Abstract
Objective
To study the protective effect of total flavonoid in rabdosia rubescens on BIT model by brain ischemic tolerance (hereinafter BIT) model of mice.
Method
BIT model is used to block bilateral common carotid arteries and to copy BIT model of mice. After 10 min of transient ischemia for rats in preconditioning group, the mice in the nimodipine group and naoluotong capsule group were given the total flavonoid in rabdosia rubescens (300 mg/kg, 150 mg/kg, 75 mg/kg) for gavage, sham operation group, ischemia/reperfusion injury (hereinafter IRI) group and BIT group were fed with the same volume of 0.5% sodium carboxymethyl cellulose (CMC) once a day for 5 days. After administration for 1 h on day 5 (120 h), the rats in the other groups except for the sham operation group were treated with blood flow block for 30 min and reperfusion for 22 h. The serum NSE level were measured and the brain NO content and NOS activity changes was measured to observe the histopathological changes of brain tissue.
Results
BIT models of mice and in rats were both successfully replicated. The total flavonoid in rabdosia rubescens can decrease the mortality of mice, decrease serum NSE level, increase the content of NO and the activity of NOS in the brain tissue of mice, and improve the pathological damage of cortex and hippocampus of mice.
Conclusion
The total flavonoid in rabdosia rubescens can stimulate an endogenous protective mechanism by inducing the release of low levels of cytokines NO and NOS, which reduces the release of serum NSE, relieves the brain tissue ischemia-reperfusion injury, and further improves the protection effect of ischemic preconditioning on brain injury. The damage of brain tissue ischemia and reperfusion, and further improve the ischemia Protective effect of preconditioning on brain injury.
Keywords: Total flavonoid in rabdosia rubescens, Ischemia Preconditioning (IPC), BIT model
1. Introduction
Rabdosia rubescens, which is slightly fragrant, bitter-sweet in taste and cold in nature, nourishing the lungs, stomach and liver, with detoxification, promoting blood circulation to arrest pain, invigorating stomach, anti-inflammatory and anti-tumor effects (Wang et al., 2016). The main components of rabdosia rubescens are diterpenoids, total flavonoids, alkaloids, organic acids, steroids, amino acids, volatile oil, etc. Studies have confirmed that rabdosia rubescens has anti-cell proliferation, inhibition of cancer cell DNA and protein synthesis as well as induction of cancer cell apoptosis and other active effects (Guo et al., 2010).
Modern medical studies have shown that a large number of oxygen free radicals (OFR) in the ischemic cascade, nitric oxide (Zhou and Zhu, 2009), excitatory amino acids, cytokines (He et al., 2010); etc. are converted to toxic substances, causing a series of electrochemical linkage reactions in brain tissue cells and irreversible injury, eventually leading to brain cell apoptosis and necrosis (Li and Huang, 2014). The body's scavenging activity of excessive toxic substances is limited, therefore, promoting blood circulation to remove blood stasis should be adopted for the general treatment of the above pathogenesis (Cheng et al., 2012). Through the summary of the literature, “promoting blood circulation to remove blood stasis while clearing heat and removing toxicity” by using traditional Chinese medicine for the prevention and treatment of cerebral ischemia is put forward in this paper (Cao et al., 2016). The approach focuses on improving the brain circulation, protecting nerve cells and scavenging free radicals to treat cerebral ischemic injury, it is based on “thermotoxic stroke”, aiming to reduce inflammatory response and the secondary reaction of cerebral ischemia. This new method has feasible preconditioning measures for clinical application, providing a new idea and a broad research prospect for the study of cerebral ischemic diseases.
2. Test materials
2.1. Drugs and reagents
Total flavonoid in rabdosia rubescens, provided by the Analytical Laboratory of Henan University of Chinese Medicine, with a total flavonoid content of 58% and LOT of 20130821.
Nimodipine tablets, LOT: 130660, manufacturer: Yabao Pharmaceutical Group Co., Ltd.
