Abstract
The leaf of Aurea helianthus (A. helianthus Jinhuakui) is popularly used in China traditional medicine, however, scientific evidence on its antioxidant properties rarely studied. In this study, biological activities of A. helianthus leave’s 80% ethanol extract (AHL) were investigated. The measured total polyphenol and flavonoid content of AHL was 184.24 ± 5.01 mg GAE/g and 102.53 ± 0.98 mg NAR/g. AHL showed the highest α, α-diphenyl-β-picrylhydrazyl (DPPH) and 2,2′-azino-bis-3-ethylbenzo-thiazoline-6-sulfonic acid (ABTS) radical scavenging activities of 98.30 ± 0.18% at 1000 µg/mL. DPPH and ABTS radical scavenging activities significantly increased in a AHL concentration-dependent manner. AHL treatment significantly suppressed the generation of pro-inflammatory mediators, including nitric oxide (NO), in lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophages. AHL demonstrated strong anti-inflammatory activity that reduced NO production in LPS-stimulated RAW 264.7 cells. To test the potential protective effect of AHL, the antioxidant capacity, on the cell growth, viability of a human hepatoma cell (HepG2) and Raw 264.7 cell were investigated. AHL also enhanced cytotoxicity on the proliferation of HepG2 cells and was capable of inhibiting 56% against LPS at 400 µg/mL. The results of this study the potential of AHL as an excellent antioxidant substance for inhibiting inflammatory mediators. Therefore, AHL may be used as a therapeutic approach to various inflammatory diseases.
Keywords: Aurea helianthus, Antioxidative, Anti-inflammatory, HepG2, Raw 264.7
1. Introduction
Antioxidant medicinal plants, including phenolic and flavonoid are considered beneficial because of their protective actions in diseases as cancer. Phenol and flavonoids have been showed a wide range of biological activities (Bravol, 1998), including anticarcinogenic actions. Most of the beneficial health effects of flavonoids are attributed to their antioxidant and chelating abilities. Over product ion of reactive oxygen species (ROS) has shown to have detrimental effects on human health leading to cell/tissue damage and degenerative disorders such as inflammation, cardiovascular and neurogenic diseases, cancer, and aging – related disorders. ROS are capable of oxidizing cellular proteins, nucleic acids, and lipids. Lipid peroxidation is a free-radical mediated propagation of oxidative insult to polyunsaturated fatty acids involving several types of free radicals, and termination occurs through enzymatic means or by free radical scavenging by antioxidants (Korkina and Afans’ev, 1997). ROS, including hydroxyl radicals, superoxide anions, singlet oxygen, and hydrogen peroxide, are generated as by-products of cellular metabolism (Crack and Taylor, 2005). If oxidative stress going on, oxidative damage to biomolecules increase and results in diverse biological damage such as mutagenesis and cell death (Ermak and Davies, 2002). Many reports suggest that ROS are principal mediators of apoptosis (Simbula et al., 2007). Antioxidants are added to food to slow the rate of oxidation and, if used properly, they can extend the length life of the food. ROS are produced by mitochondrial electron transfer processes and cytochrome P450 systems in hepatocytes (Masella et al., 2005). Human hepatoma cell line (HepG2) is quite suitable for cytotoxicity evaluation due to the quality and stability of its enzymic and metabolic background (Osseni et al., 2000). Many biological, chemical, and physical agents can generate inflammation with increased danger of human cancers (Ohshima et al., 2003). Inflammation is an important physiological defense process by infection, or exposure to endotoxins such as lipopolysaccharid (LPS) (Medzhitov, 2008). LPS is derived from gram-negative bacteria, is a strong inducer of pro-inflammatory cytokines and immune responses in animals (Choi et al., 2015). It has been involved in non-pathogenic aspects of bacterial ecology, and bacteriophage sensitivity. Overproduction of NO in response to iNOS during the inflammation process is an principal target in drug development for inflammation disease (Wilson et al., 1996). NO has harmful acts with tissue injury, septic shock and apoptosis (Kubes and McCafferty, 2000). So, many studies are currently going to develop inhibitors from medicinal plants to prevent or cure chronic inflammatory conditions for minimal side effects (Lampiasi and Montana, 2016).
