Abstract
Key points
Skeletal muscle stem cells (satellite cells) play a crucial role in repair and remodelling of muscle in response to exercise.
Satellite cells are in close spatial proximity to muscle capillaries and therefore may be influenced by them.
In this study, we describe the activation and expansion of the satellite cell pool in response to eccentric contraction‐induced muscle damage in individuals with significantly different levels of muscle capillarization.
Individuals with greater capillarization and capacity for muscle perfusion demonstrated enhanced activation and/or expansion of the satellite cell pool allowing for an accelerated recovery of muscle function.
These results provide insight into the critical relationship between muscle capillarization and satellite cells during skeletal muscle repair.
Abstract
Factors that determine the skeletal muscle satellite cell (SC) response remain incompletely understood. It is known, however, that SC activation status is closely related to the anatomical relationship between SCs and muscle capillaries. We investigated the impact of muscle fibre capillarization on the expansion and activation status of SCs following a muscle‐damaging exercise protocol in healthy young men. Twenty‐nine young men (21 ± 0.5 years) performed 300 unilateral eccentric contractions (180 deg s−1) of the knee extensors. Percutaneous muscle biopsies from the vastus lateralis and blood samples from the antecubital vein were taken prior to (Pre) exercise and at 6, 24, 72 and 96 h of post‐exercise recovery. A comparison was made between subjects who had a relative low mixed muscle capillary‐to‐fibre perimeter exchange index (CFPE; Low group) and high mixed muscle CFPE index (High group) at baseline. Type I and type II muscle fibre size, myonuclear content, capillarization, and SC response were determined via immunohistochemistry. Overall, there was a significant correlation (r = 0.39; P < 0.05) between the expansion of SC content (change in total Pax7+ cells/100 myofibres) 24 h following eccentric exercise and mixed muscle CFPE index. There was a greater increase in activated SCs (MyoD+/Pax7+ cells) in the High as compared to the Low CFPE group 72 h following eccentric exercise (P < 0.05). The current study provides further evidence that muscle fibre capillarization may play an important role in the activation and expansion of the SC pool during the process of skeletal muscle repair.
Keywords: skeletal muscle, Satellite cells, Capillaries, Muscle damage
Key points
Skeletal muscle stem cells (satellite cells) play a crucial role in repair and remodelling of muscle in response to exercise.
Satellite cells are in close spatial proximity to muscle capillaries and therefore may be influenced by them.
In this study, we describe the activation and expansion of the satellite cell pool in response to eccentric contraction‐induced muscle damage in individuals with significantly different levels of muscle capillarization.
Individuals with greater capillarization and capacity for muscle perfusion demonstrated enhanced activation and/or expansion of the satellite cell pool allowing for an accelerated recovery of muscle function.
These results provide insight into the critical relationship between muscle capillarization and satellite cells during skeletal muscle repair.
Introduction
Skeletal muscle satellite cells (SCs) are indispensable for muscle regeneration and repair following injury (Lepper et al. 2011; McCarthy et al. 2011; Sambasivan et al. 2011). In response to a physiological cue (e.g. exercise), SCs activate, proliferate and differentiate, donating nuclei to existing muscle fibres to aid in repair/adaptation, or return to a state of quiescence to replenish the basal SC pool (Bentzinger et al. 2012; Yin et al. 2013). The process of SC activation through terminal differentiation is orchestrated by a transcriptional network, known as the myogenic regulatory factors (MRFs), and is collectively referred to as the myogenic programme. Expansion of the SC pool following a single bout of exercise or muscle fibre contraction‐induced damage has been well characterized in humans (McKay et al. 2008, 2009, 2012, 2013; Bellamy et al. 2014; Nederveen et al. 2017) with appreciable expansion occurring by 24 h and peaking 72 h post‐stimulus (Snijders et al. 2015).
A number of cytokines and growth factors including, but not limited to, interleukin‐6 (IL‐6), insulin‐like growth factor‐1 (IGF‐1), myostatin and hepatocyte growth factor (HGF) are known regulators of SC progression through the myogenic programme (McKay et al. 2008, 2009; O'Reilly et al. 2008). Many of these factors are produced by skeletal muscle in its function as an ‘endocrine organ’ (Steensberg et al. 2000; Pedersen & Febbraio, 2008), or by other organs, tissues or cells (Velloso, 2008) and then delivered to the SC niche via the vasculature. Therefore, delivery of these factors to the SC niche may be a requirement of the myogenic response. Indeed, the importance of extrinsic factors in regulating SC function has been demonstrated using parabiotic pairings of old and young rodents (Conboy et al. 2005; Brack & Rando, 2007).
Muscle capillaries function as the delivery mechanism for oxygen, fuel, cytokines and growth factors that may regulate SCs, but may also act as an important modulator of the SC response. We and others have reported an anatomical relationship between muscle SCs and the microvasculature, with activated SCs situated closer to capillaries than quiescent SCs (Christov et al. 2007; Nederveen et al. 2016, 2017). Consequently, it has been proposed that SC content (Emslie‐Smith & Engel, 1990; Christov et al. 2007) and/or SC activation status (Chazaud et al. 2003; Christov et al. 2007; Nederveen et al. 2016) may be related to the extent of muscle fibre capillarization as a result of exposure of SCs to circulating factors or direct communication between endothelial cells and SCs during muscle repair (Chazaud et al. 2003; Ochoa et al. 2007; Joanisse et al. 2017). However, to what extent the muscle fibre microvascular bed may dictate the acute muscle SC response during muscle repair in humans remains unknown. Therefore, in the present study, we assessed the expansion and activation status of the SC pool following a single bout of exercise‐induced muscle fibre damage in a group of healthy young men with varying degrees of muscle fibre capillarization. We hypothesized that individuals with a greater degree of muscle fibre capillarization would demonstrate a more rapid and pronounced SC response following a single bout of eccentric exercise.
Methods
Ethical approval
The study was approved by the Hamilton Health Sciences Integrated Research Ethics Board, and conformed to the guidelines outlined in the Declaration of Helsinki. Participants gave their informed written consent prior to inclusion in the study.
Participants
Twenty‐nine healthy young men (YM: 22 ± 0.5 years; mean ± SEM) were recruited to participate in this study. Exclusion criteria included smoking, diabetes, the use of non‐steroidal anti‐inflammatory drugs (NSAIDs) and/or statins, and a history of respiratory disease and/or any major orthopaedic disability. Subjects were told to refrain from exercising throughout the duration of the study, and refrain from the use of NSAIDs (Mackey et al. 2016).
test and anthropometric measurements
During an initial visit to the laboratory, participants performed a test on a cycle ergometer (model: H‐300‐R Lode; Lode BV, Groningen, The Netherlands) and had anthropometric measurements recorded. The test consisted of load‐less pedalling for 1 min, followed by a step‐wise increase to 50 W for 2 min. After the increase to 50 W, work rate was increased by 30 W min−1 until the participant reached volitional fatigue (determined by the inability of the participant to maintain a minimum cadence of 60 rpm). Gas exchange was collected throughout the test using a metabolic cart (Moxus, AEI Technologies, Pittsburgh, PA, USA) and was calculated using the highest 30 s average during the final stage of the ramp protocol. Work rate (WR) was collected continuously throughout the test and peak aerobic power (WRpeak) was calculated using the average WR from the last 30 s of the test. In order the limit the impact of a bout of exercise on SC activation status and content (Arentson‐Lantz et al. 2016; Snijders et al. 2016), the test was performed at least 96 h prior to the Pre biopsy.
Muscle biopsy sampling
Percutaneous needle biopsies were taken, after an (∼10 h) overnight fast (Pre), from the mid‐portion of the vastus lateralis under local anaesthetic using a 5 mm Bergstrom needle adapted for manual suction. Subjects had not participated in any physical activity for at least 96 h before muscle biopsy collection prior to the single bout of eccentric exercise. The muscle biopsy procedure was repeated at 6 h, and in the fasted condition (∼10 h) at 24, 72 and 96 h of post‐exercise recovery. Incisions for the repeated muscle biopsy sampling were spaced approximately 3 cm apart to minimize any effect of the previous biopsy. Upon excision, muscle samples were immediately mounted in optimal cutting temperature (OCT) compound, frozen in liquid nitrogen‐cooled isopentane, while another part was directly frozen in liquid nitrogen, and stored at −80°C until further analysis.
Blood sampling
Blood samples were obtained from the antecubital vein immediately prior to the muscle biopsy sampling procedure before and after 6, 24, 72 and 96 h of the single bout of eccentric exercise. Blood (∼10 mL) samples were collected in EDTA‐containing tubes and centrifuged at 1500 rpm for 10 min at 4°C. Aliquots of plasma were frozen in liquid nitrogen and stored at −80°C. Plasma samples were analysed for IL‐6 protein and creatine kinase activity using commercially available enzyme‐linked immunosorbant assay (ELISA) (R&D Systems, Inc., Minneapolis, MN, USA) and activity assay kits (Abcam Inc., Toronto, Canada), respectively, following the manufacturer's instructions. Statistics were performed on the raw values, and expressed as a percentage change from baseline.
Eccentric muscle damage protocol
Maximal isokinetic unilateral muscle‐lengthening contractions of the quadriceps were performed using the Biodex dynamometer (Biodex‐System 3, Biodex Medical Systems, Inc., Shirley, NY, USA) at 180 deg s−1. For each subject, one leg was selected randomly to perform the exercise protocol described below. Movement at the shoulders, hips and thigh was restrained with straps in order to isolate the knee extensors during the protocol. Immediately prior to the intervention, subjects underwent a brief familiarization with the equipment, involving 5–10 submaximal lengthening contractions of the leg to be exercised. Subjects were required to perform 30 sets of 10 maximal knee extensions with 1 min rest between sets, for a total of 300 lengthening contractions. During each set, investigators provided verbal encouragement for the subjects to complete and exert maximal force during each contraction. This protocol has been previously shown to induce a significant level of skeletal muscle damage (Beaton et al. 2002).
