Abstract
Microbial production of plant-derived natural products by engineered microorganisms has achieved great success thanks to large extend to metabolic engineering and synthetic biology. Anthocyanins, the water-soluble colored pigments found in terrestrial plants that are responsible for the red, blue and purple coloration of many flowers and fruits, are extensively used in food and cosmetics industry; however, their current supply heavily relies on complex extraction from plant-based materials. A promising alternative is their sustainable production in metabolically engineered microbes. Here, we review the recent progress on anthocyanin biosynthesis in engineered bacteria, with a special focus on the systematic engineering modifications such as selection and engineering of biosynthetic enzymes, engineering of transportation, regulation of UDP-glucose supply, as well as process optimization. These promising engineering strategies will facilitate successful microbial production of anthocyanins in industry in the near future.
Keywords: Anthocyanin, Enzyme engineering, Metabolic engineering, Microbial production
Abbreviations: ANS, anthocyanidin synthase; CHI, chalcone isomerase; CHS, chalcone synthase; 4CL, 4-coumaroyl-CoA ligase; DFR, dihydroflavonol 4-reductase; DSSC, dye-sensitized solar cell; F3H, flavanone 3-hydroxylase; F3′H, flavonoid 3′-hydroxylase; F3′5′H, flavonoid 3′, 5′-hydroxylase; FGT, flavonoid glucosyltransferase; F3GT, flavonoid 3-O-glucosyltransferase; UV, ultraviolet
1. Introduction
As a member of the flavonoid group of polyphenols, anthocyanins are important chemicals in the plant kingdom as pigments, antioxidants, and antimicrobials (Fig. 1). With the rising interest in natural nutraceuticals, there is an increasing preference for natural food colorants such as anthocyanins, thus stimulating high demand for these compounds mainly as colorants and cosmetic additives [1], [2], [3], [4].
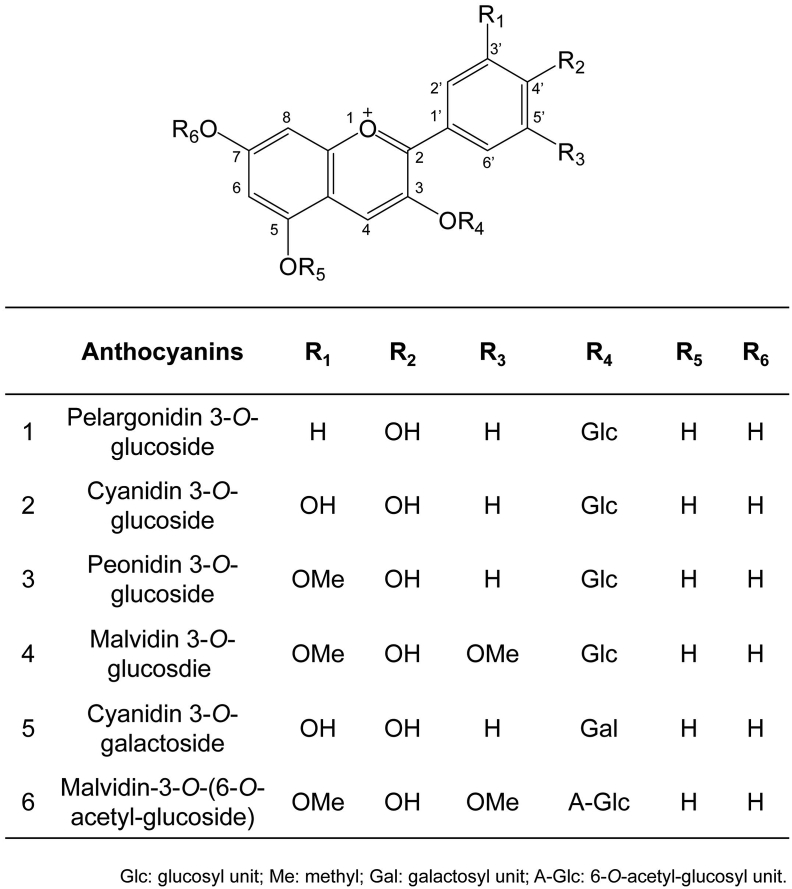
Fig. 1.
The structure of anthocyanins. Modifications (such as glycosylation, hydroxylation, methylation, and acylation) at C3’ (R1), C4’ (R2), C5’ (R3), C3 (R4), C5 (R5) and C7 (R6) generate structural analogs. R1-R5 are functional groups derived from glycosyl, hydroxyl, methyl, and acyl units. Representative anthocyanins and their structures are listed.
The traditional means of producing anthocyanins is by extraction and purification from fruits, flowers, and other tissues of plants [5], [6], [7]. This approach has a couple of advantages such as diverse sources of readily available cheap feedstocks, sophisticated extraction and purification techniques, and decent market recognition (non-GMO products). And great success has been achieved in the development of novel extraction and purification procedures, understanding of anthocyanin biosynthesis in plants, and genetic engineering of plants for anthocyanin production [8], [9], [10]. However, anthocyanins isolated from plants exist as a heterogeneous mixture of multiple types of molecules with diverse chemical structures. In addition, anthocyanin production through plant extraction is neither stable nor sustainable, since plants produce anthocyanins with varied productivity, and the production relies on farmland and irrigation, and fluctuates depending on seasonal and environmental conditions [11], [12], [13]. An alternative is microbial production, which has already demonstrated great potential in the biosynthesis of plant-derived natural compounds. The intrinsic characteristics of microorganisms, such as fast growth and easy cultivation, and the sophisticated microbial manipulation techniques, including convenient genetic modifications and readily available bioinformatics tools, render microbial production of natural products facile, controllable, and cost-effective [14], [15], [16]. So far, many plant-derived compounds have been produced, including terpenoids, alkaloids, and flavonoids, in prokaryotic and eukaryotic microorganisms [15], [17], [18]. Microbial biosynthesis of natural flavonoids dates back to 2003 [19], and various flavonoid compounds, from flavanones to the more complicated anthocyanins, have been generated in engineered microorganisms [14]. Meanwhile, microbial production of non-natural flavonoids has also been made possible through the feeding of specific substrates [20], [21]. In all these cases, the producing microbes are metabolically engineered for the optimal synthesis of the target products. Coupled with the advances in genome sequencing and DNA synthesis, structural biology and enzymology, and modeling of metabolic networks, metabolic engineering is playing a powerful and indispensable role in microbial synthesis of on-demand compounds with biochemical functions or industrial significance [22], [23].
