualization of the differentially expressed proteins within exercise training adaptation
The molecular characterization of the skeletal muscle adaptations to endurance exercise training was carried out through the review of specialized scientific literature. A total of 76 effector proteins were identified and classified into four groups according to their cellular function, such as adaptive muscle growth (14 proteins), muscle angiogenesis (8 proteins), glucose and lipid metabolism (12 proteins) and mitochondrial biogenesis and remodelling (48 proteins), with four of these 76 proteins belonging to two groups (angiogenesis + mitochondrial biogenesis and remodelling) at the same time, namely cyclic AMP‐dependent transcription factor (ATF2), steroid hormone receptor ERR1 (ESRRA, or also ERRa), mitogen‐activated protein kinase 12 (MAPK12) and myocyte‐specific enhancer factor 2A (MEF2A, or MEF2), and one, PGC‐1α, belonging to three groups (adaptive muscle growth + angiogenesis + mitochondrial biogenesis and remodelling) (Appendix, Table A1).
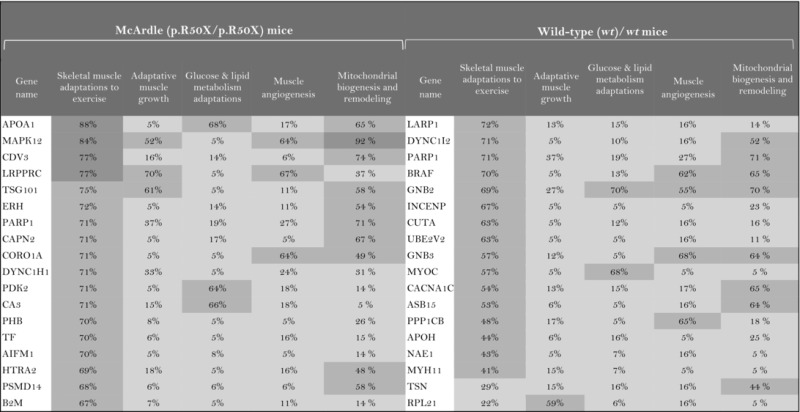
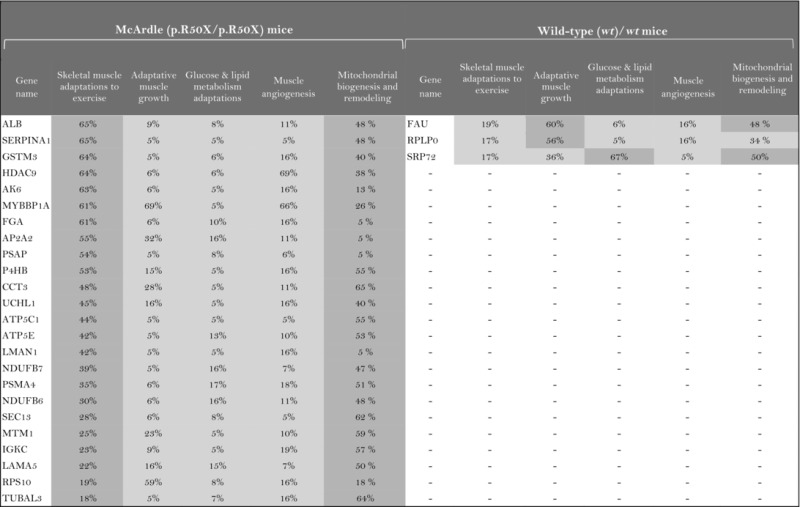
The role of the differentially expressed proteins in adaptation to endurance exercise training was evaluated according to the molecular characterization performed. The results of the analysis revealed that only one of the differentially expressed proteins in McArdle mice, MAPK12, had been previously related to skeletal muscle adaptations to exercise training (Table 1). MAPK12 is involved both in mitochondrial biogenesis and remodelling and muscle angiogenesis. By contrast, none of the differentially expressed proteins in wild‐type mice has previously been reported to play a direct role in skeletal muscle adaptations to endurance exercise training (Table 2). However, while only one differentially expressed protein from the two lists (McArdle and wild‐type mice) matches the effector proteins defined in the molecular characterization, it is remarkable that several of them interact with many effectors (Tables 1 and 2). In McArdle mice, ATP synthase H+ transporting mitochondrial F1 complex γ polypeptide 1 (ATP5C1), MAPK12 and ATP synthase and H+ transporting mitochondrial F1 complex ε subunit (ATP5E) were related to more than 10 effectors, and proteasome 26S subunit, non‐ATPase 14 (PSMD14), integrin‐linked kinase (ILK) and chaperonin containing TCP1 subunit 3 (CCT3) interacted with nine different effectors, while in wild‐type mice, we found that poly(A) binding protein cytoplasmic 1 (PABPC1), protein kinase DNA‐activated catalytic polypeptide (PRKDC), ribosomal protein lateral stalk subunit P0 (RPLP0), mitogen‐activated protein kinase kinase 4 (MAP2K4) and protein kinase N1 (PKN1) interacted with more than 10 effectors, followed by ribosomal protein L21 (RLP21) interacting with up to nine different effectors. Additionally, the common differentially expressed protein between the two groups, PARP1, interacted with seven different effectors, all of them involved in mitochondrial biogenesis and remodelling.
Table 1.
McArdle (p.R50X/p.R50X) mice: Summary of the interactions found between the differentially expressed proteins (in exercise vs. control groups) and of the protein effectors of skeletal muscle adaptations to endurance exercise training
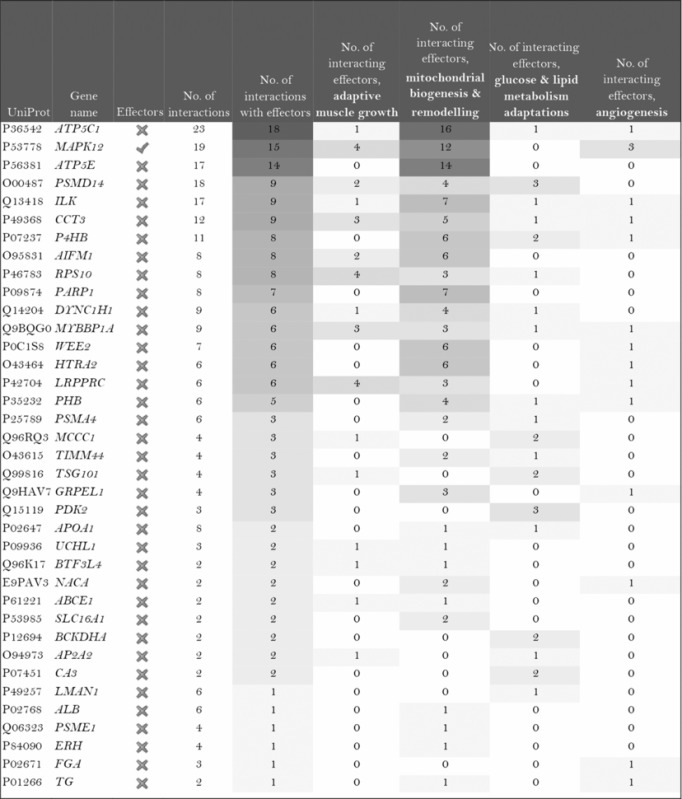
| ||||||||
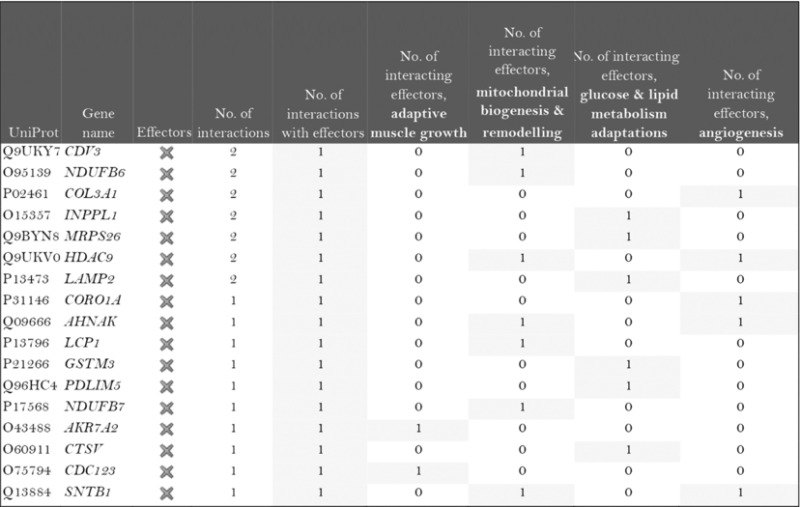
|
Symbols: non‐effectors (×) and effectors (✓) of skeletal muscle adaptations to endurance exercise training among the differentially expressed proteins.