Quantification of force production
Maximal voluntary isometric contraction (MVC) was performed using the Biodex dynamometer (Biodex‐System 3), set at a knee angle of 90 deg. Participants were situated in the system as described above, and testing consisted of three 5 s unilateral knee extension MVCs with 1 min of rest between contractions. A knee angle of 90 deg was used. The average of the recorded torque for the set was taken as the MVC. The MVC for the Pre time point was performed at least 96 h prior to the Pre biopsy, in order to minimize the influence of maximal contraction on the expansion and/or activation of the SC pool. This measurement was performed following the muscle biopsy and blood draw procedures, so as to minimize the influence of the strength test on systemic factors. The MVC for the Pre time point was performed at least 96 h prior to the Pre biopsy, in order to minimize the influence of maximal contraction on the expansion and/or activation of the SC pool.
Immunofluorescence
Muscle cross sections (7 μm) were prepared from unfixed OCT embedded samples, allowed to air dry for 30 min and stored at −80°C. Samples were stained with appropriate primary and secondary antibodies against specific antigens, found in Table 1, as previously described (Nederveen et al. 2016). Nuclei were labelled with 4′,6‐diamidino‐2‐phenylindole (DAPI; 1:20000; Sigma‐Aldrich, Oakville, ON, Canada), prior to coverslipping with fluorescent mounting media (DAKO, Burlington, ON, Canada). The staining procedures were verified using negative controls, in order to ensure appropriate specificity of staining. Slides were viewed with the Nikon Eclipse Ti Microscope (Nikon Instruments, Inc., Melville, NY, USA), equipped with a high‐resolution Photometrics CoolSNAP HQ2 fluorescent camera (Nikon Instruments). Images were captured and analysed using the Nikon NIS Elements AR 3.2 software (Nikon Instruments). All images were obtained with the ×20 objective, and ≥200 muscle fibres per subject per time point were included in the analyses for SC content/activation status (i.e. Pax7+/MyoD− or Pax7+/MyoD−), and fibre cross sectional area (CSA) and perimeter. The activation status of SCs was determined via the colocalization of Pax7+ and DAPI (Pax7+/MyoD−) and/or the colocalization of Pax7, MyoD and DAPI (i.e. Pax7+/MyoD+). Cell membranes were labelled with peroxidase conjugated wheat germ agglutinin (WGA) (1 μg mL−1, PL‐1026, Vector, Burlington, ON, Canada) and realized with a substrate kit (Vector, SK‐4700) as per manufacturer's instructions. Slides were blinded for both group and time point. The quantification of muscle fibre capillaries was performed on 50 muscle fibres per subject per time point (Porter et al. 2002). Based on the work of Hepple and colleagues (Hepple, 1997; Hepple & Mathieu‐Costello, 2001), quantification was performed for (i) capillary contacts (CC; the number of capillaries around a fibre), (ii) the capillary‐to‐fibre ratio on an individual fibre basis (C/Fi), (iii) the number of fibres sharing each capillary (i.e. the sharing factor) and (iv) the capillary density (CD). The CD was calculated by using the cross sectional area (μm2) as the reference space. The capillary‐to‐fibre perimeter exchange index (CFPE) was calculated as an estimate of the capillary‐to‐fibre surface area (Hepple, 1997). The SC‐to‐capillary distance measurements were performed on all SCs that were enclosed by other muscle fibres, and has been described previously (Nederveen et al. 2016). All immunofluorescence analyses were completed in a blinded fashion.
Table 1.
Detailed information on primary and secondary antibodies and dilutions used for immunofluorescent staining of the frozen muscle cross sections
| Antibody | Species | Source | Details | Primary | Secondary |
|---|---|---|---|---|---|
| Anti‐Pax7 | Mouse | DSHB | Pax7 | 1:1 | Alexa 594, 488 goat‐anti mouse 1:500 |
| Anti‐Laminin | Rabbit | Abcam | ab11575 | 1:500 | Alexa Fluor 488, 647 goat anti‐rabbit, 1:500 |
| Anti‐MHCI | Mouse | DSHB | A4.951 Slow isoform | 1:1 | Alexa Fluor 488 goat anti‐mouse, 1:500 |
| Anti‐CD31 | Rabbit | Abcam | ab28364 | 1:30 | Alexa Fluor 647 goat anti‐rabbit, 1:500 |
| Anti‐MyoD | Mouse | Dako | 5.8A | 1:50 | Goat anti‐mouse biotinylated secondary antibody, 1:200; streptavidin‐594 fluorochrome, 1:250 |
RNA isolation
RNA was isolated from 15–25 mg of muscle tissue using the Trizol/RNeasy method. All samples were homogenized with 1 mL of Trizol Reagent (Life Technologies, Burlington, ON, Canada), in Lysing Maxtrix D tubes (MP Biomedicals, Solon, OH, USA), with the FastPrep‐24 Tissue and Cell Homogenizer (MP Biomedicals) for a duration of 40 s at a setting of 6 m s−1. Following a 5 min room temperature incubation, homogenized samples were stored at −80°C for 1 month until further processing. After thawing on ice, 200 mL of chloroform (Sigma‐Aldrich) was added to each sample, mixed vigorously for 15 s, incubated at room temperature for 5 min, and spun at 12,000 g for 10 min at 4°C. The RNA (aqueous) phase was purified using the EZNA Total RNA Kit 1 (Omega Bio‐Tek, Norcross, GA, USA) as per the manufacturer's instructions. RNA concentration (ng mL−1) and purity (260/280) was determined with the Nano‐Drop 1000 Spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA).
Reverse transcription
Samples were reverse transcribed using a high capacity cDNA reverse transcription kit (Thermo Fisher Scientific, Waltham MA, USA) in 20 μL reaction volumes, as per the manufacturer's instructions, using an Eppendorf Mastercycler epGradient Thermal Cycler (Eppendorf, Mississauga, ON, Canada) to obtain cDNA for gene expression analysis.
Quantitative RT‐PCR
All quantitative RTPCR reactions were run in duplicate in 25 μL volumes containing RT Sybr Green qPCR Master Mix (Qiagen Inc., Valencia, CA, USA), prepared with the epMotion 5075 Eppendorf automated pipetting system (Eppendorf, Mississauga, ON, Canada), and carried out using an Eppendorf Realplex2 Master Cycler epgradient. Primers are listed in Table 2 and were re‐suspended in 1× TE buffer (10 mm Tris–HCl and 0.11 mm EDTA) and stored at −20°C prior to use. Messenger RNA expression was calculated using the method, and fold changes from baseline were calculated using the ∆∆C t method (Livak & Schmittgen, 2001). Briefly, C t values were first normalized to the housekeeping gene glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) (Table 2). C t values normalized to GAPDH were expressed as ΔC t values. These were then normalized to Pre values, expressed as ΔΔC t. Values were then transformed out of the logarithmic scale using the formula: fold change = (Livak & Schmittgen, 2001). Thus, mRNA values are expressed as a fold change from Pre (mean ± SEM). GAPDH expression was not different from Pre at any of the post‐intervention time points.
Table 2.
Primer sequences for quantitative real‐time PCR
| Gene name | Forward sequence (5′–3′) | Reverse sequence (5′–3′) |
|---|---|---|
| Myf5 | 5′‐ATGGACGTGATGGATGGCTG‐3′ | GCGGCACAAACTCGTCCCCAA |
| MyoD | 5′‐GGTCCCTCGCGCCCAAAAGAT‐3′ | CAGTTCTCCCGCCTCTCCTAC |
| MRF4 | 5′‐CCCCTTCAGCTACAGACCCAA‐3′ | CCCCCTGGAATGATCGGAAAC |
| Myogenin | 5′‐CAGTGCACTGGAGTTCAGCG‐3′ | TTCATCTGGGAAGGCCACAGA |
| GAPDH | 5′‐CCTCCTGCACCACCAACTGCTT‐3′ | GAGGGGCCATCCACAGTCTTCT |
MyoD, myogenic determination factor; Myf5, myogenic factor‐5; MRF4, myogenic regulatory factor‐4; GAPDH, glyceraldehyde 3‐phosphate dehydrogenase.
Statistical analysis
Statistical analysis was performed using SigmaStat 3.1.0 analysis software (Systat Software, San Jose, CA, USA).
Stratification of individuals based on capillarization
To assess the impact of muscle fibre capillarization on the muscle SC response following a single bout of eccentric exercise and subsequent muscle damage, participants were assigned to one of two groups (n = 10 per group) based on mixed muscle fibre capillarization (corrected for capillary sharing factor and muscle fibre perimeter, also known as capillary‐to‐fibre perimeter exchange (CFPE) index) for non‐correlative statistical analysis. In order to ensure that we selected groups that were sufficiently separated on the basis of mixed muscle CFPE, we divided the overall group of healthy young men into tertiles. The average of the Low group mixed muscle CFPE was 5.2 ± 0.5 with a range of 4.4–5.8 capillaries · 1000 μm−1, whereas the High group mixed muscle CFPE was 7.6 ± 1.0 with a range of 6.6–10.1 capillaries · 1000 μm−1. The average of the ‘non‐grouped’ or ‘mid‐group’ was 6.4 ± 0.1 with a range of 6.3–6.5 capillaries · 1000 μm−1. Stratification of participants resulted in a ‘middle’ group (n = 9) who were not used in non‐correlative statistical analysis, with the intent to create a clear separation between the Low and High group.
Baseline comparisons
Comparisons of participant demographics between the High CPFE and the Low CFPE groups are found in Table 3, and were performed with Student's t test. Baseline comparisons of muscle fibre type specific characteristics between the High CFPE and the Low CFPE group were performed using a two‐way ANOVA (group × fibre type).