In this review, the recent progress on anthocyanin production in genetically modified microorganisms will be described, with a main focus on metabolic engineering strategies on improving in vivo production. Also, process optimization and in vitro storage of anthocyanins are overviewed, providing a comprehensive understanding of the engineering and bioproduction processes of these molecules.
2. Industrial applications of anthocyanins
Anthocyanins have wide applications in pharmaceutical industry, food processing, cosmetic manufacturing, and solar cell development. Anthocyanins help to suppress neuroinflammation, neurodegradation and brain aging by blocking interleukin-1β, tumor necrosis factor α, and nuclear factor-κβ [24], and are promising in the prevention of cancer, cardiovascular diseases, neurodegenerative diseases, obesity and diabetes according to trials in animal models and humans, although the underlying mechanisms are still not very clear [24], [25], [26].
Certain types of anthocyanins strongly absorb visible and ultraviolet (UV) spectra owing to the specific polyphenol structure and side groups with phenolic compounds [27], and, when applied externally, protect human skin from aging and UV-induced damage [28], such as inflammation and oxidative damage in the epidermis, dermis, and adnexal organs of the skin [29]. These discoveries have fueled the elevated application of anthocyanins in cosmetics and skin care products [29].
In addition, anthocyanins are widely used as food colorants because of their diverse colors and nutritional properties. In the US, four anthocyanin-based colorants are exempt from FDA certification. The acylated anthocyanins are also commonly applied in industry because of the improved color stability [30]. As a dye, anthocyanins are also exploited as sensitizers in dye-sensitized solar cells (DSSCs), to replace the toxic, complicated, and costly transition metal coordination complexes, for the conversion of solar energy to electricity, with slightly lower yet acceptable efficiencies compared to the traditional silicon solar cells [31], [32], [33], [34].
3. Biosynthesis of anthocyanins in plants
Naturally produced in plants, anthocyanins serve to attract pollinating insects and seed dispersers, and to protect plants against irradiation damages and pathogens [10], [35]. Anthocyanins in plants are synthesized via the general flavonoid pathway, whereby three molecules of malonyl-CoA and one molecule of 4-coumaroyl-CoA derived from phenylalanine or tyrosine are condensed by the key enzyme chalcone synthase (CHS) to form naringenin chalcone (Fig. 2), which is further converted to its isomer naringenin by chalcone isomerase (CHI). Naringenin is modified through hydroxylation by enzymes such as flavonoid 3′-hydroxylase (F3′H) and flavonoid 3′, 5′-hydroxylase (F3′5′H), giving rise to different dihydroflavonols (dihydroquercetin, dihydrokaempferol, and dihydromyricetin). These molecules are reduced by dihydroflavonol 4-reductase (DFR) to form leucoanthocyanidins (leucocyanidin, leucopelargonidin, and leucodelphinidin). Oxidation of leucoanthocyanidins by anthocyanidin synthase (ANS) generates the unstable flavylium cation anthocyanidins, which are then linked to a monosaccharide residue at C3 in ring C or other positions through flavonoid glucosyltransferase (FGT)-catalyzed glycosylation (Fig. 1). The most common sugar unit is glucose, whereas galactose and xylose are also found in natural anthocyanins [24]. Other modifications such as methylation on the hydroxyl groups of ring B and acylation are also possible [25]. These intrinsic modifications are aimed at improving anthocyanin stability or diversifying colors of anthocyanins [36].
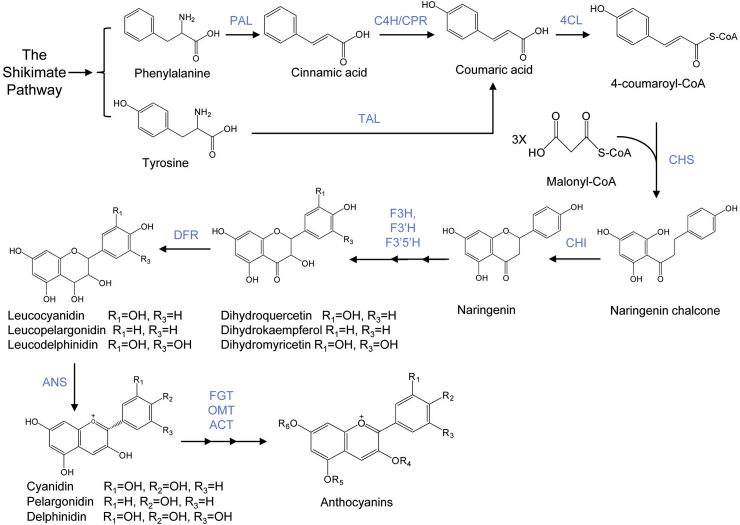
Fig. 2.
The pathway of anthocyanin biosynthesis in plants. The general precursor phenylalanine or tyrosine derived from the shikimate pathway is converted to 4-coumaroyl-CoA through the phenylpropanoid pathway or the combined effect of TAL and 4CL, respectively. One molecule of 4-coumaroyl-CoA is condensed with three molecules of malonyl-CoA to form one molecule of naringenin chalcone, which is subsequently converted to naringenin by CHI. Naringenin, the major intermediate compound, undergoes various hydroxylation to form diverse anthocyanidins. Further glycosylation and other decorations act on the anthocyanidin compounds to generate anthocyanins. Abbreviations: PAL, phenylalanine ammonia lyase; C4H, cinnamate 4-hydroxylase; CPR, cytochrome P450 reductase; TAL, tyrosine ammonia lyase; 4CL, 4-coumaroyl-CoA ligase; CHS, chalcone synthase; CHI, chalcone isomerase; F3H, flavanone 3-hydroxylase; F3′H, flavonoid 3′-hydroxylase; F3′5′H, flavonoid 3′, 5′-hydroxylase; DFR, dihydroflavonol reductase; ANS, anthocyanidin synthase; FGT, flavonoid glucosyltransferase; OMT, O-methyltransferase; ACT, anthocyanin acyltransferase.
Anthocyanins in plants are transferred to and stored in vacuoles after biosynthesis. Depending on the pH in the vacuoles of different species, anthocyanins present vastly diverse colors with distinct stability [2], [24]. Being quite unstable at neutral and basic pH, anthocyanins can be stabilized through structural decorations, lowered pH, and co-pigmentation in vacuoles [10]. Because of the complexity of anthocyanin biosynthesis and their instability, commercial production of anthocyanins in controlled systems is still a daunting challenge.