A. helianthus is a short-lived perennial herb in Abelmoschus and the tropics but an annual in cooler climates. Its main effects are antipyretic, anti-inflammatory, analgesic and it has the functions of blood lipid regulation, tumor cells inhibition, immune regulation, and anti-oxidation and so on (Lu and Jia, 2015). The young leaves and stems may be eaten raw, steamed, boiled, stir-fried or added to soups. As the leaves cook quickly, add them last to steamed vegetable or stir-fry. The leaves contain mucilage, which can give a slightly slimy feel in the mouth. A. helianthus is a very nutritious vegetable; the leaves are high in vitamins A, C, iron, and 2% protein by dry weight. However the importance of this plant is that it is one of the world's most nutritious leafy vegetables because of its high protein content. A paste of the bark is used to treat wounds. In Nepal, the root juice is applied to sprains and the flower juice is used to treat chronic bronchitis and tooth ache (Manandhar, 2002). The results are estimated to show a scientific support to the antioxidant properties of A. helianthus leave extract in vitro.
2. Materials and methods
A. helianthus leaves were collected from market, 2015. 05., China. For analysis, each 2 g of A. helianthus leaves was extracted with 80% EtOH, (3 × 250 mL) by reflux and evaporated in vacuo. Evaporation was conducted using an evaporator system under reflux in vacuo from BÜCHI, Meierseggstrasse, Flawil, Switzerland. UV/VIS spectrometer was performed by Synergy H1 Hybrid Reader purchased from BioTek Instruments Winooski, VT, USA. Human liver (HepG2) and macrophage (Raw264.7) cells were purchased ATCC (Rockvilie, MD), and stored at 4 °C until used.
2.1. Reagents
All reagents were purchased from Sigma Aldrich, St. Louis, MO, USA, except indicated. Dulbecco’s modified eagle’s medium (DMEM) and fetal bovine serum (FBS) were purchased Gibco, NY, USA. Penicillin/streptomycin and trpysin/EDTA were obtained Welgene, Gyeongsan-si, Gyeongsangbuk-do, South Korea. The reagents 1,1-diphenyl-1-picrylhydrazyl (DPPH), 2,2′-azinibis 3-ethyl benzothiazoline-6-sulfonic acid (ABTS), α-tocopherol, 2,4,6-tris(2-pyridyl)-s-triazine (TPTZ), iron (III) chloride hexahydrate, gallic acid, folin and ciocalteu’s phenol reagent, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and LPS were purchased from Sigma-Aldrich (St. Louis, MO, USA). Acetic acid was purchased from Junsei (Tokyo, Japan).
2.2. Determination of total phenol content
The concentration of total phenols in plant extracts was estimated by Folin-Denis's phenol method (Vernon et al., 1999). 100 μL of the samples with 400 μL of water were mixed with 50 μL of a 2 N folin-ciocalteu reagent. After 3 min, the Na2CO3 solution was added. The absorbance was determined after 1 h at 20 °C, versus a zero-absorbance reagent blank. The blue color produced was measured at 725 nm using a UV/VIS spectrometer. The concentration of total phenolic compounds in extracts was calculated by comparison with a standard curve similarly prepared with 0–1 mg/mL garlic acid. Total phenolic content values were determined using an equation that was obtained from the standard curve of garlic acid graph (r2 = 0.992). The total phenolic content of the sample was expressed as garlic acid equivalents which reflected the phenolic content as the amount of garlic acid (mg/g) of extracts.
2.3. Determination of total flavonoid content
The total flavonoid content was determined using the method (Chae, 2002). 1 mL of glycol in methanol was mixed with the 100 μL of the samples and 100 μL 1 N NaOH. Absorption readings at 420 nm using a UV/VIS spectrometer were taken after 1 h against a blank sample consisting of a 100 μL samples with 1.1 mL water. The total flavonoid content was determined using a curve of naringin (r2 = 0.979) as the standard. Total flavonoid content is expressed as mg of naringin equivalents CAE/g of extract.