Table 3.
Demographics and group characteristics
| Variable | Overall (n = 29) | High CFPE (n = 10) | Low CFPE (n = 10) |
|---|---|---|---|
| Age (years) | 22 ± 1 | 22 ± 1 | 21 ± 0 |
| Height (cm) | 179.2 ± 1.3 | 176.8 ± 1.1 | 179.5 ± 0.9 |
| Weight (kg) | 80.9 ± 2.4 | 72.5 ± 1.6* | 83.9 ± 2.3 |
| BMI (kg m−2) | 25.1 ± 0.6 | 23.2 ± 0.5 | 26.0 ± 0.6 |
| Force production (N m) | 319.4 ± 13.8 | 272.6 ± 8.8 | 314.4 ± 13.3 |
| (mL kg min−1) | 49.9 ± 2.3 | 63.1 ± 1.5* | 40.5 ± 1.4 |
| Peak work rate (W) | 339.2 ± 11.8 | 385.3 ± 8.9* | 290.6 ± 11.0 |
Values are means ± SEM. *Significant effect for group.
Response to eccentric contractions
One‐way repeated measures ANOVA was performed separately for each of the ‘Overall’ group, for the High CFPE group and for the Low CFPE group, with time (Pre, 6, 24, 72 and 96 h) as a within group factor. In this one‐way repeated measures ANOVA design for the acute response, post‐exercise time points were only compared with baseline (Pre) and Bonferonni corrections were applied to account for multiple comparisons. These tests were performed to assess the following outcomes, following the bout of eccentric exercise induced muscle damage: the acute change in SC activity status (i.e. Pax7+/MyoD+ cells); the acute change in SC content (i.e. mixed muscle, type I and/or type II Pax7+ cells); the acute change in plasma IL‐6 content; the acute change in plasma creatine kinase activity; the acute change in quadriceps muscle force production; and the acute change in MRF mRNA expression. For correlations, Pearson's correlation analyses were performed where appropriate between indices of muscle fibre capillarization and the SC response following eccentric exercise. Statistical significance was accepted at P < 0.05. All results are presented as means ± standard error of the mean (SEM).
Results
Subject characteristics
Overall
Complete subject characteristics are reported in Table 3.
Low vs. high CFPE group
There were no differences in age or height between the groups (Table 3). There was a significant difference in bodyweight (P < 0.05; Table 3) and a trend for BMI to be significantly different (P = 0.06, Table 3) between the groups. Both the (mL−1 kg−1 min−1) and WRpeak (W) were significantly greater in the High as compared to the Low group (P < 0.05; Table 3). There was no significant difference in force production prior to single bout eccentric exercise in the High (272.6 ± 8.8 N m) compared to the Low (314.4 ± 13.3 N m) group (Table 3).
Indices of muscle damage following repeated eccentric contractions
Overall
Following eccentric contractions, force production (N m) was significantly reduced at 6 h (253 ± 15 N m), 24 h (233 ± 16 N m), 72 h (261 ± 18 N m) and 96 h (270 ± 17 N m), as compared to Pre (319 ± 14 N m) (P < 0.05). Following the eccentric contraction protocol, plasma creatine kinase activity was significantly increased at 24 h (103.7 ± 8.2 mU mL−1, P < 0.05) compared to Pre (75.4 ± 6.1 mU mL−1), and returned back to baseline at 72 h (76.3 ± 6.7 mU mL−1) and 96 h (79.1 ± 6.6 mU mL−1)
High CFPE vs. low CFPE group
In the High group, force production was significantly reduced at 6 h (211 ± 15 N m), 24 h (196 ± 17 N m) and 72 h (230 ± 21 N m) as compared to Pre (272 ± 15 N m) and was back at baseline levels again at 96 h (241 ± 23 N m) (P < 0.05). In the Low group, force production was significantly reduced at 6 h (248 ± 29 N m), 24 h (215 ± 34 N m), 72 h (242 ± 37 N m) and 96 h (257 ± 32 N m) as compared to Pre (314 ± 23 N m) (P < 0.05). In the High group, plasma creatine kinase activity was significantly increased 24 h following eccentric exercise (93.3 ± 13.8 mU mL−1, P < 0.05), but was not significantly different at 72 h (56.0 ± 5.4 mU mL−1) and 96 h (66.4 ± 8.6 mU mL−1) as compared to Pre (60.3 ± 8.4 mU mL−1) (P < 0.05). In the Low group, plasma creatine kinase activity was significantly increased 24 h following eccentric exercise (107.9 ± 10.7 mU mL−1, P < 0.05), but was not significantly different at 72 h (92.9 ± 12.4 mU mL−1) and 96 h (91.1 ± 14.3 mU mL−1) as compared to Pre (88.4 ± 11.2 mU mL−1) (P < 0.05). Prior to the intervention, there were no significant differences in creatine kinase activity in the High compared to the Low group; there were no differences in creatine kinase activity changes following eccentric exercise.
Skeletal muscle fibre characteristics
Overall
Muscle fibre CSA was significantly greater in type II (7500 ± 355 μm2) compared to type I fibres (6326 ± 205 μm2, P < 0.05). Muscle fibre perimeter was significantly greater in type II (326 ± 6 μm) compared to type I fibres (306 ± 5 μm, P < 0.05). The number of myonuclei per fibre was not different between type II as compared to type I (3.7 ± 0.2 vs. 3.6 ± 0.2 myonuclei per fibre, respectively). Myonuclear domain size was significantly greater in type II as compared to type I muscle fibres (2019 ± 88 vs. 1805 ± 53 μm2, respectively, P < 0.05). Muscle C/Fi (2.08 ± 0.1 vs. 1.94 ± 0.1 capillaries per fibre, respectively), CFPE index (6.86 ± 0.2 vs. 6.03 ± 0.23 capillaries · 1000μm−1, respectively) and CD (655 ± 26 vs. 478 ± 29 capillaries mm−2, respectively) were significantly greater in type I compared to type II muscle fibres (P < 0.05). SC distance to nearest capillary was significantly greater in type II compared to type I muscle fibres (15.8 ± 0.7 vs. 13.9 ± 0.7 μm, respectively, P < 0.05) at baseline (Pre). Total Pax7+ cell distance to nearest capillary at baseline was negatively correlated to mixed muscle CFPE index (r = −0.49, P < 0.05) across all participants. Type II muscle fibre‐associated SC distance to nearest capillary at baseline was negatively correlated to type II fibre CFPE index (r = −0.51, P < 0.05; see Fig. 4 D).
Figure 4. The spatial relationship between satellite cells (SC) and muscle fibre capillaries impacts SC response to eccentric damage.
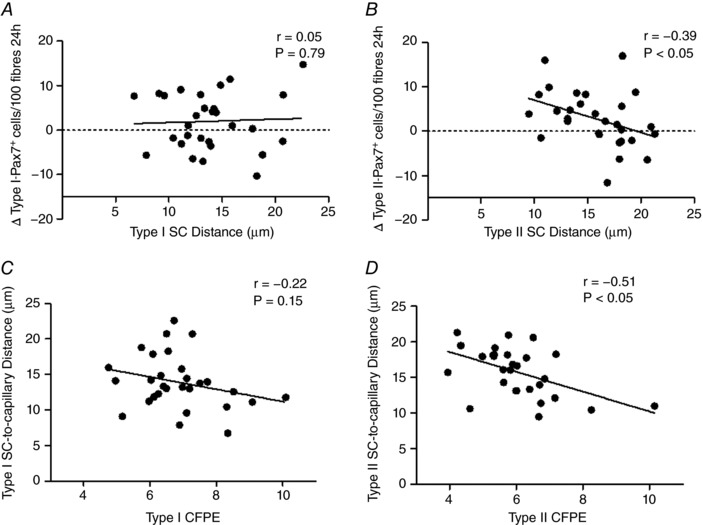
A and B, relationship between the expansion of the satellite cell (SC) pool from Pre to 24 h post‐eccentric exercise in a fibre type specific manner and fibre type specific CFPE for type I‐associated SC (r = 0.05, P = 0.79; A) and type II‐associated SC (r = −0.39, P < 0.05; B) across all participants. C and D, relationship between fibre type specific capillary to fibre exchange (CFPE) index and distance of Pax7+ SC to nearest capillary following eccentric exercise prior to eccentric damage for type I‐associated SC (r = −0.22, P = 0.15; C) and type II‐associated SC (r = −0.51, P < 0.05; D) across all participants.
High CFPE vs. low CFPE group
Type II muscle fibre CSA and perimeter were significantly greater compared with type I muscle fibre in both groups, with no difference between group (P < 0.05; Table 4). Interestingly, the proportion of type I muscle fibres was significantly higher in the High group compared with the Low group (P < 0.05, Table 4). Both type I and type II muscle fibre C/Fi, CFPE index and CC were significantly higher in the High as compared to the Low group (P < 0.05; Table 4). There were no significant differences (P > 0.05) in type I or type II myonuclei per fibre and/or myonuclear domain size between groups.
Table 4.