Naoluotong capsules, LOT: 130901, manufacturer: Jilin Jinbao Pharmaceutical Co., Ltd.
Penicillin sodium for injection, North China Pharmaceutical Group Corporation, LOT: C1206807;
Sodium chloride injection, Henan Shuanghe Huari Pharmaceutical Co., Ltd., LOT: 130629;
Coomassie brilliant blue assay kit, Nanjing Jiancheng Bioengineering Institute, LOT: 20130914;
Nitric oxide (NO) one-step kit, Nanjing Jiancheng Bioengineering Institute, LOT: 20130921;
Nitric Oxide Synthase (NOS) kit, Nanjing Jiancheng Bioengineering Institute, LOT: 20130916;
Mouse NSE ELISA Kit, R&D, LOT: 20130902B.
2.2. Laboratory apparatus
VIS-7220 N visible spectrophotometer, Beijing Ruili Analytical Instrument Co., Ltd.;
KDC-160HR high-speed refrigerated centrifuge, Zhongjia Branch of USTC Chuangxin Co., Ltd.;
BIORAD-680 microplate reader, 680Microplate Reader, Bio-Rad Laboratories;
2.3. Laboratory animals
Kunming mice, SPF level, male, weight of 25–30 g, provided by Henan Experimental Animal Center, animal certificate: NO.41003100000258; laboratory certificate No.: SYXK (Yu) 2010-001.
3. Test method
3.1. Grouping and administration
Take 128 male mice with a weight of 25–30 g, and divide into 8 groups, each group of 16. Nimodipine group (30 mg/kg, 0.1 ml/10 g, 15 times the clinical dose); naoluotong capsule group (750 mg/kg, 0.1 ml/10 g, 15 times the clinical dose); large, medium and small doses of total flavonoid in rabdosia rubescens group (300 mg/kg, 150 mg/kg, 75 mg/kg); sham operation group, IRI group, BIT model group (gavage with the same volume of 0.5% CMC).
3.2. Modeling method (Ma et al., 2012, Wang et al., 2011)
All mice were conducted with 0.03 ml/10 g abdominal anesthesia by 10% chloral hydrate, supinely fixed on the operating table, followed by neck disinfection using alcohol, and then use the scalpel for a middle incision, leaving blunt separation of bilateral common carotid arteries. In addition to the mice in sham operation group and IRI group, the bilateral common carotid arteries of mice in other groups were clamped with micro-arterial clamp for 10 min of blood flow block, and then restored perfusion. The mice in sham operation group and IRI group were exposed only their bilateral carotid arteries, with no blood flow block. After the animals were awakened, the corresponding drugs were given. The mice in BIT model group, IRI group and sham operation group were given the same volume of 0.5% CMC, once daily for 5 days, after administration for 1 h at day 5 (120 h) after modeling, all mice were conducted with anesthesia by 10% chloral hydrate, supinely fixed on the operating table, followed by neck disinfection using alcohol, and then use the scalpel for a middle incision, leaving blunt separation of bilateral common carotid arteries. In addition to the mice in sham operation group, the bilateral common carotid arteries of mice in other groups were clamped with micro-arterial clamp for 30 min of blood flow block, then restored perfusion. The mice in sham operation group were exposed only their bilateral common carotid arteries, with no blood flow block. Intraoperative ambient temperature was maintained at 25–26 °C
4. Statistical processing approach
SPSS 17.0 for windows is adopted for statistical analysis of data, measurement data are expressed by using the mean ± standard deviation ( ± s). The one-way ANOVA analysis is adopted for comparison between groups. The least significant difference (LSD) method is adopted for regular variances, and Games-Howell test is adopted for irregular variances. Grade data adopt Ridit test, it is statistically significant when P < .05.