4. Metabolic engineering of anthocyanin production in microorganisms
Engineering microorganisms for the production of natural chemicals is a promising and sustainable way of satisfying industrial needs. As the most commonly used workhorse in metabolic engineering, E. coli has been extensively engineered for the biosynthesis of natural flavonoids such as naringenin, kaempferol, and catechin [25], [37], [38], [39]. Later on, the host cell range has been expanded to Saccharomyces cerevisiae and Streptomyces venezuelae [25], [40]. Recently, the amino acid-producing bacterium Corynebacterium glutamicum has also been reported for the production of stilbenes and flavanones [41].
As important members of the flavonoid class of polyphenols, anthocyanins have been drawing much focus due to their tremendous industrial and commercial values. In 2005, Yan et al. cloned and expressed in E. coli the genes of flavanone 3-hydroxylase (F3H) and ANS from Malus domestica, DFR from Anthurium andraeanum, and flavonoid 3-O-glucosyltransferase (F3GT) from Petunia hybrida [42]. The recombinant E. coli strain produced 6.0 μg L−1 of cyanidin 3-O-glucoside and 5.6 μg L−1 pelargonidin 3-O-glucoside using naringenin and eriodictyol as precursors, respectively. Subsequent selection of plant-derived enzymes, optimization of UDP-glucose pool, regulation of precursor uptake and optimization of the production process resulted in dramatically enhanced production of pelargonidin 3-O-glucoside and cyanidin 3-O-glucoside, with their titers reaching 113 mg L−1 and 350 mg L−1, using afzelechin and catechin precursors, respectively [43], [44], [45]. The engineered microbial production of other anthocyanins is summarized in Table 1 with the schematic illustration shown in Fig. 3. All reported recombinant producing hosts are currently limited to E. coli derivatives.
Table 1.
Summary of anthocyanin production in engineered bacteria.
|
E. coli strain |
Genetic modifications | Substrate | Product | Fermentation conditions | Titer/μM | Ref. |
|---|---|---|---|---|---|---|
| JM109 | MdF3H/AaDFR/MdANS/PhF3GT | 0.25 mM Naringenin | Pelargonidin 3-O-glucoside | M9 minimal medium (pH 7) plus UDP-glucose; IPTG induction at RT |
0.012 | [63] |
| 0.1 mM Eriodictyol | Cyanidin 3-O-glucoside | 0.012 | ||||
| BL21* (DE3) | MdF3H/AaDFR/At3GT/PhANS | 0.2 mM Naringenin | Pelargonidin 3-O-glucoside | M9 minimal medium (pH 7) with 2-oxoglutarate, sodium ascorbate and UDP-glucose; IPTG induction at 30 °C |
1.34 | [39] |
| 0.2 mM Eriodictyol | Cyanidin 3-O-glucoside | 3.88 | ||||
| MdF3H/AaDFR/At3GT/PhANS/DuLAR | 0.2 mM Naringenin | Pelargonidin 3-O-glucoside | 2.09 | |||
| 0.2 mM Eriodictyol | Cyanidin 3-O-glucoside | 4.27 | ||||
| MdF3H/AaDFR/At3GT/PhANS/DuLAR/Dv3MaT | 0.2 mM Naringenin | Pelargonidin 3-O-6″-O-malonylglucoside | 0.18 | |||
| 0.2 mM Eriodictyol | Cyanidin 3-O-6″-O-malonylglulcoside | 0.21 | ||||
| BL21* (DE3) | At3GT/PhANS | 0.75 mM Catechin | Cyanidin 3-O-glucoside | 3 h IPTG induction at 30 °C; 5-fold concentration in M9 minimal medium as listed above without UDP-glucose | 5.16 | |
| BL21* (DE3) | At3GT/PhANS | 0.75 mM Catechin | Cyanidin 3-O-glucoside | Concentrated in modified M9 minimal medium (pH 5) | 80 | |
| At3GT/PhANS/galU/pgm | 0.75 mM Catechin | Cyanidin 3-O-glucoside | Same as above. | 127 | ||
| Fusion of At3GT and PhANS/galU/pgm | 0.75 mM Catechin | Cyanidin 3-O-glucoside | 146 | |||
| 0.75 mM afzelechin | Pelargonidin 3-O-glucoside | 168 | ||||
| BL21* (DE3) | Fusion of At3GT and PhANS/galU/pgm/ndk/Δudg/galE/T(inactive) | Catechin | Cyanidin 3-O-glucoside | Modified M9 medium (pH 5) with orotic acid (0.1 mM) | 215 | [21] |
| afzelechin | Pelargonidin 3-O-glucoside | 241 | ||||
| BL21* (DE3) | At3GT/PhANS/galU/pgm/cmk/ndk/YadH/ΔtolC | 2.5 mM Catechin | Cyanidin 3-O-glucoside | Modified M9 medium (pH 5) with 1% glucose, 5 mM IPTG, 2-oxoglutarate, sodium ascorbate, and orotic acid. | 260 | [43] |
| At3GT/PhANS/galU/pgm/cmk/ndk/ycjU | 2.5 mM Catechin | Cyanidin 3-O-glucoside | 252 | |||
| At3GT/PhANS/cmk/ndk/ycjU | 2.5 mM Catechin | Cyanidin 3-O-glucoside | Feed glucose and catechin | 421 | ||
| BL21* (DE3) | At3GT/PhANS/galU/pgm/cmk/ndk/ycjU | 2.5 mM Catechin | Cyanidin 3-O-glucoside | Induced at stationary phase; modified M9 medium (pH 5) with 1% glucose, 5 mM IPTG, 2-oxoglutarate, sodium ascorbate, and orotic acid; feed glucose and catechin | 722 | |
| BL21* (DE3) | MBP-At3GT/MBP-PhANS/VvAOMT/MetJ↓ (CRISPRi) |
3.44 mM Catechin | Peonidin 3-O-glucoside | Semi-rich medium AMM with 2% glucose, IPTG induction (1 mM) and production process at 30 °C | 112 | [48] |
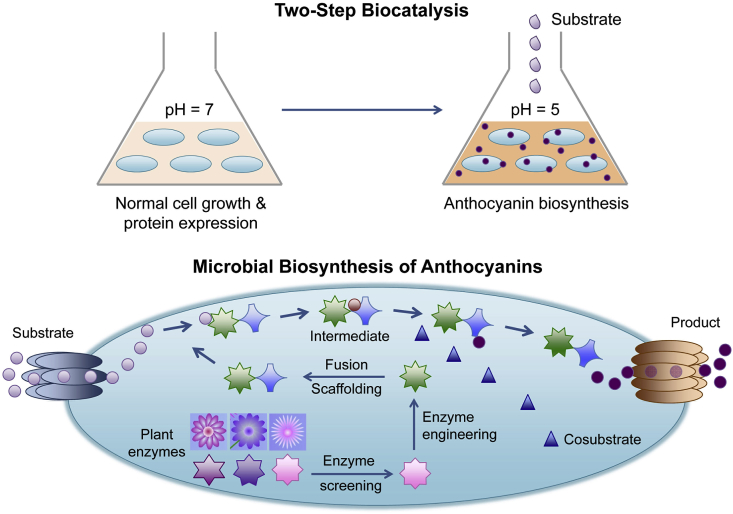
Fig. 3.