Muscle fibre capillarization and muscle characteristics
| Variable | High CFPE group (n = 10) | Low CFPE group (n = 10) |
|---|---|---|
| CC | ||
| Type I | 4.19 ± 0.23† | 3.97 ± 0.29 |
| Type II | 4.53 ± 0.28† | 2.31 ± 0.11 |
| C/Fi | ||
| Type I | 2.41 ± 0.10† | 1.66 ± 0.07 |
| Type II | 2.32 ± 0.11† | 1.50 ± 0.06 |
| CFPE (capillaries · 1000 μm−1) | ||
| Type I | 7.97 ± 0.34*, † | 5.75 ± 0.17* |
| Type II | 7.24 ± 0.35† | 4.91 ± 0.17 |
| Fibre type proportion (%) | ||
| Type I | 56.0 ± 3.1† | 33.9 ± 2.1 |
| Type II | 42.2 ± 3.3 | 65.8 ± 2.1† |
| Muscle fibre CSA (μm2) | ||
| Type I | 6185 ± 231 | 5937 ± 376 |
| Type II | 7121 ± 445* | 6626 ± 568* |
| Myonuclear domain (μm2) | ||
| Type I | 1817 ± 109 | 1736 ± 85 |
| Type II | 1896 ± 157 | 1969 ± 104 |
| Myonuclei per fibre | ||
| Type I | 3.5 ± 0.2 | 3.5 ± 0.5 |
| Type II | 3.9 ± 0.2 | 3.3 ± 0.2 |
| Muscle fibre perimeter | ||
| Type I | 306 ± 5 | 293 ± 11 |
| Type II | 327 ± 8* | 308 ± 12* |
| Satellite cell distance to nearest capillary (μm) | ||
| Type I | 12.1 ± 0.8† | 13.9 ± 0.9 |
| Type II | 13.3 ± 0.9† | 17.3 ± 0.9 |
| Satellite cell per 100 myofibres | ||
| Type I | 11.6 ± 1.6 | 10.5 ± 1.9 |
| Type II | 11.8 ± 1.3 | 10.9 ± 1.4 |
Data are means ± SEM. CC, capillary contacts; C/Fi, individual capillary‐to‐fibre ratio; CFPE, capillary‐to‐fibre perimeter exchange (CFPE) index; CSA, cross sectional area. *Significant effect within group for fibre type; †significant effect for group.
SC distance to nearest capillary across both type I and type II was significantly lower in the High group as compared to the Low group (P < 0.05, Table 4).
Mixed muscle SC response
Overall
Following the eccentric contraction protocol, total mixed muscle Pax7+ cells/100 myofibres tended to increase significantly at 6 h (14.4 ± 1.1 cells/100 myofibres (P = 0.056), increased significantly at 24 h (14.9 ± 1.1 cells/100 myofibres; P < 0.05) and 72 h (15.8 ± 1.0 cells/100 myofibres; P < 0.05), but was no longer significantly elevated at 96 h (11.4 ± 0.8 cells/100 myofibres) compared to Pre (11.8 ± 0.7 cells/100 myofibres). The change in total mixed muscle Pax7+ cells/100 myofibres between Pre and 24 h (r = 0.39, P < 0.05; Fig. 1 G) was positively correlated to mixed muscle CFPE index across all participants. The activation status of the SC pool was assessed by colocalizing SCs with MyoD before and after the eccentric contraction protocol. Mixed muscle Pax7+/MyoD+ cells/100 myofibres was significantly elevated at 6 h (1.8 ± 0.3 cells/100 myofibres, P < 0.05), 24 h (2.2 ± 0.2 cells/100 myofibres, P < 0.05), 72 h (1.9 ± 0.4 cells/100 myofibres, P < 0.05) and 96 h (1.1 ± 0.2 cells/100 myofibres, P < 0.05) as compared to Pre (0.4 ± 0.1; Fig. 2 F). The change in total mixed muscle MyoD+/Pax7+ cells/100 myofibres between Pre and 6 h (r = 0.40, P < 0.05; Fig. 1 G) and Pre and 72 h (r = 0.37, P < 0.05; Fig. 1 H) was positively correlated to mixed muscle CFPE index across all participants.
Figure 1. Fibre type specific satellite cell staining with muscle capillaries.
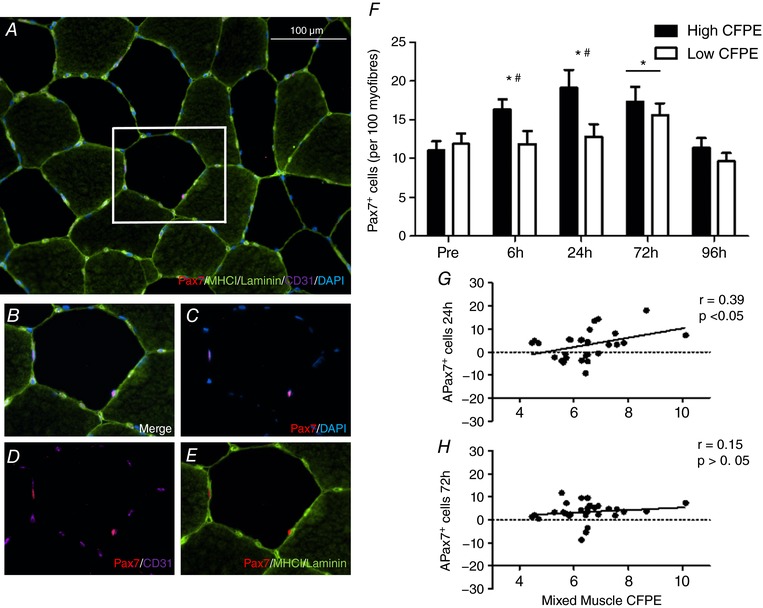
A, representative image of a MHCI/laminin/CD31/Pax7/DAPI stain of a muscle cross section. B–D and E, channel views of Merge (B), Pax7/DAPI (C), Pax7/CD31 (D) and Pax7/MHCI/Laminin (E). F, characterization of the expansion of the total mixed muscle satellite cell (SC) pool before and 6, 24, 72 and 96 h following eccentric contractions in the group with a High capillary to fibre exchange (CFPE) index and the group with Low CFPE index. *Significantly different compared with Pre (P < 0.05); bar indicates that effect of time is present for both groups. #Significantly greater increase with time High vs. Low group (P < 0.05). Data are expressed as mean ± SEM. G and H, relationship between the expansion of the total SC pool and mixed muscle CFPE following ∆24 h post‐eccentric exercise (r = 0.39, P < 0.05; G) and ∆72 h post‐exercise (r = 0.15, P > 0.05; H) across all participants.
Figure 2. Mixed muscle staining of satellite cell (SC) activation with muscle capillaries.
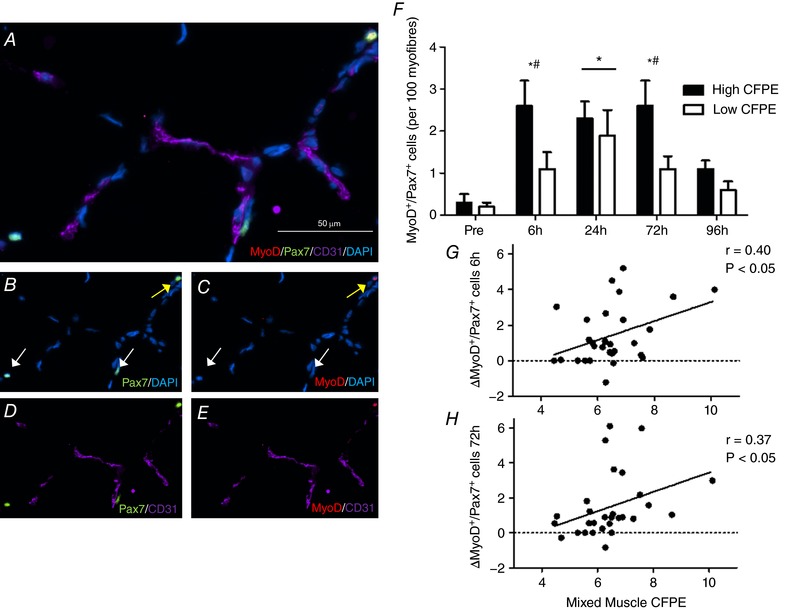
A, representative image of a CD31/Pax7/MyoD/DAPI stain of a muscle cross section). B–D and E, channel view of Pax7/DAPI (B), MyoD/DAPI (C), Pax7/CD31 (D) and MyoD/CD31 (E). F, characterization of the activation status of the SC pool before and 6, 24, 72 and 96 h following eccentric contractions in the group with a High capillary to fibre exchange (CFPE) index and the group with Low CFPE index. *Significantly different compared with Pre (P < 0.05); bar indicates that effect of time is present for both High and Low group. †Significantly greater increase with time High vs. Low group (P < 0.05). Data are expressed as means ± SEM. G and H, relationship between the activation of the SC pool (∆MyoD+/Pax7+ cells) and mixed muscle CFPE following ∆6 h post‐eccentric exercise (r = 0.40, P < 0.05; G) and ∆72 h post‐exercise (r = 0.37, P < 0.05; H) across all participants.
High vs. low CFPE group
Prior to the intervention, there were no differences in mixed muscle total Pax7+ cells/100 myofibres (P > 0.05) between the High (11.0 ± 1.2 cells/100 myofibres) and the Low (11.9 ± 1.3 cells/100 myofibres) group (Table 5). Compared to baseline, total mixed muscle Pax7+ cells/100 myofibres was significantly increased at 6 h (P < 0.05), 24 h (P < 0.05) and 72 h (P < 0.05) after the single bout of eccentric exercise in the High group (Fig. 1 F and Table 5). In contrast, total mixed muscle Pax7+ cells/100 myofibres was only significantly increased at 72 h (P < 0.05) during post‐exercise recovery in the Low group compared to baseline (Fig. 1 F and Table 5). There was a significantly greater increase in mixed muscle total Pax7+ cells/100 myofibres from Pre to 6 h (P < 0.05) and Pre to 24 h (P < 0.05) and a trend for Pre to 72 h to be significantly different (P = 0.052) following eccentric contractions in the High compared with the Low Group.
Table 5.
