Abstract
As shown earlier, raft-like domains resembling those thought to be present in natural cell membranes can be formed in supported planar lipid monolayers. These liquid-ordered domains coexist with a liquid-disordered phase and form in monolayers prepared both from synthetic lipid mixtures and lipid extracts of the brush border membrane of mouse kidney cells. The domains are detergent-resistant and are highly enriched in the glycosphingolipid GM1. In this work, the properties of these raft-like domains are further explored and compared with properties thought to be central to raft function in plasma membranes. First, it is shown that domain formation and disruption critically depends on the cholesterol density and can be controlled reversibly by treating the monolayers with the cholesterol-sequestering reagent methyl-β-cyclodextrin. Second, the glycosylphosphatidylinositol-anchored cell-surface protein Thy-1 significantly partitions into the raft-like domains. The extent of this partitioning is reduced when the monolayers contain GM1, indicating that different molecules can compete for domain occupation. Third, the partitioning of a saturated phospholipid analog into the raft phase is dramatically increased (15% to 65%) after cross-linking with antibodies, whereas the distribution of a doubly unsaturated phospholipid analog is not significantly affected by cross-linking (≈10%). This result demonstrates that cross-linking, a process known to be important for certain cell-signaling processes, can selectively translocate molecules to liquid-ordered domains.
Keywords: membrane domains, receptor cross-linking, cholesterol, glycosylphosphatidylinositol-anchored proteins, signal transduction
Treatment of cell lysates with cold nonionic detergent and subsequent sucrose gradient centrifugation allows extraction of a detergent-resistant membrane fraction (DRM) (1, 2). The finding that this fraction is enriched in cholesterol and sphingolipids that form a liquid-ordered phase (3, 4) has led to the hypothesis that the DRM arises directly from discrete liquid-ordered domains in the plasma membrane termed lipid rafts (5, 6). Such domains could be important for membrane trafficking and sorting (1, 7). Moreover, cell signaling molecules are enriched in the DRM, and the partitioning of these molecules into the DRM is altered during signaling (8–12). Extraction of cholesterol from plasma membranes affects the abundance of molecules found in the DRM and impairs processes like membrane trafficking (13, 14) and cell activation (15, 16). These findings suggest that lipid rafts on plasma membranes are maintained by proper cholesterol levels as functional, preassembled signal transduction complexes (17–19).
Although there is accumulating evidence suggesting that some form of lipid domains or clusters are present in cell membranes, there is still considerable controversy surrounding the basic physical properties of these domains (compositional diversity, size, structure, and dynamics) and their relation to the DRM (for review see refs. 20 and 21). The raft hypothesis proposes that naturally occurring lipids such as sphingomyelin (SM), cholesterol, glycosphingolipids, and perhaps saturated phospholipids specifically aggregate in the plane of the membrane, driven solely or primarily by distinct lipid–lipid interactions (5, 22, 23). We have previously demonstrated that detergent-resistant, liquid-ordered domains can be directly imaged in the free-standing lipid lamella of giant unilamellar vesicles and in planar-supported bilayers and monolayers, formed both from artificial lipid mixtures approximating the plasma membrane lipid composition and natural lipid extracts from the brush border membrane (BBM) of mouse kidney cells (24). These results are consistent with other work showing that liquid-disordered and liquid-ordered phases can coexist in model membranes (25–27) and that the liquid-ordered phase is partially resistant to detergent (28, 29). These studies lend strong support to the notion that raft domains in cell membranes can arise primarily from lipid–lipid interactions.
In the present work, fluorescence microscopy is used to explore several key properties of raft-like domains reconstituted in model systems consisting of Langmuir monolayers transferred from the water-air interface to silanized glass supports. The results are interpreted in terms of their relevance to the underlying physical factors governing how rafts in cell membranes might mediate cell signaling events. Because cholesterol is known to strongly influence the abundance and chemical composition of DRM isolated from cell membranes, the effects of cholesterol extraction and repletion on raft-like domains in the model system have been examined. Because glycosphingolipids and glycosylphosphatidylinositol (GPI)-linked proteins are thought to preferentially associate with rafts on plasma membranes, the relative partitioning of the glycosphingolipid GM1 and the GPI-linked protein Thy-1 between the liquid-ordered and liquid-disordered regions in supported monolayers has been investigated. Finally, in that molecular cross-linking is a key step in the initiation of certain signal transduction events and is known to influence receptor occupation in the DRM of certain cell types, the partitioning of phospholipid analogs between the two phases, in the model system, before and after cross-linking with antibodies also has been examined.
Materials and Methods
Materials.
