Abstract
Non-small cell lung cancer (NSCLC) is a major type of lung cancer, with the highest mortality rate in all cancers. For all stages of NSCLC, the five-year survival is less than fifteen percent. Epithelial-mesenchymal transition (EMT) is a significant process in tumor occurrence and development, in which microRNAs may play an important role. In many cancers, microRNA-15's family member can act as suppressors or oncogenes of tumors; however, the relation between these microRNAs and EMT in lung cancer remains unclear. According to our study, miR-15a expression decreased in tumor tissues compared with than that in adjacent tissue samples. Knocking down miR-15a expression in NSCLC cells inhibited apoptosis and facilitated cell proliferation and invasion, and. Moreover, down-regulating miR-15a decreased the expression of an EMT-associated protein, E-cadherin, while increased those of vimentin, N-cadherin, and slug.
Keywords: miR-15a, EMT, NSCLC
1. Introduction
Primary lung cancer, which is categorized into small and non-small cell types (Ferlay, 2010); is considered to be the most common malignant tumor after nonmelanocytic skin cancer (Jemal, 2011). Approximately 1.59 million people die of lung cancer worldwide annually, and this trend is predicted to continue until 2030 (Sun, 2016). NSCLC contributes to approximately eighty percent of all cases of lung cancer, including squamous cell carcinoma and adenocarcinoma (Siegel, 2012). The five-year survival for all stages of NSCLC remains less than fifteen percent because of lack of symptoms for early diagnosis and the highly malignant potential of this cancer (Heist and Engelman, 2012). Treatment of lung cancer patients remain unsatisfactory despite the promising results of standard treatment regimens with neoadjuvant and adjuvant strategies. Therefore, early screening and diagnosis of biomarker for NSCLC must be identified aiming to prolong affected patients’ life.
The leading causes of death of patients with NSCLC are chemotherapy resistance and metastasis, whose underlying mechanisms remain unclear (Gao, 2012, Mallini et al., 2014). Aberrant cell proliferation and apoptosis significantly contribute to chemotherapy resistance, and EMT plays a momentous role in tumor progression toward cancer cell invasion and metastasis (Singh and Settleman, 2010). EMT is a completely differentiated epithelial cell that undergoes a transition to a mesenchymal phenotype that produces fibroblasts and myofibroblasts (Kasai et al., 2005). Oncogenic EMT, who is specifically related to tumor invasiveness, refers to malignant cells to obtain migratory phenotypes (Smith and Bhowmick, 2016).
MicroRNAs (miRNAs), with sizes of 17–25 nucleotides, are a sort of extremely conservative, endogenous, non-coding, and small RNAs (Bartel, 2004, Wightman et al., 1993, Lee et al., 1993). In human cancers; miRNAs often cause abnormal expression, acting as essential modulators of chemotherapy resistance, carcinogenesis, and metastasis (Esquela-Kerscher et al., 2006). MicroRNA-15 family are clustered on three separate chromosomes (Porrello, 2011). miR-15a/16-1 cluster is the first miRNAs associated with cancer by Croce and colleagues (Calin, 2002). After this discovery, there comes a myriad studies trying to define the role of miRNAs in the pathogenesis of cancer. The microRNA-15 family functions as tumor suppressors or oncogenes in many cancers (Aqeilan et al., 2010, Cimmino, 2005); but the functions of miR-15a in NSCLC and its relation to EMT remains unclear.
In our research, we illustrated that miR-15a expression level was down-regulated in tumor tissues compared with adjacent tissue. To functionally characterize miR-15a in NSCLC, miR-15a’s expression in NSCLC cells were knocked down. As a result, knocking down miR-15a inhibited apoptosis, while promoting cell proliferation and invasion and. Moreover, down-regulating miR-15a decreased E-cadherin’s expression, which is an EMT-associated protein, as well as increased those of vimentin, N-cadherin, and slug.
2. Materials and methods
2.1. Clinical tissue samples
NSCLC tissues (n = 15) and adjacent tissues (n = 15) were obtained from Peking Union Medical College Hospital from May 2013 to June 2015. All samples were stored in liquid nitrogen for RNA extraction. Patients undergoing surgery signed written authorization to donate tissues for analysis.