Satellite cell response following repeated eccentric contractions in the High CFPE (n = 10) and Low CFPE (n = 10) groups
| Time and group Group | Total muscle Pax7+ SCs (per 100 fibres) | Total muscle activated SCs (MyoD+/Pax7+) (per 100 fibres) | Type I Pax7+ SCs (per 100 fibres) | Type II Pax7+ SCs (per 100 fibres) |
|---|---|---|---|---|
| Pre | ||||
| High CFPE | 11.0 ± 1.2 | 0.3 ± 0.2 | 11.6 ± 1.6 | 10.5 ± 1.9 |
| Low CFPE | 11.9 ± 1.3 | 0.2 ± 0.1 | 11.8 ± 1.3 | 10.9 ± 1.3 |
| 6 h | ||||
| High CFPE | 16.3 ± 1.3*, † | 2.6 ± 0.6*, † | 13.9 ± 1.7 | 18.8 ± 2.4*, † |
| Low CFPE | 11.9 ± 1.7 | 1.1 ± 0.4 | 12.3 ± 2.2 | 11.4 ± 1.3 |
| 24 h | ||||
| High CFPE | 19.1 ± 2.3*, † | 2.3 ± 0.4* | 17.7 ± 2.1 | 20.6 ± 3.5*, † |
| Low CFPE | 12.8 ± 1.6 | 1.9 ± 0.5* | 12.7 ± 1.5 | 12.9 ± 1.3 |
| 72 h | ||||
| High CFPE | 17.3 ± 2.0* | 2.6 ± 0.6*, † | 14.0 ± 1.3 | 20.5 ± 2.7* |
| Low CFPE | 15.6 ± 1.5* | 0.8 ± 0.2 | 15.6 ± 1.9 | 14.4 ± 1.2* |
| 96 h | ||||
| High CFPE | 11.4 ± 0.8 | 1.1 ± 0.2* | 9.5 ± 1.5 | 13.2 ± 2.3 |
| Low CFPE | 9.6 ± 1.2 | 0.6 ± 0.2 | 9.1 ± 1.3 | 10.2 ± 2.0 |
Data are means ± SEM. CFPE, capillary‐to‐fibre perimeter exchange (CFPE) index. *Significant as compared to Pre. †Significantly greater increase from Pre compared to Low.
Prior to the intervention, there were no differences in total MyoD+/Pax7+ cells/100 myofibres in mixed muscle (P > 0.05) between the High (0.3 ± 0.2 cells/100 myofibres) and the Low (0.2 ± 0.1 cells/100 myofibres) groups. Mixed muscle Pax7+/MyoD+ cells/100 myofibres was significantly higher in the High group at 6 h (P < 0.05), 24 h (P < 0.05), 72 h (P < 0.05) and 96 h (P < 0.05) as compared to Pre (Fig. 2 F and Table 5). In the Low group, Pax7+/MyoD+ cells/100 myofibres in mixed muscle was only significantly elevated at 24 h (P < 0.05) as compared to Pre (Fig. 2 F and Table 5). In comparing the Low and the High mixed muscle SCs activation (Pax7+/MyoD+ cells) response to eccentric exercise, we observed that there was a significantly greater increase in the number of mixed muscle Pax7+/MyoD+ cells/100 myofibres from Pre to 6 h, and from Pre to 72 h post‐exercise recovery in the High as compared to the Low group (P < 0.05; Fig. 2 F). No significant correlation was observed between mixed muscle SC to nearest capillary at baseline and change in the number of activated SC (MyoD+/Pax7+ cells) at any post‐exercise recovery time point compared to Pre.
Type I and type II muscle fibre SC response
Overall
Prior to the intervention, there was no significant difference between type I‐associated (11.5 ± 0.9 cells/100 myofibres) and type II‐associated Pax7+ cells/100 myofibres (11.8 ± 1.0 cells/100 myofibres) across all participants (P > 0.05). Type I‐associated Pax7+ cells/100 myofibres remained unchanged at 6, 24 and 96 h (14.0 ± 1.4, 14.2 ± 1.1, 10.4 ± 0.9 Pax7+ cells/100 myofibres, respectively) but trended towards a significant increase at 72 h (14.2 ± 0.9 Pax7+ cells/100 myofibres, P = 0.09), as compared to Pre (11.5 ± 0.9 Pax7+ cells/100 myofibres).
Type II‐associated Pax7+ cells/100 myofibres remained unchanged at 6 h (14.7 ± 1.2 Pax7+ cells/100 myofibres) and 24 h (15.6 ± 1.6 Pax7+ cells/100 myofibres) but increased significantly at 72 h (17.4 ± 1.5 Pax7+ cells/100 myofibres; P < 0.05), returning to basal levels at 96 h (12.4 ± 1.3 Pax7+ cells/100 myofibres) as compared to Pre (12.1 ± 1.1 Pax7+ cells/100 myofibres) (Fig. 3 A).
Figure 3. Type II fibre‐associated satellite cell (SC) response.
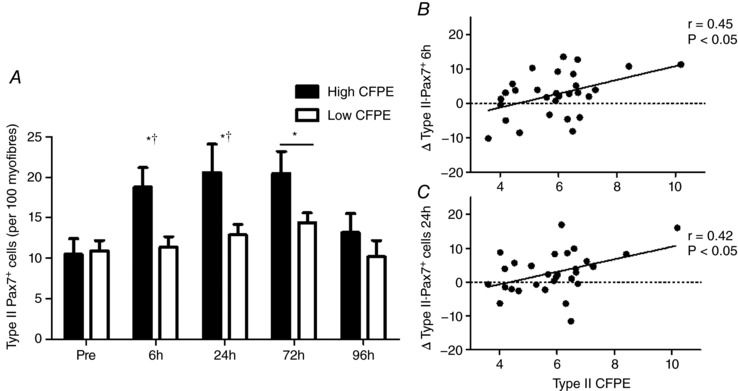
(A) Characterization of the expansion of type II fibre‐associated SC pool following eccentric contractions before and after 6 h, 24 h, 72 h and 96 h following eccentric contractions in the group with a High capillary to fibre exchange (CFPE) index and the group with Low CFPE index. *Significantly different compared with Pre (P < 0.05), bar indicates that effect of time is present for both groups (P < 0.05). †indicates a significantly greater increase with time High vs Low group (P < 0.05). Data are expressed as mean ± sem. Relationship between the expansion of the type II SC pool and type II CFPE following (B) ∆6 h post‐eccentric exercise (r = 0.45, P < 0.05) and (C) ∆24 h‐post exercise (r = 0.42, P < 0.05) across all participants.
The change in type II‐associated Pax7+ cells/100 myofibres between Pre and 6 h (r = 0.45, P < 0.05; Fig. 3 B) and Pre and 24 h (r = 0.42, P < 0.05; Fig. 3 C) following eccentric exercise was positively correlated with type II CFPE index across all participants.
The change in type II associated SC Pax7+ cells/100 myofibres from Pre to 24 h (r = −0.37, P < 0.05; Fig. 4 B) following eccentric exercise was negatively correlated to type II SC distance to nearest capillary at baseline across all participants. There were no relationships between type I associated SC and type I SC distance to nearest capillary at baseline across all participants.
High vs. low CFPE group
Prior to the intervention, there were no differences in type I‐associated Pax7+ cells/100 myofibres or type II‐associated Pax7+ cells/100 myofibres between Low and High groups. (P > 0.05; Table 4). Type I Pax7+ cells/100 myofibres was not significantly changed at 6, 24, 72, or 96 h following eccentric contractions compared to Pre in the High (P > 0.05) or Low group (P > 0.05, Table 5). In comparing the Low group with the High group following eccentric exercise, there were no differences between type I Pax7+/100 myofibres between Pre and any post‐exercise time point. Type II Pax7+ cells/100 myofibres was significantly increased at 6 h (P < 0.05), 24 h (P < 0.05) and 72 h (P < 0.05) following eccentric contractions in the High group, as compared to Pre (Fig. 3 A and Table 5).
In the Low group, type II Pax7+ cells/100 myofibres was only significantly elevated at 72 h (P < 0.05), as compared to Pre (Fig. 3 A and Table 5). In comparing the Low and the High muscle fibre type specific SC response to eccentric exercise, we observed that there was a greater change in the number of type II Pax7+ cells/100 myofibres from Pre to 6 h, and from Pre to 24 h post‐exercise in the High group as compared to the Low (P < 0.05; Fig. 3 A and Table 5).
SC distance to nearest capillary response following eccentric
Overall
SC distance to nearest capillary in mixed muscle fibres and/or type I/II‐associated SC did not change in response to the single bout of eccentric exercise.
High vs. low CFPE group
Type I SC distance to nearest capillary did not change (P > 0.05) following eccentric contractions in either the High (Pre: 12.2 ± 0.8; 6 h: 13.0 ± 0.9; 24 h: 12.4 ± 0.8; 72 h: 12.6 ± 0.7; 96 h: 11.9 ± 1.2 μm) or the Low group (Pre: 13.9 ± 0.9; 6 h: 12.1 ± 0.7; 24 h: 17.0 ± 1.1; 72 h: 17.5 ± 1.8; 96 h: 15.0 ± 0.9 μm) as compared to baseline values. Type II SC distance to nearest capillary did not change (P > 0.05) following eccentric contractions in either the High (Pre: 13.3 ± 0.8; 6 h: 13.1 ± 1.3; 24 h: 15.1 ± 1.2; 72 h: 16.0 ± 1.0; 96 h: 12.5 ± 1.3 μm) or the Low group (Pre: 17.3 ± 0.9; 6 h: 17.0 ± 1.0; 24 h: 16.9 ± 0.8; 72 h: 20.0 ± 1.5; 96 h: 16.8 ± 1.1 μm) as compared to baseline values.
Myogenic regulatory factor response
Overall
MyoD, MRF4 and Myogenin mRNA expression were significantly increased at 6 h (2.2‐, 1.8‐ and 4.4‐fold change, respectively), 24 h (1.4‐, 2.1‐ and 4.0‐fold change, respectively), 72 h (1.6‐, 1.6‐ and 2.0‐fold change, respectively) and 96 h (1.4‐, 2.3‐ and 1.9‐fold change, respectively) after the single bout of eccentric exercise. Myf5 mRNA expression was only significantly increased at 24 h (1.6‐fold change), 72 h (1.6‐fold change) and 96 h (1.9‐fold change) following exercise.