Table 2.
Wild‐type (wt/wt) mice: summary of the interactions found between the differentially expressed proteins (in exercise vs. control groups) and of the protein effectors of skeletal muscle adaptations to endurance exercise training
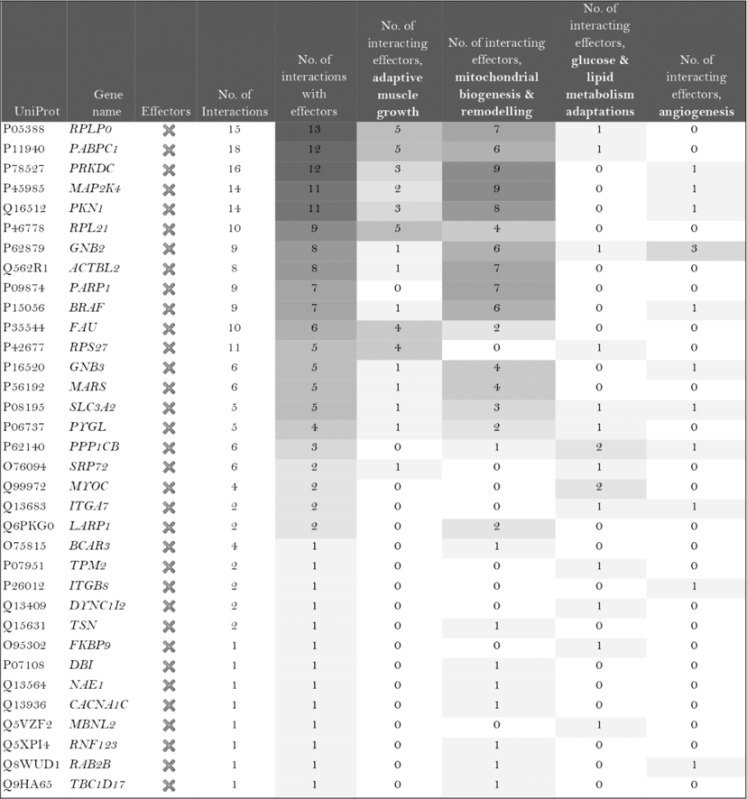
|
Symbol: ×, non‐effectors of skeletal muscle adaptations to exercise training among the differentially expressed proteins.
Mechanistic evaluation of the relationship with endurance exercise training: analysis of artificial neuronal networks
The potential molecular relationships between the differentially expressed proteins and skeletal muscle adaptations to endurance exercise training were evaluated through the analysis of mathematical models, to determine the possible activity relationships between protein sets or regions inside the network (i.e. ANN); this allowed us to provide a predictive score that quantifies the probability of the existence of a relationship between the evaluated differential proteins and network region (i.e. the different pathophysiological signatures characterized and adaptation to endurance exercise training as a whole). Each score is associated with a P‐value that describes the probability of the result being a true positive result. To better understand the results, we divided the ranking score into four categories: strong (P < 0.05), medium‐strong (P 0.05–0.25), weak (P < 0.25) and not assessed. As shown in Appendix, Table A4, it was not possible to evaluate 5 of 116 proteins for the McArdle mouse groups, and 5 of 73 for the wild‐type groups. Nevertheless, the possible relationship between 111 proteins for McArdle mice and 68 proteins for wild‐type mice was successfully assessed. When considering the differential proteins individually, ∼28% and 22% of the proteins showed a significant relationship (strong or medium‐strong category) with skeletal muscle adaptations to endurance exercise training in McArdle and wild‐type mice, respectively. Moreover, when analysing the differential proteins in sets of two proteins, ∼30% of the protein combinations showed a significant association with skeletal muscle adaptations to endurance exercise training, both in the McArdle and wild‐type mice.
Relationships between the differentially expressed proteins and skeletal muscle adaptations to endurance exercise training: individual protein evaluation
As a result of the analysis of the relationship between individual proteins and skeletal muscle adaptations to endurance exercise training, a total of 33 proteins were reported to present a significant relationship with McArdle's disease mice and 16 with wild‐type mice (Table 3). One of the differentially expressed proteins common to both mouse groups, PARP1, appeared to be significantly related to skeletal muscle adaptations to endurance exercise training. Moreover, the assessment of the potential relationship between the proteins of interest and skeletal muscle adaptations to endurance exercise training signatures allowed the evaluation of specific subsets of the physiological processes that in some cases were not detected by considering the whole context. For instance, nine proteins of interest in McArdle mice and five in wild‐type mice that were predicted to be related to at least one of the signatures of skeletal muscle adaptations to endurance exercise training were actually not found to be related to the general characterization of skeletal muscle adaptations to endurance exercise training (Table 3).
Table 3.
Differentially expressed proteins significantly linked to skeletal muscle adaptations in McArdle and wild‐type mice
| |||||||||||
|
The higher the artificial neuronal network (ANN) predictive score, the stronger is the relationship between the protein and skeletal muscle adaptations to endurance exercise training or its motives.  ANN score (strong relationship) > 76% P‐value < 0.05
ANN score (strong relationship) > 76% P‐value < 0.05  ANN score 40–76 (strong‐medium) P‐value 0.05‐0.25
ANN score 40–76 (strong‐medium) P‐value 0.05‐0.25  ANN score (weak) < 40% P‐value < 0.25.
ANN score (weak) < 40% P‐value < 0.25.
Relationships between the differentially expressed proteins and skeletal muscle adaptations to endurance exercise training: association in combination of two proteins
As a result of the analysis of the relationship between combinations of two proteins and skeletal muscle adaptations to endurance exercise training (Appendix, Table A4), a total of 2035 protein combinations were reported to present a significant relationship in McArdle mice and 719 in wild‐type mice. When considering only the combinations with a significant synergic effect (synergic effect is considered when the overall effect is >20% of the amount of the individual effect of the two proteins), a total of 199 and 57 protein combinations were found for McArdle and wild‐type mice, respectively. One of the common differentially expressed proteins in both groups, LIMCH1, appeared to be significantly related to skeletal muscle adaptations to endurance exercise training in combination with other proteins.
In order to represent the intermolecular relationships obtained regarding protein interactions (Tables 1 and 2 and Appendix, Table A1) and mechanistic evaluation (Appendix, Table A4), a visual protein interaction network map was generated for both McArdle (Fig. 2) and wild‐type (Fig. 3) mice. Only the proteins with a significant relationship with skeletal muscle adaptations to endurance exercise training (based on ANNs score) and their direct interactions appear on the representations. However, closer analysis of the signalling networks revealed that PARP1 was more profoundly overexpressed in trained McArdle than in trained wild‐type mice (Figs 2 and 3, respectively).
Figure 2. Visual protein interaction network of the proteins significantly related to skeletal muscle adaptations to endurance exercise training according to the artificial neuronal network score (ANNs), and their interacting proteins, in McArdle (p.R50X/p.R50X) mice.
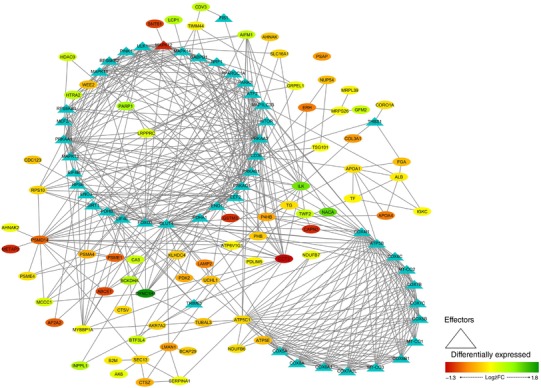
The network includes three representations of the following interactions: (i) within the differentially expressed proteins (ovals), (ii) between the differentially expressed proteins and skeletal muscle adaptions to endurance exercise effectors (ovals and triangles), and (iii) within the skeletal muscle adaptations to endurance exercise effectors (triangles). Note: all the interactions, from both the significantly associated proteins and the non‐significantly associated proteins according to the ANN score, are reported.
