Abstract
Background
The in-vitro study indicated that ERK/MAPK and PI3K/AKT signal channels may play an important role in reparative regeneration process after peripheral nerve injury. But, relevant in-vivo study was infrequent. In particular, there has been no report on simultaneous activation of ERK/MAPK and PI3K/AKT signal channels in facial nerve cell and axon after facial nerve injury.
Results
The expression of P-ERK enhanced in nerve cells at the injury side on the 1 d after the rat facial nerve was cut and kept on a higher level until 14 d, but decreased on 28 d. The expression of P-AKT enhanced in nerve cells at the injury side on 1 d after injury, and kept on a higher level until 28 d. The expression of P-ERK enhanced at the near and far sections of the injured axon on 1 d, then increased gradually and reached the maximum on 7 d, but decreased on 14 d, until down to the level before the injury on 28 d. The expression of P-AKT obviously enhanced in the injured axon on 1 d, especially in the axon of the rear section, but decreased in the axon of the rear section on 7 d, while the expression of axon in the far section increased to the maximum and kept on till 14 d. On 28 d, the expression of P-AKT decreased in both rear and far sections of the axon.
Conclusion
The facial nerve simultaneously activated ERK/MAPK and PI3K/AKT signal channels in facial nerve cells and axons after the cut injury, but the expression levels of P-ERK and P-AKT varied as the function of the time. In particular, they were quite different in axon of the far section. It has been speculated that two signal channels might have different functions after nerve injury. However, their specific regulating effects should still be testified by further studies in regenerative process of peripheral nerve injury.
Keywords: ERK/MAPK, PI3K/AKT, Nerve cell, Axon, Facial nerve injury
1. Introduction
A series of physiological changes occur after peripheral nerve injury, such as degeneration of nerve cell, disintegration of axon, dedifferentiation and proliferation of Schwann cell, extension of axon, dedifferentiation of Schwann cell and so on. Finally, axon regeneration and partial recovery of nerve functions can be realized to a certain extent (Rich et al., 1989, Koeppen, 2004, Grinsell and Keating, 2014, Sulaiman and Gordon, 2000). At present, the molecular mechanism for such a complicated physiological process is still unclear, but the study for molecular mechanism of the regeneration of the peripheral nerve injury can provide a new strategy for rehabilitation therapy after peripheral nerve injury. Unlike the regeneration of peripheral nerve, it is difficult for the injured central nerve to regenerate, of which one important reason is that the grown cells of the central nerve lose endogenous axon growing capability. Although recent studies had obtained pleasing results in improving the growth potential of the injured axon by regulating transcription and interpretation (Park et al., 2008, PTEN deletion enhances the regenerative ability of adult corticospinal neurons), the regenerative effect of these nerves is still inferior to natural regenerative results of axon after peripheral nerve injury. It means that understanding the molecular mechanism of axon’s natural regeneration should be a main point for studying neural regeneration and the first step is to explain the possible signal channel, which regulates natural regeneration of axon.
PI3K/AKT and ERK/MAPK signal channels are important intracellular signal channels, which are known in regulating cell survival, growth, splitting and proliferation at present (Schmid et al., 2000, Forcet et al., 2002, Carter and Downes, 1992). The literature reported that PI3K/AKT and ERK/MAPK signal channels could be activated at multiple sites of the injured nerve (Klimaschewski et al., 2013, Liu and Snider, 2001, Klesse and Parada, 1998), including facial nerve cell, axon, Schwann cell, etc. and possibly play an important role in repair and regeneration processes after nerve injury. However, most previous studies are vitro studies, while in-vivo studies are infrequent, especially aging studies for P-AKT and P-ERK expressions after facial nerve injury. In the process of peripheral nerve regeneration, it is not a single signal channel that plays the role of regulating, but various signal channels are activated simultaneously or in turn and the crosstalk among different signal channels is significant in regulating nerve regeneration. Therefore, understanding the time-variation features of P-AKT and P-ERK expressions after nerve injury is helpful for use to explore the regulating function of the signal channel after nerve injury.
In this thesis, Western and immunohistochemistry means were adopted to study the expression and time limit of P-ERK and P-AKT in facial nerve cell and axon, after rate facial nerve is cut, so as to assist in exploring the functional mechanism of PI3K/AKT and ERK/MAPK signal channels in the repair and regenerative process after facial nerve injury.
2. Materials and methods
2.1. Animal treatment and facial nerve axotomy injury model
30 male adult SD rats with body weights of 200–250 g were divided into 6 groups at random with 5 ones per group, which were named as the 1st, 3rd, 7th, 14th and 28th groups and one blank control group. The rats were provided by the Animal Experiment Center of Dalian Medical University and approved by the Ethic Committee.