5. Test results
5.1. The effect of total flavonoid in rabdosia rubescens on the mortality of BIT mice
As can be seen from Table 1, the mortality of BIT model group decreased compared with the IRI group, to some extent it proves that ischemic preconditioning induced BIT production. The mortality of mice in nimodipine group, naoluotong capsule group, large- and medium-dose total flavonoid in rabdosia rubescens group reduced.
Table 1.
The effect of total flavonoid in rabdosia rubescens on the mortality of BIT model mice.
| Group | Dose (mg/kg) | n1 (At the beginning) | n2 (After test) | Mortality (%) |
|---|---|---|---|---|
| Sham operation group | – | 16 | 16 | 0 |
| IRI group | – | 16 | 11 | 31.25 |
| BIT model group | – | 16 | 13 | 18.75 |
| Nimodipine group | 30 | 16 | 12 | 25.00 |
| Naoluotong capsule group | 750 | 16 | 14 | 12.50 |
| High-dose total flavonoid in rabdosia rubescens group | 300 | 16 | 14 | 12.5 |
| Medium-dose total flavonoid in rabdosia rubescens group | 150 | 16 | 12 | 25 |
| Small-dose total flavonoid in rabdosia rubescens group | 75 | 16 | 11 | 31.25 |
5.2. The effect of total flavonoid in rabdosia rubescens on serum NSE level in BIT model mice
As can be seen from Table 2, NSE level in sham operation group mice serum was low. Compared with the sham operation group, the level of NSE in IRI group was significantly increased (P < .01). Compared with the IRI group, the ischemic preconditioning groups can significantly decrease the serum NSE level (P < .01). Compared with the BIT model group, serum NSE levels were significantly decreased in each administration group (P < .01).
Table 2.
The effect on serum NSE level of BIT model mice ().
| Group | n | Dose (mg/kg) | NSE (ng/ml) |
|---|---|---|---|
| Sham operation group | 12 | – | 1.45 ± 0.33**△△ |
| IRI group | 11 | – | 3.50 ± 0.27 |
| BIT model group | 13 | – | 2.35 ± 0.18△△ |
| Nimodipine group | 12 | 30 | 1.58 ± 0.21**△△ |
| Naoluotong capsule group | 14 | 750 | 1.83 ± 0.33**△△ |
| High-dose total flavonoid in rabdosia rubescens group | 14 | 300 | 1.52 ± 0.42**△△ |
| Medium-dose total flavonoid in rabdosia rubescens group | 12 | 150 | 1.95 ± 0.27**△△ |
| Small-dose total flavonoid in rabdosia rubescens group | 11 | 75 | 1.78 ± 0.18**△△ |
Note: Compared with the IRI group, △P < .05, △△P < .01; compared with the BIT model group, *P < .05, **P < .01.
5.3. The effect of total flavonoid in rabdosia rubescens on NO and NOS activity in brain homogenate of BIT model mice
It can be seen from Table 3 that the NO content in brain homogenate in IRI group was significantly higher than that in sham operation group (P < .01). Compared with IRI group, the content of NO in brain homogenate was significantly increased by preconditioning group (P < .01). Compared with BIT model group, NO content in brain homogenate in high-dose total flavonoid in rabdosia rubescens group was significantly increased (P < .05). There was no significant difference between NO content in the brain homogenate by comparing nimodipine group, naoluotong capsule group, medium- and small-dose total flavonoid in rabdosia rubescens group and BIT model group (P > .05). Compared with IRI group, the preconditioning groups could significantly enhance the activity of NOS in brain homogenate (P < .01, P < .05). Compared with BIT model group, nimodipine group, large- and medium-dose total flavonoid in rabdosia rubescens group can significantly attenuate the activity of NOS in brain homogenate (P < .01), and small-dose total flavonoid in rabdosia rubescens group can significantly attenuate the activity of NOS in brain homogenate (P < .05), NOS in brain homogenate in naoluotong capsule group was not significantly abated (P > .05).
Table 3.