Figure 3. Visual protein interaction network of the proteins significantly related to skeletal muscle adaptations to endurance exercise training according to the artificial neuronal network score (ANNs), and their interacting proteins, in wild‐type (wt/wt) mice.
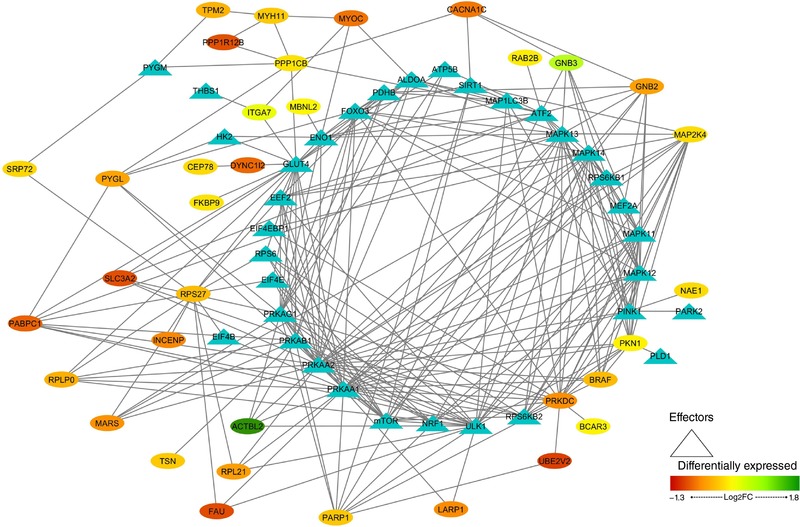
The network includes include three representations of the following interactions: (i) within the differentially expressed proteins (ovals), (ii) between the differentially expressed proteins and skeletal muscle adaptions to endurance exercise effectors (ovals and triangles), and (iii) within the skeletal muscle adaptations to endurance exercise effectors (triangles).
Enrichment analysis
To analyse the expression data related to signalling pathways and clinical conditions, we undertook a hypergeometric enrichment analysis approach within the differentially expressed proteins in the trained and the control groups of McArdle and wild‐type mice. As shown in Appendix, Table A5, 121 and 79 protein sets were found enriched in the comparison of control vs. exercise groups, both in McArdle and in wild‐type mice. Subsequently, a pathway comparison between the results obtained from both conditions was performed, obtaining four common pathways and 117 and 75 proteins specifically enriched in McArdle and wild‐type groups, respectively. The enriched protein sets common to McArdle and wild‐type conditions corresponded to those related to high blood glucose levels and gene expression (BEDVES database: induction of oxidative stress diabetic neuropathies and induction of oxidative stress diabetic neuropathies and retinopathies; GOFUNTION database: term poly(A) RNA binding and the GOLOCATION referring to cytosol).
The enriched protein sets reported within the differentially expressed proteins in exercise vs. control McArdle mice (Appendix, Table A5) reflect changes related to muscular adaptations to endurance exercise training that may be related to improvement in exercise tolerance, as well as to processes associated with the pathophysiology of the condition. Regarding enriched protein sets related to muscular adaptations to endurance exercise training, there were several pathways referring to mitochondria and their functions (Appendix, Table A6), including GO location terms referring to several mitochondrial structures such as the proton‐transporting ATP synthase complex (responsible for ATP synthesis), and GO processes such as the respiratory electron transport chain. Furthermore, several pathways and GO terms related to lipid and protein metabolism are also reported in Appendix, Table A6, and enriched protein sets related to muscle structure and growth are described in Appendix, Table A7.
Conversely, the enriched protein sets within the wild‐type groups were mainly related to endurance exercise training‐induced physiological changes due to focal adhesion, actin cytoskeleton reorganization and phosphatidylinositol 3‐kinase (PI3K) signalling pathway involvement (Appendix, Table A8). Moreover, several pathways referring to β‐blocker activity also appeared to be enriched, possibly due to changes in catecholamine modulation (Appendix, Table A9).
Discussion
Although McArdle's disease causes ‘exercise intolerance’ (Lucia et al. 2012), under carefully controlled conditions, McArdle's patients may perform acute exercise safely, especially if carbohydrate solutions are taken before exercise to bypass the metabolic blockade that occurs upstream of the uptake of glucose by muscle fibres (Vissing & Haller, 2003). They may also adapt favourably to moderate‐intensity endurance exercise training (Haller et al. 2006; Mate‐Munoz et al. 2007). Yet, no study has assessed in depth the molecular signals associated with muscle exercise adaptations in McArdle's patients and how they compare with those of healthy individuals. Accordingly, in the present study we aimed to identify, with no a priori hypothesis, the key proteins and protein networks linked to the effects of moderate‐intensity endurance exercise training in a mouse model that closely mimics the phenotype of McArdle's patients (Nogales‐Gadea et al. 2012). Unravelling the muscle metabolic adaptations to endurance exercise training with no muscle glycogen availability might help to gain insight into the proteome profile that characterizes muscle adaptations to such a type of training. This is an important question given that the skeletal muscle contains 50–75% of all the body proteins and is responsible for 30–50% of the whole body protein translation process (Frontera & Ochala, 2015). The majority of proteins identified here both in McArdle and wild‐type mice control the expression of myriad genes related to angiogenesis, carbohydrate, lipid and protein metabolism, mitochondrial biogenesis and remodelling, and muscle growth.
In addition to reporting the first non‐pharmacological intervention in this mouse model, a novel finding of our study was that, like patients, McArdle mice adapt favourably to an individualized moderate‐intensity endurance exercise training regimen (albeit without reaching the performance capacity of healthy mice). Yet, our results revealed a remarkable difference in the protein networks involved in the muscle tissue adaptations that occur with endurance exercise training with normal glycogen availability (wild‐type mice) as compared with those that occur in conditions of blocked glycogenolysis (McArdle mice). Indeed, endurance exercise training promoted the expression of only three proteins common to both McArdle and wild‐type mice: LIMCH1, PARP1 and TIGD4. Likely, all three proteins play a prominent role in the relationship between skeletal muscle plasticity and endurance exercise training, independent of muscle glycogen availability, which warrants further investigation. It is well known that during exercise, numerous stress signals are transduced to activate intracellular signalling pathways controlling skeletal muscle gene transcription and translation (Bassel‐Duby & Olson, 2006; Koulmann & Bigard, 2006; Favier et al. 2008; Russell, 2010). Against this background, both LIMCH1 and PARP1 could participate in stress pathways controlled by exercise. Indeed, cell contraction accelerates when LIMCH1 promotes the assembly of actin stress fibres during cell spreading. Moreover, the absence of LIMCH1 expression impacts the formation of actin stress fibres as well as the stability of focal adhesions (Lin et al. 2017), which are fundamental structures in the union of muscle fibres to ensure optimal contraction and muscular distention. TIGD4 protein belongs to the tigger subfamily of the pogo superfamily of DNA‐mediated transposons in humans. The latter proteins are related to DNA transposons found in fungi and nematodes and more distantly to transposases Tc1 and mariner, and they are very similar to the major mammalian B centromere protein. However, the exact function of TIGD4 remains to be clarified.
PARP1 is the most characterized member of the PARP family of nuclear enzymes. These proteins are sensitive to changes in intracellular redox pathways, positioning the poly ADP‐ribosylation (PARylation) reaction as an important biochemical marker of oxidative stress (Jungmichel et al. 2013). Whereas basal activity of PARP1 is crucial to maintain cellular homeostasis, its over‐activity leads to an increase in protein PARylation, which in turn depletes intracellular NAD+ levels, leading to cell death (Ha & Snyder, 1999). Thus, PARP1 activity is a key sensor for cell survival. Interestingly, we found that endurance exercise training mediates the expression of PARP1 in both McArdle and wild‐type mice, although it was higher in trained McArdle than in trained wild‐type mice. This could be due, at least partly, to high muscle damage that characterizes this disease.