After rats were anaesthetized by injecting 10% chloral hydrate 3.5 ml/kg into their abdominal cavities, the curved incisions were made at the lower parts of right auricles and separated up to the stylomastoid foramen to expose the trunk of facial nerve. The trunk of facial nerve was cut at 5 mm place of the stylomastoid foramen 5 mm, the facial nerve anastomosis was carried out with 10/0 sutures under an operating microscope and then the wound was seamed up with 4–0 nylon sutures.
2.2. Tissue preparation
In 7 days after the operation, the rats were anaesthetized by injecting 10% chloral hydrate 3.5 ml/kg, the former facial incision was separated to expose facial nerve, which was separately cut from stylomastoid foramen of the facial nerve to 5 mm far end of anastomotic stoma and the obtained facial nerve specimen was about 1 cm long. The facial nerve specimens of 5 rats were taken for each group and separately cut at the anastomosis places into a 5 mm-log rear section and a 5 mm-long far section and the 5 mm facial nerve taken from the same position of the blank control group was used for Western detection. Via the left ventricle, a cannula was inserted to the ascending aorta, the normal saline 150 ml was filled quickly until the liver turned white, then 4% paraformaldehyde (4 °C, pH7.4) 300 ml was filled for fixing quickly early and slowly lately until the limbs of animals became stiff. Then, rat heads were cut off and opened to take brain stem. With the cerebellum bulbopontine sulcus as the center, about 2 mm-thick brain stem tissues, containing facial nerve core, were cut horizontally from left and right sides and put into 4% paraformaldehyde for fixing overnight (4 °C). After being bleached and dehydrated, the tissues were embedded with paraffin, the facial nerve cores were positioned according to the three-dimensional cerebrum positioning spectrum of the rats and serial sections, about 20 μm thick, were prepared and used for immunohistochemistrical dying.
2.3. Antibodies
Anti-p-Akt1/2/3(Ser473) IgG, anti-Akt1/2/3 IgG, anti-β-actin mouse IgG were from Abcam (Cambridge, MA, USA), anti-p-p44/42MAPK(Erk1/2)(Thr202/Tyr204)IgG, anti-Erk1/2 IgG were from Cell Signaling Technology (Beverly, MA, USA), goat anti-rabbit IgG - horse radish peroxidase, rabbit anti-goat IgG-horse radish peroxidase, goat anti-mouse IgG-horse radish peroxidase were from Santa CruzBiotechnology (Santa Cruz, CA, USA).
2.4. Immunohistochemistry
The paraffin sections were dewaxed, hydrated, rinsed with PBS, put into a pressure cooking kettle containing citric acid buffer solution (pH6.0) and heated for 3 min; then cooled to room temperature, rinsed with PBS again, drippily added with peroxidase blocking agent and incubated for 10 min under room temperature; drippily added with the first antibody (P-ERK 1:400, P-AKT 1:200), incubated for 60 min under room temperature and rinsed with PBS again; drippily added with MAXVision/HRP agent (Fujian MXB Biotechnologies Co., Ltd.), incubated for 15 min under room temperature and colorated with DAB and rinsed with tap water until color development stopped; redyed with hematoxyllin, re-blued with PBS, dehydrated, vitrified with xylene and sealed up with neutral resin. Then, the Image-Pro Plus software was used to measure the mean optical density, of positive nerve cells, which represented the immune dyeing strength of the facial nerve cell.
2.5. Western blotting
After the facial nerve had been cut into pieces, RIPA buffer solution was added and the mixture was cracked ultrasonically and centrifuged for 10 min with a low-temperature ultracentrifuge (10,000 rpm). Then, the supernatant was sucked and the BCA protein concentration determination kit was used to determine the protein concentration of the sample. The 5× loading buffer was added and the mixture was heated for 5 min under 100 °C, treated with SDS-PAGE gel electrophoresis and transferred to PVDF film for 1 h sealing under room temperature. p-Akt (Thr473), Akt, p-p44/42MAPK (Erk1/2)(Thr202/Tyr204), p44/42MAPK (Erk1/2)(Thr202/Tyr204) and β-actin (dilution ratio was 1: 1000 for all) were added separately and then the mixture was incubated overnight under 4 °C. After film rinsing, HRP rabbit anti-goat IgG, HRP goat anti-rabbit IgG and HRP rabbit anti-mouse IgG (dilution ratio was 1:2000 for all) were added separately and the mixture was incubated for 1 h under room temperature. After being rinsed, the PVDF film was soaked in the ECL illuminant solution for 3 min and then taken out for development. According to the calibrated marker, Gelpro 32 densitometric scanning and protein analysis software was used to analyze protein strips.