High vs. Low CFPE group
In the High group, MyoD mRNA expression was significantly (P < 0.05) increased at 6 h (2.4‐fold change), 24 h (1.5‐fold change), 72 h (2.1‐fold change) and 96 h (1.6‐fold change) post‐exercise, as compared to Pre. In the Low group, MyoD mRNA expression was significantly (P < 0.05) increased at 6 h (2.4‐fold change), but not different from Pre (1.2‐fold change) at 24 h post‐exercise. MyoD mRNA expression was significantly increased (P < 0.05) 72 h (1.4‐fold change) and 96 h (1.9‐fold change) post‐exercise compared to pre‐exercise. Myf5 mRNA expression was not significantly different from Pre following 6 h (1.1‐fold change) or 24 h (1.4‐fold change), but was significantly increased (P < 0.05) 72 h (1.4‐fold change) and 96 h (1.8‐fold change) post‐exercise recovery in the High group. In the Low group, Myf5 mRNA expression was not different 6 h (1.1‐fold change) but was significantly increased (P < 0.05) 24 h (2.1‐fold change), 72 h (1.9‐fold change) and 96 h (2.7‐fold change) following eccentric exercise. In both the High and the Low group, MRF4 mRNA expression was significantly (P < 0.05) increased from pre‐exercise at 6 h (2.1‐ and 2.4‐fold change, respectively), 24 h (1.9‐ and 3.1‐fold change, respectively), 72 h (1.8‐ and 1.8‐fold change, respectively) and 96 h (1.9‐ and 3.2‐fold change, respectively). In both the High and the Low group, myogenin mRNA expression was significantly (P < 0.05) increased from pre‐exercise at 6 h (4.5‐ and 4.5‐fold change, respectively), 24 h (2.0‐ and 7.9‐fold change, respectively), 72 h (2.1‐ and 2.1‐fold change, respectively) and 96 h (2.2‐ and 2.5‐fold change, respectively). In comparing the Low and the High myogenic gene mRNA expression in response to eccentric exercise, we observed that there was a greater change in MyoD mRNA expression from Pre to 72 h in the High group as compared to the Low group (P < 0.05). We also observed that there was a trend for a smaller increase in myogenin mRNA gene expression from Pre to 24 h, in the High group as compared to the Low (P = 0.055).
Cytokine response to repeated eccentric contractions
Overall
Plasma IL‐6 concentration was significantly increased at 6 h (2.2 ± 0.2 pg mL−1, P < 0.05) and 24 h (1.6 ± 0.1 pg mL−1, P < 0.05) but at 72 h (1.2 ± 0.1 pg mL−1) was no longer different from Pre (1.1 ± 0.1 pg mL−1). The change in plasma IL‐6 between Pre and 6 h (r = 0.42, P < 0.05), as well as Pre and 72 h (r = −0.42, P < 0.05), following eccentric contractions was negatively correlated to mixed muscle CFPE index across all participants.
High CFPE vs. low CFPE group
Prior to the intervention, there were no significant differences in plasma IL‐6 concentrations in the High compared to the Low group. In the High group, plasma IL‐6 concentration was significantly increased at 6 h (1.9 ± 0.3 pg mL−1, P < 0.05) and 24 h (1.8 ± 0.2 pg mL−1, P < 0.05) but was not significantly different at 72 h (1.2 ± 0.2 pg mL−1) as compared to Pre (1.2 ± 0.2 pg mL−1). In the Low group, plasma IL‐6 concentration was significantly increased at 6 h (2.7 ± 0.2 pg mL−1, P < 0.05) and 24 h (1.7 ± 0.1 pg mL−1, P < 0.05) but was not significantly different at 72 h (1.3 ± 0.1 pg mL−1) as compared to Pre (1.2 ± 0.2 pg mL−1). In comparing the IL‐6 to eccentric exercise in the High CFPE as compared to the Low CFPE group, we observed that there was a greater change in plasma IL‐6 concentrations from Pre to 6 h in the Low group as compared to the High group (P < 0.05).
Discussion
In the present study, we observed that there was an enhanced expansion and activation of the SC pool in individuals with high as compared to low capacity for muscle perfusion following eccentric contractions. Therefore muscle fibre capillarization may be a critical factor for the activation and expansion of the SC pool in response to muscle damage in humans. Furthermore, individuals with a higher capacity for muscle perfusion experienced a more rapid force recovery following muscle damage as compared to the group with a lower capacity for muscle perfusion.
SCs are indispensable for the repair and/or regeneration of damaged muscle in rodents (Lepper et al. 2011; McCarthy et al. 2011; Sambasivan et al. 2011). In humans, a single bout of high‐velocity eccentric contractions results in increased plasma creatine kinase, reduced force production and myofibrillar ultrastructual damage (Beaton et al. 2002; Clarkson & Hubal, 2002; Paulsen et al. 2012). Consequently, eccentric contractions are an effective tool for expansion of the muscle SC pool (Crameri et al. 2004; Dreyer et al. 2006; McKay et al. 2008, 2009; Cermak et al. 2013), though the degree of expansion is dependent on many factors (Snijders et al. 2015). However, the specific factors that determine the degree of activation and expansion of the SC pool are not well understood. In agreement with previous literature, we report that there is an expansion in the SC pool (as determined by total Pax7+ cells/100 myofibres) and an increase in SC pool activation (as determined by MyoD+/Pax7+ cells/100 myofibres) in the days following a single bout of eccentric contractions. To better understand factors that determine the degree of activation and expansion of the SC pool we examined whether muscle fibre capillarization may be a determining factor following an acute bout of eccentric contractions in young men. Skeletal muscle capillarization and perfusion are necessary for the delivery of oxygen, growth factors and macronutrients to muscle fibres and resident cell populations alike. We and others have previously already reported an anatomical relationship between muscle SCs and capillaries (Christov et al. 2007; Nederveen et al. 2016, 2017), suggesting that the proximity of SCs to their nearest capillary may be a determining factor in their activation status (Christov et al. 2007; Nederveen et al. 2016). In the present study, there was a positive correlation between the expansion of the total SC pool 24 h post‐eccentric exercise and mixed muscle CFPE, an index of muscle perfusion, suggesting that the greatest SC pool size expansion was experienced by subjects with the highest capacity for muscle fibre perfusion. When participants were retrospectively divided based on their mixed muscle CFPE index into a High CFPE and Low CFPE group, we observed that there was a greater expansion of the total Pax7+ SC pool in the group with high CFPE (High; CFPE index 7.6 ± 1.0 capillaries · 1000 μm−1) as compared to low CFPE (Low; CFPE index 5.2 ± 0.5 capillaries · 1000 μm−1). This observation was made 6 h (∼48% vs. ∼1% Pax7+ cells/100 myofibres, respectively) and 24 h (∼73% vs. ∼10% Pax7+ cells/100 myofibres, respectively) post‐eccentric contractions. Work by Christov and colleagues (2007) supports these findings as they observed a correlation between fibre capillarization and SC content in human deltoid muscle in the resting state, regardless of muscle fibre type. Furthermore, we observed that the High group had a greater activation of the SC pool at 6 h (∼750% vs. ∼450% MyoD+/Pax7+ cells/100 myofibres, respectively) and 72 h (∼750% vs. ∼300% ± MyoD+/Pax7+ cells/100 myofibres, respectively). It is important to note that while a large number (i.e. >200 fibres) were quantified for MyoD+/Pax7+ cells/100 myofibres analysis, the relative rarity of this population in the basal state may increase sampling bias. However, the data here support the notion that there is a limited quantity of activated SCs at rest (Joanisse et al. 2015; Arentson‐Lantz et al. 2016), suggesting that quantification of large numbers of fibres for SC analysis is important for accuracy (Mackey et al. 2009).
Interestingly, in both the High and Low CFPE groups, creatine kinase activity was increased at 24 h following eccentric contractions, and force production was significantly reduced at 6, 24 and 72 h post‐exercise recovery. However, we observed that the force production returned to baseline again at 96 h post‐exercise recovery in the High CFPE group, whereas this was not the case in the Low CFPE group. Together with the greater activation and expansion of the SC pool size observed in the High group during post‐exercise recovery, these data indicate that individuals with a high CFPE index also appear to have force production recovery as soon as 96 h following an acute bout of damaging exercise
Type I muscle fibres are more oxidative, associated with more capillaries and/or are perfused to a greater degree than their type II counterparts. In the present study we observed that participants in the High group had a significantly greater percentage of type I muscle fibres as compared with the Low group (∼56% vs. ∼34% type I fibres, respectively). The extensive expansion of the type II SC pool in the High CFPE group may be, in part, due to the relatively small type II fibre type distribution in these individuals. Eccentric exercise predominately targets damage‐susceptible type II fibres (Friden & Lieber, 1992; Brockett et al. 2002; Lynch et al. 2008). Thus, it is possible that these type II fibres were damaged to a greater extent in participants with a lower distribution of these fibres (i.e. the High CFPE group). However, considering that muscle fibre capillaries are shared amongst a mosaic of fibre types in humans, a greater percentage of type I muscle fibres may result in enhanced perfusion of neighbouring type II muscle fibres, ultimately exposing SCs to greater systemic signals and/or interactions with endothelial cells (Christov et al. 2007). A greater association with shared muscle fibre capillaries amongst the muscle fibre type mosaic may contribute not only to a greater type II muscle fibre perfusion, but also to a closer proximity between type II Pax7+ SCs and the nearest capillary observed in the High group. Consistent with this notion, we observed a negative correlation between type II muscle fibre CFPE index and type II Pax7+ SC distance to their nearest capillary. Considering that SC distance to nearest capillary in type II muscle fibres was negatively correlated with a greater change in type II Pax7+ cells/100 myofibres from Pre to 24 h following eccentric exercise, we propose that the link between a greater SC activation/expansion in response to muscle fibre damage may be the reduced spatial proximity to microvascular capillaries. In line with this, we have previously observed that type II SCs are located at a further distance from capillaries in older men as compared to their young counterparts (Nederveen et al. 2016). Older men typically exhibit an impaired expansion and/or activation response to exercise (McKay et al. 2012; Snijders et al. 2014), as well as lower basal SC content (Verdijk et al. 2007) concomitant with a loss of muscle capillarization (Proctor et al. 1995). Taken together, these data support a relationship between muscle capillarization and functional SC in humans.