In general, the low overlap between groups (74 and 123 differentially expressed proteins with training in wild‐type and McArdle mice, respectively) suggests considerable differences in the physiological adaptations to chronic endurance exercise between McArdle and wild‐type mice, at a mechanistic level. Indeed, when we compared the protein profile between McArdle and wild‐type mice after training, we found that McArdle mice presented a specific and strong expression of MAPK12 and a potentially substantial number of effector interactions. MAPK12 is a serine/threonine kinase that acts as an essential component of the MAP kinase signal transduction pathway. Also known as p38MAPKγ, MAPK12 is one of the four p38 MAPKs that play an important role in the cellular response cascade induced by extracellular stimuli such as proinflammatory cytokines or physical stress (Cuenda & Rousseau, 2007). MAPK12 plays a role in myoblast differentiation, as it is involved in the regulation of the expression of the solute carrier family 2 (facilitated glucose transporter), member 1 (SLC2A1). Further, basal glucose uptake in L6 myotubes and MAPK12 signalling positively regulate the expansion of transient amplifying myogenic precursor cells during muscle growth and regeneration (Ho et al. 2004). Our analyses suggest that MAPK12 is also related to mitochondrial biogenesis and remodelling, with such potentially MAPK12‐mediated improvement in mitochondrial function perhaps endeavouring to compensate for the deficit in energy supply owing to glycogen unavailability. By contrast, we detected a lower endurance exercise training‐induced increase in MPAK12 protein expression in wild‐type mice.
Pathway enrichment analysis of the highly and significantly expressed proteins in McArdle and wild‐type mice (121 and 79 proteins, respectively) provided an enrichment in clinical processes that are related to the induction of oxidative stress, neuropathies and retinopathies. When McArdle and wild‐type mice were analysed separately, we observed that after endurance exercise training in McArdle mice the most widely represented functions were those involved in ATP synthesis and electron transport chain processes, β‐oxidation of lipids, and different steps in lipid and protein catabolism. In addition, cell death and regulation of necrotic processes were also reflected in this analysis. In fact, the overexpression of PARP1 could be associated with rhabdomyolysis, and PARP activation is linked to necrosis, as shown in PARP‐deficient mouse fibroblasts (Ha & Snyder, 1999). All of the aforementioned adaptations found in McArdle mice after endurance exercise training may be related to the metabolic strategies that the skeletal muscle tissue of McArdle mice deploys to obtain energy. Conversely, after endurance exercise training the functions that were most represented in wild‐type mice were those related to cytoskeleton regulation and focal adhesions, and also PI3K and mTOR signalling pathway activation related to maintenance of skeletal muscle cell survival. Thus, from an overall perspective, it seems that the main muscle molecular adaptations to endurance exercise training in McArdle mice are more oriented to obtain energy for tissue regeneration in a state of energetic deficit, whereas wild‐type muscle adaptations seem to be more related to support tissue maintenance while coping with exercise stress stimuli.
Our study is not without limitations. In contrast to human research, where performing repeated muscle biopsy sampling may be feasible, a first technical limitation is the impossibility to determine muscle glycogen content before and after a training session in the wild‐type mice as a proof‐of‐concept for glycogen utilization. Of note, owing to the total inability to utilize glycogen as a fuel, massive muscle glycogen accumulation occurs in McArdle mice, a phenomenon much more remarkable than in patients, with muscle glycogen levels being several orders of magnitude higher than in CB7Bl/6J wild‐type mice as used here (Nogales‐Gadea et al. 2012). Moreover, we chose forced treadmill running rather than wheel running as a model of endurance exercise training. Although wheel running is a less stressful, more natural form of activity in rodents, it does not allow for the establishment of predetermined training loads, which was a crucial aspect of our design to ensure they were similar in the exercise groups. Nevertheless, researchers in charge of mouse training were well experienced in mouse handling such as to minimize stress in these animals. Indeed, only gentle tail touching was used to prompt the mice to run and no electrical stimulation was applied during the training sessions. Another potential limitation comes from the use of a single muscle type, quadriceps (with a highly glycolytic phenotype) for proteome analyses. Future research should determine whether comparable findings are obtained in a more oxidative muscle like the soleus or even in other glycolytic muscles. In this regard, although there are differences between muscles (even between those with a similar metabolic phenotype and with only subtle differences in proportion of fibre types, such as the quadriceps and tibialis anterior) overall, both fast‐ and slow‐twitch muscles in the untrained state are affected by both structural degeneration and energy deficiency in McArdle mice (Krag et al. 2016a). Along this line, recent research has identified the quadriceps muscle as having more compensatory adaptations to counterbalance energetic deficiency in the untrained state (i.e. in terms of expression of proteins involved in glucose uptake, glycogen synthesis and glycolysis) than other studied muscles, whether they were predominantly glycolytic (tibialis anterior, extensor digitorum longus) or more oxidative (soleus) (Krag et al. 2016b). Finally, we did not report blood serum creatine kinase levels (as an indirect marker of muscle damage) over the study period.
In conclusion, we have determined that, akin to patients, McArdle mice responded favourably to moderate‐intensity endurance exercise training, although their maximal aerobic capacity is clearly lower than that of normal peers. This suggests that glycogen availability is crucial for ensuring maximal endurance performance in mammals, with unavailability of this substrate resulting in muscles adopting a remarkably different ‘molecular strategy’ to cope with the training loads while ensuring cellular homeostasis during a state of energetic deficit. In this regard, we have identified for the first time the signalling strategies (in terms of signalling molecules and protein networks) that skeletal muscle employs to overcome blockade of glycogen breakdown during endurance exercise training, demonstrating that the protein network involved in muscle adaptations to such a type of training greatly differs depending on glycogen availability (see also Fig. 4 for a summary). Our findings provide a framework for future studies aimed at elucidating the molecular mechanisms associated with the most relevant identified proteins here. It would also be interesting to study in depth some of the proteins sets identified by the enrichment analysis; among which, PARP1, LIMCH1 and MAPK12 emerge as good candidates.
Figure 4. Integrative model summarizing the main study findings.
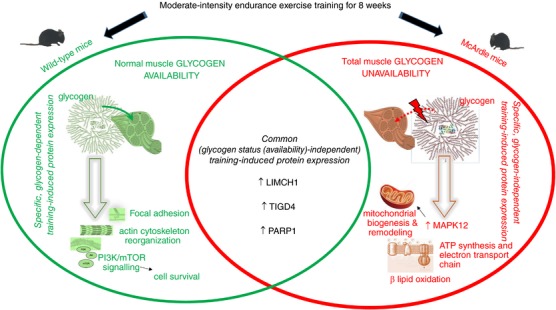
Abbreviations: LIMCH1, LIM and calponin homology domains‐containing protein 1; MAPK12, mitogen‐activated protein kinase 12; PARP1, poly (ADP‐ribose) polymerase 1; TIGD4, tigger transposable element derived 4.
Additional information
Competing interests
All the authors declare they have no conflict of interesting.
Author contributions
The experiments were performed in the Centre of Energy, Environment and Technical Research, CIEMAT (mouse genotyping, housing and training); and Centro Nacional de Investigaciones (Cardiovasculares): proteomics. C.F.L.: conception and design of the work, acquisition, analysis and interpretation of data for the work, and drafting the work; A.S.L. and J.L.Z.: design of the work, analysis and interpretation of data for the work, and drafting the work; F.L., J.A., M.A.M., A.L.A., T.P., B.G.B. and J.V.: interpretation of data for the work and revising the work critically for important intellectual content; G.N.G., J.D.B., R.C. and A.G.M.: acquisition of data for the work and revising the work critically for important intellectual content; C.B.: analysis and interpretation of data for the work and revising the work critically for important intellectual content; J.A.L.: analysis and interpretation of data for the work and revising the work critically for important intellectual content; A.L.: conception and design of the work, analysis and interpretation of data for the work, drafting the work. All authors qualify for authorship, approved the final version of the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
The CNIC is supported by the Ministry of Economy, Industry and Competitiveness (MEIC) and the Pro CNIC Foundation, and is a Severo Ochoa Centre of Excellence (SEV‐2015‐0505). This study was funded by grants from Fondo de Investigaciones Sanitarias (PI15/01756, PI15/00558, PI12/00914, and PI14/00903), cofinanced by FEDER. G.N.G. is supported by a Miguel Servet research contract (ISCIII CD14/00032 and FEDER) and C.F.L. by a Sara Borrell post doc contract (CD14/00005). Miguel A. Martín is supported by Fondo de Investigaciones Sanitarias (FIS 15/00432). Tomâs Pinós is supported by Fondo se Investigaciones Sanitarias (FIS PI16/01492).