2.6. Statistical analysis
The measurement data were expressed by mean value ± standard deviation (Mean ± SD) and should be analyzed with ANOVA variance by SPSS 19.0 statistical software and P < 0.05 indicated that the deviation was statistically significant.
3. Results
3.1. Expression of P-ERK I facial nerve cell
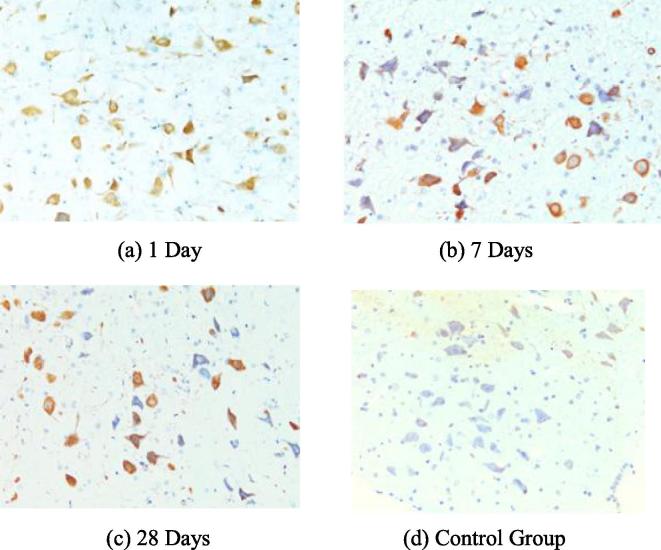
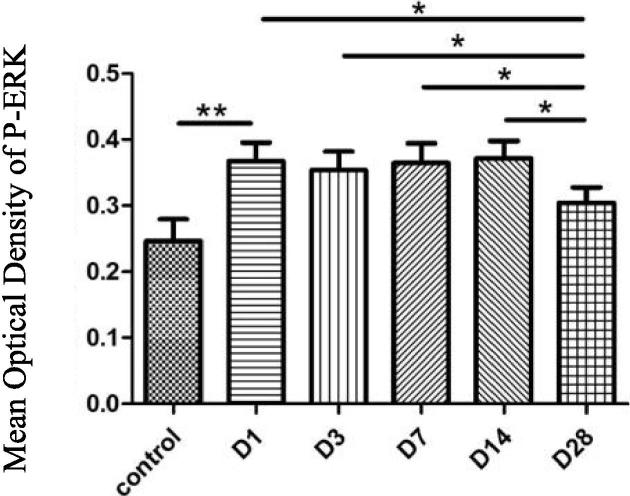
The expression of P-ERK was relatively weak in facial nerve nucleus of the blank control group, but obviously enhanced in the injured facial nerve nucleus. The mean optical density of P-ERK in the facial nerve nucleus at the injury side raised more obviously than that in the blank control group in 1d after the injury (P < 0.01), but the differences among 3d, 7d and 14d were not significant statistically (P > 0.05). At 28d, the mean optical density of P-ERK decreased and the difference was statistically significant while compared with other time points (P < 0.05). See Figs. 1 and 2.
Fig. 1.
The expressions of P-ERK under different time points.
Fig. 2.
Mean optical density of P-ERK, ** P < 0.01, * P < 0.05.