We also observed that enhanced capacity for muscle perfusion (i.e. muscle CFPE) or a reduction in the distance of a SC to its nearest capillary was associated with an enhanced activation and expansion of the SC pool in response to eccentric contraction‐induced muscle fibre damage in mixed, type I and type II muscle fibres. Previously, we reported that following resistance training, there was an increase in muscle capillarization and also an enhanced activation of SCs in response to an acute bout of resistance exercise (Nederveen et al. 2017). Taken together, this suggests that muscle capillarization and the ability of the SC pool to activate and expand following exercise/damage are closely linked. Indeed, it is now well established that activated SCs are found at closer proximity to capillaries than their quiescent counterparts (Chazaud et al. 2003; Christov et al. 2007; Nederveen et al. 2016). However, the specific cues for induction of the myogenic programme in response to muscle fibre damage remain to be elucidated. The process of SC activation, proliferation and/or differentiation is regulated by a multitude of cytokines and growth factors (e.g. IL‐6, IGF‐1, myostatin, HGF) (Kadi et al. 2005). Considering that the CFPE index is regarded as a proxy measure of microvascular perfusion (Hepple & Mathieu‐Costello, 2001; Weber et al. 2006), variations in the CPFE index could modify delivery of circulating nutrients and/or growth factors and presumably change the local environment of a SC post‐exercise/damage (Conboy et al. 2005; Brack & Rando, 2007). In this capacity, few growth factors have been as extensively investigated as the cytokine IL‐6, a well‐characterized member of the interleukin family. IL‐6 is known to respond to various forms of exercise (Pedersen & Febbraio, 2008), but importantly known to play a role in SC function (Toth et al. 2011; McKay et al. 2013). Furthermore, elevation of IL‐6 concentration has been shown to be associated specifically with SC proliferation in response to muscle fibre injury (Pedersen & Febbraio, 2008; Toth et al. 2011; McKay et al. 2013). In the current study, we observed that individuals with a lower mixed muscle CFPE index had a greater increase in plasma concentration of IL‐6 from Pre to 6 h following eccentric exercise. In line with this, we observe that the Low as compared to the High group had a greater change in circulating IL‐6 from Pre to 6 h (increase of ∼163% vs. ∼66%, respectively). Interestingly, the greater plasma concentration of IL‐6 observed in the Low group occurred simultaneously with a lesser activation of muscle SC (i.e. MyoD+/Pax7+ cells/100 myofibres) over this same time period. Diminished activation of SC in the Low group despite an elevated systemic plasma IL‐6 response in comparison to the High group suggests that there may be other mechanisms that regulate the impact of systemic IL‐6 concentration upon SC activation and/or proliferation. Previous work has established that the presence of IL‐6 can reduce endothelial signalling in some physiological situations (Yuen et al. 2009). Increased plasma IL‐6 concentrations may have implications for an increased local SC niche concentration, and may therefore interfere with the observed cellular cross‐talk between SC and endothelial cells (Chazaud et al. 2003; Ochoa et al. 2007). Previous work suggests that IL‐6 is produced by various resident cell types such as macrophages (Zhang et al. 2013), fibroblasts (Joe et al. 2010) or endothelial cells (Sironi et al. 1989; Yan et al. 1995), the exercising muscle (Steensberg et al. 2000; Pedersen & Febbraio, 2008) as well as SCs themselves (Kami & Senba, 2002). Future work should continue to address the paracrine and autocrine functions of increased IL‐6 within the local SC niche.
Given the positive relationship between muscle capillarization and the activation and expansion of the SC pool, we conclude that the SC response is modulated by cross‐talk with endothelial cells within the microvasculature, exposure to circulating signals, or a combination of both. In the future, attention should be focused on study populations that are compromised with respect to muscle capillarization Coggan et al. 1992; Proctor et al. 1995), and/or impaired SC content at rest and in response to exercise (McKay et al. 2012; Snijders et al. 2014) such as the elderly. These future studies may provide insight into whether the blunted post‐exercise SC response in elderly individuals can be improved by increasing muscle fibre capillarization. In conclusion, the present study shows that skeletal muscle fibre capillarization is a significant factor for muscle SC activation and pool size expansion, thereby accelerating the muscle repair response following muscle damage in healthy young men.
Additional information
Competing interests
There are no conflict of interests.
Author contributions
J.P.N., S.J., T.S., D.K., G.P., G.P., conceived and designed the experiments; J.P.N., S.J., T.S., A.C.Q., D.K., G.P., collected samples; J.P.N., S.J., T.S., A.C.Q. performed experiments. J.P.N., S.J., T.S., A.C.Q., G.P., analyzed data; J.P.N., S.J., T.S., G.P., interpreted results of experiments; J.P.N., prepared figures; J.P.N., G.P. drafted manuscript; J.P.N., S.J., T.S., A.C.Q., D.K., G.P. approved final version of the manuscript and agree to be accountable for all aspects of the work. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
Funding
G.P. was supported by a Natural Sciences and Engineering Research Council of Canada (NSERC) Grant (1455843), J.P.N by a NSERC Canadian Graduate Scholarship (CGS‐D).
Acknowledgements
The Pax7 hybridoma cells developed by Dr A. Kawakami, and the A4.951 developed by Dr H. Blau were obtained from the Developmental Studies Hybridoma Bank, created by the NICHD of the NIH and maintained at The University of Iowa, Department of Biology, Iowa City, IA 52242, USA.
Edited by: Michael Hogan & Troy Hornberger
This is an Editor's Choice article from the 15 March 2018 issue.
References
- Arentson‐Lantz EJ, English KL, Paddon‐Jones D & Fry CS (2016). Fourteen days of bed rest induces a decline in satellite cell content and robust atrophy of skeletal muscle fibers in middle‐aged adults. J Appl Physiol (1985) 120, 965–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaton LJ, Tarnopolsky MA & Phillips SM (2002). Variability in estimating eccentric contraction‐induced muscle damage and inflammation in humans. Can J Appl Physiol 27, 516–526. [DOI] [PubMed] [Google Scholar]
- Bellamy LM, Joanisse S, Grubb A, Mitchell CJ, McKay BR, Phillips SM, Baker S & Parise G (2014). The acute satellite cell response and skeletal muscle hypertrophy following resistance training. PLoS One 9, e109739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzinger CF, Wang YX & Rudnicki MA (2012). Building muscle: molecular regulation of myogenesis. Cold Spring Harb Perspect Biol 4, a008342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brack AS & Rando TA (2007). Intrinsic changes and extrinsic influences of myogenic stem cell function during aging. Stem Cell Rev 3, 226–237. [DOI] [PubMed] [Google Scholar]
- Brockett CL, Morgan DL, Gregory JE & Proske U (2002). Damage to different motor units from active lengthening of the medial gastrocnemius muscle of the cat. J Appl Physiol (1985) 92, 1104–1110. [DOI] [PubMed] [Google Scholar]
- Cermak NM, Snijders T, McKay BR, Parise G, Verdijk LB, Tarnopolsky MA, Gibala MJ & Van Loon LJ (2013). Eccentric exercise increases satellite cell content in type II muscle fibers. Med Sci Sports Exerc 45, 230–237. [DOI] [PubMed] [Google Scholar]
- Chazaud B, Sonnet C, Lafuste P, Bassez G, Rimaniol AC, Poron F, Authier FJ, Dreyfus PA & Gherardi RK (2003). Satellite cells attract monocytes and use macrophages as a support to escape apoptosis and enhance muscle growth. J Cell Biol 163, 1133–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christov C, Chretien F, Abou‐Khalil R, Bassez G, Vallet G, Authier FJ, Bassaglia Y, Shinin V, Tajbakhsh S, Chazaud B & Gherardi RK (2007). Muscle satellite cells and endothelial cells: close neighbors and privileged partners. Mol Biol Cell 18, 1397–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson PM & Hubal MJ (2002). Exercise‐induced muscle damage in humans. Am J Phys Med Rehabil 81, S52–S69. [DOI] [PubMed] [Google Scholar]
- Coggan AR, Spina RJ, King DS, Rogers MA, Brown M, Nemeth PM & Holloszy JO (1992). Histochemical and enzymatic comparison of the gastrocnemius muscle of young and elderly men and women. J Gerontol 47, B71–B76. [DOI] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL & Rando TA (2005). Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433, 760–764. [DOI] [PubMed] [Google Scholar]
- Crameri RM, Langberg H, Magnusson P, Jensen CH, Schroder HD, Olesen JL, Suetta C, Teisner B & Kjaer M (2004). Changes in satellite cells in human skeletal muscle after a single bout of high intensity exercise. J Physiol 558, 333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer HC, Blanco CE, Sattler FR, Schroeder ET & Wiswell RA (2006). Satellite cell numbers in young and older men 24 hours after eccentric exercise. Muscle Nerve 33, 242–253. [DOI] [PubMed] [Google Scholar]
- Emslie‐Smith AM & Engel AG (1990). Microvascular changes in early and advanced dermatomyositis: a quantitative study. Ann Neurol 27, 343–356. [DOI] [PubMed] [Google Scholar]
- Friden J & Lieber RL (1992). Structural and mechanical basis of exercise‐induced muscle injury. Med Sci Sports Exerc 24, 521–530. [PubMed] [Google Scholar]