1,2-Dioleoyl-sn-glycero-3-phosphocholine (DOPC), cholesterol, brain SM, ovine ganglioside GM1 (GM1), and 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-fluorescein (FL-DOPE) were purchased from Avanti Polar Lipids. 1,2-Dipalmitoyl-sn-glycero-3-phosphoethanolamine-fluorescein (FL-DPPE), 1,2- dipalmitoyl-sn-glycero-3-phospho-ethanol-amine-x-Texas red (TR-DPPE), and Alexa594-conjugated rabbit polyclonal antifluorescein antibodies were obtained from Molecular Probes. Fluorescein-conjugated cholera toxin B subunit (FL-CTB), the cholesterol-sequestering agent methyl-β-cyclodextrin (MβCD), and water-soluble cholesterol (i.e., MβCD loaded with cholesterol) were obtained from Sigma. Rhodamine-conjugated CTB was obtained by conjugating lissamine rhodamine B sulfonyl chloride (Molecular Probes) to CTB (Sigma). Lipids were extracted from BBMs, and the GPI-linked protein Thy-1 was purified from a T cell lymphoma and labeled with fluorescein isothiocyanate (F-Thy-1), as described in the supporting information, which is published on the PNAS web site, www.pnas.org. PBS was prepared from 50 mM sodium phosphate, 150 mM NaCl, and 0.01% NaN3 (pH 7.4). A purification system provided water with a specific resistance of 17 MΩ⋅cm.
Preparation of Planar-Supported Lipid Monolayers.
Supported lipid monolayers were prepared by transfer from the water-air interface of a Langmuir trough to silanized glass coverslips as described (24). Host lipids were either lipid extracts from BBM (see supporting information), DOPC/SM (1:1, mol/mol), or DOPC/SM/cholesterol (1:1:1, mol/mol/mol). Fluorescent lipid probes and/or GM1 were added to these solutions at concentrations less than 1 mol%. Coverslip-supported monolayers were mounted on glass slides with spacers to form chambers containing ≈90 μl of PBS and kept at 24°C for all subsequent preparation steps. To change the composition of the fluid adjacent to supported monolayers, 100 μl of the desired solution was gently injected while for flushing steps 300–400 μl of buffer was applied.
F-Thy-1 was reconstituted into supported lipid monolayers by first diluting 250–300 μg/ml F-Thy-1 in a buffered solution of 40 mM n-octyl-β-d-glucopyranoside (see supporting information) 10-fold with PBS. The final detergent concentration was 4 mM, well below the critical micelle concentration of 20 mM (30, 31). One hundred microliters of diluted F-Thy-1 was then immediately injected into the fluid adjacent to a supported monolayer. Samples were allowed to equilibrate for 2 h before flushing the chamber with 400 μl PBS.
Fluorescence Microscopy.
Fluorescence imaging, video fluorescence recovery after photobleaching (FRAP), and spot FRAP measurements were carried out as described in supporting information.
Cross-Linking Fluorescein-Conjugated Phospholipid Analogs.
Alexa594-conjugated antifluorescein antibodies were dissolved in 200 μl PBS (15 μg/ml) and clarified for 15 min at 11,400 g. The top 100 μl of the supernatant was carefully removed and injected into the volume adjacent to a supported lipid monolayer containing 0.5 mol% FL-DOPE or FL-DPPE. After 15 min, unbound antibody was removed by washing with 300 μl PBS. Samples were allowed to equilibrate for an additional 45 min before acquiring fluorescence micrographs. Treating monolayers that did not contain FL-DPPE or FL-DOPE with Alexa594-conjugated antifluorescein antibodies produced only a faint signal in the red channel, which was without significance (≈5%) when compared with the fluorescence obtained for monolayers containing fluorescein-conjugated lipids. In the latter case, the fluorescence signal was significantly quenched in the green channel, as expected after specific antibody binding (32, 33). The reduction in intensity (≈40%) was similar for both FL-DOPE and FL-DPPE, suggesting that approximately equivalent densities of antibodies were bound for monolayers containing the two fluorescent lipid analogs.
Relative Partitioning of Molecules into the Raft Phase.
To quantify the partitioning of fluorescent probe molecules between raft and nonraft phases, the average intensities of areas corresponding to these phase regions were calculated from 12-bit digitized charge-coupled device fluorescence micrographs. The intensities were corrected for dark counts and stray light by subtracting the average background intensity measured for samples not containing transferred lipid layers. The relative partition coefficient, R, was defined as R = Ir/(Ir + In) where Ir and In were the background-corrected average intensities measured in the raft and nonraft regions, respectively. Under ideal conditions, the quantum efficiencies of the fluorophores attached to the probe molecules and the lipid matrix densities are identical for both lipid phases. In this case, the background-corrected fluorescence intensities accurately reflect the relative concentrations of the probe molecules in the two lipid phases.
Results
Cholesterol Critically Controls the Abundance of the Raft-Like Phase.