3.2. Expression of P-AKT in facial nerve cell
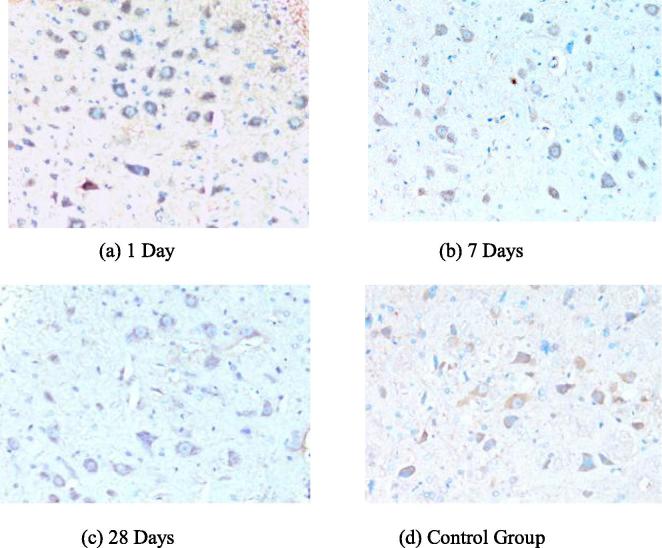
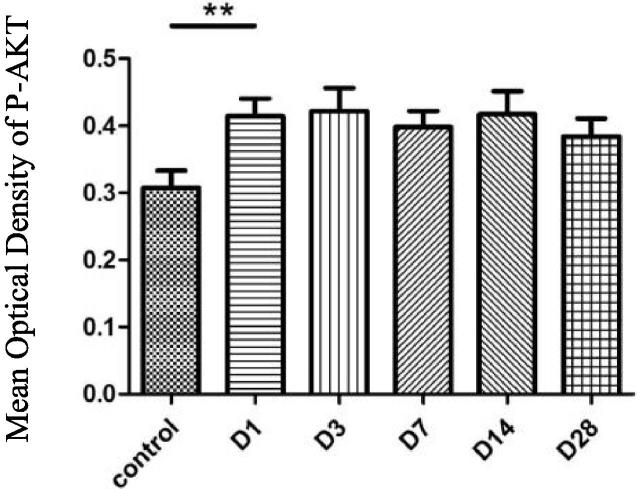
The expression of P-AKT was found in facial nerve nucleus at the control side and was more distinct in facial nerve nucleus at the injury side. The mean optical density of P-AKT in the facial nerve nucleus at the injury side raised more obviously than that in the blank control group in 1d after the injury and the difference was statistically significant (P < 0.01). It was kept at a close expression level until 28d and the differences among all time points were not statistically significant (P < 0.05). See Figs. 3 and 4.
Fig. 3.
The expressions of P-ERK under different time points.
Fig. 4.
Mean optical density of P-AKT, ** P < 0.01.
3.3. Expression of P-ERK in axon
In the blank control group, only a little expression of P-ERK in axon could be found. On 1 d after the facial nerve was cut and sutured, the expression of P-ERK in axon started to rise up, especially in the rear section. The expression of P-ERK rose more predominantly on 3 d while compared with the control group, reached the maximum value on 7 d, but decreased on 14 d while compared with that on 7 d. The expression of P-ERK in axon was closed to that of the blank control group on 28 d. At various time points after the facial nerve was cut and sutured, the expression of P-ERK in rear section of axon was higher than that in the far section. See Fig. 5.
Fig. 5.
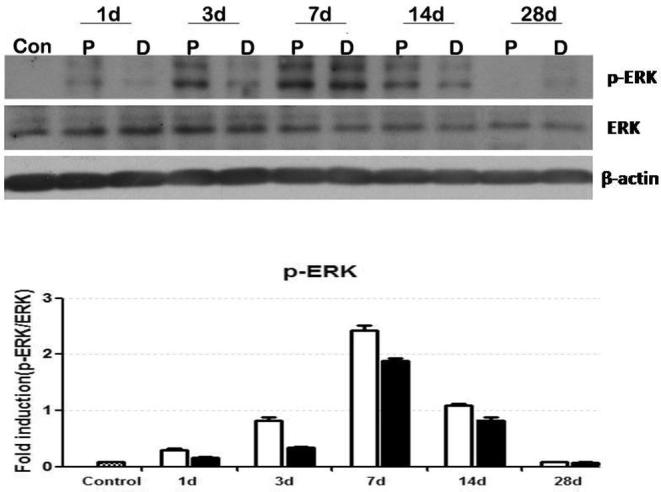
Expressions of P-ERK at different time points after axon injury.
3.4. Expression of P-AKT in axon
In the blank control group, only a little expression of P-AKT in axon could be found. On 1d after the facial nerve was cut and sutured, the expression of P-AKT in axon started to rise up, especially in the rear axon section. The expression of P-AKT on 3 d was close to that on 1d, but decreased in the rear axon section on 7 d. However, the expression reached to the maximum in the far section and kept on until 14d. The expression of P-AKT decreased in near, far and middle sections of the axon on 28 d. See Fig. 6.
Fig. 6.
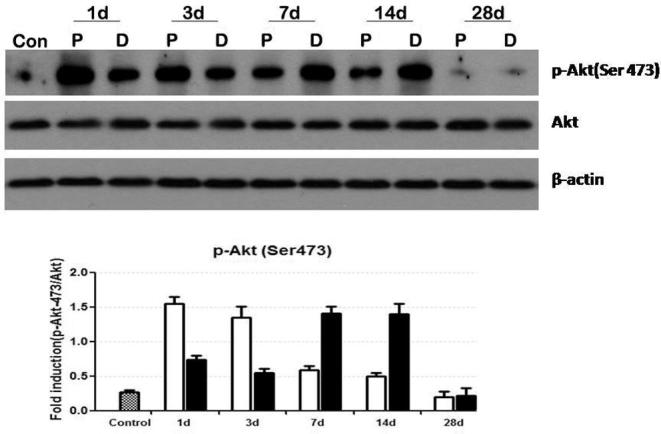
Expressions of P-AKT at different time points after axon injury.