The strategies applied in metabolic engineering of E. coli for the biosynthesis of anthocyanins. The modifications of the anthocyanin-producing strains focus on the enzymes in the metabolic pathways, the transport of substrates and products, and the supply of cosubstrate UDP-glucose. The current production process is based on dividing the whole biocatalysis into two stages, i.e., cell growth and enzyme production at normal pH in the first stage, and anthocyanin production and accumulation at a lower pH in the second stage.
4.1. Enzyme screening and engineering for anthocyanin production
Construction and engineering of the anthocyanin pathway in microbes involves co-expression of plant enzymes. Gene orthologs encode enzymes catalyzing the same reaction in different plants that exhibit diverse kinetic and thermodynamic activities, and can thus lead to varied metabolic behaviors and distinct production levels of the target compounds when they are heterologously expressed in microorganisms. Hence, enzyme screening and selection from diverse species is an important way of improving the production of anthocyanins and other flavonoids [38]. Yan et al. compared the in vivo activities of ANS from four plants, and achieved maximal cyanidin production in E. coli by P. hybrida ANS [39]. Similarly, naringenin production in E. coli was optimized by comparing different homologs for each of the upstream pathway enzymes, i.e., 4-coumaroyl-CoA ligase (4CL), CHS and CHI, and enzyme combinations of different sources showed drastic variation in naringenin titer [37]. The same approach was also adopted for resveratrol production in E. coli [46].
With a suitable enzyme system containing the optimal grouping of enzymes from proper sources, the high yield microbial production of anthocyanins is still not always guaranteed, and the challenge lies in the heterologous expression of these plant-based enzymes in prokaryotic cells. Typically, the genes encoding these enzymes need engineering modifications prior to functional expression (Fig. 3). For example, to achieve functional prokaryotic expression of a P450 F3′5′H from Catharanthus roseus, the four codons at the 5′-end were deleted to remove the N-terminal membrane anchor, the fifth one was replaced with the start codon, and the sixth was changed from leucine to alanine to create an anchor suitable for bacterial expression [47]. The new F3′5′H was fused to a shortened P450 reductase with C. roseus origin to form a chimeric hydroxylase that catalyzed the formation of quercetin.
Apart from modifications of individual enzymes in the metabolic pathway, fused expression of multiple enzymes in successive steps is another effective method of improving anthocyanin production. It has been shown that upon fusion of F3GT from Arabidopsis thaliana to the N-terminus of ANS from P. hybrida with a pentapeptide linker, a higher titer of cyanidin 3-O-glucoside was achieved with the chimeric enzyme than with the uncoupled ANS and F3GT. The chimeric enzyme complex was proposed to catalyze the successive biochemical reactions more efficiently than the independent enzymes, probably by creating a higher local concentration of the unstable intermediate cyanidin, and by fueling it rapidly to F3GT for glycosylation, without causing much cyanidin degradation [39].
4.2. Regulation of cofactor/cosubstrate supply for anthocyanin overproduction
Efficient biosynthesis of anthocyanins requires sufficient yet balanced supply of cofactors and/or cosubstrates for electron transfer and enzyme activation/stabilization. For example, ferrous ions and sodium ascorbate as cofactors, and 2-oxoglutarate as a cosubstrate, are necessary for ANS to conduct a two-electron oxidation of its substrates [39], [42], and an equimolar amount of UDP-glucose is essential as a cosubstrate for the glycosylation of cyanidin at the C3 position.
Supply of UDP-glucose is indispensable for the overproduction of glycosylated anthocyanins. Since UDP-glucose is also involved in many other metabolic pathways for the generation of energy, cofactors, and metabolic precursors and intermediates, the global adjustment of its biosynthesis is crucial. The commonly used regulation schemes are overexpression of the biosynthetic genes and partial inhibition of the degradation pathways for a higher level of intracellular UDP-glucose. Attempts of such metabolic adjustments in E. coli involved the upregulation of one or more genes responsible for UDP-glucose biosynthesis from orotic acid (pyrE, pyrR, cmk, ndk, pgm, galU) and inhibition of the competitive UDP-glucose consumption pathways. These modifications resulted in cyanidin 3-O-glucoside production increasing by more than 20-fold (from 4 mg L−1 to 97 mg L−1) [21], [39]. In another case, the overexpression of intracellular genes pgm and galU along with the expression of ANS and 3GT led to a 57.8% increase in cyanidin 3-O-glucoside production [39].
S-Adenosyl-l-methionine is also a necessary cosubstrate for the production of methylated anthocyanins such as peonidin 3-O-glucoside. By increasing the availability of S-adenosyl-l-methionine through the CRISPR interference (CRISPRi)-mediated silencing of the transcriptional repressor MetJ, a twofold improvement of peonidin 3-O-glucoside production was achieved in E. coli with a final titer of 56 mg L−1 [48].
Beyond UDP-glucose and S-adenosyl-l-methionine, sodium ascorbate is another necessary ingredient to sustain the overproduction of anthocyanins. It has been found that the addition of sodium ascorbate significantly increases the consumption of the substrate catechin and the production of anthocyanin 3-O-glucoside [39]. But for the cosubstrate 2-oxoglutarate, extra addition is generally unnecessary, which may be due to 2-oxoglutarate being an intermediate compound in the Krebs cycle and its supply being commonly abundant [39].
4.3. Engineering of specific transporters for anthocyanin production
Many target products in engineered microorganisms are toxic to the host strains, thereby inhibiting their high-titer production. A feasible strategy to continuously biosynthesize these compounds at acceptable levels is to transfer the products from cytoplasm to extracellular environments, where the harmful products are attenuated, through specific efflux pumps. First reported by Dunlop et al. for improved production of biofuels in E. coli upon introduction of an array of efflux pumps responsible for the biofuel export from the producing cells [44], this approach has been extended to many systems, including the biosynthesis of anthocyanins for higher titers. A cyanidin 3-O-glucoside-associated efflux pump YadH has been identified and its overexpression led to 15% more production of anthocyanins [43]. Moreover, deletion of another efflux pump TolC, probably responsible for the secretion of the substrate catechin, further promoted the titer of cyanidin 3-O-glucoside.