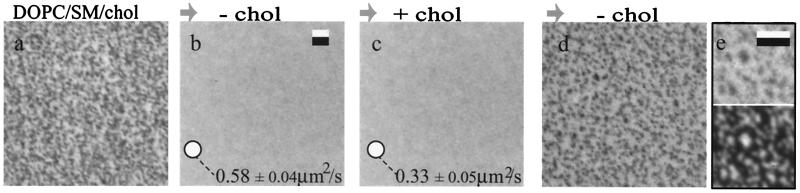
As described (24), planar-supported monolayers prepared from synthetic raft-lipid mixtures (i.e., equimolar amounts of unsaturated phospholipids, cholesterol, and SM) or from lipid extracts of BBMs contain coexisting fluid–fluid domains at room temperature. Because liquid-ordered phases depend on the presence of cholesterol, the response of supported lipid monolayers containing coexisting liquid-ordered and liquid-disordered phases to cholesterol depletion was examined. In what follows, we refer to the liquid-ordered phases as raft-like domains or phases. Fig. 1a shows a fluorescence image of a DOPC/ SM/cholesterol monolayer with 1 mol% GM1 and 0.5 mol% FL-DPPE after transfer at 35 dyne/cm onto a silanized glass coverslip. In these monolayers, FL-DPPE partitions into the liquid-disordered phase (24). Microdomains are not present within optical resolution after treatment with 10 mM MβCD (Fig. 1b), a reagent that has been used in a number of studies to extract cholesterol from phospholipid membranes (e.g., refs. 15, 34, and 35). The homogeneous distribution of FL-DPPE is not visually changed after resupplementing cholesterol by subsequent treatment with 30 μg/ml water-soluble cholesterol (Fig. 1c), but FL-DPPE mobility is significantly reduced by a factor of ≈2, as measured by spot FRAP. After further treatment with 5 mM MβCD, monolayers again exhibit a heterogeneous FL-DPPE distribution (Fig. 1d) very similar in appearance to that of the original film state. To confirm that the nonfluorescent regions that were produced in the monolayers after cholesterol extraction, repletion, and final extraction (Fig. 1d) were not lipid-depleted defects caused by the extended preparation steps, monolayers were treated with rhodamine-conjugated CTB (RH-CTB) to probe for the presence of GM1. As shown in Fig. 1e, RH-CTB identifies the GM1-containing raft-like domains (Lower), which appear dark with respect to FL-DPPE fluorescence (Upper). These results demonstrate that the presence of the coexisting phases critically depends on the cholesterol density: Repleting cholesterol took the monolayer into a uniform high-cholesterol density phase and only after partial redepletion did the raft phase reappear. Supported monolayers prepared without cholesterol (DOPC/SM) were in a homogeneous fluid crystalline state as judged by video FRAP measurements (data not shown).
Figure 1.
Effect of cholesterol (chol) on the raft-like phase in monolayers prepared from synthetic lipids. DOPC/SM/cholesterol monolayers were transferred at 35 dyne/cm onto silanized glass coverslips. The synthetic lipid mixture also contained 1 mol% GM1 and 0.5 mol% FL-DPPE. The distribution of FL-DPPE was imaged after (a) exchange of the bulk solution from water to PBS and subsequent treatments for 15 min with PBS containing (b) 10 mM MβCD, (c) 30 μg/ml water-soluble cholesterol, and (d) 5 mM MβCD. GM1 was visualized (e Lower) by incubating the lipid layer for 5 min with rhodamine-conjugated CTB (2.5 μg/ml) and its distribution was compared with that of FL-DPPE (e Upper). The diffusion coefficients of FL-DPPE were measured by spot FRAP. The beam size is indicated as ○ (Ø 2 μm). T = 24°C. (Bars are 2 μm.)
Coexisting raft-like and liquid-disordered domains are present not only in monolayers prepared from synthetic lipids but also in monolayers prepared from BBM lipid extracts (Fig. 2a Left). The raft-like domains exclude TR-DPPE (Fig. 2a Left) but are highly enriched in GM1 when this glycosphingolipid is added to the lipid extracts at 1 mol% and labeled with FL-CTB (24). Both phases are liquid crystalline, as readily seen by conducting video FRAP for TR-DPPE in regions containing both an enclosed GM1-enriched domain and a smaller TR-DPPE-enriched inclusion within this domain (Fig. 2b). TR-DPPE in the small inclusion readily exchanges with TR-DPPE from the region surrounding the GM1-enriched domain, indicating that TR-DPPE mobility in the GM1-enriched raft-like domains is significant. Together with the observation that extraction of cholesterol causes domain “melting” (Fig. 2a Center), we conclude that the original domains are in a cholesterol-enriched liquid-ordered phase state. Repletion of cholesterol leads to the formation of a liquid-ordered phase (Fig. 2a Right) that covers most of the monolayer and coexists with small domains enriched in TR-DPPE, which are presumably in a liquid-disordered state. Consistent with this interpretation, spot FRAP indicates that TR-DPPE in the more abundant phase has a diffusion coefficient that is ≈2-fold lower than the coefficient in the cholesterol-depleted lipid monolayer (Fig. 2 Center and Right).
Figure 2.
Effect of cholesterol (chol) on the raft-like phase in monolayers prepared from BBM lipid extracts. Monolayers composed of BBM lipids extracts with 0.4 mol% TR-DPPE and either (a) containing or (c) depleted of cholesterol (see Materials and Methods) were transferred at 32 dyne/cm onto silanized glass coverslips. Fluorescence micrographs show monolayers (Left) immediately after transfer onto supports; (Center) after being treated with 10 mM MβCD for 15 min; and (Right) after subsequent incubation with 30 μg/ml water-soluble cholesterol for 15 min. Video FRAP measurements, carried out before MβCD treatment, are depicted for monolayers prepared from (b) BBM and (d) cholesterol-depleted BBM lipid extracts. These images show TR-DPPE fluorescence before bleaching in the areas indicated by the ○ and after allowing fluorescence recovery to occur. T = 24°C. (All bars are 2.5 μm.)