The effect on NO and NOS activity in brain homogenate of BIT model mice ().
| Group | n | Dose (mg/kg) | NO (μmol/gprot) | NOS (U/mgprot) |
|---|---|---|---|---|
| Sham operation group | 12 | – | 4.84 ± 1.59**△△ | 7.15 ± 2.57**△△ |
| IRI group | 11 | – | 8.25 ± 2.04 | 17.08 ± 4.21 |
| BIT model group | 13 | – | 12.04 ± 2.52△△ | 28.66 ± 5.16△△ |
| Nimodipine group | 12 | 30 | 13.67 ± 2.13△△ | 22.54 ± 3.69**△△ |
| Naoluotong capsule group | 14 | 750 | 11.54 ± 2.62△△ | 25.47 ± 4.71△△ |
| High-dose total flavonoid in rabdosia rubescens group | 14 | 300 | 14.13 ± 2.26*△△ | 20.89 ± 4.62**△ |
| Medium-dose total flavonoid in rabdosia rubescens group | 12 | 150 | 13.15 ± 2.99△△ | 22.82 ± 4.28**△△ |
| Small-dose total flavonoid in rabdosia rubescens group | 11 | 75 | 12.25 ± 1.93△△ | 24.10 ± 5.24*△ |
Note: Compared with IRI group, △P < .05, △△P < .01; compared with BIT model group, *P < .05, **P < .01.
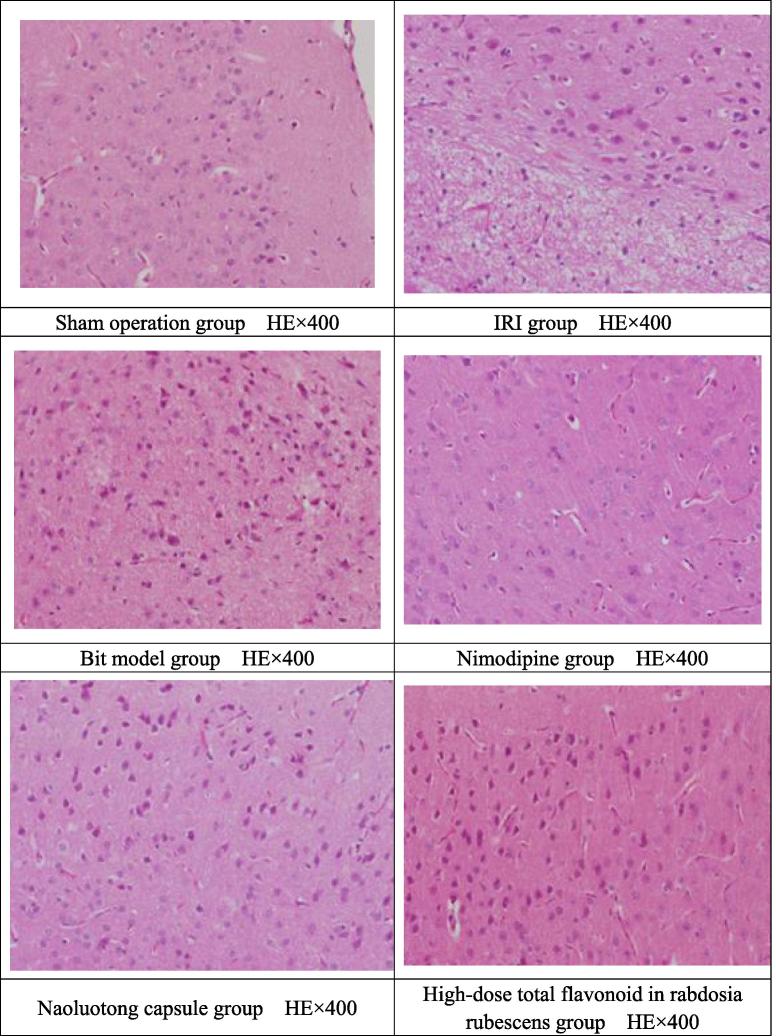
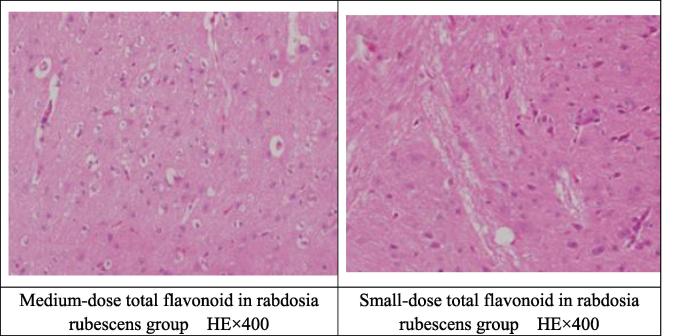
5.4. The effect of total flavonoid in rabdosia rubescens on pathological changes of cortex and hippocampus in BIT model mice
The effect of total flavonoid in rabdosia rubescens on brain histopathology of BIT model mice was observed as follows. As for sham operation group, brain tissue structure was basically normal; IRI group: neurons in the cerebral cortex and hippocampus had significant edema and stained cytoplasm, with tabular necrosis in most of the cortical neurons; BIT model group: most of the cerebral cortex and hippocampus neuronal cell structure were blurred, with cell edema, cytoplasm pale staining and necrosis in most neuronal cells, a very small number of focal necrosis; nimodipine group: neurons in the cerebral cortex and hippocampus had cell edema, cytoplasm pale staining and necrosis in some neuronal cells; naoluotong capsule group: cerebral cortex and hippocampus neuronal cell structure had cell edema, cytoplasm pale staining and necrosis in some neuronal cells, a very small number of focal