Apart from engineering of the transporters naturally present in host microorganisms, introduction of transporters from plants is also a possible route to enhancing the transportation of substrates and products for improved anthocyanin production in engineered microbes. Anthocyanins in their natural plant hosts are transported to and accumulate in vacuoles by specific transporters [40], [45]. In maize, anthocyanin transport is largely dependent on an ATP-binding cassette transporter ZmMRP3 present in the tonoplast [49], and the deposition of anthocyanins in vacuoles is achieved by glutathione S-transferase encoded by Bronze-2 [41]. In other plants, H+-gradient-dependent transporters are also related to anthocyanin transportation. The gene Transparent Testa12 in Arabidopsis encodes a secondary transporter-like protein belonging to the MATE family, which is predicted to participate in the vacuolar transport of anthocyanins via an H+-antiport mechanism. Indeed, the mutant lacking this gene exhibits greatly reduced proanthocyanidin accumulation in vacuoles [50].
These transporters have not been tested in bacterial strains for their functions in the efflux of anthocyanins or in the improvement of anthocyanin production. Given that transfer of anthocyanins into plant vacuoles and outside microbial hosts both involve delivery across membrane bilayers that are composed of ordered lipids with anchored integral proteins, it would be highly interesting to investigate the potential potency of plant-derived transporters and combine them with bacterial transporters in microbial hosts.
4.4. Optimization of culture processes for anthocyanin production
The highly unstable nature of anthocyanins is a problematic issue for their microbial production. Unlike in plants where naturally synthesized anthocyanins are stored and stabilized in vacuoles [10], [51], there is a lack of anthocyanin stabilization mechanisms in bacterial cells that serve as artificial producing hosts. And since the intracellular pH is around 7 for most bacteria commonly used for metabolic engineering under their normal growth conditions, the synthesized anthocyanins are extremely labile. To stabilize anthocyanins in microbial hosts, a two-step biocatalysis has been proposed [39]. In the first step, cells are cultured in medium at pH 7, where normal cell growth and expression of heterologous enzymes are maintained. In the second step, cells at a certain growth stage are transferred to fresh medium with pH adjusted to 5 to minimize anthocyanin degradation (Fig. 3). Protective agents such as glutamate can be added to protect cells under low pH. Such a strategy helped to elevate the titer of cyanidin 3-O-glucoside in E. coli by ∼15-fold–38.9 mg L−1 compared to the traditional single-step production (2.5 mg L−1) [39].
Apart from pH, several other factors also play important roles in anthocyanin production, such as induction time-point, substrate feeding, amount of dissolved oxygen, and temperature. Lim and coworkers found that induction at the stationary phase was optimal for cyanidin 3-O-glucoside production in engineered E. coli, and that pulsing of glucose and catechin improved anthocyanin production [43]. The effect of induction point could be a consequence of differential protein expression at different growth stages. The improvement from glucose feeding may lie in the increased supply of UDP-glucose, whereas the additional supplementation of catechin forms a driving force for the bioconversion.
Dissolved oxygen has a dual effect on anthocyanin production. On the one hand, oxygen is key to the function of ANS; on the other hand, oxygen may oxidize anthocyanins. Thus, an optimal supply of oxygen is critical. In a study of microbial eriodictyol conversion, improved catechin synthesis was achieved by increasing the concentration of dissolved oxygen, which might be related to elevated NADPH supply [38]. However, currently there are no reports on the effect of oxygen on anthocyanin production.
Temperature is a global and key factor affecting cell viability and expression of heterologous proteins. Fluctuations in temperature often lead to highly variable folding behaviors and production yields of certain proteins, and thus imposing indirect influence on microbial production of useful compounds. In a co-culture system producing afzelechin, the induction temperature of 20 °C gave rise to the highest titer of 22.9 mg L−1 among different co-cultures, whereas the titer was only 6.1 mg L−1 at 10 °C [37].
5. Future perspectives
At present, the cost of anthocyanins from recombinant bacteria is still much higher than that from plant extraction, and one of the most important causes is the relatively low titer, which is attributed to low efficiencies and poor capabilities of the introduced pathways. Thus, to maximize the potential of the biosynthetic pathways while minimizing the side effects of anthocyanin overproduction on microbial hosts through a series of regulation and optimization will be the key task for microbial supply of anthocyanins. The rapid expansion of genome sequencing and DNA synthesis capabilities, leading to the discoveries of new enzymes and pathways, as well as redesign of key enzymes based on present knowledge, has already resulted in the construction of new biosynthetic pathways that can be expressed in microorganisms for the production of known anthocyanins. Meanwhile, further optimization of anthocyanin production in microorganisms will continue to also focus on flexible regulation of UDP-glucose supply, as well as the identification of efficient and specific transporters for anthocyanins and relative substrates. Systematic engineering of UDP-glucose supply is a great challenge for further optimization given that UDP-glucose is also a precursor for the biosynthesis of other bioactive compounds such as trehalose and glycogen, and is involved in the biosynthesis of glycopolymers that are vital for cell metabolism, cell signaling, and defense systems. Recently, a dCas9-based toolbox has been developed to regulate the expression of multiple genes simultaneously in E. coli [52], [53]. This approach can be exploited to fine-tune the levels of a variety of enzymes, transporters and cofactors associated directly or indirectly with anthocyanin production, for the identification of commitment steps, rate-limiting factors, and potential regulation points.
Pathway balancing is another important factor that should be taken into consideration for process optimization of anthocyanin production. A balanced pathway reduces metabolic burden imposed on host cells during the overproduction of anthocyanins while maintaining normal cell growth and metabolism to the most extent. Many techniques and tools are currently available to balance metabolic pathways, such as ePathBrick vectors, the ePathOptimize scheme, combinatorial promoter engineering, synthetic RNA switches, and biosensor-based dynamic regulation of key intracellular metabolites [54], [55], [56], [57], [58], [59], [60].
Besides pathways-associated factors, inefficient transportation and lack of storage organelles of anthocyanins can also lead to compromised production. Microbial hosts lack the machinery of anthocyanin transport and stabilization inherent in plant hosts, where anthocyanins are moved from the cytoplasm to the vacuole and stored stably [61]. Currently, the path of anthocyanin movement inside microbial cells and across cell membranes is poorly understood, and there is no research on the interactions between anthocyanins and structural components of the producing microbes. The elucidation of these mechanisms, together with the introduction or engineering of specific transporters, will benefit the optimal production of anthocyanins to a great extent.