In contrast to the homogeneous monolayers composed of the synthetic lipid mixture DOPC/SM, domains that exclude TR-DPPE form in monolayers prepared from cholesterol-depleted BBM lipid extracts (more than 95% cholesterol removal) (Fig. 2c Left). [The reason for the difference between Fig. 2 a and c is most likely that to see the apparent gel phase in BBM monolayers, almost all of the cholesterol (>95%) must be removed. With MβCD extraction, this condition cannot be met.] However, several lines of evidence indicate that these domains are in a gel-phase state. First, video FRAP measurements (Fig. 2d) indicate that the small inclusions enriched in TR-DPPE do not recover after large-area bleaching, even after several hours. Second, treatment with MβCD to reduce cholesterol levels does not melt the domains, but instead leaves them essentially unchanged (Fig. 2c Center). Third, the addition of water-soluble cholesterol into the bulk phase melts the domains (Fig. 2c Right).
The GPI-Linked Protein Thy-1 Significantly Partitions into the Raft-Like Phase.
The DRM from a variety of cell types is enriched in GPI-anchored proteins, suggesting that these proteins preferentially associate with lipid rafts in cell membranes (36–39). Therefore, a GPI-anchored protein (F-Thy-1) was reconstituted into supported lipid monolayers by detergent dilution and its distribution and dynamics were examined with fluorescence microscopy. These reconstitution studies were carried out on monolayers prepared both from BBM lipid extracts and DOPC/SM/cholesterol. The lateral mobility of F-Thy-1 was comparable to that of fluorescent phospholipid analogs (e.g., D = 0.52 ± 0.18 μm2/s measured by spot FRAP for DOPC/SM/cholesterol raft mixture). In control measurements with TR-DPPE-labeled supported monolayers, treatment for 2 h with 4 mM n-octyl-β-d-glucopyranoside in PBS did not cause measurable changes either in relative area coverage or relative fluorescence intensities of coexisting lipid phases.
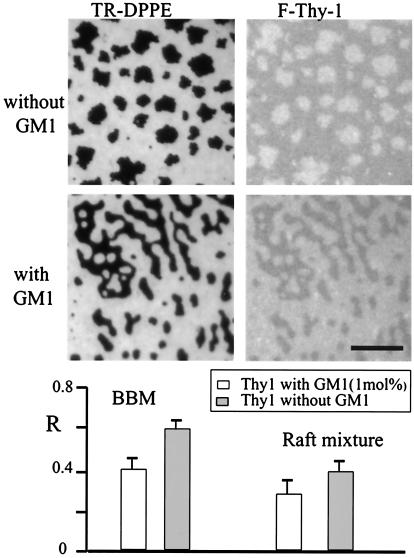
Fig. 3 shows a supported lipid monolayer prepared from BBM lipid extracts without fluorescent lipids or GM1 added, into which F-Thy-1 was incorporated. The image contrast, based on the fluorescence intensities of F-Thy-1, indicates the presence of two distinct lipid phases. As shown, video FRAP measurements demonstrated that both phases are fluid and that exchange of F-Thy-1 rapidly occurs between the two phases. In a set of measurements with monolayers prepared from BBM lipid extracts or synthetic raft-lipid mixtures, the relative partitioning of F-Thy-1 into the raft phase was examined, both in the absence and presence of GM1 (1 mol%). To unambiguously identify the coexisting phases, small amounts of TR-DPPE (0.2 mol%) were incorporated. This lipid brightly stains the liquid-disordered phase while being depleted from the raft-like phase (R ≈0.05 for all lipid compositions used). Fig. 4 shows typical fluorescence micrographs for BBM monolayers either not containing (Top) or containing (Middle) GM1. Fluorescence intensities are shown for TR-DPPE (Left, red channel) and F-Thy-1 (Right, green channel). In preparations without GM1, raft-like domains appear bright in F-Thy-1 fluorescence, whereas the contrast is inverted for preparations containing GM1. This result indicates that the tendency of F-Thy-1 to partition into the liquid-ordered phase (dark in TR-DPPE fluorescence image) is reduced in the presence of 1 mol% GM1. As illustrated with the R values measured for F-Thy-1 (Fig. 4), the same effect was observed for monolayers prepared from synthetic raft-lipid mixtures (DOPC/SM/cholesterol). For both natural and synthetic lipid mixtures, the relative partitioning value of F-Thy-1 significantly drops when GM1 is present, suggesting that the two molecules compete for raft domain occupation.
Figure 3.
Distribution and dynamics of the GPI-linked protein Thy-1 in supported lipid monolayers. F-Thy-1 was reconstituted into a BBM lipid monolayer by detergent dilution. Fluorescence image panels (green channel) show a video FRAP sequence. T = 24°C.
Figure 4.