2.4. DPPH scavenging assay
This assay was carried out as described by Brand-Williams et al. (1995)with some modifications. 40 μL of various dilutions of the test materials (pure antioxidants or plant extracts) were mixed with 160 μL of a 0.25 mM DPPH solution. After 30 min, the absorbance at 517 nm of maximum absorbance of DPPH, was recorded as Asample, using a UV/VIS spectrophotometer. A blank experiment was also carried out applying the same procedure to a solution without the test material and the absorbance was recorded as Ablank. Ascorbic acid was used as a positive control. The radical scavenging activity of each solution was then calculated as percent according to the following equation:
Radical scavenging activity (%) = (Ablank − Asample)/Ablank × 100, where Ablank is the absorbance of the control reaction (containing all reagents except the test sample), and Asample is the absorbance of the test sample. Tests were carried out in triplicate. The experiment was performed in triplicate.
2.5. ABTS scavenging assay
Radical cation scavenging capacity of extract was examined against ABTS generated by the method (Re et al., 1999). ABTS was dissolved in water to a 7 mM concentration. ABTS radical cation was produced by reacting ABTS stock solution with 2.45 mM potassium peroxodisulfate and allowing the mixture to stand in the dark at room temperature for 12–16 h before use. The ABTS+ reaction mixture contained 180 μL of ABTS+ and 20 μL of antioxidant testing sample with an absorbance at 734 nm, recorded as Asample. The radical scavenging activity of each solution was then calculated as percent according to the following equation:Radical scavenging activity (%) = (Ablank − Asample)/Ablank × 100. Ascorbic acid was used as a positive control.
2.6. Reducing power assay
To determine the reducing capacity of extracts, use the method of Yen and Chen (1995). Extracts (tested from 125 to 1000 g/mL) 250 μL were added in 200 mM sodium phosphate buffer 250 μL. For a 500 μL sample solution, 250 μL of 1% potassium ferricyanide were added and incubation was carried out for 20 min at 50 °C. After added 250 μL of 10% trichloroacetic acid (TCA) to sample solution, centrifuge at 3000 rpm. Then, 500 μL supernatants of sample solutions added 100 μL of 0.1% ferric chloride (FeCl3). After incubation at room temperature for 10 min, optical density was determined using a microplate reader set at 700 nm.
2.7. Cell culture
HepG2 and Raw 264.7 cells were cultured in DMEM medium supplemented with 10% FBS and 1% penicillin/streptomycin. Cells were maintained in a humidified atmosphere at 37 °C, 5% CO2. Cells were subcultured at 70–80% confluence.
2.8. Cell viability
The cell viability was measured using MTT assay (Angius and Floris, 2015). HepG2 cells were seeded in 96 well plates at 2 × 104 cell/well. After overnight growth, cells were treated with AHL for 1 h, followed in the presence or absence of H2O2 (20 mM) for the next 24 h. And Raw 264.7 cells were seeded in 96 well plates at 2 × 104 cell/well. After overnight growth, cells were treated with AHL for 1 h, followed in the presence or absence of LPS (1 mg/mL) for the next 24 h MTT solution (5 mg/mL) was added and the cells were further cultured for 2 h. After the incubation, the formazan crystals in each well were dissolved in 125 µl of DMSO for 15 min. The absorbance at 570 nm was measured with microplate reader.
2.9. Intercellular reactive oxygen species (ROS) genenration
Intercellular ROS generation was measured by DCF-DA (2′,7′-dichlorodihydrofluorescein diacetate) with temperature maintained at 37 °C (Wang and Joseph, 1999). After 24 h cultured HepG2 cells with extract in black 96 well plate, treated with 20 μM DCF-DA in serum free medium (SFM) for 50 min and treated with 200 μM H2O2 in Krebs-Henseleit buffer (KHB) for 30 min and 1 h. The fluorescence at the excitation filter at 485 nm and the emission filter at 535 nm was measured by a microplate reader.
2.10. Nitro oxide (NO) assay
Nitrite concentration as an index for NO synthesis was determined using griess reagent as the manual directs (Kyung et al., 2012). After 24 h cultured Raw 264.7 cells treated extracts with or without LPS, supernatants 50 μL and griess reagent 50 μL were added to 96 well plates to each well. After incubation at room temperature for 10 min, optical density was determined using a microplate reader set at 540 nm. The nitric oxide content was determined using a curve of sodium nitrite (r2 = 0.998) as the standard.