In addition, the feeding of expensive flavonoid precursors increases the overall cost of anthocyanin production. The de novo production of pelargonidin 3-O-glucoside (10 mg L−1) from glucose has been accomplished with a poly-culture system [62]. Leveraging this strategy in optimized bacteria would significantly decrease the cost and make the production process commercially viable.
Optimization of anthocyanin production in microbes is a complicated and time-consuming process. Up till now, researchers have identified multiple factors that have significant effects on anthocyanin bioconversion, including host and substrate selection, enzyme expression, cofactor supply, mass transfer, and growth/induction conditions. However, there is still a plethora of questions unanswered by the current studies. For example, how do the turnover and kinetics of different enzymes influence the final production of the target products? Do normal cellular metabolites activate or inhibit the enzymes involved in the engineered pathways? How do the introduction of the anthocyanin pathway and the overproduction of anthocyanins influence the host cell genetics, epigenetics, and physiology? Without such information, the microbes are similar to black boxes, and controlled regulation and optimization of microbial anthocyanin production is difficult, or even impossible in some circumstances. In addition, with specific substrate feeding and excessive or limited supply of certain nutrients for anthocyanin production, the basic cell metabolism can be altered while the microbial hosts switch to uncommon rescue pathways, and the intermediate metabolites that are designed to be at restricted levels can be generated in large amounts in the real case. So a combinatorial and multidisciplinary strategy, which could evaluate multiple aspects simultaneously, is particularly crucial for the delicate control and optimization of the anthocyanin pathway in engineered microorganisms.
6. Conclusions
Microbial production of anthocyanins is a promising means to supplement current methods of producing these molecules for industrial applications. In this review, we summarized recent progress on metabolic engineering of anthocyanin production in microorganisms, with particular emphasis on E. coli, the only microorganism that has been used for the recombinant production of these molecules. We presented the strategies that have been applied in engineering the biosynthetic pathways, the host strains, and the bioreaction processes. By introducing these modifications, the production of cyanidin 3-O-glucoside reached over 300 mg L−1, which is promising for industrial applications. However, many difficulties still remain to be overcome to further improve the production and to increase the commercial competitiveness of the microbial production, such as poor expression and improper balancing of genes involved in the microbial biosynthetic pathways of anthocyanins, and engineering of transporters for the efficient secretion of these molecules into the extracellular medium. There is, however, little doubt, given the great strides that have been made in optimizing recombinant microorganisms in the past 15 years, that engineered microorganisms will become one of the most competitive sources of anthocyanin molecules in the near future.
Conflict of interest
The authors declare no commercial or financial conflict of interest.
Acknowledgement
Funding for some of the work reviewed in this manuscript was provided by the National Science Foundation grant number IIP-1549767.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Tanaka Y., Ohmiya A. Seeing is believing: engineering anthocyanin and carotenoid biosynthetic pathways. Curr Opin Biotechnol. 2008;19:190–197. doi: 10.1016/j.copbio.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Ananga A., Georgiev V., Ochieng J., Phills B., Tsolova V. Production of anthocyanins in grape cell cultures: a potential source of raw material for pharmaceutical, food, and cosmetic industries. In: Poljuha D., Sladonja B., editors. The mediterranean genetic code - grapevine and olive. InTech; 2013. [Google Scholar]
- 3.Aberoumand A. A review article on edible pigments properties and sources as natural biocolorants in foodstuff and food industry. World J Dairy Food Sci. 2011;6:71–78. [Google Scholar]
- 4.Bhan N., Xu P., Koffas M.A.G. Pathway and protein engineering approaches to produce novel and commodity small molecules. Curr Opin Biotechnol. 2013;24:1137–1143. doi: 10.1016/j.copbio.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 5.Corrales M., Toepfl S., Butz P., Knorr D., Tauscher B. Extraction of anthocyanins from grape by-products assisted by ultrasonics, high hydrostatic pressure or pulsed electric fields: a comparison. Innov Food Sci Emerg Technol. 2008;9:85–91. [Google Scholar]
- 6.Mora-Pale M., Sanchez-Rodriguez S.P., Linhardt R.J., Dordick J.S., Koffas M.A.G. Biochemical strategies for enhancing the in vivo production of natural products with pharmaceutical potential. Curr Opin Biotechnol. 2014;25:86–94. doi: 10.1016/j.copbio.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Mora-Pale M., Sanchez-Rodriguez S.P., Linhardt R.J., Dordick J.S., Koffas M.A.G. Metabolic engineering and in vitro biosynthesis of phytochemicals and non-natural analogues. Plant Sci. 2013;210:10–24. doi: 10.1016/j.plantsci.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Shi M.Z., Xie D.Y. Biosynthesis and metabolic engineering of anthocyanins in Arabidopsis thaliana. Recent Pat Biotechnol. 2014;8:47–60. doi: 10.2174/1872208307666131218123538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dixon R.A., Liu C., Jun J.H. Metabolic engineering of anthocyanins and condensed tannins in plants. Curr Opin Biotechnol. 2013;24:329–335. doi: 10.1016/j.copbio.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y., Butelli E., Martin C. Engineering anthocyanin biosynthesis in plants. Curr Opin Plant Biol. 2014;19:81–90. doi: 10.1016/j.pbi.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 11.Zhu L., Huang Y., Zhang Y., Xu C., Lu J., Wang Y. The growing season impacts the accumulation and composition of flavonoids in grape skins in two-crop-a-year viticulture. J Food Sci Technol. 2017;54:2861–2870. doi: 10.1007/s13197-017-2724-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryan J.M., Revilla E. Anthocyanin composition of cabernet sauvignon and tempranillo grapes at different stages of ripening. J Agric Food Chem. 2003;51:3372–3378. doi: 10.1021/jf020849u. [DOI] [PubMed] [Google Scholar]