2.2. Cell culture and transfection
H1299 and A549 cell lines were provided by the Chinese Academy of Sciences of Shanghai. H1299 and A549 cells were transfected with inhibitor negative control (NC) or the hsa-miR-15a-5p inhibitor (Ribobio, Guangzhou, China) by using Lipofectamine 2000 (Invitrogen Life Technologies, USA) under the instruction. Then cells were cultured for 48 h and collected for further study.
2.3. Total RNA extraction and qPCR
We used the TRIzol method (Invitrogen, USA) to extract the total RNAs of samples. A miRNA cDNA Synthesis Kit (Takara, Japan) is used to reverse transcription. miR-15a quantification assays were conducted using a miRNA qPCR Assay Kit (Cwbiotech, Beijing, China). As miRNA internal control, we used U6 RNA. U6 snRNA qPCR Primer and Bulge-Loop™ hsa-miR-15a-5p qPCR Primer were obtained from Ribobiotech Company (Ribobio, Guangzhou, China). The reaction conditions: Pre-denaturation, 95 °C for 3 min, a total of 36 cycles of amplification were performed and each cycle consisted of 5 s at 94 °C and 40 s at 65 °C. Each sample was analyzed in triplicate, and 2-ΔCt method was used to calculate the results.
2.4. Western blot
Cells collected from different treatment groups were washed twice in the precooled PBS solution. After the washing procedure, cells were lysed for twenty minutes by using RIPA buffer solution with phosphatase inhibitors and protease (Roche Diagnostics, USA). Approximately 100 µg of the protein was separated by SDS-PAGE and then transferred to membrane (Millipore, USA). The polyvinylidene fluoride membranes were blocked in five percent nonfat dry milk at indoor temperature in the first place for one hour, and were subsequentially incubated with primary antibodies at 4 °C overnight. Monoclonal antibodies against Bcl-2, Bax, activated caspase 3, E-cadherin, total ERK and p-ERK, vimentin, total AKT and p-AKT, N-cadherin, and slug were diluted at a ratio of 1:1000 (Abcam, USA). GAPDH monoclonal antibody was diluted by the ratio of 1:5000 (PTG, USA) and used as internal control. The polyvinylidene fluoride membranes were washed and then incubated with secondary antibody (1:5000, PTG, USA) for ECL detection.
2.5. Cell-proliferation assay
Cell counting kit-8 method was used to determine the effect of miR-15a on cell growth (Solarbio Biotechnology, Beijing, China). Cells transfected with NC or the miR-15a inhibitor for 24 h, cultured to the 96-well plate with the density of 5 × 103 cells/well and allowed to grow overnight. We assessed cell proliferation every 24 h according to the instructions of the kit. 1.5 h before the end of the incubation period, 10 μl of the CCK8 reagent was added to each well. The miniature microplate reader were used to determine the optical density (OD) at 450 nm. The reproducibility of our study was guaranteed by repeating all experiments at least three times.
2.6. Transwell invasion assay
Transwell inserts (8 μm pore size, BD, USA) were used to perform cell invasion assay, coated with Matrigel (dilute 1:8, BD, USA). Cells in serum-free media were cultured in the upper chamber (4 × 105 cells) at 24 h after transfection, and media containing ten percent fetal bovine serum were added to the lower chamber. After 14–16 h of incubation, cells that did not invade the membrane were wiped. The membranes were fixed for thirty minutes with stained with zero point five percent crystal violet and four percent paraformaldehyde. Numbers of penetrating cells was calculated using a microscope at a magnification of ×200 in five random fields.
2.7. Migration analysis
Analysis of cell migration is performed by a wound healing assay. At 24 h after the cells’ transfection with miR-15a inhibitor or NC in a six-well plate and when the cells grew to 90–95% confluence, the cell monolayer was scratched using a sterile 10 µl pipette tip. The detached cells were washed and removed, and the plates were incubated at 37 °C for further culture. The wounds were photographed every 24 h. At least five different wounds were analyzed.
2.8. Apoptosis assay
At 24 h after transfection, the cells were cultured with serum-free media for 24 h to induce apoptosis. The cells were harvested after digestion using trypsin without EDTA and the cell suspension was diluted at a density of 1 × 106/ml using a binding buffer. Cells were stained with PI and Annexin-V by using an Annexin V-FITC Apoptosis Detection Kit (Abcam, USA). Flow Cytometry (Beckman Coulter, USA) and FlowJo software were used to analyze the specimens
2.9. Statistical analysis
All experiment data were normalized and presented as mean ± SEM. Data were analyzed using SPSS 16.0. Two-tail student’s t-test and ANOVA were implemented to determine differences.