- Hepple RT ( 1997). A new measurement of tissue capillarity: the capillary‐to‐fibre perimeter exchange index. Can J Appl Physiol 22, 11–22. [DOI] [PubMed] [Google Scholar]
- Hepple RT & Mathieu‐Costello O (2001). Estimating the size of the capillary‐to‐fiber interface in skeletal muscle: a comparison of methods. J Appl Physiol (1985) 91, 2150–2156. [DOI] [PubMed] [Google Scholar]
- Joanisse S, McKay BR, Nederveen JP, Scribbans TD, Gurd BJ, Gillen JB, Gibala MJ, Tarnopolsky M, Parise G (2015). Satellite cell activity, without expansion, after nonhypertrophic stimuli. Am J Physiol Regul Integr Comp Physiol 309, R1101–R1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joanisse S, Nederveen JP, Snijders T, McKay BR & Parise G (2017). Skeletal muscle regeneration, repair and remodelling in aging: the importance of muscle stem cells and vascularization. Gerontology 63, 91–100. [DOI] [PubMed] [Google Scholar]
- Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA & Rossi FM (2010). Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol 12, 153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadi F, Charifi N, Denis C, Lexell J, Andersen JL, Schjerling P, Olsen S & Kjaer M (2005). The behaviour of satellite cells in response to exercise: what have we learned from human studies? Pflugers Arch 451, 319–327. [DOI] [PubMed] [Google Scholar]
- Kami K & Senba E (2002). In vivo activation of STAT3 signaling in satellite cells and myofibers in regenerating rat skeletal muscles. J Histochem Cytochem 50, 1579–1589. [DOI] [PubMed] [Google Scholar]
- Lepper C, Partridge TA & Fan CM (2011). An absolute requirement for Pax7‐positive satellite cells in acute injury‐induced skeletal muscle regeneration. Development 138, 3639–3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ & Schmittgen TD (2001). Analysis of relative gene expression data using real‐time quantitative PCR and the 2− ΔΔ C (T) method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Lynch GS, Faulkner JA & Brooks SV (2008). Force deficits and breakage rates after single lengthening contractions of single fast fibers from unconditioned and conditioned muscles of young and old rats. Am J Physiol Cell Physiol 295, C249–C256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey AL, Rasmussen LK, Kadi F, Schjerling P, Helmark IC, Ponsot E, Aagaard P, Durigan JL & Kjaer M (2016). Activation of satellite cells and the regeneration of human skeletal muscle are expedited by ingestion of nonsteroidal anti‐inflammatory medication. FASEB J 30, 2266–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy JJ, Mula J, Miyazaki M, Erfani R, Garrison K, Farooqui AB, Srikuea R, Lawson BA, Grimes B, Keller C, Van Zant G, Campbell KS, Esser KA, Dupont‐Versteegden EE & Peterson CA (2011). Effective fiber hypertrophy in satellite cell‐depleted skeletal muscle. Development 138, 3657–3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay BR, De Lisio M, Johnston AP, O'Reilly CE, Phillips SM, Tarnopolsky MA & Parise G (2009). Association of interleukin‐6 signalling with the muscle stem cell response following muscle‐lengthening contractions in humans. PLoS One 4, e6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay BR, Ogborn DI, Baker JM, Toth KG, Tarnopolsky MA & Parise G (2013). Elevated SOCS3 and altered IL‐6 signaling is associated with age‐related human muscle stem cell dysfunction. Am J Physiol Cell Physiol 304, C717–C728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay BR, Ogborn DI, Bellamy LM, Tarnopolsky MA & Parise G (2012). Myostatin is associated with age‐related human muscle stem cell dysfunction. FASEB J 26, 2509–2521. [DOI] [PubMed] [Google Scholar]
- McKay BR, O'Reilly CE, Phillips SM, Tarnopolsky MA & Parise G (2008). Co‐expression of IGF‐1 family members with myogenic regulatory factors following acute damaging muscle‐lengthening contractions in humans. J Physiol 586, 5549–5560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nederveen JP, Joanisse S, Snijders T, Ivankovic V, Baker SK, Phillips SM & Parise G (2016). Skeletal muscle satellite cells are located at a closer proximity to capillaries in healthy young compared with older men. J Cachexia Sarcopenia Muscle 7, 547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nederveen JP, Snijders T, Joanisse S, Wavell CG, Mitchell CJ, Johnston LM, Baker SK, Phillips SM & Parise G (2017). Altered muscle satellite cell activation following 16 wk of resistance training in young men. Am J Physiol Regul Integr Comp Physiol 312, R85–R92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa O, Sun D, Reyes‐Reyna SM, Waite LL, Michalek JE, McManus LM & Shireman PK (2007). Delayed angiogenesis and VEGF production in CCR2−/− mice during impaired skeletal muscle regeneration. Am J Physiol Regul Integr Comp Physiol 293, R651–R661. [DOI] [PubMed] [Google Scholar]
- O'Reilly C, McKay B, Phillips S, Tarnopolsky M & Parise G (2008). Hepatocyte growth factor (HGF) and the satellite cell response following muscle lengthening contractions in humans. Muscle Nerve 38, 1434–1442. [DOI] [PubMed] [Google Scholar]
- Paulsen G, Mikkelsen UR, Raastad T & Peake JM (2012). Leucocytes, cytokines and satellite cells: what role do they play in muscle damage and regeneration following eccentric exercise? Exerc Immunol Rev 18, 42–97. [PubMed] [Google Scholar]
- Pedersen BK & Febbraio MA (2008). Muscle as an endocrine organ: focus on muscle‐derived interleukin‐6. Physiol Rev 88, 1379–1406. [DOI] [PubMed] [Google Scholar]
- Porter MM, Koolage CW & Lexell J (2002). Biopsy sampling requirements for the estimation of muscle capillarization. Muscle Nerve 26, 546–548. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Sinning WE, Walro JM, Sieck GC & Lemon PW (1995). Oxidative capacity of human muscle fiber types: effects of age and training status. J Appl Physiol (1985) 78, 2033–2038. [DOI] [PubMed] [Google Scholar]
- Sambasivan R, Yao R, Kissenpfennig A, Van Wittenberghe L, Paldi A, Gayraud‐Morel B, Guenou H, Malissen B, Tajbakhsh S & Galy A (2011). Pax7‐expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development 138, 3647–3656. [DOI] [PubMed] [Google Scholar]
- Sironi M, Breviario F, Proserpio P, Biondi A, Vecchi A, Van Damme J, Dejana E & Mantovani A (1989). IL‐1 stimulates IL‐6 production in endothelial cells. J Immunol 142, 549–553. [PubMed] [Google Scholar]
- Snijders T, Nederveen JP & Parise G (2016). Are satellite cells lost during short‐term disuse‐induced muscle fiber atrophy? J Appl Physiol (1985) 120, 1490. [DOI] [PubMed] [Google Scholar]
- Snijders T, Nederveen JP, McKay BR, Joanisse S, Verdijk LB, van Loon LJ & Parise G (2015). Satellite cells in human skeletal muscle plasticity. Front Physiol 6, 283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijders T, Verdijk LB, Smeets JS, McKay BR, Senden JM, Hartgens F, Parise G, Greenhaff P & van Loon LJ (2014). The skeletal muscle satellite cell response to a single bout of resistance‐type exercise is delayed with aging in men. Age (Dordr) 36, 9699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steensberg A, van Hall G, Osada T, Sacchetti M, Saltin B & Klarlund Pedersen B (2000). Production of interleukin‐6 in contracting human skeletal muscles can account for the exercise‐induced increase in plasma interleukin‐6. J Physiol 529, 237–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth KG, McKay BR, De Lisio M, Little JP, Tarnopolsky MA & Parise G (2011). IL‐6 induced STAT3 signalling is associated with the proliferation of human muscle satellite cells following acute muscle damage. PLoS One 6, e17392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velloso CP (2008). Regulation of muscle mass by growth hormone and IGF‐I. Br J Pharmacol 154, 557–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdijk LB, Koopman R, Schaart G, Meijer K, Savelberg HH & van Loon LJ (2007). Satellite cell content is specifically reduced in type II skeletal muscle fibers in the elderly. Am J Physiol Endocrinol Metab 292, E151–E157. [DOI] [PubMed] [Google Scholar]
- Weber MA, Krakowski‐Roosen H, Delorme S, Renk H, Krix M, Millies J, Kinscherf R, Kunkele A, Kauczor HU & Hildebrandt W (2006). Relationship of skeletal muscle perfusion measured by contrast‐enhanced ultrasonography to histologic microvascular density. J Ultrasound Med 25, 583–591. [DOI] [PubMed] [Google Scholar]
- Yan SF, Tritto I, Pinsky D, Liao H, Huang J, Fuller G, Brett J, May L & Stern D (1995). Induction of interleukin 6 (IL‐6) by hypoxia in vascular cells. Central role of the binding site for nuclear factor‐IL‐6. J Biol Chem 270, 11463–11471. [DOI] [PubMed] [Google Scholar]
- Yin H, Price F & Rudnicki MA (2013). Satellite cells and the muscle stem cell niche. Physiol Rev 93, 23–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen DY, Dwyer RM, Matthews VB, Zhang L, Drew BG, Neill B, Kingwell BA, Clark MG, Rattigan S & Febbraio MA (2009). Interleukin‐6 attenuates insulin‐mediated increases in endothelial cell signaling but augments skeletal muscle insulin action via differential effects on tumor necrosis factor‐α expression. Diabetes 58, 1086–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Li Y, Wu Y, Wang L, Wang X & Du J (2013). Interleukin‐6/signal transducer and activator of transcription 3 (STAT3) pathway is essential for macrophage infiltration and myoblast proliferation during muscle regeneration. J Biol Chem 288, 1489–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]