4. Discussion and conclusion
Various vitro studies thought that ERK/MAPK and PI3K/Akt were the most important signal channels promoting the survival of nerve cells (Creedon et al., 1996, Edström and Ekström, 2003). Some studies confirmed that exogenous Ras could reduce the death of facial nerve cells due to the cutting of facial nerve and the quantity of the injured facial nerve cells was not statically significant while compared with the control group (Heumann et al., 2000). In addition, some studies indicated that PI3K/Akt means played an important role in protecting the damaged nerve cell (Crowder and Freeman, 1998). It was found in the in-vitro culture of DRG nerve ganglion that inhibiting the activity of PI3K channel might result in the death of DRG nerve cell (Edström and Ekström, 2003). Some studies showed that activating the ERK channel could promote spontaneous survival of nerve cells after nerve injury and the protective effect of neurotrophic factors (e.g. BDNF) for nerve cells was also subject to the ERK means (Obata et al., 2003). Moreover, the effects of NGF, GDNF and other cell factors in promoting survival of nerve cells should also depend on the PI3K means. Even if the neurotrophic factors NGF and GDNF existed, inhibiting the activity of PI3K or Akt also led to the death of nerve cells (Yao and Cooper, 1995).
Various vitro studies indicated that ERK could promote axon growth of DRG, SCG and other nerve cells and the growth rate of axon was correlated with the duration and strength of ERK signals (Atwal et al., 2000, Teow and Ali, 2017). For example, the growth of PC12 pheochromocytoma axon required continuous activation of ERK. The ERK channel might also play an important role in axon growth process stimulated by NGF, GDNF and other neurotrophic factors, which indicated that the ERK channel was quite important for autogenic and exogenous stimulus regeneration of axon (Traverse et al., 1992). The difference was that inhibiting ERK had no effect on autogenic axon growth of adult DRG, but might produce obvious inhibition for axon growth induced by growth factors. Like the functions of ERK, the enhancement of Akt phosphorylation level could promote the axon growth of in-growth and adult DRG nerve cells (Liu and Snider, 2001). In addition, it might also promote axon growth of NGF-induced sensory nerve. Different from the effect of ERK, the in-vitro culture revealed that AKT could promote not only prolongation of axon but also branch growth of axon, and inhibiting PI3K could reduce both autogenic axon growth and NGF and GDNF induced axon growth (Sjögreen et al., 2000, Alghadeer and Hornsby, 2017).
Compared with in-vitro studies, there were less in-vivo studies for ERK/MAPK and PI3K/Akt signal channels activated after peripheral nerve injury (Jones et al., 2003). In particular, there was no study for signal channel activation simultaneously in nerve cell and axon (Gallo and Letourneau, 1998), but the expression positions of P-ERK and P-AKT after peripheral nerve injury might be correlated with their functions, which was the basis for studying regeneration action of signal channel regulating nerve. On the other hand, the relation between ERK and AKT phosphorylation levels and injury times should be clarified further. The time variation of P-ERK and PAKT expression levels might represent the harmonious effects among different signal channels in nerve regeneration process (Markus et al., 2003). These studies are quite important for understanding the active regenerative mechanism after peripheral nerve injury (Edström and Ekström, 2003).
This study has discovered that the p-ERK and p-AKT expression levels in facial nerve cell rose on 1d after cutting of facial nerve while compared with the blank control group and the elevated expression levels could sustain 14 days and 28 days and even longer, respectively. Although this study did not involve the active mechanism of ERK/MAPK and PI3K/Akt signal channels, it was predicted in combination of the results of previous in-vitro studies that they might participate in regulating the survival after injury of facial nerve cell. Some scholars had found during studying sublingual nerve injury that Akt phosphorylation level was obviously improved in the adult sports nerve cell with the enhanced activity of nerve cells, but decreased in new sports nerve cell with more death of nerve cells. Therefore, the death of the new sports nerve cell after injury might be caused by inactivated Akt, which indicated that the Akt channel was quite important in activity of nerve cell after nerve injury.