2.11. Statistical analysis
All statistical analyses were performed using Graphpad prism 6 software. Results were compared by one-way analysis of variance (ANOVA). A P-value of <.05 was considered to be significant.
3. Results and discussion
Flavonoids and polyphenolics in general depending on their glycoside, isoprenoids and aliphatic ethers content can acquire almost and polarity. The content of extractable polyphenolics in solvent extracts of AHL, determined from regression equation of calibration curve y = (x − 0.0047)/0.0051, were expressed as garlic acid (GA) equivalent (GAE). Polyphenols were present in A. helianthus (186.24 ± 5.01). Flavonoids have been reported to indicate a wide range of biological effects containing antibacterial, antiviral, anti-inflammatory, and anti-allergic among others (Cao and Prior, 1999). The content of extractable flavonoids in solvent extracts of AHL, determined from regression equation of calibration curve y = (x + 0.0099)/0.0039, were expressed as naringin equivalent (Table 1). It is important that flavonoid content of plants may diverse in plant species and the morphology or plant part used. No other studies have shown that flavonoids are the important bioactivity compounds in A. helianthus and are responsible for their antioxidant activity. The flavonoids present in AHL may contribute to the antioxidant activity (radical scavenging activity) in HepG2 cells and Raw 264.7 cells.
Table 1.
Extract yield, total phenol and flavonoid contents in ethanol extract of A. helianthus LEAVES.
| Sample | Extract yield (%) | Total phenol (mg GAE/g)* | Total flavonoid (mg NAR/g)# |
|---|---|---|---|
| AHL | 23.3 | 186.24 ± 5.01 | 102.53 ± 0.98 |
All values are mean ± SD of triplicates analysis. SD: Standard deviations, GAE: gallic acid, NAR: naringin, AHL: Extract of Aurea helianthus leaves.
Total phenol content analyzed as GAE equivalents.
Total flavonoid content analyzed as NAR equivalents.
The DPPH free radical scavenging capacity of AHL is showed in Table 2. AHL showed the highest DPPH scavenging percentage 60.35% at 1000 mg/mL, Thanks to polyphenolic compounds, the most plants have antioxidant effects and more effective antioxidants in vitro than ascorbate (Vitamin C) (Shahidi, 2000). The correlation between the scavenging activity and the polyphenol content of AHL were highly significant scavenging capacity against DPPH free radical. So it can be assumed that the polyphenols in AHL is major contributors to its DPPH scavenging capacity, hence its antioxidant activity.
Table 2.
DPPH and ABTS scavenging activity of ethanol extract of A. helianthus LEAVES.
| Sample | Assay | Concentration (μg/mL) | RSA (%) |
|---|---|---|---|
| AHL | ABTS | 100 | 19.85 ± 0.33 |
| 250 | 38.32 ± 0.09 | ||
| 500 | 65.39 ± 0.56 | ||
| 750 | 86.94 ± 0.48 | ||
| 1000 | 98.30 ± 0.18 | ||
| Ascorbic acid | 1000 | 99.78 ± 0.09 | |
| DPPH | 100 | 7.92 ± 0.11 | |
| 250 | 14.92 ± 0.11 | ||
| 500 | 34.06 ± 0.28 | ||
| 750 | 51.13 ± 0.11 | ||
| 1000 | 60.35 ± 0.29. | ||
| Ascorbic acid | 1000 | 99.35 ± 0.39 |
All values are mean ± SD of triplicates analysis. SD: Standard deviations, RSA: Radical scavenging activity, DPPH: 1,1-diphenyl-2-picrylhydrazyl, ABTS: 2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid), L: Extract of Aurea helianthus leaves.
Table 3 shows reducing power based on their ability to reduce ferric (Fe3+) to ferrous (Fe2+) ion through the donation of an electron, with the resulting Fe2+ formation monitored spectrophotometrically at 700 nm (Hassas-Roudsari et al., 2009). Overall, result indicates that AHL had the strongest reducing power of the antioxidants activity at any given concentration. AHL results from the present investigation (125–1000 µg/mL) exhibited higher reducing power due to the presence of higher concentration. So, absorbance increased dependent on concentration.