Acknowledgements
We thank Dr Kenneth McCreath for his editorial assistance during manuscript preparation. The authors also thank Anaxomic Biotech (Spain) for bioinformatic support in the proteomic data analysis.
Table A1.
Molecular effectors of skeletal muscle adaptations to endurance exercise training determined through the review of specialized scientific literature
| MOTIVE ID | Effector protein (name) | Effector protein (short name) |
|---|---|---|
| Adaptive muscle growth | 40S ribosomal protein S6 | RPS6 (rpS6) |
| E3 ubiquitin‐protein ligase TRIM63 | TRIM63 | |
| Elongation factor 2 | EEF2 | |
| Eukaryotic translation initiation factor 4B | IF4B | |
| Eukaryotic translation initiation factor 4E | EIF4E (eIF4E ) | |
| Eukaryotic translation initiation factor 4E‐binding protein 1 | EIF4EBP1 (4EBP1) | |
| Forkhead box protein O3 | FOXO3 | |
| Growth/differentiation factor 8 | MSTN | |
| Mechanistic target of rapamycin | mTOR | |
| Peroxisome proliferator‐activated receptor γ coactivator 1‐α | PGC‐1α | |
| Phospholipase D1 | PLD1 | |
| Phospholipase D2 | PLD2 | |
| Ribosomal protein S6K 1 | RPS6KB1 (p70S6K1) | |
| Ribosomal protein S6K 2 | RPS6KB2 (p70S6K2) | |
| Angiogenesis | Cyclic AMP‐dependent transcription factor ATF‐2 | ATF2 |
| Mitogen‐activated protein kinase 12 | MAPK12 (p38g) | |
| Myocyte‐specific enhancer factor 2A | MEF2A (MEF2) | |
| Nitric oxide synthase, endothelial | NOS3 | |
| Peroxisome proliferator‐activated receptor γ coactivator 1‐α | PGC‐1α | |
| Steroid hormone receptor ERR1 | ESRRA (ERRa) | |
| Thrombospondin‐1 | TSP‐1 | |
| Vascular endothelial growth factor A | VEGFA | |
| Glucose and lipid metabolism adaptations | α‐Enolase | ENO1 |
| β‐Enolase | ENO3 | |
| Carnitine O‐palmitoyltransferase 1, muscle isoform | CPT1B | |
| Fatty acid‐binding protein, heart | FABP3 (FABPH) | |
| Glycogen phosphorylase, muscle form | PYGM | |
| Glyoxalase I (lactoylglutathione lyase) | GLO1 | |
| Muscle form hexokinase | HK2 | |
| Muscle‐type aldolase | ALDOA | |
| Platelet glycoprotein 4 | FAT/CD36 | |
| Pyruvate dehydrogenase E1 component subunit α, somatic form, mitochondrial | PDHA1 (PDHa) | |
| Pyruvate dehydrogenase E1 component subunit β, mitochondrial | PDHB (PDHb) | |
| Solute carrier family 2, facilitated glucose transporter member 4 | SLC2A4 (GLUT4) | |
| Mitochondrial biogenesis and remodelling | 5‐Aminolevulinate synthase, non‐specific, mitochondrial | ALAS1 |
| 5′‐AMP‐activated protein kinase catalytic subunit α‐1 | PRKAA1 (AMPKa1) | |
| 5′‐AMP‐activated protein kinase catalytic subunit α‐2 | PRKAA1 (AMPKa2) | |
| 5′‐AMP‐activated protein kinase subunit β‐1 | PRKAB1 (AMPKb) | |
| 5′‐AMP‐activated protein kinase subunit γ‐1 | PRKAG1 (AMPKg) | |
| ATP synthase subunit β, mitochondrial | ATP5B | |
| BCL2/adenovirus E1B 19 kDa protein‐interacting protein 3 | BNIP3 | |
| BCL2/adenovirus E1B 19 kDa protein‐interacting protein 3‐like | BNIP3L | |
| Cyclic AMP‐dependent transcription factor 2 | ATF2 | |
| Cytochrome c oxidase subunit 1 | MT‐CO1 | |
| Cytochrome c oxidase subunit 2 | MT‐CO2 | |
| Cytochrome c oxidase subunit 3 | MT‐CO3 | |
| Cytochrome c oxidase subunit 4 isoform 1, mitochondrial | COX4I1 | |
| Cytochrome c oxidase subunit 4 isoform 2, mitochondria | COX4I2 | |
| Cytochrome c oxidase subunit 5A, mitochondrial | COX5A | |
| Cytochrome c oxidase subunit 5B, mitochondrial | COX5B | |
| Cytochrome c oxidase subunit 6A1, mitochondrial | Cox6a1 | |
| Cytochrome c oxidase subunit 6A2, mitochondrial | Cox6a2 | |
| Cytochrome c oxidase subunit 6B1 | Cox6b1 | |
| Cytochrome c oxidase subunit 6B2 | Cox6b2 | |
| Cytochrome c oxidase subunit 6C | Cox6c | |
| Cytochrome c oxidase subunit 7A‐related protein, mitochondrial | Cox7r | |
| Cytochrome c oxidase subunit 7A1, mitochondrial | Cox7a1 | |
| Cytochrome c oxidase subunit 7A2, mitochondrial | Cox7a2 | |
| Cytochrome c oxidase subunit 7B, mitochondrial | Cox7b | |
| Cytochrome c oxidase subunit 7C, mitochondrial | Cox7c | |
| Cytochrome c oxidase subunit 8A, mitochondrial P | Cox8a | |
| Cytochrome c oxidase subunit 8C, mitochondrial | Cox8c | |
| E3 ubiquitin‐protein ligase parkin | PARK2 (PARKIN) | |
| FUN14 domain‐containing protein 1 | FUNDC1 | |
| Microtubule‐associated proteins 1A/1B light chain 3B | MAP1LC3B (LC3) | |
| Mitochondrial fission 1 protein | FIS1 | |
| Mitofusin‐1 | MFN1 | |
| Mitofusin‐2 | MFN2 | |
| Mitogen‐activated protein kinase 11 | MAPK11 (p38b) | |
| Mitogen‐activated protein kinase 12 | MAPK12 (p38g) | |
| Mitogen‐activated protein kinase 13 | MAPK13 (p38d) | |
| Mitogen‐activated protein kinase 14 | MAPK14 (p38a) | |
| Myocyte‐specific enhancer factor 2A | MEF2A (MEF2 ) | |
| NAD‐dependent protein deacetylase sirtuin‐1 | SIRT1 | |
| Nuclear respiratory factor 1 | NRF‐1 | |
| Nuclear respiratory factor 2 (GA‐binding protein subunit β‐1) | NRF‐2 | |
| Peroxisome proliferator‐activated receptor γ coactivator 1‐α | PGC‐1α | |
| PTEN‐induced putative kinase protein 1 | PINK1 | |
| Putative cytochrome c oxidase subunit 7A3, mitochondrial | Cox7a3 | |
| Serine/threonine‐protein kinase ULK1 | ULK1 (ATG1 ) | |
| Steroid hormone receptor ERR1 | ESRRA (ERRa) | |
| Transcription factor A, mitochondrial | TFAM |
Table A2.