necrosis; total flavonoid in rabdosia rubescens administration group: most of the cerebral cortex and hippocampus neuronal cell structure had slight cell edema, cytoplasm pale staining and necrosis in some neuronal cells; where, high-dose total flavonoid in rabdosia rubescens group: some of the cerebral cortex and hippocampus neuronal cells returned to normal functions. Medium- and small-dose total flavonoid in rabdosia rubescens group had necrosis in some neuronal cells and a very small number of focal necrosis. Specific pathology is shown in Table 4.
Table 4.
The effect on brain tissue histology changes in BIT model of mice.
| Group | Dose (mg/kg) | n | Level |
P | |||
|---|---|---|---|---|---|---|---|
| − | + | ++ | +++ | ||||
| Sham operation group | – | 12 | 12 | 0 | 0 | 0 | **△△ |
| IRI group | – | 11 | 0 | 1 | 4 | 6 | |
| BIT model group | – | 13 | 0 | 4 | 8 | 1 | △ |
| Nimodipine group | 30 | 12 | 0 | 6 | 6 | 0 | △△ |
| Naoluotong capsule group | 750 | 14 | 0 | 6 | 6 | 2 | △ |
| High-dose total flavonoid in rabdosia rubescens group | 300 | 14 | 2 | 5 | 7 | 0 | △△ |
| Medium-dose total flavonoid in rabdosia rubescens group | 150 | 12 | 0 | 2 | 8 | 2 | |
| Small-dose total flavonoid in rabdosia rubescens group | 75 | 11 | 0 | 3 | 7 | 1 | △ |
Note: Compared with IRI group, △P < .05, △△P < .01; compared with BIT model group, *P < .05, **P < .01.
By Ridit test, compared with sham operation group, IRI group had significant statistical significance (P < .01), indicating that modeling was successful. Compared with IRI group, BIT model group, naoluotong capsule group and small-dose total flavonoid in rabdosia rubescens group could significantly improve the pathological damage of brain tissue of mice (P < .05). Nimodipine group and high-dose total flavonoid in rabdosia rubescens group could significantly improve the pathological changes of the brain tissue of the mice (P < .01). The comparison between BIT model group and other administration groups was not statistically significant (P < .05).