Table 3.
Reducing power of AHL A. helianthus leaves.
| Sample | Concentration (μg/mL) | Absorbance (700 nm) |
|---|---|---|
| AHL | 125 | 0.12 ± 0.01 |
| 250 | 0.18 ± 0.03 | |
| 500 | 0.27 ± 0.02 | |
| 1000 | 0.47 ± 0.03 |
All values are mean ± SD of triplicates analysis. SD: Standard deviations, AHL: Extract of Aurea helianthus leaves.
As shown in Fig. 1 cell viability was maintained more than 99% after treatment of AHL at concentration of 2 mg/mL. Therefore, our AHL not showed cell toxicity even the concentration of 1 mg/mL in H2O2 treated HepG2 cells. The cytotoxicity can be measured by assessing cellular damage (Tsuchiya, 2010). Cytotoxic effect of AHL on the proliferation of human liver hepatoma cells (Hep G2) was assessed with the MTT assay. Results showed the AHL capacity to inhibit the proliferation of the cancer cells in a concentration dependent manner (Fig. 1A). At 5 mg/mL, the AHL was capable of scavenging 40% of cancerous cells. So it can be assumed that the cytotoxic effect of AHL on the proliferation of the HepG2 cancer cells is due to their antioxidant capacity which interfere with important biological processes in cell development. Polyphenols owe their antioxidant properties to their ability to pair the unpaired electron of the free radical.
Fig. 1.
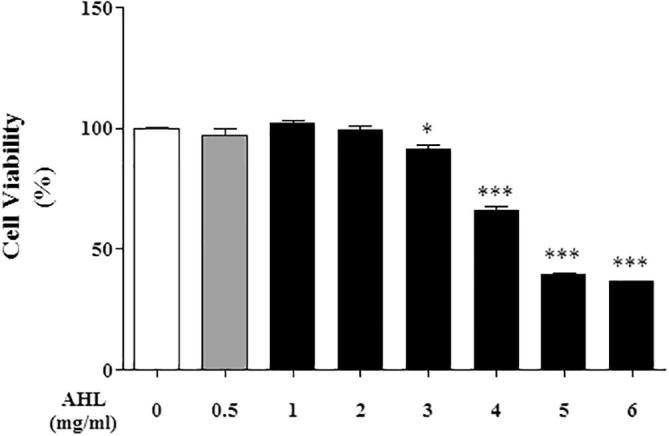
Effect of AHL on cell viability in HepG2 cells.
Cell viability in HepG2 cells treated with AHL for 24 h. All values are means ± SD from three independent experiments. (∗P < .01, ∗∗∗P < .001; significant effect between the control and treated extract) AHL: Extract of Aurea helianthus leaves.
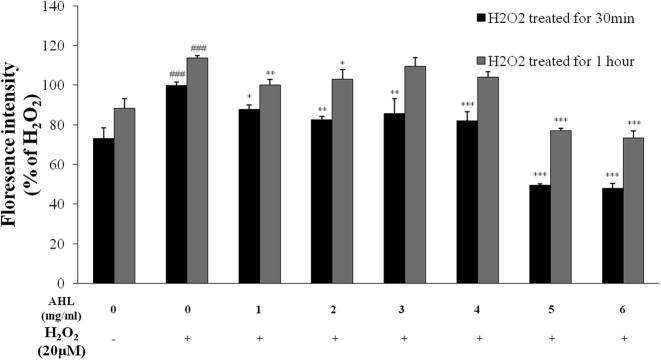
We performed DCF-DA assay for measuring ROS production in HepG2 cells. H2O2-induced ROS generation was increased up to 1.4-fold when compared with control group (Fig. 2). In contrast AHL effectively reduced intracellular ROS production to more than 20 μM of H2O2 treated cells.
Fig. 2.
Intercellular ROS generation in H2O2 – induced HepG2 cells.
Intercellular ROS generation were measured by DCF-DA treated with AHL for 30 min and 1 h with H2O2 (20 μM) in HepG2 cells. All values are means ± SD from three independent experiments.  H2O2 treated for 30 min
H2O2 treated for 30 min  H2O2 treated for 1 h (#P < .01, ###P < .0001; significant effect between the control without H2O2 and control with H2O2, ∗P < .01, ∗P < .001, ∗∗P < 0.0001; significant effect between the control with H2O2 and treated extract with H2O2) AHL: Extract of A. helianthus leaves.