In this study, the Western test revealed that the expressions of P-ERK and P-AKT enhanced in axon after the facial nerve was cut, especially in axon of the near section. It was conferred that they might take part in axon regeneration of the injured facial nerve. Some scholars had verified that the peripheral nerve injury might result in activation of ERK/MAPK signal channels in the axon and it had been deduced that it might participate in the process of axon regeneration. It was found in study of rat’s sciatic nerve injury that Akt phosphorylation level was enhanced obviously. In addition, some studies indicated that the distance of rat nerve regeneration was much lower than that of the control group after ERK had been inhibited, which indicated that ERK channel should be activated during regeneration after axon injury (Namikawa et al., 2000).
Western test revealed that P-ERK and P-AKT expressions enhanced in axon, which might derive from axon nerve fiber and Schwann cell around the axon, but pure Western test was difficult to distinguish their accurate expression positions. Previous studies verified that Schwann cell was statistically significant for regeneration after peripheral nerve injury, but ERK/MAPK and PI3K/Akt might be the important signal channel in regulating differentiation of Schwann cell (Agthong et al., 2006). The in-vitro studies indicated that activating the ERK/MAPK signal channel might induce Schwann cells to differentiate and proliferate (Yamazaki et al., 2009, Gao et al., 2017). Meanwhile, the in-vivo studies also indicated that P-ERK could be activated in Schwann cells after nerve injury, especially in Schwann cells of the far axon, so that the Schwann cell could be dedifferentiated into the precursor state and promote the proliferation of Schwann cell (Agthong et al., 2009). The above studies indicated that ERK/MAPK activation induced by nerve injury was quite important for dedifferentiation of Schwann cell (Agthong et al., 2006). In addition, some scholars found that only in-vivo activation of ERK channels could be sufficient to dedifferentiate myelinized Schwann cell into the precursor state, resulting in severe loss of sport functions, even if there was no nerve injury (Harrisingh et al., 2004). It indicated that the dedifferentiation of Schwann cells did not rely on axon degradation, but was dependent on persistence of P-ERK activity (Napoli et al., 2012). When P-ERK signal activity was lost, Schwann cell might be myelinized quickly and the nerve functions recovered. Contrary to P-ERK effect, some reports said that the activated PI3K/AKT signal channel could promote differentiation of Schwann cells and also believed that the significantly activated ERK and the activity-enhanced AKT joint played important roles in inducing activation and proliferation process of Schwann cells at the early state of nerve injury (Ogata et al., 2004). In this study, the Western test revealed that the expression level of P-ERK rose up gradually at the far end of axon, reached the peak value on 7d and then decreased subsequently, until down to the level before injury on 28 d. However, P-AKT was always at the activation state at the far end of axon after injury and its expression level was highest on 7 d and 14 d and then reduced accordingly but kept on a certain level on 28 d. According to the above results, it could be conferred that early ERK phosphorylation of nerve injury was activated and could neutralize P-AKT effect, so that Schwann cells were maintained at the immature precursor state and could keep the capacity of continuous differentiation; at the later period of nerve regeneration, P-ERK level decreased and kept at a higher level after the new axon gradually grew into the Büngner zone at the far end, so that myelinization of new axon could be promoted again. It indicated that the balance among different signal channels played an important role in regulating the functions of Schwann cells. Moreover, its regulating function was dependent on different stages of tissue effect.
Although this study could not certify which regulating effect the ERK/MAPK and PI3K/Akt signal channels brought into play during regeneration process after the facial nerve injury, but the immune grouping and Western tests could at least verify that both were activated in nerve cell and axon of facial nerve after injury. Moreover, P-ERK and P-AKT expressions had respective time effect and different signal channels were activated at the same position on different times, which might be significantly related to its regulating of nerve regeneration.
Acknowledgments
The work is financially supported by National Natural Science Foundation of China (30872898) and Beijing Municipal National Science Foundation (7132173).
Footnotes
Peer review under responsibility of King Saud University.