Effect of GM1 on F-Thy-1 partitioning between the liquid-disordered and raft-like phases. Lipid monolayers, prepared either from BBM lipid extracts or synthetic lipid raft mixtures, were labeled with 0.1 mol% TR-DPPE, and either did not contain or contained 1 mol% GM1. F-Thy-1 was incorporated into the monolayers by detergent dilution. Fluorescence micrographs show BBM preparations without GM1 (Top) and with GM1 (Middle) imaged in the red (Left, TR-DPPE) and green (Right, F-Thy-1) channels. The plot (Bottom) shows the relative partition coefficient R, for F-Thy-1 into the liquid-ordered phase, for both lipid compositions and in the absence and presence of GM1. T = 24°C. (Bar = 10 μm.)
Cross-Linking by mAbs Can Translocate Fluorescent Phospholipid Analogs from the Liquid-Disordered to the Liquid-Ordered Phase.
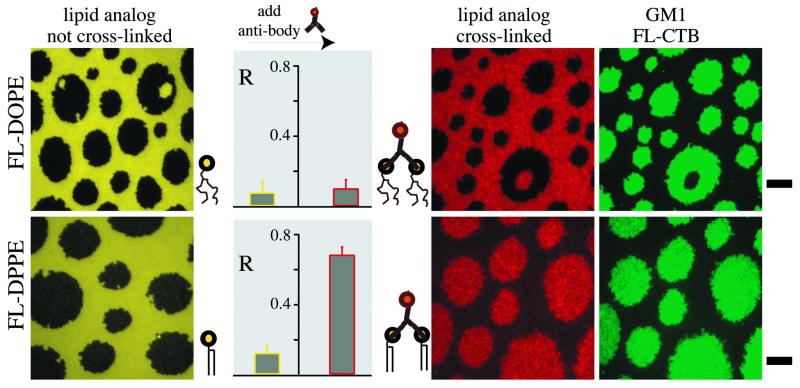
Receptor cross-linking is a key step in the initiation of signal transduction and can influence receptor occupation in the DRM of certain cell types (39, 40). Therefore, the distributions of two fluorescent phospholipid analogs (FL-DOPE and FL-DPPE) before and after cross-linking by an Alexa594-labeled antifluorescein polyclonal antibody were examined. Fig. 5 shows two typical sets of measurements, carried out for supported lipid monolayers composed of synthetic raft-lipid mixtures containing 1 mol% GM1 and 0.5 mol% of either FL-DOPE (Upper) or FL-DPPE (Lower). Both fluorescent lipids, in the monomeric state before antibody treatment, exhibit only a small tendency to partition into the raft-like phase, with R values of ≈0.07 for FL-DOPE and ≈0.14 for FL-DPPE. In these images, the raft-like domains appear dark (yellow panels). Cross-linked unsaturated phospholipids remained in the liquid-disordered phase and the image contrast (red panel, Upper) and R values (plot) were not significantly altered by antibody binding. Remarkably, the image contrast was inverted for cross-linked FL-DPPE (R ≈ 0.65, Fig. 5), and the originally dark raft-like domains (yellow panel, Lower) appeared bright with Alexa594 imaging (red panel, Lower). To clearly identify the raft-like phase, green-channel images were recorded for antibody-treated monolayers stained with FL-CTB (green panels). In these images, the fluorescein-conjugated lipids contributed only moderately to the fluorescence intensity (≈10%), because of the high affinity of FL-CTB for GM1 and the fact that the antifluorescein antibodies significantly quench the fluorescein fluorescence upon binding (see Materials and Methods). Longer antibody incubation times (up to 6 h) or equilibration times (up to 24 h) did not alter the results obtained for 15-min incubation and 45-min equilibration times. This result suggests that the recorded micrographs reflect an equilibrium or quasi-equilibrium distribution of the cross-linked lipids. Cross-linked complexes remained mobile in both phases as measured by video FRAP (not shown).
Figure 5.
Effect of cross-linking on the distribution of fluorescent phospholipid analogs between the liquid-disordered and raft-like phases. Monolayers were prepared from DOPC/SM/cholesterol containing 1 mol% GM1 and 0.5 mol% FL-DOPE or 0.5 mol% FL-DPPE, and transferred onto silanized glass coverslips at 32 dyne/cm. Typical fluorescence micrographs are shown for monolayers containing (Upper) FL-DOPE and (Lower) FL-DPPE. The yellow images show the distributions of the fluorescent lipids as monitored by measuring fluorescein fluorescence in the green channel. The red images illustrate the distributions of complexes of fluorescent lipids and Alexa594-conjugated antifluorescein polyclonal antibodies as determined by measuring Alexa594 fluorescence in the red channel. The green images show the corresponding distribution of GM1 as indicated by measuring the fluorescence in the green channel for monolayers after treatment with antibodies and then FL-CTB. To achieve accurate representations of FL-CTB densities, the offsets in the green panels were determined as the average intensities measured in the green channel before FL-CTB binding. The plots depict the relative partitioning, R, into the raft-like phase for fluorescent lipid analogs before and after cross-linking with antibodies before FL-CTB incubation. T = 24°C. (Bars are 5 μm.)
Discussion
Raft Domain Formation in Planar-Supported Monolayers.
