Abstract
The purpose of this study was to evaluate the antifeedant and oviposition-deterring activity of total ginsenosides against P. rapae. Total ginsenosides exhibited increased antifeedant effects against P. rapae. The highest nonselective and selective antifeedant activity were observed at 2.0% concentration where ginsenosides caused antifeedant percentages of 86.09 and 88.90, respectively. The total ginsenosides showed significantly oviposition-deterring activity of 77.78% against oviposition of P. rapae at 1.0% concentration. Total ginsenosides had antifeeding activity against P. rapae and inhibitory effects on its oviposition. Ginsenosides could be used as an agent to prepare botanical new pesticidal formulations.
Keywords: Total ginsenosides, Pieris rapae, Feeding behavior, Ooviposition-deterring activity
1. Introduction
Panax ginseng C.A. Meyer is one of the most traditional medicinal herb in East Asia. Triterpene glycoside saponin, named ginsenoside, the major active component in ginseng which have various biological and pharmacological activities (Liu et al., 2010). However, while the pharmacological properties of ginsenosides have been extensively studied, their ecological role has not been systematically investigated. Secondary metabolites of some plants were well known that may act as kairomones, allomones, stimulants or deterrents of feeding and oviposition, antifeedants, insecticides and insect hormones (Nawrot et al., 1986). Saponins are generally considered to be part of defense systems to counter pathogens and pest attacks (Francis et al., 2002, Sparg et al., 2004, Augustin et al., 2011) and can be anticipated to influence herbivore feeding behavior (Kubo, 2006, Goławska et al., 2008, Goławska et al., 2010). The bitter taste of ginsenosides makes them either insecticides or antifeedants to phytophagous insects (Ikbal et al., 2007, Saha et al., 2010). Although many researches have widely studied the effects of saponins and flavonoids on insects, little is known about ginsenosides affecting insect behavior, especially feeding behavior. Therefore, in this paper, the effects of ginsenosides on feeding behavior and oviposition-deterring activity of P. rapae are examined in detail.
2. Materials and methods
Insects—Third instar larvae of P. rapae were collected from the cabbage experimental fields in Jilin Agricultural University, Changchun, China. The laboratory colony of P. rapae was maintained as previously described (Zhang et al., 2005). Briefly, P. rapae larvae were raised on fresh cabbage leaves in a plastic cage (55 cm × 55 cm × 55 cm, 1.0 mm × 1.0 mm mesh) at 25 ± 1 °C and 65 ± 5% RH under a L16:D8 photoperiod. The adults were then kept in a cage in the greenhouse until used in the experiments.
Chemicals--The total ginsenosides (purity ≥ 80%, UV method) were purchased from Jilin Hongjiu Biotech Co., Ltd. These were dissolved in distilled water.
2.1. Bioassay for feeding behavior and oviposition-deterring activity
A leaf disc bioassay was used to test insect for evaluation of antifeedant activity (Yan, 2011, Wen, 2010). The ginsenoside concentrations were 2.0%, 1.0% and 0.5% (mass fraction (MF)). These concentrations are within the range of ginsenoside levels normally found in P. ginseng. Leaf discs(Φ = 2.0 cm) were cut from fresh cabbage leaves (Brassica oleracea). Six excised cabbage leaf discs (Φ = 2.0 cm), soaked in solutions with different concentrations of ginsenosides for 30 min (control discs received distilled water only) then dried at room temperature. It was treated and control discs were placed in each compartment of a plastic test tray. The third instar larvae were starved for 8 h and gently introduced into the center of each compartment. The amount of larvae was 20 per treatment, and each experiment was repeated three times. The area of feeding on the leaves was measured every 3 h using squared grid paper. The selective antifeeding rate (%) formula was [(C − T)/(C + T)] × 100, and the non-selective antifeeding rate (%) formula was [(C − T)/C)] × 100, where C and T are the areas consumed by the control and treated leaf disks, respectively. The data were analyzed by variance analysis and means were compared for significance by a Student–Newman–Keuls test at the 5% level (P < .05) (Cohort Software Inc., 1985).