3. Results
3.1. miR-15a expression is reduced in human lung cancer tissues
We determined miR-15a expression in NSCLC samples as well as corresponded adjacent tissues, aiming to investigate the role of miR-15a in NSCLC of human beings. Based on the qRT-PCR analysis data (Fig. 1), miR-15a’s expression was significantly lower (p < .01) in NSCLC samples (n = 15) than that in corresponding adjacent tissues (n = 15). Hence, low miR-15a expression may contribute to NSCLC development.
Fig. 1.
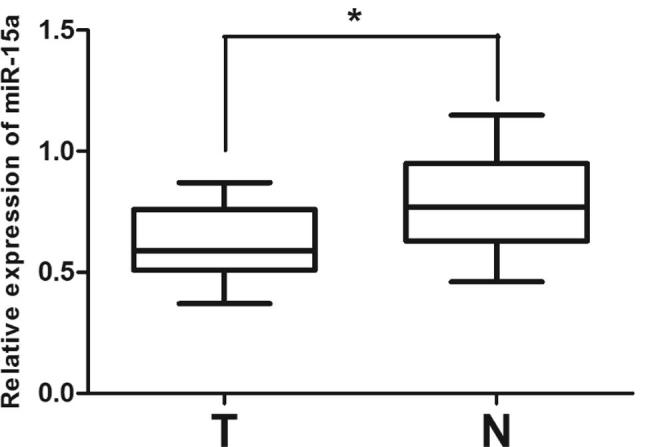
miR-15a expression decreases in patients with NSCLC. (A) miR-15a mRNA expression in NSCLC specimens (n = 15) and corresponding adjacent tissues (n = 15) relative to U6 detected using qRT-PCR. T refers to tumor tissues, and N refers to normal corresponding adjacent tissues. *P < .01, Statistical analysis was conducted using student t-test.
3.1.1. Effect of gene silencing using siRNA in NSCLC cells in vitro
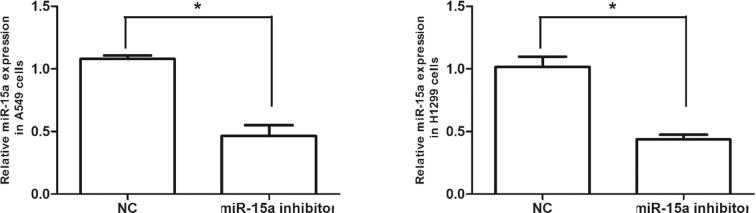
The effects of gene silencing using siRNA in NSCLC cells in vitro were analyzed. H1299 and A549 cells were transfected with the miR-15a inhibitor. After 48 h, miR-15a mRNA expression level in siRNA-transfected groups was significantly lower than that in NC group. The expression level of miR-15a were 48.5% and 47.6% in H1299 and A549 cells transfected with the miR-15a inhibitor (Fig. 2). Thus, the miR-15a inhibitor can effectively knock down miR-15a expression level in H1299 and A549 cells.
Fig. 2.
miR-15a is knocked down in lung cancer cells. A549 and H1299 cells were transfected with the miR-15a inhibitor; after 48 h, the mRNA expression of miR-15a was measured using qPCR analysis and normalized to U6 expression; miR-15a expression in the group transfected with the miR-15a inhibitor decreased significantly compared with that in the NC group. Data are represented as mean ± SEM of three independent experiments, *P < .01.