The model membrane systems investigated here are prepared by transferring Langmuir monolayers from the water-air interface to silanized glass supports. The short acyl chain of the silane is covalently bound to the glass, and we assume that the structures observed by fluorescence microscopy are based on the molecular interactions present in the distal lipid leaflet. The strong contrast for molecules partitioning between the observed two liquid crystalline phases (CTB-labeled GM1, R ≈ 0.95; TR-DPPE, R ≈ 0.05) indicates that this model system is able to preserve interactions that cause and stabilize domain formation. The lateral pressure at which lipid monolayers were transferred onto planar supports (≈32 dyne/cm) was selected to mimic the physiological density of phospholipid bilayers (41). Indeed, the translational diffusion coefficients and fractional mobilities of fluorescent lipid probes in the monolayers are similar to those in planar-supported phospholipid bilayers (42–44) and the plasma membrane of cells (45).
Raft Domains Critically Depend on the Cholesterol Content.
The reversible disruption and formation of domains upon changing cholesterol levels in the lipid monolayers (Figs. 1 and 2) demonstrate that the lipid preparations are in thermodynamic exchange with the bulk phase and presumably close to equilibrium with respect to their phase composition. The observation that treating the supported monolayers with MβCD did not significantly change the intensities (±10%) or fractional recovery (0.92 ± 0.04) of incorporated fluorescent lipid probes (FL-DPPE or TR-DPPE) indicates that cholesterol can be added or extracted without impairing the integrity of the lipid leaflet, similar to lipid monolayers at the water-air interface (34). The very effective and prompt disruption of the model raft domains upon MβCD treatment (Figs. 1b and 2a) lends strong credence to the notion that certain signaling and sorting events in cells are based on cholesterol-enriched membrane domains termed rafts.
GPI-Anchored Proteins Partition into Raft Domains.
Significant amounts of F-Thy-1, used as a representative GPI-anchored protein, partition into the raft-like phase in supported lipid monolayers (Figs. 3 and 4). The highest F-Thy-1 partition coefficient was found for BBM preparations without GM1 (R ≈ 0.60); for Thy-1 partitioning into monolayers made from synthetic lipid mixtures, R was lower (R ≈ 0.40). The extent to which GPI-anchored proteins are recruited into rafts in cell membranes and the conditions for which this lateral sorting occurs are not yet clearly understood (20, 46, 47). GM1, which does not significantly affect the partitioning of the lipid analog TR-DPPE between the liquid-disordered and liquid-ordered phases, reduces the abundance of F-Thy-1 in the raft phase when added in small amounts (1 mol%, Fig. 4). The competition by GM1 and F-Thy-1 for raft residency could be because both molecules have large extracellular domains capable of ordering the aqueous face of the raft domains even when these molecules are present at relatively low densities. It is also possible that GM1 alters raft-like domain structure, reducing Thy-1 partitioning into them. Indeed, a recent study has shown that increased levels of GM1 displace GPI-anchored proteins from sphingolipid-cholesterol microdomains in living cells (48).
Selective Partitioning of Cross-Linked Components into Raft Domains.
The fact that physical cross-linking results in movement from the liquid-disordered to the liquid-ordered phase is quite striking given that the initial event in signal transduction, for many receptors, is cross-linking and that lipid rafts on cell membranes are thought to function as preassembled signal transduction complexes. A possible physical explanation for the result that cross-linking FL-DPPE with antifluorescein antibodies results in translocation to the raft-phase regions is that protein binding orders the FL-DPPE acyl chains. This notion is consistent with previous work showing that protein binding to lipids in phospholipid monolayers and bilayers can decrease hydrocarbon chain flexibility (49) and reduce lipid translation mobility (50, 51). An additional factor might be the increased ability of the larger head groups with their bound antibody to shield cholesterol from water (52). At this point it is important to note that CTB forms stable pentamers (53), offering binding sites for five GM1 lipids. Because we detect the distribution of CTB-GM1 complexes, the possibility that monomeric GM1 partitions differently into the coexisting phases cannot be excluded. Our results indicate that a delicate interplay of head-related and tail-related interactions determine the tendency to partition into the cholesterol-dependent liquid-ordered (“raft”) phases. Overall, it is striking how many tenets of the raft hypothesis can be confirmed in model membranes.
Supplementary Material
Acknowledgments
We thank Prof. Gerald J. Spangrude of the University of Utah for his generous gift of the 31–11 hybridoma. This work was supported by National Institutes of Health Grant GM41402 (K.J.), National Science Foundation Grant MCB9728116 (N.L.T.), a Veterans Administration Merit Review Grant (M.L.), and Deutsche Forschungsgemeinschaft Scholarship Di 691/1–1 (C.D.).
Abbreviations
- GPI
glycosylphosphatidylinositol
- BBM
brush border membrane
- DRM
detergent-resistant membrane fraction
- DOPC
1,2-dioleoyl-sn-glycero-3-phosphocholine
- SM
sphingomyelin
- FL-DOPE
1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-fluorescein
- FL-DPPE
1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-fluorescein
- TR-DPPE
1,2- dipalmitoyl-sn-glycero-3-phospho-ethanol-amine-x-Texas red
- CTB
cholera toxin B
- FL-CTB
fluorescein-conjugated CTB
- MβCD
methyl-β-cyclodextrin
- F-Thy-1
Thy-1 labeled with fluorescein isothiocyanate
- FRAP
fluorescence recovery after photobleaching
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
See commentary on page 10517.