6. Conclusion
By observing the mortality, serum NSE level, NO and NOS activity in brain homogenate and pathological changes of brain tissue injury in BIT model of mice, it suggests that total flavonoid in rabdosia rubescens can reduce animal mortality and serum NSE level, elevate NO and NOS content in inflammatory cells of brain homogenate. The low concentration of NO do not harm the nerve cells, instead they can stimulate the body's own defense system to produce anti-injury, improve the brain tissue cortex and hippocampus neuronal cell damage and protect brain tissue.
In this paper, the BIT model of mice was successfully copied. The test results show that the number of animal deaths after drug preconditioning is reduced, stimulating the body to produce endogenous protective effect and improving the ischemia-reperfusion injury of brain tissue. Thus, ischemic preconditioning has a protective effect on brain tissue. After preconditioning, total flavonoid in rabdosia rubescens had a protective effect on brain tissue.
Acknowledgements
The work was financially supported by ‘National Natural Science Foundation of China (81274154)’ – ‘China’, ‘Henan Science and Technology Key Project (132102310104)’ – ‘China’, and ‘Henan Excellent Scientific and Technical Innovation Group’ – ‘China’ (TCJ2014-391).
Footnotes
Peer review under responsibility of King Saud University.
Appendix
Histopathological changes of brain tissue in BIT model mice with total flavonoid in rabdosia rubescens
References
- Cao Lihua, Zheng Yan, Xin Weiyun, Xu Kun, Miao Mingsan. The effect of total flavonoid in ilex pubesceus on mice with blood stasis combined with cerebral ischemic tolerance model. China J. Chin. Mater. Med. 2016;41(18):3419–3424. doi: 10.4268/cjcmm20161817. [DOI] [PubMed] [Google Scholar]
- Cheng Xiao, Zhang Xiaoli, Bai Ming. Prevention and treatment characteristics and analysis of cerebral ischemia by promoting blood circulation to remove blood stasis and by clearing heat and removing toxicity. World J. Chin. Med. 2012;27(5):615–619. [Google Scholar]
- Guo Ping, Li Yushan, Guo Yuanqiang. Study on chemical constituents and pharmacological activities of rabdosia rubescens. Drug Eval. Res. 2010;33(2):144–147. [Google Scholar]
- Hu Yonghong, Zhou Aimin, Tian Ye. Effect of ligustrazine on cerebral ischemic tolerance and its mechanism. Chin. J. Basic Med. Trad. Chin. Med. 2010;16(8):671–674. [Google Scholar]
- Li Jinying, Huang Qihui. Research progress on the prevention and treatment of cardiovascular and cerebrovascular disease by clearing heat and removing toxicity. Chin. J. Integrative Med. Cardio-/Cerebrovas. Dis. 2014;12(1):87–90. [Google Scholar]
- Ma Xiao, Wang Zhaohui, Miao Mingtao. Study on the dose for the induction of cerebral ischemic tolerance of mice. J. Henan Med. College Staff Workers. 2012;24(1):1–3. [Google Scholar]
- Wang Can, Fang Xiaoyan, Miao Mingsan. Determination of total flavonoids in effective parts of rabdosia rubescens. World J. Chin. Med. 2016;31(07) 1018-1020+1035. [Google Scholar]
- Wang Zhaohui, Yan Guohua, Ma Xiao. Study on time window of BIT induced by Mice. Heilongjiang Sci. Technol. Inform. 2011;34:80. [Google Scholar]
- Zhou L., Zhu D.Y. Neuronal nitric oxide synthase: structure, subcellular localization, regulation, and clinical implications. Nitric Oxide Biol. Chem. 2009;20(4):223–230. doi: 10.1016/j.niox.2009.03.001. [DOI] [PubMed] [Google Scholar]