- 13.Tsai P.J., Wu S.C., Cheng Y.K. Role of polyphenols in antioxidant capacity of napiergrass from different growing seasons. Food Chem. 2008;106:27–32. [Google Scholar]
- 14.Pandey R.P., Parajuli P., Koffas M.A.G., Sohng J.K. Microbial production of natural and non-natural flavonoids: pathway engineering, directed evolution and systems/synthetic biology. Biotechnol Adv. 2016;34:634–662. doi: 10.1016/j.biotechadv.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 15.Chemler J.A., Koffas M.A.G. Metabolic engineering for plant natural product biosynthesis in microbes. Curr Opin Biotechnol. 2008;19:597–605. doi: 10.1016/j.copbio.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 16.Staniek A., Bouwmeester H., Fraser P.D., Kayser O., Martens S., Tissier A. Natural products – learning chemistry from plants. Biotechnol J. 2014;9:326–336. doi: 10.1002/biot.201300059. [DOI] [PubMed] [Google Scholar]
- 17.Li Y., Smolke C.D. Engineering biosynthesis of the anticancer alkaloid noscapine in yeast. Nat Commun. 2016;7:12137. doi: 10.1038/ncomms12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paddon C.J., Keasling J.D. Semi-synthetic artemisinin: a model for the use of synthetic biology in pharmaceutical development. Nat Rev Microbiol. 2014;12:355–367. doi: 10.1038/nrmicro3240. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y., Chen S., Yu O. Metabolic engineering of flavonoids in plants and microorganisms. Appl Microbiol Biotechnol. 2011;91:949–956. doi: 10.1007/s00253-011-3449-2. [DOI] [PubMed] [Google Scholar]
- 20.Fowler Z.L., Shah K., Panepinto J.C., Jacobs A., Koffas M.A.G. Development of non-natural flavanones as antimicrobial agents. PLoS One. 2011;6:e25681. doi: 10.1371/journal.pone.0025681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leonard E., Yan Y., Fowler Z.L., Li Z., Lim C.G., Lim K.H. Strain improvement of recombinant Escherichia coli for efficient production of plant flavonoids. Mol Pharm. 2008;5:257–265. doi: 10.1021/mp7001472. [DOI] [PubMed] [Google Scholar]
- 22.Woolston B.M., Edgar S., Stephanopoulos G. Metabolic engineering: past and future. Annu Rev Chem Biomol Eng. 2013;4:259–288. doi: 10.1146/annurev-chembioeng-061312-103312. [DOI] [PubMed] [Google Scholar]
- 23.Yadav V.G., De Mey M., Giaw Lim C., Kumaran Ajikumar P., Stephanopoulos G. The future of metabolic engineering and synthetic biology: towards a systematic practice. Metab Eng. 2012;14:233–241. doi: 10.1016/j.ymben.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsuda T. Dietary anthocyanin-rich plants: biochemical basis and recent progress in health benefits studies. Mol Nutr Food Res. 2012;56:159–170. doi: 10.1002/mnfr.201100526. [DOI] [PubMed] [Google Scholar]
- 25.de Pascual-Teresa S., Moreno D.A., García-Viguera C. Flavanols and anthocyanins in cardiovascular health: a review of current evidence. Int J Mol Sci. 2010;11:1679–1703. doi: 10.3390/ijms11041679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burton-Freeman B., Linares A., Hyson D., Kappagoda T. Strawberry modulates LDL oxidation and postprandial lipemia in response to high-fat meal in overweight hyperlipidemic men and women. J Am Coll Nutr. 2010;29:46–54. doi: 10.1080/07315724.2010.10719816. [DOI] [PubMed] [Google Scholar]
- 27.Giusti M.M., Wrolstad R.E. Current protocols in food analytical chemistry. John Wiley & Sons, Inc; 2001. Characterization and measurement of anthocyanins by UV-visible spectroscopy. [Google Scholar]
- 28.Chan C.F., Lien C.Y., Lai Y.C., Huang C.L., Liao W.C. Influence of purple sweet potato extracts on the UV absorption properties of a cosmetic cream. J Cosmet Sci. 2010;61:333–341. [PubMed] [Google Scholar]
- 29.Rojo L.E., Roopchand D.E., Graf B., Cheng D.M., Ribnicky D., Fridlender B. Role of anthocyanins in skin aging and UV-induced skin damage. In: Wallace T.C., Giusti M.M., editors. Anthocyanins in health and disease. CRC Press; 2013. pp. 309–322. [Google Scholar]
- 30.Giusti M.M., Wrolstad R.E. Acylated anthocyanins from edible sources and their applications in food systems. Biochem Eng J. 2003;14:217–225. [Google Scholar]
- 31.Calogero G., Marco G.D. Red Sicilian orange and purple eggplant fruits as natural sensitizers for dye-sensitized solar cells. Sol Energy Mater Sol Cells. 2008;92:1341–1346. [Google Scholar]
- 32.Wongcharee K., Meeyoo V., Chavadej S. Dye-sensitized solar cell using natural dyes extracted from rosella and blue pea flowers. Sol Energy Mater Sol Cells. 2007;91:566–571. [Google Scholar]
- 33.Ramamoorthy R., Radha N., Maheswari G., Anandan S., Manoharan S., Victor Williams R. Betalain and anthocyanin dye-sensitized solar cells. J Appl Electrochem. 2016;46:929–941. [Google Scholar]
- 34.Calogero G., Yum J.-H., Sinopoli A., Di Marco G., Grätzel M., Nazeeruddin M.K. Anthocyanins and betalains as light-harvesting pigments for dye-sensitized solar cells. Sol Energy. 2012;86:1563–1575. [Google Scholar]
- 35.Zhao J., Dixon R.A. The 'Ins' and 'outs' of flavonoid transport. Trends Plant Sci. 2010;15:72–80. doi: 10.1016/j.tplants.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 36.Chalker-Scott L. Environmental significance of anthocyanins in plant stress responses. Photochem Photobiol. 1999;70:1–9. [Google Scholar]
- 37.Jones J.A., Vernacchio V.R., Sinkoe A.L., Collins S.M., Ibrahim M.H.A., Lachance D.M. Experimental and computational optimization of an Escherichia coli co-culture for the efficient production of flavonoids. Metab Eng. 2016;35:55–63. doi: 10.1016/j.ymben.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 38.Zhao S., Jones J.A., Lachance D.M., Bhan N., Khalidi O., Venkataraman S. Improvement of catechin production in Escherichia coli through combinatorial metabolic engineering. Metab Eng. 2015;28:43–53. doi: 10.1016/j.ymben.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 39.Yan Y., Li Z., Koffas M.A. High-yield anthocyanin biosynthesis in engineered Escherichia coli. Biotechnol Bioeng. 2008;100:126–140. doi: 10.1002/bit.21721. [DOI] [PubMed] [Google Scholar]