Summary of training loads in the two exercise groups (McArdle and wild‐type) during the endurance exercise training programme
| Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | Week 6 | Week 7 | Week 8 | |
|---|---|---|---|---|---|---|---|---|
| Training load variable | (5 sessions) | (5 sessions) | (5 sessions) | (5 sessions) | (5 sessions) | (5 sessions) | (5 sessions) | (3 sessions) |
| Intensity (%)* | 55 | 56 | 61 | 56 | 59 | 65 | 66 | 62 |
| (50, 60) | (50, 60) | (55, 65) | (50, 70) | (50, 65) | (55, 70) | (55, 75) | (50, 75) | |
| Treadmill inclination (%) | 0% | 3 | 9 | 9 | 13 | 14 | 14 | 12 |
| (0, 5) | (5, 15) | (5, 15) | (10, 15) | (10, 15) | (10, 15) | (5, 15) | ||
| Session duration (min) | 32 | 35 | 41 | 40 | 43 | 44 | 45 | 40 |
| (30, 35) | (30, 40) | (35, 45) | (40, 40) | (35, 50) | (35, 50) | (40, 50) | (30, 50) | |
| Distance/session (McArdle, m) | 227 | 264 | 328 | 337 | 341 | 372 | 389 | 396 |
| (207, 255) | (219, 305) | (292, 364) | (324, 362) | (280, 386) | (349, 412) | (261, 462) | (219, 455) | |
| Distance/session (wild‐type, m) | 303 | 339 | 423 | 432 | 440 | 446 | 463 | 468 |
| (277, 344) | (283, 392) | (371, 473) | (385, 447) | (355, 492) | (434, 471) | (383, 481) | (411, 493) |
Data are mean and range (min, max) for the core part of all the sessions within each week. *Determined as percentage of the velocity reached at the end of the maximal performance test performed at the start of the study (abbreviated as V max in the text). Of note, training loads decreased in the two days of the 8th week (Wednesday and Thursday) that preceded the final performance test (Friday).
Table A3.
List of the differentially expressed proteins between the trained and sedentary McArdle mice (n = 123) and the trained and sedentary wild‐type mice (n = 74)
Table A4.
Number of proteins reported for each artificial neuronal network (ANN) category in relation to skeletal muscle adaptations to endurance exercise training in McArdle and wild‐type mice; information is given for proteins individually (‘individual’), in combination (‘combined’) and both (‘total’)
| McArdle | Wild‐type | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ANN category | Individual | % | Combined | % | Total | % | Individual | % | Combined | % | Total | % | Common proteins |
| Strong (P < 0.05) | 4 | 4% | 135 | 2% | 139 | 2% | 0 | 0% | 1 | 0% | 1 | 0% | 0 |
| Medium–strong (P = 0.05–0.25) | 29 | 25% | 1900 | 34% | 1 929 | 33% | 16 | 22% | 718 | 32% | 734 | 31% | 1 |
| Weak (P > 0.25) | 78 | 67% | 3 636 | 64% | 3 714 | 64% | 52 | 71% | 1 559 | 68% | 1 611 | 69% | 2 |
| Not assessed | 5 | 4% | 0 | 0% | 5 | 0% | 5 | 7% | 0 | 0% | 5 | 0% | 0 |
| Total | 116 | 100% | 5 671 | 100% | 5 787 | 100% | 73 | 100% | 2 278 | 100% | 2 351 | 100% | 3 |
Table A5.
Summary of all the enriched protein sets (the corresponding database is also indicated)
| Source | McArdle mice | Wild‐type mice |
|---|---|---|
| BED | 7 | 0 |
| BED Motives | 9 | 5 |
| BED Pathways | 5 | 3 |
| GO Function | 7 | 3 |
| GO Location | 35 | 1 |
| GO Process | 55 | 2 |
| PharmGKB | 2 | 1 |
| TRRUST | 1 | 0 |
| KEGG | 0 | 13 |
| SMPDB | 0 | 51 |
| Total | 121 | 79 |
Abbreviations: BED, Biological Effectors Database; GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes; PharmGKB, Pharmacogenomics Knowledgebase; SMPDB, Small Molecule Pathway Database; TRRUST, Transcriptional Regulatory Relationships Unraveled by Sentence‐based Text‐mining database.
Table A6.
Enriched protein sets related to mitochondrial function in McArdle mice
| Source | Set name |
|---|---|
| BED pathways | Oxidative phosphorylation (OXPHOS) |
| GO function ID | Proton‐transporting ATP synthase activity, rotational mechanism |
| GO location | Mitochondrial crista junction |
| GO location | Mitochondrial inner membrane |
| GO location | Mitochondrial intermembrane space |
| GO location | Mitochondrial matrix |
| GO location | Mitochondrial proton‐transporting ATP synthase complex |
| GO location | Mitochondrial proton‐transporting ATP synthase complex, catalytic core F(1) |
| GO location | Mitochondrion |
| GO process | Mitochondrion organisation |
| GO process | Protein targeting to mitochondrion |
| GO process | Respiratory electron transport chain |
Abbreviations: BED, Biological Effectors Database; GO, Gene Ontology;
Table A7.
Enriched protein sets related to muscle structure and growth
| Source | Set name |
|---|---|
| GO location | Costamere |
| GO location | Cytoskeleton |
| GO location | Focal adhesion |
| GO location | Membrane |
| GO location | Actin filament |
| GO process | Cell‐matrix adhesion |
| GO process | Integrin‐mediated signalling pathway |
| GO process | Positive regulation of Rho protein signal transduction |
| GO process | Regulation of actin cytoskeleton organization |
| GO process | Mitotic cell cycle |
| GO process | Positive regulation of ubiquitin‐protein ligase activity involved in regulation of mitotic cell cycle transition |
| GO process | Regulation of ubiquitin‐protein ligase activity involved in mitotic cell cycle |
Abbreviations: GO, Gene Ontology.
Table A8.
Summary of the enriched protein sets related to increase in muscle size and function, and neuromodulation
| SOURCE | SET_NAME |
|---|---|
| KEGG | 04510 _Focal adhesion |
| KEGG | 04810 _Regulation of actin cytoskeleton |
| KEGG | 04151 _PI3K‐Akt signalling pathway |
| BED pathways | Gastrointestinal smooth muscle sustained contraction |
| BED pathways | Smooth muscle relaxation |
| BED pathways | Vascular smooth muscle contraction |
| KEGG | 04270 _Vascular smooth muscle contraction |
| GO function | poly(U) RNA binding |
| GO function | Translation activator activity |
| GO process | Small GTPase mediated signal transduction |
| GO process | Translational initiation |
| KEGG | 03015 _mRNA surveillance pathway |
| BED motives | Nervous impluse generation deffect |
| KEGG | 04713 _Circadian entrainment |
| KEGG | 04151 _PI3K‐Akt signalling pathway |
| KEGG | 04720 _Long‐term potentiation |
| KEGG | 04726 _Serotonergic synapse |
| KEGG | 04727 _GABAergic synapse |
| KEGG | 04728_Dopaminergic synapse |
Abbreviations: BED, Biological Effectors Database; GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes.
Table A9.