3.2. miR-15a regulates the invasion and migration of NSCLC cells in vitro
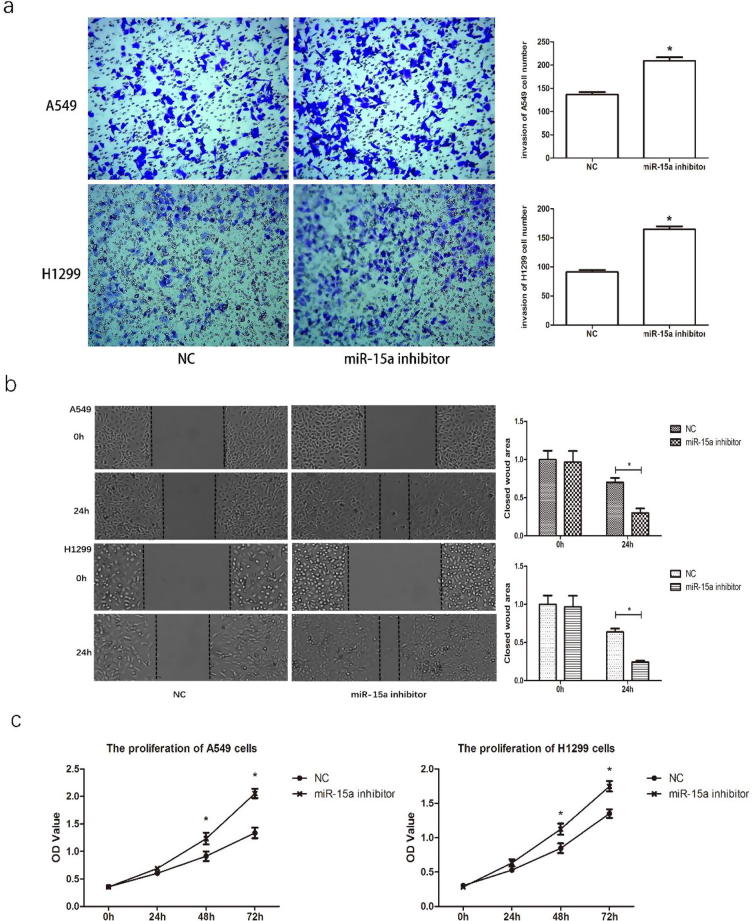
The effects of down-regulating miR-15a on cell invasion were evaluated to explore the role of miR-15a expression in NSCLC cancer metastasis, using Transwell invasion assay. The results indicated that down-regulating miR-15a could significantly promote the invasion of H1299 and A549 cells (Fig. 3a). Moreover, the wound-healing assay results indicated that down-regulating miR-15a in lung cancer cells could promote cell migration (Fig. 3b). Collectively, our data imply that down-regulating miR-15a might greatly contribute to the development of breast cancer metastasis and invasion.
Fig. 3.
Downregulation of miR-15a promotes invasion, migration, and proliferation of NSCLC cells in vitro. A549 and H1299 cells were transfected with 50 nM miR-15a inhibitor or control mimics. a. Transwell assay was performed to analyze cell invasion. Invasive cells at the bottom of the membrane stained with crystal violet are visualized; and the quantifications of cell invasion are presented with a histogram. b. Wound healing assay was performed to analyze cell migration. The cloud wound area is presented with a histogram. c. CCK8 assay was performed at different time points. The OD value reflects the proliferation of cells. Data are presented as the mean ± SEM of three independent experiments, *P < .01.
3.3. Knocking down miR-15a expression promotes cell proliferation
We used a CCK8 assay to investigate the effects of miR-15a on cell proliferation, aiming to figure out whether miR-15a could regulate NSCLC cell proliferation in vitro. Distinct differences in cell proliferation were observed after down-regulating miR-15a in both A549 and H1299 cells at 48 and 72 h time points (Fig.3c). The proliferation assay showed that knocking down miR-15a expression promoted the proliferation of lung cancer cells.
3.4. Knocking down miR-15a expression suppresses apoptosis
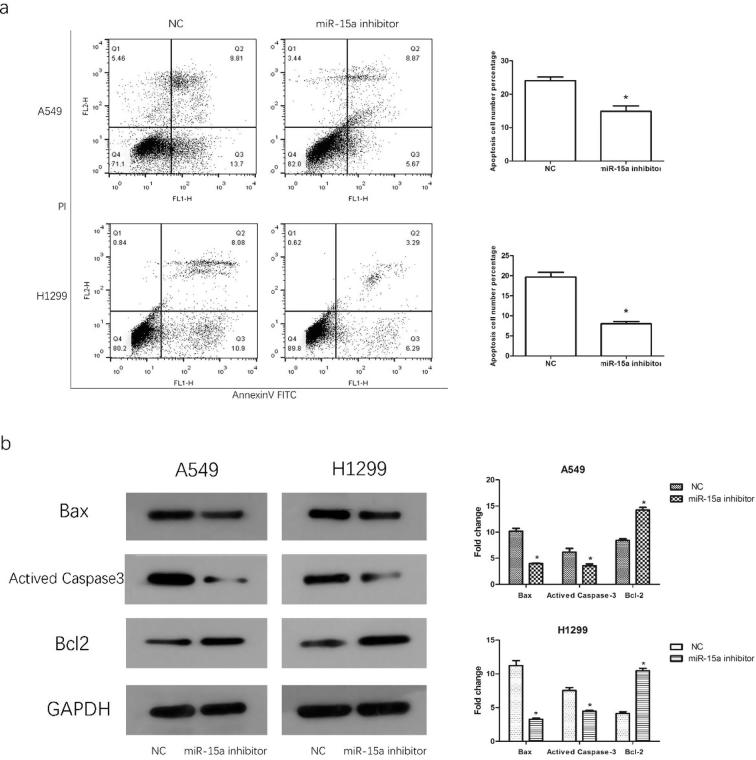
We performed apoptosis assay to investigate whether aberrantly expressed miR-15a influences NSCLC cell apoptosis. Flow cytometry analysis showed that knocking down miR-15a expression inhibited apoptosis induced by starvation (Fig. 4a). Bcl2 acts a significant role in the process of cell apoptosis and is the central regulator of caspase activation, previous study showed that bcl2 was regulated by miR-15a in B cell chronic lymphocytic leukemia (Cimmino, 2005). To further explore the mechanism through which miR-15a affects apoptosis in NSCLC cells, we detected the expression level of activated Caspase3, Bax, and Blc-2 by using Western blot analysis. As is shown in Fig. 4b, down-regulating miR-15a inhibited the expression of apoptotic proteins but promoted the expression level of anti-apoptotic proteins.