H2O2 treated for 1 h (#P < .01, ###P < .0001; significant effect between the control without H2O2 and control with H2O2, ∗P < .01, ∗P < .001, ∗∗P < 0.0001; significant effect between the control with H2O2 and treated extract with H2O2) AHL: Extract of A. helianthus leaves.
We studied AHL cytotoxic effect on cell growth and viability in RAW 264.7 macrophage cells with MTT assay. Macrophage cells are important immune cells in the host defense against infections and cancer cells and they activated when they encounter antigens like as pathogens or cancer cells (Medzhitov and Janeway, 1997). RAW 264.7 cells were used to assess the effect of AHL on inflammatory mediator synthesis. AHL did not affect normal cell growth and various concentrations of AHL (0, 50, 10, 200, 400, 800 and 1000 µg/mL) were applied to RAW 264.7 macrophages for 24 h (Fig. 3).
Fig. 3.
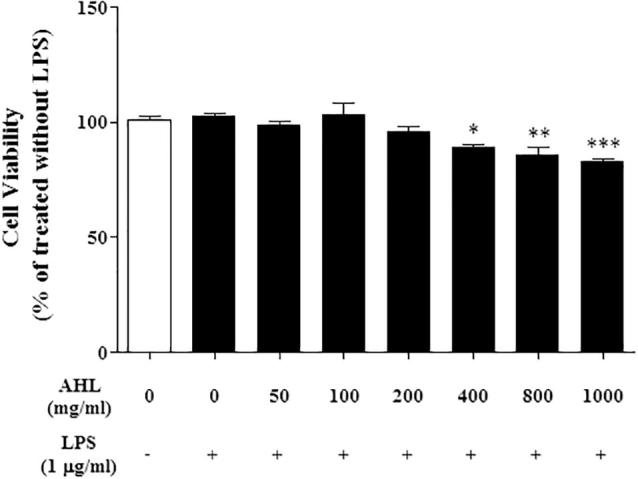
Effect of AHL on cell viability with or without LPS in Raw 264.7 cell.
Cell viability of AHL in Raw 264.7 cells treated with or without LPS (1 μg/mL) for 24 h. All values are means ± SD from three independent experiments. (∗P < 0.1, ∗P < .05, ∗∗P < .001; significant effect between the control with LPS and treated extract with LPS.) AHL: Extract of A. helianthus leaves.
Cell viability assays were subsequently performed and the viability showed was greater than 95% for all the treatment groups. Moreover the cell viability observed for the AHL was similar to that of the non-treated controls. These results indicate that AHL at the concentrations tested are not cytotoxic to RAW 264.7 cells macrophages (Fig. 3). Macrophages produce several pro-inflammatory mediators interleukin-1b, tumor necrosis factor-a, or NO (Beutler, 1999). The process of acute inflammation is initiated by resident immune cells already present in the involved tissue, mainly resident macrophages, dendritic cells, histiocytes, Kupffer cells and mast cells. We first investigated the inhibitory influence of A. helianthus and assessed the anti-inflammatory effect in LPS-stimulated RAW 264.7 cells as an in vitro model. Production of NO was detected following the treatment with various concentrations of AHL. AHL showed dose-dependent inhibition of LPS-induced NO production at 200 μg/mL (44%). In contrast, pretreatment of cells with AHL led to a significant decrease in LPS-induced NO production (Fig. 4).
Fig. 4.
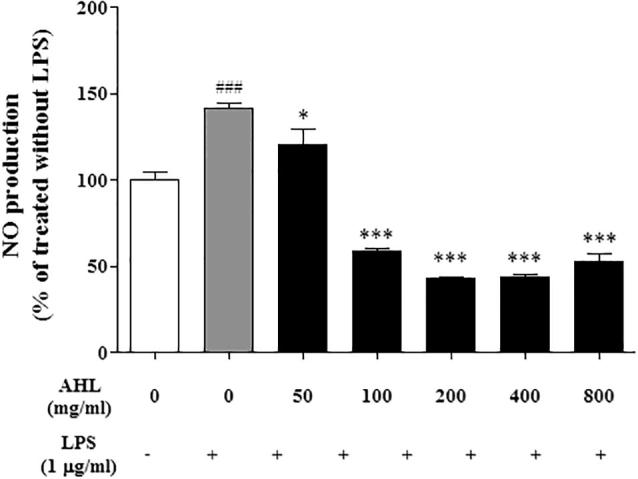
NO production in LPS – induced Raw 264.7 cells.