Summary of the enriched protein set related to catecholamine modulation within wild‐type (wt/wt) mice; set sources are also indicated
| Source | Set name | Source | Set name |
|---|---|---|---|
| PHARMKB | [PA2024] Beta‐agonist/beta‐blocker pathway, pharmacodynamics | SMPDB | [SMP00367] Carvedilol action pathway |
| SMPDB | [SMP00296] Acebutolol action pathway | SMPDB | [SMP00368] Labetalol action pathway |
| SMPDB | [SMP00297] Alprenolol action pathway | SMPDB | [SMP00375] Verapamil action pathway |
| SMPDB | [SMP00298] Atenolol action pathway | SMPDB | [SMP00376] Amlodipine action pathway |
| SMPDB | [SMP00299] Betaxolol action pathway | SMPDB | [SMP00377] Felodipine action pathway |
| SMPDB | [SMP00300] Bisoprolol action pathway | SMPDB | [SMP00378] Isradipine action pathway |
| SMPDB | [SMP00301] Esmolol action pathway | SMPDB | [SMP00379] Nifedipine action pathway |
| SMPDB | [SMP00302] Metoprolol action pathway | SMPDB | [SMP00380] Nimodipine action pathway |
| SMPDB | [SMP00303] Nadolol action pathway | SMPDB | [SMP00381] Nisoldipine action pathway |
| SMPDB | [SMP00304] Oxprenolol action pathway | SMPDB | [SMP00382] Nitrendipine action pathway |
| SMPDB | [SMP00305] Penbutolol action pathway | SMPDB | [SMP00588] Muscle/heart contraction |
| SMPDB | [SMP00306] Pindolol action pathway | SMPDB | [SMP00619] Felodipine metabolism pathway |
| SMPDB | [SMP00307] Propranolol action pathway | SMPDB | [SMP00657] Bopindolol action pathway |
| SMPDB | [SMP00320] Intracellular signalling through adenosine receptor A2a and adenosine | SMPDB | [SMP00658] Carteolol action pathway |
| SMPDB | [SMP00321] Intracellular signalling through adenosine receptor A2b and adenosine | SMPDB | [SMP00659] Timolol action pathway |
| SMPDB | [SMP00323] Quinidine action pathway | SMPDB | [SMP00660] Sotalol action pathway |
| SMPDB | [SMP00324] Procainamide (antiarrhythmic) action pathway | SMPDB | [SMP00661] Epinephrine action pathway |
| SMPDB | [SMP00325] Disopyramide action pathway | SMPDB | [SMP00662] Dobutamine action pathway |
| SMPDB | [SMP00326] Fosphenytoin (antiarrhythmic) action pathway | SMPDB | [SMP00663] Isoprenaline action pathway |
| SMPDB | [SMP00328] Lidocaine (antiarrhythmic) action | SMPDB | [SMP00664] Arbutamine action pathway |
| SMPDB | [SMP00329] Mexiletine action pathway | SMPDB | [SMP00665] Amiodarone action pathway |
| SMPDB | [SMP00330] Tocainide action pathway | SMPDB | [SMP00666] Levobunolol action pathway |
| SMPDB | [SMP00331] Flecainide action pathway | SMPDB | [SMP00667] Metipranolol action pathway |
| SMPDB | [SMP00332] Ibutilide action pathway | SMPDB | [SMP00668] Bevantolol action pathway |
| SMPDB | [SMP00359] Diltiazem action pathway | SMPDB | [SMP00669] Practolol action pathway |
| SMPDB | [SMP00366] Nebivolol action pathway | SMPDB | [SMP00670] Bupranolol action pathway |
Edited by: Scott Powers & Bruno Grassi
C. Fiuza‐Luces and A. Santos‐Lozano contributed equally to this work.
J. L. Zugaza and A. Lucia share senior authorship.
References
- Altschul SF, Gish W, Miller W, Myers EW & Lipman DJ (1990). Basic local alignment search tool. J Mol Biol 215, 403–410. [DOI] [PubMed] [Google Scholar]
- Ayala JE, Bracy DP, James FD, Julien BM, Wasserman DH & Drucker DJ (2009). The glucagon‐like peptide‐1 receptor regulates endogenous glucose production and muscle glucose uptake independent of its incretin action. Endocrinology 150, 1155–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett JD, Hawley JA & Morton JP (2015). Carbohydrate availability and exercise training adaptation: too much of a good thing? Eur J Sport Sci 15, 3–12. [DOI] [PubMed] [Google Scholar]
- Bassel‐Duby R & Olson EN (2006). Signaling pathways in skeletal muscle remodeling. Annu Rev Biochem 75, 19–37. [DOI] [PubMed] [Google Scholar]
- Berenbaum MC (1989). What is synergy? Pharmacol Rev 41, 93–141. [PubMed] [Google Scholar]
- Bergstrom J, Hermansen L, Hultman E & Saltin B (1967). Diet, muscle glycogen and physical performance. Acta Physiol Scand 71, 140–150. [DOI] [PubMed] [Google Scholar]
- Blake JA, Eppig JT, Kadin JA, Richardson JE, Smith CL, Bult CJ & the Mouse Genome Database Group (2017). Mouse Genome Database (MGD) – 2017: community knowledge resource for the laboratory mouse. Nucleic Acids Res 45, D723–D729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonzon‐Kulichenko E, Garcia‐Marques F, Trevisan‐Herraz M & Vazquez J (2015). Revisiting peptide identification by high‐accuracy mass spectrometry: problems associated with the use of narrow mass precursor windows. J Proteome Res 14, 700–710. [DOI] [PubMed] [Google Scholar]
- Brull A, de Luna N, Blanco‐Grau A, Lucia A, Martin MA, Arenas J, Marti R, Andreu AL & Pinos T (2015). Phenotype consequences of myophosphorylase dysfunction: insights from the McArdle mouse model. J Physiol 593, 2693–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona M, Lopez JA, Serafin A, Rongvaux A, Inserte J, Garcia‐Dorado D, Flavell R, Llovera M, Canas X, Vazquez J & Sanchis D (2015). Executioner caspase‐3 and 7 deficiency reduces myocyte number in the developing mouse heart. PLoS One 10, e0131411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuenda A & Rousseau S (2007). p38 MAP‐kinases pathway regulation, function and role in human diseases. Biochim Biophys Acta 1773, 1358–1375. [DOI] [PubMed] [Google Scholar]
- Favier FB, Benoit H & Freyssenet D (2008). Cellular and molecular events controlling skeletal muscle mass in response to altered use. Pflugers Arch 456, 587–600. [DOI] [PubMed] [Google Scholar]
- Fiuza‐Luces C, Nogales‐Gadea G, García‐Consuegra I, Pareja‐Galeano H, Rufián‐Vázquez L, Pérez LM, Andreu AL, Arenas J, Martín MA, Pinós T, Lucia A & Morán M (2016). Muscle signaling in exercise intolerance: insights from the McArdle mouse model. Med Sci Sports Exerc 48, 1448–1458. [DOI] [PubMed] [Google Scholar]
- Fiuza‐Luces C, Soares‐Miranda L, Gonzalez‐Murillo A, Palacio JM, Colmenero I, Casco F, Melen GJ, Delmiro A, Moran M, Ramirez M & Lucia A (2013). Exercise benefits in chronic graft versus host disease: a murine model study. Med Sci Sports Exerc 45, 1703–1711. [DOI] [PubMed] [Google Scholar]
- Frolkis A, Knox C, Lim E, Jewison T, Law V, Hau DD, Liu P, Gautam B, Ly S, Guo AC, Xia J, Liang Y, Shrivastava S & Wishart DS (2010). SMPDB: The Small Molecule Pathway Database. Nucleic Acids Res 38, D480–D487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frontera WR & Ochala J (2015). Skeletal muscle: a brief review of structure and function. Calcif Tissue Int 96, 183–195. [DOI] [PubMed] [Google Scholar]
- Garcia‐Marques F, Trevisan‐Herraz M, Martinez‐Martinez S, Camafeita E, Jorge I, Lopez JA, Mendez‐Barbero N, Mendez‐Ferrer S, Del Pozo MA, Ibanez B, Andres V, Sanchez‐Madrid F, Redondo JM, Bonzon‐Kulichenko E & Vazquez J (2016). A novel systems‐biology algorithm for the analysis of coordinated protein responses using quantitative proteomics. Mol Cell Proteomics 15, 1740–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy D (2015). Principles and standards for reporting animal experiments in The Journal of Physiology and Experimental Physiology . J Physiol 593, 2547–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha HC & Snyder SH (1999). Poly(ADP‐ribose) polymerase is a mediator of necrotic cell death by ATP depletion. Proc Natl Acad Sci USA 96, 13978–13982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller RG, Wyrick P, Taivassalo T & Vissing J (2006). Aerobic conditioning: an effective therapy in McArdle's disease. Ann Neurol 59, 922–928. [DOI] [PubMed] [Google Scholar]
