Abstract
Objectives
Comparison of Ticagrelor vs clopidogrel in antiplatelet therapeutic effect of acute myocardial infarction patients undergoing percutaneous coronary intervention.
Methods
The study focused on 2000 acute myocardial infarction patients undergoing percutaneous coronary intervention (PCI) in our hospital from January 2013 to December 2015. To reduce the formation of acute stent thrombosis caused by clopidogrel resistance, we had two options, one was to double the dosage of clopidogrel, and the other was to substitute ticagrelor for clopidogrel. Based on random number table method, the 2000 patients were divided into experimental group and control group, each containing 1,000 patients. The patients in experimental group took 180 mg ticagrelor before PCI and 90 mg ticagrelor twice a day after PCI (Gu, 2016). In contrast, the patients control group took 600 mg clopidogrel before PCI and 150 mg clopidogrel once a day after PCI. Both groups were drawn 2.7 ml of fasting venous blood for platelet aggregation rate test before PCI and 2 h, 24 h, 7 days after PCI respectively. Turbidimetric method was used to measure the ADP-induced platelet aggregation rate and observe change of platelet aggregation rate and success rate. Incidence of liver and kidney malfunction and adverse actions were monitored. All patients accepted a 6-month of follow-up examination to record and compare incidences of major adverse cardiac and cerebrovascular events. The statistical results of both groups are analyzed and compared.
Results
The platelet aggregation rate of experimental group before PCI and 2 h, 24 h, 7 days after PCI was 59.71% ± 7.24%, 59.20% ± 7.70%, 48.66% ± 7.80% and 43.39% ± 8.28%; The control group was 58.04% ± 5.61%, 56.25% ± 6.02%, 55.68% ± 3.14%, 53.94% ± 5.30%; Comparing the platelet aggregation rate of different time, P was less than 0.05. The success rate of platelet aggregation of experimental group and control group was 80.56% and 46.86% respectively. There were significant differences between the two groups and the P was less than .05. The postoperative serum creatinine level of experimental group was higher than that in the control group (P < .05). The incidence of adverse reactions in the experimental group was significantly lower than that of the control group. There were significant differences between the two groups and the difference was of statistical significance (P < .05). According to the 5-month follow-up examination: the incidence of major adverse cardiac and cerebrovascular events in experimental group was 2.60% (52/2000) ,while the control group was 13.00% (260/2000) . There were significant differences between the two groups and the difference was of statistical significance (P < .05).
Conclusions
Compared with clopidogrel, ticagrelor can achieve better n antiplatelet effect for patients with acute myocardial infarction undergoing percutaneous coronary intervention (PCI). It can effectively reduce the incidence of postoperative adverse cardiac and cerebrovascular events and control the rate of adverse reactions within the acceptable range.
Keywords: Ticagrelor, Clopidogrel, Percutaneous coronary intervention (PCI), Adverse reactions
1. Introduction
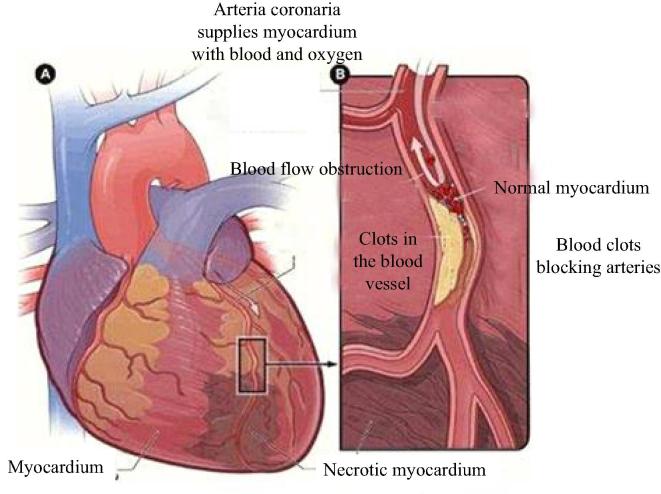
Percutaneous coronary intervention (PCI) is currently an effective method (Wei et al., 2015) used in clinical treatment of acute myocardial infarction (see Fig. 1). Scientific and effective antiplatelet therapy can significantly reduce the incidence of myocardial ischemia and inner frame thrombosis and other adverse events in patients (see Fig. 2) undergoing percutaneous coronary intervention (PCI). This study analyzed the antiplatelet therapeutic effect of ticagrelor vs clopidogrel for acute myocardial infarction patients undergoing percutaneous coronary intervention (PCI).