2.2. Repellent effect of ginsenosides on oviposition
When the seedlings reached the 6–8-leaf stage, ten potted cabbage plants were sprayed with the 0.5% concentration of ginsenosides 20 mL, while ten control pots were sprayed with 20 mL distilled water, then they were placed in three large outdoor cages (70 cm × 55 cm × 60 cm) made of aluminium bracket and woven nylon mesh (1.5 mm mesh). The cages were directly placed in a glass greenhouse and a bunch of cotton soaked in 10% honey aqueous solution were placed at the centre of each cage. Five gravid cabbage moth females that had been ovipositing for 24 h and five males were put into each cage. The males were introduced to make the experimental environment as far as possible to natural environment. The quantity of eggs on the cabbages was recorded after 24 h treatment. The oviposition rate was calculated by the formula:
(C: The number of eggs on the untreated cabbage leaf, T: The number of eggs on the ginsenosides treated cabbage leaf). Each experiment was repeated 3 times.
2.3. Statistical analysis
All the results from experimental replicates were expressed as the means (±S.E.M.) and analyzed by one-way analysis of variance (ANOVA) and t-test using SPSS 17.0 for Windows software.
3. Results
In the present study, the total ginsenosides had nonselective antifeedant activity against P. rapae at various concentrations and the activity was statistically significant over control. The nonselective antifeeding ratios of third instar P. rapae at 3, 6 and 9 h are shown in Table 1. Nonselective antifeedant activity and ginsenoside concentration had a significantly correlation. As the total ginsenoside concentration increased, the nonselective antifeeding activity was enhanced. Total ginsenosides showed a strong antifeedant activity of 86.09% against P. rapae at 0.2% concentration and the activity was statistically significant over control (Table 1).
Table 1.
Nonselective antifeedant ratio of total ginsenoside on third instar larvae of P. rapae.
| Concentration of total ginsenosides (%) | 3 h |
6 h |
9 h |
|||
|---|---|---|---|---|---|---|
| Average feeding area (cm2) | Nonselective antifeeding ratio (%) | Average feeding area (cm2) | Nonselective antifeeding ratio (%) | Average feeding area (cm2) | Nonselective antifeeding ratio (%) | |
| Control | 3.76 ± 0.37 | – | 3.67 ± 0.24 | – | 3.73 ± 0.31 | – |
| 0.5 | 1.87 ± 0.20 | 50.38c | 2.16 ± 0.26 | 41.24c | 2.24 ± 0.25 | 40.16c |
| 1.0 | 1.19 ± 0.12 | 68.29b | 1.79 ± 0.35 | 51.14b | 2.16 ± 0.31 | 42.11b |
| 2.0 | 0.52 ± 0.19 | 86.09a | 0.78 ± 0.12 | 78.75a | 1.19 ± 0.35 | 68.06a |
Data are presented as the means ± SE (P < .05).
The selective antifeeding ratios of the third instar P. rapae larvae are shown in Table 2. The third instar P. rapae larvae preferred to eat the leaves of the control; however, they did consume a small amount of the treated leaves. The total ginsenosides had significant inhibitory effect on third instar P. rapae larvae, and this effect increased with an increase in total ginsenoside concentration decreased as the time prolonged. The total ginsenosides showed a strong antifeedant activity of 88.90%, 81.02% and 76.58% against P. rapae larvae at 2.0% concentration at 3 h, 6 h and 9 h, respectively and the activities statistically significant over control.
Table 2.