- Han H, Shim H, Shin D, Shim JE, Ko Y, Shin J, Kim H, Cho A, Kim E, Lee T, Kim H, Kim K, Yang S, Bae D, Yun A, Kim S, Kim CY, Cho HJ, Kang B, Shin S & Lee I (2015). TRRUST: a reference database of human transcriptional regulatory interactions. Sci Rep 5, 11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrando‐Grabulosa M, Mulet R, Pujol A, Mas JM, Navarro X, Aloy P, Coma M & Casas C (2016). Novel neuroprotective multicomponent therapy for amyotrophic lateral sclerosis designed by networked systems. PLoS One 11, e0147626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho RC, Alcazar O, Fujii N, Hirshman MF & Goodyear LJ (2004). p38gamma MAPK regulation of glucose transporter expression and glucose uptake in L6 myotubes and mouse skeletal muscle. Am J Physiol Regul Integr Comp Physiol 286, R342–R349. [DOI] [PubMed] [Google Scholar]
- Hoydal MA, Wisloff U, Kemi OJ & Ellingsen O (2007). Running speed and maximal oxygen uptake in rats and mice: practical implications for exercise training. Eur J Cardiovasc Prev Rehabil 14, 753–760. [DOI] [PubMed] [Google Scholar]
- Iborra‐Egea O, Galvez‐Monton C, Roura S, Perea‐Gil I, Prat‐Vidal C, Soler‐Botija C & Bayes‐Genis A (2017). Mechanisms of action of sacubitril/valsartan on cardiac remodeling: a systems biology approach. NPJ Syst Biol Appl 3, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isern J, Martin‐Antonio B, Ghazanfari R, Martin AM, Lopez JA, del Toro R, Sanchez‐Aguilera A, Arranz L, Martin‐Perez D, Suarez‐Lledo M, Marin P, Van Pel M, Fibbe WE, Vazquez J, Scheding S, Urbano‐Ispizua A & Mendez‐Ferrer S (2013). Self‐renewing human bone marrow mesenspheres promote hematopoietic stem cell expansion. Cell Rep 3, 1714–1724. [DOI] [PubMed] [Google Scholar]
- Jungmichel S, Rosenthal F, Altmeyer M, Lukas J, Hottiger MO & Nielsen ML (2013). Proteome‐wide identification of poly(ADP‐ribosyl)ation targets in different genotoxic stress responses. Mol Cell 52, 272–285. [DOI] [PubMed] [Google Scholar]
- Kanehisa M, Goto S, Sato Y, Kawashima M, Furumichi M & Tanabe M (2014). Data, information, knowledge and principle: back to metabolism in KEGG. Nucleic Acids Res 42, D199–D205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koulmann N & Bigard AX (2006). Interaction between signalling pathways involved in skeletal muscle responses to endurance exercise. Pflugers Arch 452, 125–139. [DOI] [PubMed] [Google Scholar]
- Krag TO, Pinos T, Nielsen TL, Brull A, Andreu AL & Vissing J (2016a). Differential muscle involvement in mice and humans affected by McArdle disease. J Neuropathol Exp Neurol 75, 441–454. [DOI] [PubMed] [Google Scholar]
- Krag TO, Pinós T, Nielsen TL, Duran J, García‐Rocha M, Andreu AL & Vissing J (2016b). Differential glucose metabolism in mice and humans affected by McArdle disease. Am J Physiol Regul Integr Comp Physiol 311, R307–R314. [DOI] [PubMed] [Google Scholar]
- Lane SC, Camera DM, Lassiter DG, Areta JL, Bird SR, Yeo WK, Jeacocke NA, Krook A, Zierath JR, Burke LM & Hawley JA (2015). Effects of sleeping with reduced carbohydrate availability on acute training responses. J Appl Physiol (1985) 119, 643–655. [DOI] [PubMed] [Google Scholar]
- Lin YH, Zhen YY, Chien KY, Lee IC, Lin WC, Chen MY & Pai LM (2017). LIMCH1 regulates nonmuscle myosin‐II activity and suppresses cell migration. Mol Biol Cell 28, 1054–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucia A, Nogales‐Gadea G, Perez M, Martin MA, Andreu AL & Arenas J (2008). McArdle disease: what do neurologists need to know? Nat Clin Pract Neurol 4, 568–577. [DOI] [PubMed] [Google Scholar]
- Lucia A, Ruiz JR, Santalla A, Nogales‐Gadea G, Rubio JC, Garcia‐Consuegra I, Cabello A, Perez M, Teijeira S, Vieitez I, Navarro C, Arenas J, Martin MA & Andreu AL (2012). Genotypic and phenotypic features of McArdle disease: insights from the Spanish national registry. J Neurol Neurosurg Psychiatry 83, 322–328. [DOI] [PubMed] [Google Scholar]
- Martinez‐Bartolome S, Navarro P, Martin‐Maroto F, Lopez‐Ferrer D, Ramos‐Fernandez A, Villar M, Garcia‐Ruiz JP & Vazquez J (2008). Properties of average score distributions of SEQUEST: the probability ratio method. Mol Cell Proteomics 7, 1135–1145. [DOI] [PubMed] [Google Scholar]
- Mate‐Munoz JL, Moran M, Perez M, Chamorro‐Vina C, Gomez‐Gallego F, Santiago C, Chicharro L, Foster C, Nogales‐Gadea G, Rubio JC, Andreu AL, Martin MA, Arenas J & Lucia A (2007). Favorable responses to acute and chronic exercise in McArdle patients. Clin J Sport Med 17, 297–303. [DOI] [PubMed] [Google Scholar]
- Navarro P, Trevisan‐Herraz M, Bonzon‐Kulichenko E, Nunez E, Martinez‐Acedo P, Perez‐Hernandez D, Jorge I, Mesa R, Calvo E, Carrascal M, Hernaez ML, Garcia F, Barcena JA, Ashman K, Abian J, Gil C, Redondo JM & Vazquez J (2014). General statistical framework for quantitative proteomics by stable isotope labeling. J Proteome Res 13, 1234–1247. [DOI] [PubMed] [Google Scholar]
- Navarro P & Vazquez J (2009). A refined method to calculate false discovery rates for peptide identification using decoy databases. J Proteome Res 8, 1792–1796. [DOI] [PubMed] [Google Scholar]
- Nogales‐Gadea G, Pinos T, Lucia A, Arenas J, Camara Y, Brull A, de Luna N, Martin MA, Garcia‐Arumi E, Marti R & Andreu AL (2012). Knock‐in mice for the R50X mutation in the PYGM gene present with McArdle disease. Brain 135, 2048–2057. [DOI] [PubMed] [Google Scholar]
- Pache RA, Zanzoni A, Naval J, Mas JM & Aloy P (2008). Towards a molecular characterisation of pathological pathways. FEBS Lett 582, 1259–1265. [DOI] [PubMed] [Google Scholar]
- Pernow B & Saltin B (1971). Availability of substrates and capacity for prolonged heavy exercise in man. J Appl Physiol 31, 416–422. [DOI] [PubMed] [Google Scholar]
- Rivals I, Personnaz L, Taing L & Potier MC (2007). Enrichment or depletion of a GO category within a class of genes: which test? Bioinformatics 23, 401–407. [DOI] [PubMed] [Google Scholar]
- Russell AP ( 2010). Molecular regulation of skeletal muscle mass. Clin Exp Pharmacol Physiol 37, 378–384. [DOI] [PubMed] [Google Scholar]
- Santalla A, Nogales‐Gadea G, Ortenblad N, Brull A, de Luna N, Pinos T & Lucia A (2014). McArdle disease: a unique study model in sports medicine. Sports Med 44, 1531–1544. [DOI] [PubMed] [Google Scholar]
- Sonnhammer EL & Ostlund G (2015). InParanoid 8: orthology analysis between 273 proteomes, mostly eukaryotic. Nucleic Acids Res 43, D234–D239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The UniProt Consortium (2017). UniProt: the universal protein knowledgebase. Nucleic Acids Res 45, D158–D169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk WR, Heller SL, Norris BJ & Nemeth PM (1990). Increased muscular β‐hydroxyacyl CoA dehydrogenase with McArdle's disease. Muscle Nerve 13, 607–612. [DOI] [PubMed] [Google Scholar]
- Vissing J & Haller RG (2003). The effect of oral sucrose on exercise tolerance in patients with McArdle's disease. N Engl J Med 349, 2503–2509. [DOI] [PubMed] [Google Scholar]
- Webster CC, Noakes TD, Chacko SK, Swart J, Kohn TA & Smith JA (2016). Gluconeogenesis during endurance exercise in cyclists habituated to a long‐term low carbohydrate high‐fat diet. J Physiol 594, 4389–4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whirl‐Carrillo M, McDonagh EM, Hebert JM, Gong L, Sangkuhl K, Thorn CF, Altman RB & Klein TE (2012). Pharmacogenomics knowledge for personalized medicine. Clin Pharmacol Ther 92, 414–417. [DOI] [PMC free article] [PubMed] [Google Scholar]