Fig. 1.
Schematic diagram of acute myocardial infarction.
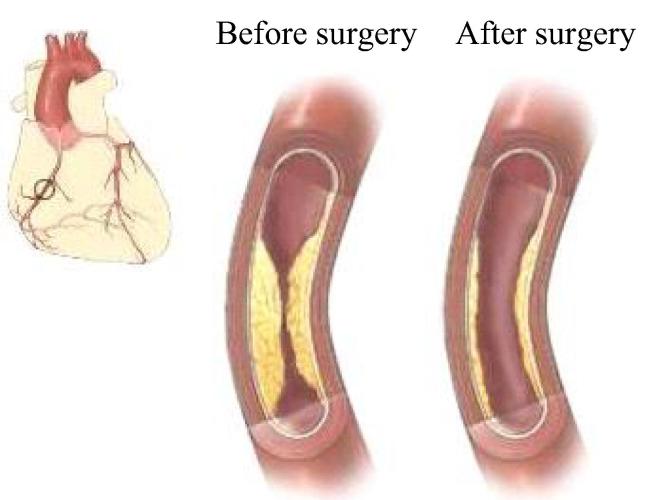
Fig. 2.
Schematic diagram of PCI.
2. Materials and methods
2.1. General reference
2000 patients who had been treated by undergoing percutaneous coronary intervention in our hospital from January 2013 to December 2015 were selected as research objects. To reduce the formation of acute stent thrombosis caused by clopidogrel resistance, we had two options, one was to double the dosage of clopidogrel, and the other was to substitute ticagrelor for clopidogrel. Among the 2000 patients, 1100 were male patients, 900 were female patients. Their age ranged from 62 to 78, with average age of (68.34 ± 6.34). Based on random number table method, the 2000 patients were divided into experimental group and control group, each containing 1,000 patients.
The experimental group included 550 male patients and 450 female patients, with age ranging from 62 to 78, with average value at (60.51 ± 9.45). Complicating diseases in experiment group included 270 cases of hypertension, 220 cases of diabetes, 210 cases of hyperlipidemia; platelet aggregation rate wass (58.04 ± 5.61). By comparing the general information of experimental group and control group, we can find that there were no significant differences in case numbers, gender, age, other associated diseases, and platelet aggregation rate. Statistical analysis showed that P was greater than 0.05, therefore both groups can be observed as controlled trials.
2.2. Including criteria
The criteria of clopidogrel resistance: the change rate of ADP-induced platelet aggregation is less than 30% or over 70% (Yang, 2015). The patients who were excluded include: patients who were administered with warfarin, dipyridamole, and cilostazol simultaneously; patients with neoplastic disease; patients with autoimmune disease; patients whose hepatic and renal functions were damaged; patients with history of cerebral hemorrhage; patients with other serious disease and expected lifetime was less than 1 year (Zhang et al., 2016).
2.3. Treatment method
Patients in both groups took 300 mg of aspirin orally and were administered with 100U/kg heparin before PCI. After PCI, both groups took 100 mg aspirin once a day (Gu, 2016).
-
(1).
Experimental group
The patients in experimental group took 180 mg ticagrelor before PCI and 90 mg ticagrelor twice a day after PCI (Marin et al., 2016).
-
(2).
Control group
The patients in control group took 600 mg clopidogrel before PCI and 150 mg clopidogrel once a day.
Curative effect of both groups was assessed after 7-day of medicine administration. Relevant data of both groups were recorded in detail.