Fig. 4.
Knocking down miR-15a expression suppresses NSCLC cell apoptosis. At 24 h after transfection, the cells were cultured with serum-free media for 24 h to induce apoptosis. a. Apoptotic cells were stained with PI and Annexin-V and analyzed by flow cytometry. b. Apoptotic proteins were detected by Western blot analysis, and the results are presented with a histogram. Data are presented as mean ± SEM of three independent experiments, *P < .01.
3.5. Knocking down miR-15a expression increased the phosphorylation level of ERK and Akt
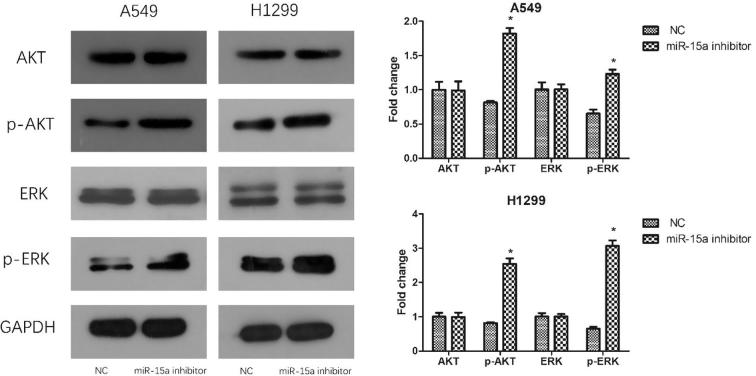
The activation of the ERK and Akt signaling pathways are the major signaling pathway that regular cell survival, proliferation and apoptosis by affecting the activity of down-tream effector molecules (Vara, 2004, Kohno and Pouyssegur, 2006). To explore the connection between miR-15a and the two signaling pathways, we analyzed the phosphorylation level of ERK and Akt. Western blot indicated that the expression level of p-ERK and p-Akt were improved (Fig. 5) after miR-15a was down-regulated.
Fig. 5.
Knocking down miR-15a expression increased the phosphorylation of Akt and ERK of NSCLC cells in vitro. The proteins of AKT and ERK were detected by Western blot analysis after cells were transfected with miR-15a inhibitor. The results are presented with a histogram. Data are presented as mean ± SEM of three independent experiments, *P < .01.