References
- 1.Brown D A, Rose J. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- 2.Brown D A, London E. Biochem Biophys Res Commun. 1997;240:1–7. doi: 10.1006/bbrc.1997.7575. [DOI] [PubMed] [Google Scholar]
- 3.Fridriksson E K, Shipkova P A, Sheets E D, Holowka D, Baird B, McLafferty F W. Biochemistry. 1999;38:8056–8063. doi: 10.1021/bi9828324. [DOI] [PubMed] [Google Scholar]
- 4.Ge M, Field K A, Aneja R, Holowka D, Baird B, Freed J H. Biophys J. 1999;77:925–933. doi: 10.1016/S0006-3495(99)76943-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simons K, Ikonen E. Nature (London) 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 6.Simons K, Ikonen E. Science. 2000;290:1721–1726. doi: 10.1126/science.290.5497.1721. [DOI] [PubMed] [Google Scholar]
- 7.Simons K, Meer G V. Biochemistry. 1988;27:6197–6202. doi: 10.1021/bi00417a001. [DOI] [PubMed] [Google Scholar]
- 8.Patel V P, Moran M, Low T A, Miceli M C. J Immunol. 2001;166:754–764. doi: 10.4049/jimmunol.166.2.754. [DOI] [PubMed] [Google Scholar]
- 9.Khoshnan A, Bae D, Tindell C A, Nel A E. J Immunol. 2000;165:6933–6940. doi: 10.4049/jimmunol.165.12.6933. [DOI] [PubMed] [Google Scholar]
- 10.Anderson H A, Hiltbolt E M, Roche P A. Nat Immunol. 2000;1:156–162. doi: 10.1038/77842. [DOI] [PubMed] [Google Scholar]
- 11.Guo B C, Kato R M, Garcia-Lloret M, Wahl M I, Rawlings D J. Immunity. 2000;13:243–253. doi: 10.1016/s1074-7613(00)00024-8. [DOI] [PubMed] [Google Scholar]
- 12.Aman M J, Ravichandran K S. Curr Biol. 2000;10:393–396. doi: 10.1016/s0960-9822(00)00415-2. [DOI] [PubMed] [Google Scholar]
- 13.Keller P, Simons K. J Cell Biol. 1998;140:1357–1367. doi: 10.1083/jcb.140.6.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simons K, Gruenberg J. Trends Cell Biol. 2000;10:459–462. doi: 10.1016/s0962-8924(00)01847-x. [DOI] [PubMed] [Google Scholar]
- 15.Sheets E D, Holowka D, Baird B. J Cell Biol. 1999;145:877–887. doi: 10.1083/jcb.145.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manes S, del Real G, Lacalle R A, Lucas P, Gomez-Mouton C, Sanchez-Palomino S, Delgado R, Alcami J, Mira E, Martinez C. EMBO Rep. 2000;1:190–196. doi: 10.1093/embo-reports/kvd025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoessli D C, Ilangumaran S, Soltermann A, Robinson P J, Borisch B, Din N U. Glycoconjugate J. 2000;17:191–197. doi: 10.1023/a:1026585006064. [DOI] [PubMed] [Google Scholar]
- 18.Prieschl E E, Baumruker T. Immunol Today. 2000;21:555–560. doi: 10.1016/s0167-5699(00)01725-4. [DOI] [PubMed] [Google Scholar]
- 19.Cinek T, Horejsi V. J Immunol. 1992;149:2262–2270. [PubMed] [Google Scholar]
- 20.Jacobson K, Dietrich C. Trends Cell Biol. 1999;9:84–92. doi: 10.1016/s0962-8924(98)01495-0. [DOI] [PubMed] [Google Scholar]
- 21.Brown D A, London E. J Biol Chem. 2000;275:17221–17224. doi: 10.1074/jbc.R000005200. [DOI] [PubMed] [Google Scholar]
- 22.Brown D A, London E. J Membr Biol. 1998;164:103–114. doi: 10.1007/s002329900397. [DOI] [PubMed] [Google Scholar]
- 23.Thompson T E, Tillack T W. Annu Rev Biophys Biophys Chem. 1985;14:361–386. doi: 10.1146/annurev.bb.14.060185.002045. [DOI] [PubMed] [Google Scholar]
- 24.Dietrich C, Bagatolli L, Levi M, Volovyk Z, Thompson N L, Jacobson K, Gratton E. Biophys J. 2001;80:1417–1428. doi: 10.1016/S0006-3495(01)76114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang T Y, Silvius J R. Biophys J. 2000;79:1478–1489. doi: 10.1016/S0006-3495(00)76399-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mesquita R M R S, Melo E, Thompson T E, Vaz W L C. Biophys J. 2000;78:3019–3025. doi: 10.1016/S0006-3495(00)76840-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Radhakrishnan A, Anderson T G, McConnell H M. Proc Natl Acad Sci USA. 2000;97:12422–12427. doi: 10.1073/pnas.220418097. . (First Published October 24, 2000; 10.1073/pnas.220418097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schroeder R, London E, Brown D A. Proc Natl Acad Sci USA. 1994;91:12130–12134. doi: 10.1073/pnas.91.25.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmed S N, Brown D A, London E. Biochemistry. 1997;36:10944–10953. doi: 10.1021/bi971167g. [DOI] [PubMed] [Google Scholar]