NO production of AHL in Raw 264.7 cells treated with or without LPS (1 μg/mL) for 24 h. All values are means ± SD from three independent experiments. (###P < .001; significant effect between the control without LPS and control with LPS. ∗P < .1, ∗∗P < .001; significant effect between the control with LPS and treated extract with LPS.) AHL: Extract of Aurea helianthus leaves.
Previous research did not demonstrated the presence of antioxidants in different from the A. helianthus, which are being used in China as traditional and modern medicinal herbs and supplements. The yield, total polyphenolic and flavonoid content, and the antioxidant properties in vitro of AHL extract has not been reported. These results remain important as the first step in screening the antioxidant activity of A. helianthus.
4. Conclusion
In vitro antioxidant activity represents only a status in the basic assessment of A. helianthus pharmacological activity. Further evaluation of specific flavonoid present in the leaf is ensured. Evaluation of the antioxidative properties of AHL in vivo is needed to ensure functionality and long term safety.
Acknowledgments
Acknowledgement
This work was carried out with the support of Cooperative Research Program for Agriculture Science and Technology Development (PJ011582) Rural Development Administration, Republic of Korea.
Conflict of interest
The authors have declared that no competing interests exist.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
YoungOck Kim, Email: kyo9128@gmail.com.
Gi-Ho Sung, Email: sung97330@gmail.com.
References
- Angius F., Floris A. Liposomes and MTT cell viability assay: an incompatible affair. Toxicol. In Vitro. 2015;29:314–319. doi: 10.1016/j.tiv.2014.11.009. [DOI] [PubMed] [Google Scholar]
- Beutler B.A. The role of tumor necrosis factor in health and disease. J. Rheumatol. Suppl. 1999;57:16–21. [PubMed] [Google Scholar]
- Brand-Williams W., Cuvelier M.E., Berset C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995;28:25–30. [Google Scholar]
- Bravol L. Polypenols: chemistry, dietary sources, metabolism and nutritional significance. Nutr. Rev. 1998;56:317–333. doi: 10.1111/j.1753-4887.1998.tb01670.x. [DOI] [PubMed] [Google Scholar]
- Cao G., Prior R.L. Measurement of oxygen radical absorbance capacity in biological sample. Meth. Enzymol. 1999;299:50–62. doi: 10.1016/s0076-6879(99)99008-0. [DOI] [PubMed] [Google Scholar]
- Chae S.K. third ed. Jigu-moonwha Sa Publishers; Seoul (Korea): 2002. Standard Food Analysis. [Google Scholar]
- Choi E.Y., Kim H.J., Han J.S. Anti-inflammatory effects of calcium citrate in RAW 264.7 cells via suppression of NF-κB activation. Environ. Toxicol. Pharmacol. 2015;39:27–34. doi: 10.1016/j.etap.2014.11.002. [DOI] [PubMed] [Google Scholar]
- Crack P.J., Taylor J.M. Reactive oxygen species and the modulation of stroke. Free Radic. Biol. Med. 2005;38:1433–1444. doi: 10.1016/j.freeradbiomed.2005.01.019. [DOI] [PubMed] [Google Scholar]
- Ermak G., Davies K.J. Calcium and oxidative stress: from cell signaling to cell death. Mol. Immunol. 2002;38:713–721. doi: 10.1016/s0161-5890(01)00108-0. [DOI] [PubMed] [Google Scholar]
- Hassas-Roudsari M., Chang P.R., Pegg R.B., Tyler R.T. Anti-oxidant capacity of bioactives extracted from canola meal by subcritical water, ethanol and hot water extraction. Food Chem. 2009;114:717–726. [Google Scholar]
- Korkina L.G., Afans’ev I.B. Antioxidant and chelating properties of flavonoids. Adv. Pharmacol. 1997;38:151–163. doi: 10.1016/s1054-3589(08)60983-7. [DOI] [PubMed] [Google Scholar]