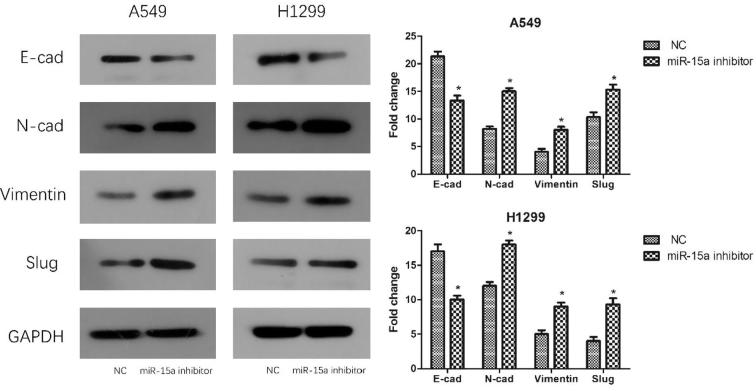
3.6. Effect of miR-15a on EMT-related proteins in NSCLC cells
EMT is involved in the development and progression of epithelial origin tumors, particularly in metastasis and invasion of cancer. E-cadherin is considered to act a momentous role in the invasion ability of abundant cancer cells. Tumor invasion, migration and a poor prognosis may be all associated with the lack of E-cadherin expression. Vimentin expression helps to enhance cancer invasion and metastasis (Zhai, 2014). As such, we investigated the correlation between EMT and miR-15a. Western blot analysis indicated that the expression level of EMT-related proteins significantly changed in NSCLC cells transfected with the miR-15a inhibitor compared with that in NC-transfected cells. Cells transfected with the miR-15a inhibitor exhibited down-regulated E-cadherin expression and up-regulated expression of N-cadherin, vimentin, and slug (Fig. 6). Hence, the experiment evidence suggested that down-regulating miR-15a in lung cancer cells in vitro could promote EMT.
Fig. 6.
Knocking down miR-15a expression promotes the EMT of NSCLC cells in vitro. After transfecting the cells with the miR-15a inhibitor, total cell proteins were harvested and EMT-related proteins were detected by Western blot analysis. The results are presented with a histogram. Data are presented as mean ± SEM of three independent experiments, *P < .01.
4. Discussion
NSCLC is responsible for more than eighty percent of lung cancer cases, making it one of the deadliest cancers worldwide. NSCLC has poor prognosis and a low overall five-year survival of eleven percent; moreover, most patients are diagnosed with the disease in the advanced stage (Siegel, 2012). Therefore, the underlying mechanism of NSCLC must be investigated.
MiRNAs are non-coding endogenous small RNAs. They can bind to 3′-UTR of the target mRNAs to regulate gene expression level, leading to inhibit the translation of target mRNAs or degradation them. MiRNAs are important in cancer initiation and progression (O'Connell et al., 2010). MiR-15a is a genomic region frequently deleted in B-cell chronic lymphocytic leukemia (Calin, 2008). The target genes of miR-15a include BCL2, CCND1, MCL1, and WNT3A. Previous study have indicated that miR-15a performed as tumor suppressor and down-regulated in B-cell chronic lymphocytic leukemia, melanoma, colorectal cancer, bladder cancer and other solid tumors (Aqeilan et al., 2010, Pekarsky and Croce, 2015). According to our study, miR-15a expression markedly decreased in NSCLC tissues suggesting that low level expression of miR-15a might contribute to the development of NSCLC. Known as the central regulator of caspase activation, Bcl2 family of intracellular proteins and its opposing factions of pro-apoptotic and anti-apoptotic members arbitrate the life-or-death decision (Cory and Adams, 2002). Previous study gave the evidence of miR-15a inducing apoptosis by targeting Bcl2 in CLLs (Cimmino, 2005). In the current research, knocking down miR-15a expression in NSCLC cells inhibited the apoptosis of NSCLC cells, the expression of anti-apoptotic protein Bcl2 improved, while the apoptotic proteins were reduced. Furthermore, knocking down miR-15a expression in NSCLC cells in vitro effectively promoted the proliferation and invasion of NSCLC cells. Meanwhile, the expression level of p-ERK and p-AKT which was the key regulator in AKt and MAPK signaling pathway respectively were enhanced. Considering their critical roles in the proliferation, metastasis and apoptosis of tumors, we speculate that miR-15a could be a novel tumor suppressor in NSCLC and regulate the physiological function through the PI3K/AKT and MAPK signaling pathway.