References
- Agthong S., Kaewsema A., Tanomsridejchai N., Chentanez V. Activation of MAPK ERK in peripheral nerve after injury. BMC Neurosci. 2006;8(7):45. doi: 10.1186/1471-2202-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agthong S., Koonam J., Kaewsema A., Chentanez V. Inhibition of MAPK ERK impairs axonal regeneration without an effect on neuronal loss after nerve injury. Neurol Res. 2009;31(10):1068–1074. doi: 10.1179/174313209X380883. [DOI] [PubMed] [Google Scholar]
- Alghadeer S., Hornsby L. Assessment of novel oral anticoagulant use within a community teaching hospital. Saudi Pharm. J. 2017;25(1):93–98. doi: 10.1016/j.jsps.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwal J.K., Massie B., Miller F.D., Kaplan D.R. The TrkB-Shc site signals neuronal survival and local axon growth via MEK and P13-kinase. Neuron. 2000;27(2):265–277. doi: 10.1016/s0896-6273(00)00035-0. [DOI] [PubMed] [Google Scholar]
- Carter A.N., Downes C.P. Phosphatidylinositol 3-kinase is activated by nerve growth factor and epidermal growth factor in PC12 cells. J. Biol. Chem. 1992;267(21):14563–14567. [PubMed] [Google Scholar]
- Creedon D.J., Johnson E.M., Lawrence J.C. Mitogen-activated protein kinase-independent pathways mediate the effects of nerve growth factor and cAMP on neuronal survival. J. Biol. Chem. 1996;271(34):20713–20718. doi: 10.1074/jbc.271.34.20713. [DOI] [PubMed] [Google Scholar]
- Crowder R.J., Freeman R.S. Phosphatidylinositol 3-kinase and Akt protein kinase are necessary and sufficient for the survival of nerve growth factor-dependent sympathetic neurons. J. Neurosci. 1998;18(8):2933–2943. doi: 10.1523/JNEUROSCI.18-08-02933.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edström A., Ekström P.A. Role of phosphatidylinositol 3-kinase in neuronal survival and axonal outgrowth of adult mouse dorsal root ganglia explants. J. Neurosci. Res. 2003;74(5):726–735. doi: 10.1002/jnr.10686. [DOI] [PubMed] [Google Scholar]
- Forcet C., Stein E., Pays L., Corset V., Llambi F., Tessier L.M., Mehlen P. Netrin-1-mediated axon outgrowth requires deleted in colorectal cancer-dependent MAPK activation. Nature. 2002;417(6887):443–447. doi: 10.1038/nature748. [DOI] [PubMed] [Google Scholar]
- Gallo G., Letourneau P.C. Localized sources of neurotrophins initiate axon collateral sprouting. J Neurosci. 1998;18(14):5403–5414. doi: 10.1523/JNEUROSCI.18-14-05403.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W., Wang Y.Q., Basavanagoud B., Jamil M.K. Characteristics studies of molecular structures in drugs. Saudi Pharm. J. 2017;25(4):580–586. doi: 10.1016/j.jsps.2017.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinsell D., Keating C.P. Peripheral nerve reconstruction after injury: a review of clinical and experimental therapies. Biomed. Res. Int. 2014 doi: 10.1155/2014/698256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrisingh M.C., Perez N.E., Parkinson D.B., Malcolm D.S., Mudge A.W., Lloyd A.C. The Ras/Raf/ERK signalling pathway drives Schwann cell dedifferentiation. Embo J. 2004;23(15):3061–3071. doi: 10.1038/sj.emboj.7600309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heumann R., Goemans C., Bartsch D., Lingenhöhl K., Waldmeier P.C., Hengerer B., Allegrini P.R., Schellander K., Wagner E.F., Arendt T., Kamdem R.H., Obst P.K., Narz F., Wahle P., Berns H. Transgenic activation of Ras in neurons promotes hypertrophy and protects from lesion-induced degeneration. J. Cell Biol. 2000;151(7):1537–1548. doi: 10.1083/jcb.151.7.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D.M., Tucker B.A., Rahimtula M., Mearow K.M. The synergistic effects of NGF and IGF-1 on neurite growth in adult sensory neurons: convergence on the PI3-kinase signaling pathway. J. Neurochem. 2003;86(5):1116–1128. doi: 10.1046/j.1471-4159.2003.01925.x. [DOI] [PubMed] [Google Scholar]
- Klesse L.J., Parada L.F. p21 ras and phosphatidylinositol-3 kinase are required for survival of wild-type and NF1 mutant sensory neurons. J. Neurosci. 1998;18(24):10420–10428. doi: 10.1523/JNEUROSCI.18-24-10420.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimaschewski L., Hausott B., Angelov D.N. The pros and cons of growth factors and cytokines in peripheral axon regeneration. Int. Rev. Neurobiol. 2013;108:137–171. doi: 10.1016/B978-0-12-410499-0.00006-X. [DOI] [PubMed] [Google Scholar]