- Kubes P., McCafferty D.M. Nitric oxide and intestinal inflammation. Am. J. Med. 2000;109:150–158. doi: 10.1016/s0002-9343(00)00480-0. [DOI] [PubMed] [Google Scholar]
- Kyung J., Kim D., Park D., Yang Y.H., Choi E.K., Lee S.P., Kim T.S., Lee Y.B., Kim Y.B. Synergistic anti-inflammatory effects of Laminaria japonica fucoidan and Cistanche tubulosa extract. Lab Anim. Res. 2012;28:91–97. doi: 10.5625/lar.2012.28.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampiasi N., Montana G. The molecular events behind ferulic acid mediated modulation of IL-6 expression in LPS-activated RAW 264.7 cell. Immunobiology. 2016;221:486–493. doi: 10.1016/j.imbio.2015.11.001. [DOI] [PubMed] [Google Scholar]
- Lu D., Jia R.B. Research progress on Chinese medicinal material aurea helianthus. Chin. J. Drug Eval. 2015;02 [Google Scholar]
- Manandhar N.P. Timber Press; 2002. Plants and People of Nepal; p. 599. [Google Scholar]
- Masella R., Di Benedetto R., Vari R., Filesi C., Giovannini C. Novel mechanisms of natural antioxidant compounds in biological systems: involvement of glutathione and glutathione-related enzymes. J. Nutr. Biochem. 2005;10:577–586. doi: 10.1016/j.jnutbio.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- Medzhitov R., Janeway C.A., Jr. Innate immunity: impact on the adaptive immune response. Curr. Opin. Immunol. 1997;9:4–9. doi: 10.1016/s0952-7915(97)80152-5. [DOI] [PubMed] [Google Scholar]
- Ohshima H., Tatemichi M., Sawa T. Chemical basis of inflammation-induced carcinogenesis. Arch. Biochem. Biophys. 2003;417:3–11. doi: 10.1016/s0003-9861(03)00283-2. [DOI] [PubMed] [Google Scholar]
- Osseni R.A., Rat P., Bogdan A., Warnet J.M., Touitou Y. Evidence of prooxidant and antioxidant action of melatonin on human liver cell line HepG2. Life Sci. 2000;68:387–399. doi: 10.1016/s0024-3205(00)00955-3. [DOI] [PubMed] [Google Scholar]
- Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS Radical cation decolorization assay. Free Radical Biol. Med. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Shahidi F. Antioxidants in food and food antioxidants. Nahrung. 2000;44:158–163. doi: 10.1002/1521-3803(20000501)44:3<158::AID-FOOD158>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Simbula G., Columbano A., Ledda-Columbano G.M., Sanna L., Deidda M., Diana A., Pibiri M. Increased ROS generation and p53 activation in α-lipoic acid-induced apoptosis of hepatoma cells. Apoptosis. 2007;12:113–123. doi: 10.1007/s10495-006-0487-9. [DOI] [PubMed] [Google Scholar]
- Tsuchiya H. Structure-dependent membrane interaction of flavonoids associated with their bioactivity. Food Chem. 2010;120:1089–1096. [Google Scholar]
- Vernon L.S., Rudolf O., Rosa M.L. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Meth. Enzymol. 1999;299:152–178. [Google Scholar]
- Wang H., Joseph J.A. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radical Biol. Med. 1999;27:612–616. doi: 10.1016/s0891-5849(99)00107-0. [DOI] [PubMed] [Google Scholar]
- Wilson K.T., Ramanujam K.S., Mobley H.L., Musselman R.F., James S.P., Meltzer S.J. Helicobacter pylori stimulates inducible nitric oxide synthase expression and activity in a murine macrophage cell line. Gastroenterology. 1996;111:1524–1533. doi: 10.1016/s0016-5085(96)70014-8. [DOI] [PubMed] [Google Scholar]
- Yen G.C., Chen H.Y. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J. Agric. Food Chem. 1995;43:27–32. [Google Scholar]