In EMT, epithelial cells decrease cellular adhesion molecules’ expression by interacting with surrounding mesenchymal cells which also promote cell dispersion. During this process, cytoskeleton’s main component altered from keratin to vimentin gradually, leading to structure rearrangement, reduced cellular adhesion, and enhanced cell mobility (Techasen, 2012). EMT is important in tumor occurrence and development. The commencement of EMT marks the beginning of tumor metastasis (Ogunwobi and Liu, 2012, Yang et al., 2011). A marked characteristic of EMT is the down-regulated expression level of the intercellular adhesion molecule, E-cadherin, and the up-regulated expression level of a series of mesenchymal markers, including fibronectin, N-cadherin, and vimentin which are important in maintaining the epithelial phenotype and stabilizing inter-cellular contact (Gheldof and Berx, 2013, Liu, 2013). The microRNA-15 family regulate the occurrence and development of tumors in various methods: Ramaiah et al. found miR-15 controls cell proliferation in breast cancer cells by targeting p70S6 kinase1 (Ramaiah, 2014). Pouliot et al. demonstrated that miR-15 sensitized cisplatin-resistant cells by controlling the expression of Wee1 and CHK1 (Pouliot, 2012). Furthermore, microRNA-15 family influences cancer cell metastasis by regulating EMT in malignant cancer cells (Renjie and Haiqian, 2015, Shi, 2014, Gao, 2017, Wang et al., 2014). According to our study, knocking down miR-15a expression in NSCLC cells up-regulated the expression level of vimentin, N-cadherin, and slug and can further down-regulate the expression of E-cadherin. These experimental evidences indicate that the regulation of E-cadherin and vimentin expression in tumor cells may be associated with the promotion effect of low miR-15a on the EMT process.
In conclusion, knocking down miR-15a expression inhibited the apoptosis of NSCLC cells, as well as promoting the proliferation and invasion. Meanwhile these effects participated in the up-regulation of Bcl2 expression, which is the down-regulation of Bax and active Caspase3 expression. In addition, Akt and ERK phosphorylation was increased. Down-regulating miR-15a decreased E-cadherin’s (an EMT-associated protein) expression, while increasing the expression level of N-cadherin, vimentin, and slug. Therefore, miR-15a may be a potential biomarker for diagnosis of NSCLC. This study provides novel insights for treatment of NSCLC.
Footnotes
Peer review under responsibility of King Saud University.
References
- Aqeilan R.I., Calin G.A., Croce C.M. miR-15a and miR-16-1 in cancer: discovery, function and future perspectives. Astron. Astrophys. 2010;17:215. doi: 10.1038/cdd.2009.69. [DOI] [PubMed] [Google Scholar]
- Bartel D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Calin G.A. Frequent deletions and down-regulation of micro- RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:15524–15529. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calin G.A. MiR-15a and miR-16-1 Cluster Functions in Human Leukemia. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:5166–5171. doi: 10.1073/pnas.0800121105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimmino A. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:13944–13949. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory S., Adams J.M. The Bcl2 family: regulators of the cellular life-or-death switch. Nat. Rev. Cancer. 2002;2:647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- Esquela-Kerscher A., Slack F.J., Esquela-Kerscher A., Slack F.J. Oncomirs-microRNAs with a role in cancer. Nat. Rev. Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- Ferlay J. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Can. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- Gao D. Microenvironmental regulation of epithelial-mesenchymal transitions in cancer. Can. Res. 2012;72:4883–4889. doi: 10.1158/0008-5472.CAN-12-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W. The first multiplication atom-bond connectivity index of molecular structures in drugs. Saudi Pharm. J. 2017;25(4):548–555. doi: 10.1016/j.jsps.2017.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gheldof A., Berx G. Cadherins and epithelial-to-mesenchymal transition. Prog. Mol. Biol. Transl. Sci. 2013;116:317. doi: 10.1016/B978-0-12-394311-8.00014-5. [DOI] [PubMed] [Google Scholar]
- Heist R.S., Engelman J.A. SnapShot: non-small cell lung cancer. Cancer Cell. 2012;21 doi: 10.1016/j.ccr.2012.03.007. 448 e442. [DOI] [PubMed] [Google Scholar]