Selective antifeedant ratio of total ginsenosides on third instar larvae of P. rapae.
| Concentration of total ginsenosides (%) | 3 h |
6 h |
9 h |
|||
|---|---|---|---|---|---|---|
| Average feeding area (cm2) | Selective antifeeding ratio (%) | Average feeding area (cm2) | Selective antifeeding ratio (%) | Average feeding area (cm2) | Selective antifeeding ratio (%) | |
| 0.5 | 0.83 ± 0.29 | 56.22c | 1.24 ± 0.21 | 45.62c | 1.09 ± 0.27 | 42.67c |
| Control | 2.97 ± 0.50 | – | 3.31 ± 0.16 | – | 2.72 ± 0.35 | – |
| 1.0 | 0.38 ± 0.13 | 80.64b | 0.60 ± 0.11 | 70.19b | 0.73 ± 0.17 | 64.62b |
| Control | 3.53 ± 0.55 | – | 3.41 ± 0.16 | – | 3.40 ± 0.28 | – |
| 2.0 | 0.20 ± 0.02 | 88.90a | 0.36 ± 0.06 | 81.02a | 0.45 ± 0.12 | 76.58a |
| Control | 3.46 ± 0.83 | – | 3.43 ± 0.06 | – | 3.39 ± 0.37 | – |
Data are presented as the means ± SE.
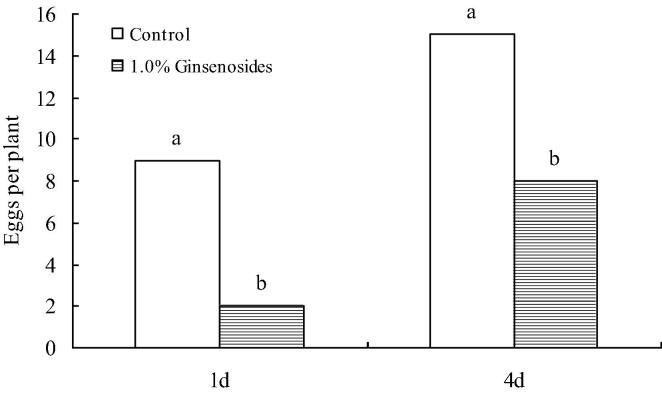
The amount of eggs for ginsenoside-treated plants were significantly lower than the controls (Fig. 1). The total ginsenosides had significant inhibitory effect on oviposition deterrence of P. rapae, and this effect decreased as the time prolonged. The total ginsenosides showed a strong oviposition-deterring activity of 77.78% and 46.67% against oviposition of P. rapae at 1.0% concentration at 1 d and 4 d, respectively and the activities statistically significant over control.
Fig. 1.
The number of eggs on plants treated with ginsenoside or control on P. rapae.
4. Discussion
Secondary metabolites of plants play an important role in insect defense against herbivores as growth regulators or by acting as antifeedants (Isman, 2006). Our research results have shown that the total ginsenosides had strong feeding deterrent effects against third instar P. rapae larvae and inhibitory effect on oviposition deterrence of P. rapae. The activities were concentration dependent. Our results are consistent with those from previous studies that triterpenoid saponins are repellent or deterrent to some insect herbivores (Gao et al., 2011, Geyter et al., 2007). In conclusion, the present results indicate that total ginsenosides possess potent antifeedant activities against P. rapae. Due to their strong feeding deterrent properties ginsenosides could be used to protect plants in the greenhouse or field. The ginsenosides might be considered as major compounds for developing safe alternative plant protection agents. Further studies on pesticidal and toxicological effects of ginsenosides are needed.
Acknowledgments
This work was funded by the National Natural Science Foundation of China (No. 31100239, No. 31200224, No. 31470420), the Funded Projects for Science and Technology Development Plan of Jilin Province (No. 20130206030YY, 20140520159JH, 20170204018YY), and the “13th Five-Year” Science and Technology Research Project supported by the Ministry of Education Department of Jilin Province (No. 2016-198).
Footnotes
Peer review under responsibility of King Saud University.
References
- Augustin J.M., Kuzina V., Anderson S.B., Bak S. Molecular activities, biosynthesis and evolution of triterpenoid saponins. Phytochemistry. 2011;72:435–457. doi: 10.1016/j.phytochem.2011.01.015. [DOI] [PubMed] [Google Scholar]
- Cohort Software Inc, 1985. Costat User's Manual. Version 3 Cohort Tucson, Arizona, USA.