- 40.Goossens A., Hakkinen S.T., Laakso I., Oksman-Caldentey K.M., Inze D. Secretion of secondary metabolites by ATP-binding cassette transporters in plant cell suspension cultures. Plant Physiol. 2003;131:1161–1164. doi: 10.1104/pp.102.016329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marrs K.A., Alfenito M.R., Lloyd A.M., Walbot V. A glutathione S-transferase involved in vacuolar transfer encoded by the maize gene Bronze-2. Nature. 1995;375:397–400. doi: 10.1038/375397a0. [DOI] [PubMed] [Google Scholar]
- 42.Turnbull J.J., Nakajima J.I., Welford R.W.D., Yamazaki M., Saito K., Schofield C.J. Mechanistic studies on three 2-oxoglutarate-dependent oxygenases of flavonoid biosynthesis: anthocyanidin synthase, flavonol synthase, and flavanone 3β-hydroxylase. J Biol Chem. 2004;279:1206–1216. doi: 10.1074/jbc.M309228200. [DOI] [PubMed] [Google Scholar]
- 43.Lim C.G., Wong L., Bhan N., Dvora H., Xu P., Venkiteswaran S. Development of a recombinant Escherichia coli strain for overproduction of plant pigment, anthocyanin. Appl Environ Microbiol. 2015:6276–6284. doi: 10.1128/AEM.01448-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dunlop M.J., Dossani Z.Y., Szmidt H.L., Chu H.C., Lee T.S., Keasling J.D. Engineering microbial biofuel tolerance and export using efflux pumps. Mol Syst Biol. 2011;7:487. doi: 10.1038/msb.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yazaki K. Transporters of secondary metabolites. Curr Opin Plant Biol. 2005;8:301–307. doi: 10.1016/j.pbi.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 46.Lim C.G., Fowler Z.L., Hueller T., Schaffer S., Koffas M.A.G. High-yield resveratrol production in engineered Escherichia coli. Appl Environ Microbiol. 2011;77:3451–3460. doi: 10.1128/AEM.02186-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leonard E., Yan Y., Koffas M.A.G. Functional expression of a P450 flavonoid hydroxylase for the biosynthesis of plant-specific hydroxylated flavonols in Escherichia coli. Metab Eng. 2006;8:172–181. doi: 10.1016/j.ymben.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 48.Cress B.F., Leitz Q.D., Kim D.C., Amore T.D., Suzuki J.Y., Linhardt R.J. CRISPRi-mediated metabolic engineering of E. coli for O-methylated anthocyanin production. Microb Cell Fact. 2017;16:10. doi: 10.1186/s12934-016-0623-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goodman C.D., Casati P., Walbot V. A multidrug resistance–associated protein involved in anthocyanin transport in Zea mays. Plant Cell. 2004;16:1812–1826. doi: 10.1105/tpc.022574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Debeaujon I., Peeters A.J.M., Léon-Kloosterziel K.M., Koornneef M. The TRANSPARENT TESTA12 gene of Arabidopsis encodes a multidrug secondary transporter-like protein required for flavonoid sequestration in vacuoles of the seed coat endothelium. Plant Cell. 2001;13:853–872. doi: 10.1105/tpc.13.4.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Passeri V., Koes R., Quattrocchio F.M. New challenges for the design of high value plant products: stabilization of anthocyanins in plant vacuoles. Front Plant Sci. 2016;7:153. doi: 10.3389/fpls.2016.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cress B.F., Jones J.A., Kim D.C., Leitz Q.D., Englaender J.A., Collins S.M. Rapid generation of CRISPR/dCas9-regulated, orthogonally repressible hybrid T7-lac promoters for modular, tuneable control of metabolic pathway fluxes in Escherichia coli. Nucleic Acids Res. 2016;44:4472–4485. doi: 10.1093/nar/gkw231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cress B.F., Toparlak ÖD Guleria S., Lebovich M., Stieglitz J.T., Englaender J.A. CRISPathBrick: modular combinatorial assembly of type II-A CRISPR arrays for dCas9-mediated multiplex transcriptional repression in E. coli. ACS Synth Biol. 2015;4:987–1000. doi: 10.1021/acssynbio.5b00012. [DOI] [PubMed] [Google Scholar]
- 54.Skjoedt M.L., Snoek T., Kildegaard K.R., Arsovska D., Eichenberger M., Goedecke T.J. Engineering prokaryotic transcriptional activators as metabolite biosensors in yeast. Nat Chem Biol. 2016;12:951–958. doi: 10.1038/nchembio.2177. [DOI] [PubMed] [Google Scholar]
- 55.Jones J.A., Vernacchio V.R., Lachance D.M., Lebovich M., Fu L., Shirke A.N. ePathOptimize: a combinatorial approach for transcriptional balancing of metabolic pathways. Sci Rep. 2015;5:11301. doi: 10.1038/srep11301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu P., Li L., Zhang F., Stephanopoulos G., Koffas M. Improving fatty acids production by engineering dynamic pathway regulation and metabolic control. Proc Natl Acad Sci. 2014;111:11299–11304. doi: 10.1073/pnas.1406401111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu P., Vansiri A., Bhan N., Koffas M.A.G. ePathBrick: a synthetic biology platform for engineering metabolic pathways in E. coli. ACS Synth Biol. 2012;1:256–266. doi: 10.1021/sb300016b. [DOI] [PubMed] [Google Scholar]
- 58.Alper H., Fischer C., Nevoigt E., Stephanopoulos G. Tuning genetic control through promoter engineering. Proc Natl Acad Sci. 2005;102:12678–12683. doi: 10.1073/pnas.0504604102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen X., Gao C., Guo L., Hu G., Luo Q., Liu J. DCEO biotechnology: tools to design, construct, evaluate, and optimize the metabolic pathway for biosynthesis of chemicals. Chem Rev. 2017 doi: 10.1021/acs.chemrev.6b00804. [DOI] [PubMed] [Google Scholar]
- 60.Liang Joe C., Bloom Ryan J., Smolke Christina D. Engineering biological systems with synthetic RNA molecules. Mol Cell. 2011;43:915–926. doi: 10.1016/j.molcel.2011.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao J. Flavonoid transport mechanisms: how to go, and with whom. Trends Plant Sci. 2015;20:576–585. doi: 10.1016/j.tplants.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 62.Jones J.A., Vernacchio V.R., Collins S.M., Shirke A.N., Xiu Y., Englaender J.A. Complete biosynthesis of anthocyanins using E. coli polycultures. mBio. 2017:8. doi: 10.1128/mBio.00621-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yan Y., Chemler J., Huang L., Martens S., Koffas M.A.G. Metabolic engineering of anthocyanin biosynthesis in Escherichia coli. Appl Environ Microbiol. 2005;71:3617–3623. doi: 10.1128/AEM.71.7.3617-3623.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]