- Jemal A. Global cancer statistics. CA: Can. J. Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- Kasai H., Allen J.T., Mason R.M., Kamimura T., Zhang Z. TGF-β1 induces human alveolar epithelial to mesenchymal cell transition (EMT) Respir. Res. 2005;6:56. doi: 10.1186/1465-9921-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno M., Pouyssegur J. Targeting the ERK signaling pathway in cancer therapy. Ann. Med. 2006;38:200–211. doi: 10.1080/07853890600551037. [DOI] [PubMed] [Google Scholar]
- Lee R.C., Feinbaum R.L., Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- Liu T. Dysregulated expression of slug, vimentin, and E-cadherin correlates with poor clinical outcome in patients with basal-like breast. Cancer. 2013;107:188–194. doi: 10.1002/jso.23240. [DOI] [PubMed] [Google Scholar]
- Mallini P., Lennard T., Kirby J., Meeson A. Epithelial-to-mesenchymal transition: what is the impact on breast cancer stem cells and drug resistance. Can. Treat. Rev. 2014;40:341–348. doi: 10.1016/j.ctrv.2013.09.008. [DOI] [PubMed] [Google Scholar]
- O'Connell R.M., Rao D.S., Chaudhuri A.A., Baltimore D. Physiological and pathological roles for microRNAs in the immune system. Nat. Rev. Immunol. 2010;10:111–122. doi: 10.1038/nri2708. [DOI] [PubMed] [Google Scholar]
- Ogunwobi O.O., Liu C. Therapeutic and prognostic importance of epithelial–mesenchymal transition in liver cancers: Insights from experimental models. Crit. Rev. Oncol./Hematol. 2012;83:319. doi: 10.1016/j.critrevonc.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Pekarsky Y., Croce C.M. Role of miR-15/16 in CLL. Cell Death Differ. 2015;22:6. doi: 10.1038/cdd.2014.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrello E.R. The miR-15 family regulates post-natal mitotic arrest of cardiomyocytes. Circ. Res. 2011;109:670–679. doi: 10.1161/CIRCRESAHA.111.248880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouliot L.M. Cisplatin sensitivity mediated by WEE1 and CHK1 is mediated by miR-155 and the miR-15 family. Can. Res. 2012;72:5945–5955. doi: 10.1158/0008-5472.CAN-12-1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaiah M.J. miR-15/16 complex targets p70S6 kinase1 and controls cell proliferation in MDA-MB-231 breast cancer cells. Gene. 2014;552:255–264. doi: 10.1016/j.gene.2014.09.052. [DOI] [PubMed] [Google Scholar]
- Renjie W., Haiqian L. MiR-132, miR-15a and miR-16 synergistically inhibit pituitary tumor cell proliferation, invasion and migration by targeting Sox5. Cancer Lett. 2015;356:568–578. doi: 10.1016/j.canlet.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Shi L. p53-induced miR-15a/16-1 and AP4 form a double-negative feedback loop to regulate epithelial-mesenchymal transition and metastasis in colorectal cancer. Can. Res. 2014;74:532. doi: 10.1158/0008-5472.CAN-13-2203. [DOI] [PubMed] [Google Scholar]
- Siegel, R., MS, D.N.M. & PhD, A.J.D. Cancer statistics, 2012 † ‡. Ca A Can. J. Clin. 62, 10–29 (2012). [DOI] [PubMed]
- Singh A., Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene. 2010;29:4741. doi: 10.1038/onc.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B.N., Bhowmick N.A. Role of EMT in metastasis and therapy resistance. J. Clin. Med. 2016;5:17. doi: 10.3390/jcm5020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C. MicroRNA-187-3p mitigates non-small cell lung cancer (NSCLC) development through down-regulation of BCL6. Biochem. Biophys. Res. Commun. 2016;471:82–88. doi: 10.1016/j.bbrc.2016.01.175. [DOI] [PubMed] [Google Scholar]
- Techasen A. Cytokines released from activated human macrophages induce epithelial mesenchymal transition markers of cholangiocarcinoma cells. Asian Pac. J. Cancer Prev. Apjcp. 2012;13(Suppl):115. [PubMed] [Google Scholar]
- Vara J.Á.F. PI3K/Akt signalling pathway and cancer ☆. Can. Treat. Rev. 2004;30:193–204. doi: 10.1016/j.ctrv.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Wang Q., Li X., Zhu Y., Yang P. MicroRNA-16 suppresses epithelial-mesenchymal transition-related gene expression in human glioma. Mol. Med. Rep. 2014;10:3310. doi: 10.3892/mmr.2014.2583. [DOI] [PubMed] [Google Scholar]
- Wightman B., Ha I., Ruvkun G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell. 1993;75:855–862. doi: 10.1016/0092-8674(93)90530-4. [DOI] [PubMed] [Google Scholar]
- Yang J.D., Nakamura I., Roberts L.R. The tumor microenvironment in hepatocellular carcinoma: current status and therapeutic targets. Semin. Cancer Biol. 2011;21:35. doi: 10.1016/j.semcancer.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai X. Abnormal expression of EMT-related proteins, S100A4, vimentin and E-cadherin, is correlated with clinicopathological features and prognosis in HCC. Med. Oncol. 2014;31:970. doi: 10.1007/s12032-014-0970-z. [DOI] [PubMed] [Google Scholar]