- Francis G., Kerem Z., Makkar H.P.S., Becker K. The biological action of saponins in animal systems: a review. Br. J. Nutr. 2002;88:587–605. doi: 10.1079/BJN2002725. [DOI] [PubMed] [Google Scholar]
- Gao G.C., Lu Z.X., Tao S.H., Zhang S., Wang F.Z. Triterpenoid saponins with antifeedant activities from stem bark of Catunaregam spinosa (Rubiaceae) against Plutella xylostella (Plutellidae) Carbohyd Res. 2011;346:2200–2205. doi: 10.1016/j.carres.2011.07.022. [DOI] [PubMed] [Google Scholar]
- Geyter E.D., Lambert E., Geelen D., Smagghe G. Novel advances with plant saponins as natural insecticides to control pest insects. Pest Tech. 2007;1:96–105. [Google Scholar]
- Goławska S., Łukasik I., Goławski A., Kapusta I., Janda B. Alfalfa (Medicago sativa L.) apigenin glycosides and their effect on the pea aphid (Acyrthosiphon pisum) Pol. J. Environ. Stud. 2010;19:913–919. [Google Scholar]
- Goławska S., Łukasik I., Leszczyński B. Effect of alfalfa saponins and flavonoids on pea aphid. Entomol. Exp. Appl. 2008;128:147–153. [Google Scholar]
- Ikbal C., Monia B.H.K., Mounir T., Wassila H., Najet R., Dorsaf B.A., Mejda D., Habib B.H.M. Pesticidal potentialities of Cestrum parqui saponins. Int. J. Agric. Res. 2007;2:275–281. [Google Scholar]
- Isman M.B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Ann. Rev. Entomol. 2006;51:45–66. doi: 10.1146/annurev.ento.51.110104.151146. [DOI] [PubMed] [Google Scholar]
- Kubo, I., 2006. New concept to search for alternate insect control agents from plants. In: Rai, M., Carpinella, M. (Eds.), Naturally Occurring Bioactive Compounds, vol. 3, pp. 61–80.
- Liu L., Zhu X.M., Wang Q.J., Zhang D.L., Fang Z.M., Wang C.Y., Wang Z., Sun B.S., Wu H., Sung C.K. Enzymatic preparation of 20(S, R)-protopanaxadiol by transformation of 20(S, R)-Rg3 from black ginseng. Phytochemistry. 2010;71:1514–1520. doi: 10.1016/j.phytochem.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Nawrot J., Bloszyk E., Harmatha J., Novotyn L., Drozdz B. Action of antifeedants of plant origin on beetles infesting stored products. Acta Ent. Bohemoslov. 1986;83:327–335. [Google Scholar]
- Saha S., Walia S., Kumar J., Dhingra S., Parmar B. Screening for feeding deterrent and insect growth regulatory activity of triterpenic saponins from Diploknema butyracea and Sapindus mukorossi. J. Agric. Food Chem. 2010;58:434–440. doi: 10.1021/jf902439m. [DOI] [PubMed] [Google Scholar]
- Sparg S.G., Light M.E., van Staden J. Biological activities and distribution of plant saponins. J. Ethnopharmacol. 2004;94:219–243. doi: 10.1016/j.jep.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Wen L.Z. Science Press; Beijing, China: 2010. Introduction to Entomology Research Methods and Techniques. [Google Scholar]
- Yan F.M. Science Press; Beijing, China: 2011. Chemical Ecology. [Google Scholar]
- Zhang Z., Ye G.Y., Cai J., Hu C. Comparative venom toxicity between Pteromalus puparum and Nasonia vitripennis (Hymenoptera: Pteromalidae) toward the hemocytes of their natural hosts, non-target insects and cultured insect cells. Toxicon. 2005;46:337–349. doi: 10.1016/j.toxicon.2005.05.005. [DOI] [PubMed] [Google Scholar]