- Koeppen A.H. Wallerian degeneration: history and clinical significance. J. Neurol. Sci. 2004;220(1–2):115–117. doi: 10.1016/j.jns.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Liu R.Y., Snider W.D. Different signaling pathways mediate regenerative versus developmental sensory axon growth. J. Neurosci. 2001;21(17) doi: 10.1523/JNEUROSCI.21-17-j0003.2001. RC164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markus A., Zhong J., Snider W.D. Raf and akt mediate distinct aspects of sensory axon growth. Neuron. 2003;35(1):65–76. doi: 10.1016/s0896-6273(02)00752-3. [DOI] [PubMed] [Google Scholar]
- Namikawa K., Honma M., Abe K., Takeda M., Mansur K., Obata T., Miwa A., Okado H., Kiyama H. Akt/protein kinase B prevents injury-induced motoneuron death and accelerates axonal regeneration. J. Neurosci. 2000;20(8):2875–2886. doi: 10.1523/JNEUROSCI.20-08-02875.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli I., Noon L.A., Ribeiro S., Kerai A.P., Parrinello S., Rosenberg L.H., Collins M.J., Harrisingh M.C., White I.J., Woodhoo A., Lloyd A.C. A central role for the ERK-signaling pathway in controlling Schwann cell plasticity and peripheral nerve regeneration in vivo. Neuron. 2012;73(4):729–742. doi: 10.1016/j.neuron.2011.11.031. [DOI] [PubMed] [Google Scholar]
- Obata K., Yamanaka H., Dai Y. Differential activation of extracellular signal-regulated protein kinase in primary afferent neurons regulates brain-derived neurotrophic factor expression after peripheral inflammation and nerve injury. J. Neurosci. 2003;23:4117–4126. doi: 10.1523/JNEUROSCI.23-10-04117.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata T., Iijima S., Hoshikawa S., Miura T., Yamamoto S., Oda H., Nakamura K., Tanaka S. Opposing extracellular signal-regulated kinase and Akt pathways control Schwann cell myelination. J. Neurosci. 2004;24(30):6724–6732. doi: 10.1523/JNEUROSCI.5520-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K.K., Liu K., Hu Y., Smith P.D., Wang C., Cai B. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Sustained axon regeneration induced by co-deletion of PTEN and SOCS3. [DOI] [PMC free article] [PubMed]
- Rich K.M., Disch S.P., Eichler M.E. The influence of regeneration and nerve growth factor on the neuronal cell body reaction to injury. J. Neurocytol. 1989;18(5):569–576. doi: 10.1007/BF01187077. [DOI] [PubMed] [Google Scholar]
- Schmid R.S., Pruitt W.M., Maness P.F. A MAP kinase-signaling pathway mediates neurite outgrowth on L1 and requires Src-dependent endocytosis. J. Neurosci. 2000;20(11):4177–4188. doi: 10.1523/JNEUROSCI.20-11-04177.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjögreen B., Wiklund P., Ekström P.A. Mitogen activated protein kinase inhibition by PD98059 blocks nerve growth factor stimulated axonal outgrowth from adult mouse dorsal root ganglia in vitro. Neuroscience. 2000;100(2):407–416. doi: 10.1016/s0306-4522(00)00278-5. [DOI] [PubMed] [Google Scholar]
- Sulaiman O.A., Gordon T. Effects of short- and long-term Schwann cell denervation on peripheral nerve regeneration, myelination, and size. Glia. 2000;32(3):234–246. doi: 10.1002/1098-1136(200012)32:3<234::aid-glia40>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Teow S.Y., Ali S.A. Altered antibacterial activity of Curcumin in the presence of serum albumin, plasma and whole blood. Pak. J. Pharm. Sci. 2017;30(2):449–457. [PubMed] [Google Scholar]
- Traverse S., Gomez N., Paterson H., Marshall C., Cohen P. Sustained activation of the mitogen-activated protein (MAP) kinase cascade may be required for differentiation of PC12 cells. Comparison of the effects of nerve growth factor and epidermal growth factor. Biochem. J. 1992;288(Pt 2):351–355. doi: 10.1042/bj2880351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki T., Sabit H., Oya T., Ishii Y., Hamashima T., Tokunaga A., Ishizawa S., Jie S., Kurashige Y., Matsushima T., Furuta I., Noguchi M., Sasahara M. Activation of MAP kinases, Akt and PDGF receptors in injured peripheral nerves. J. Peripher Nerv. Syst. 2009;14(3):165–176. doi: 10.1111/j.1529-8027.2009.00228.x. [DOI] [PubMed] [Google Scholar]
- Yao R., Cooper G.M. Requirement for phosphatidylinositol-3 kinase in the prevention of apoptosis by nerve growth factor. Science. 1995;267(5206):2003–2006. doi: 10.1126/science.7701324. [DOI] [PubMed] [Google Scholar]