- 30.Brito R M M, Vaz W L C. Anal Biochem. 1986;152:250–255. doi: 10.1016/0003-2697(86)90406-9. [DOI] [PubMed] [Google Scholar]
- 31.Chattopadhyay A, London E. Anal Biochem. 1984;139:408–412. doi: 10.1016/0003-2697(84)90026-5. [DOI] [PubMed] [Google Scholar]
- 32.Ahlers M, Grainger D W, Herron J N, Lim K, Ringsdorf H, Salesse C. Biophys J. 1992;63:823–838. doi: 10.1016/S0006-3495(92)81645-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mummert M E, Voss E W. Mol Immunol. 1995;32:1225–1233. doi: 10.1016/0161-5890(95)00106-9. [DOI] [PubMed] [Google Scholar]
- 34.Radhakrishnan A, McConnell H M. Biochemistry. 2000;39:8119–8124. doi: 10.1021/bi0005097. [DOI] [PubMed] [Google Scholar]
- 35.Christian A E, Haynes M P, Phillips M C, Rothblat G H. J Lipid Res. 1997;38:2264–2272. [PubMed] [Google Scholar]
- 36.Millan J, Qaidi M, Alonso M A. Eur J Immunol. 2001;31:467–473. doi: 10.1002/1521-4141(200102)31:2<467::aid-immu467>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 37.Pralle A, Keller P, Florin E L, Simons K, Horber J K H. J Cell Biol. 2000;148:997–1007. doi: 10.1083/jcb.148.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benting J, Rietveld A, Ansorge I, Simons K. FEBS Lett. 1999;462:47–50. doi: 10.1016/s0014-5793(99)01501-x. [DOI] [PubMed] [Google Scholar]
- 39.Harder T, Scheiffele P, Verkade P, Simons K. J Cell Biol. 1998;141:929–942. doi: 10.1083/jcb.141.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sheets E D, Holowka D, Baird B. Curr Opin Chem Biol. 1999;3:95–99. doi: 10.1016/s1367-5931(99)80017-9. [DOI] [PubMed] [Google Scholar]
- 41.Demel R A, Guerts van Kessel W S M, Zwaal R F A, Roelofsen B, van Deenen L L M. Biochem Biophys Acta. 1975;406:97–107. doi: 10.1016/0005-2736(75)90045-0. [DOI] [PubMed] [Google Scholar]
- 42.Merkel R, Sackmann E, Evans E. J Phys (France) 1989;50:1535–1555. [Google Scholar]
- 43.Tamm L K, McConnell H M. Biophys J. 1985;47:105–113. doi: 10.1016/S0006-3495(85)83882-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wright L L, Palmer A G, Thompson N L. Biophys J. 1988;54:463–470. doi: 10.1016/S0006-3495(88)82979-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jacobson K, Hou Y, Derzko Z, Wojcieszyn J, Organisciak D. Biochemistry. 1981;20:5268–5275. doi: 10.1021/bi00521a027. [DOI] [PubMed] [Google Scholar]
- 46.Kenworthy A K, Petranova N, Edidin M. Mol Biol Cell. 2000;11:1645–1655. doi: 10.1091/mbc.11.5.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Varma R, Mayor S. Nature (London) 1998;394:789–801. doi: 10.1038/29563. [DOI] [PubMed] [Google Scholar]
- 48.Simons M, Friedrichson T, Schulz J, Pito M, Masserini M, Kurzchalia T. Mol Biol Cell. 1999;10:3187–3196. doi: 10.1091/mbc.10.10.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hart M J, Kimura K, Nakanishi M. FEBS Lett. 1988;190:249–252. doi: 10.1016/0014-5793(85)81293-x. [DOI] [PubMed] [Google Scholar]
- 50.Huang Z, Pearce K H, Thompson N L. Biochem Biophys Acta. 1992;1112:259–265. doi: 10.1016/0005-2736(92)90400-g. [DOI] [PubMed] [Google Scholar]
- 51.Timbs M M, Poglitsch C L, Pisarchick M L, Summer M T, Thompson N L. Biochem Biophys Acta. 1991;1064:219–228. doi: 10.1016/0005-2736(91)90305-r. [DOI] [PubMed] [Google Scholar]
- 52.Huang J Y, Feigenson G W. Biophys J. 1999;76:2142–2157. doi: 10.1016/S0006-3495(99)77369-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ribi H O, Ludwig D S, Mercer K L, Schoolnik G K, Kornberg R D. Science. 1988;239:1272–1276. doi: 10.1126/science.3344432. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.