2.4. Observation methods
Both groups were drawn 2.7 ml of fasting venous blood for platelet monitoring before PCI and 2 h, 24 h, 7 days after PCI respectively. Turbidimetric method was used to measure the ADP-induced platelet aggregation rate (see Fig. 3) and observe the change of platelet aggregation rate and success rate (The rate of 5 μmol/L ADP-induced platelet aggregation was less than 50%). Incidence of liver and kidney malfunction and adverse actions were also monitored (Yung et al., 2015). All Patients accepted 6-month follow-up examination to record the incidences of major adverse cardiac and cerebrovascular events and the differences of indexes between two groups were compared.
Fig. 3.
Platelet aggregation.
2.5. Statistical method
The SPSS19.0 statistical analysis software was used in the retrospective analysis. All data generated in the analysis were analyzed and processed by this software. Enumeration data were expressed in the form of ( ± s) and inter-group difference was compared by chi-square; measurement data was expressed by natural number and (n) and percentage (%) and the inter-group difference was compared by t. When P < .05, the inter-group difference is regarded significant and of statistical significance.
3. Results
3.1. Platelet aggregation rate
The platelet aggregation rate of experimental group before PCI and 2 h, 24 h, 7 days after PCI was 59.71% ± 7.24%, 59.20% ± 7.70%, 48.66% ± 7.80% and 43.39% ± 8.28%; The control group was 58.04% ± 5.61%, 56.25% ± 6.02%, 55.68% ± 3.14%, 53.94% ± 5.30%; Comparing the platelet aggregation rate between both groups, the inter-group difference was of statistical significance (P < .05). The success rates of platelet aggregations of experimental group and control group were 80.56% and 46.86%, respectively. There were significant differences between the two groups and the difference was of statistical significance (P < .05).
3.2. Other evaluating indicator
The postoperative serum creatinine level of experimental group was higher than that in the control group (P < .05). The incidence of adverse reactions in the experimental group was significantly lower than that of the control group, and the intergroup difference was of statistical significance (P < .05). According to the results of 5-month follow-up examination of both groups, the incidence of major adverse cardiac and cerebrovascular events in experimental group was 5.20% (52/21,000) ,while the control group was 26.00% (260/1000) . It can be known that there were significant differences between the two groups and the intergroup difference was of statistical significance (P < .05).
4. Discussions
In the long-term antiplatelet therapy after percutaneous coronary intervention, clopidogrel and aspirin are two common drugs (Cahill et al., 2015). Under conventional percutaneous coronary intervention with clopidogrel, some patients may still suffer arterial thrombosis event, which is clinically named as clopidogrel resistance. Clopidogrel resistance severely impairs the clinical effect of percutaneous coronary intervention (PCI). It also increases the cost of treatment and the patient's pain. Clinical personnel should actively look for solutions to improve the effectiveness and safety of PCI clinical treatment. Ticagrelor is a novel platelet P2Y12 receptor antagonist, which is capable of reversibly binding P2Y12 receptor. So it could play a role in inhibiting ADP-inducing platelet activation by adjusting its structure (Dobson et al., 2015).
From the results above, we can see that the antiplatelet therapy effect of experimental group is superior to that of control group. So, compared with clopidogrel, ticagrelor has better antiplatelet therapy effect for patients with acute myocardial infarction undergoing percutaneous coronary intervention PCI). It could effectively reduce the incidence of postoperative adverse cardiac and cerebrovascular events and control the rate of adverse reactions within the acceptable range (Ghoneum et al., 2015, Mellotte et al., 2015).
Acknowledgement
This work was supported by the National Natural Science Foundation of China (No. 81170087).
Footnotes
Peer review under responsibility of King Saud University.
References
- Cahill T., Chen X.L., Lee J.W., Weiss M., Chang V.T., Cella D. Principles of radiofrequency ablation for cancer. Asian Pac. J. Surg. Oncol. 2015;1(1):47–58. [Google Scholar]
- Dobson P.R., Brown B.L., Beck D., Yang H., Zhou J., Voon Y.L. Management of surgical oncologic emergencies. Asian Pac. J. Surg. Oncol. 2015;1(2):59–72. [Google Scholar]
- Ghoneum M., Felo N., Nwaogu O.M., Fayanju I.Y., Jeffe J.A., Margenthaler D.B. Clinical trials in surgical oncology. Asian Pac. J. Surg. Oncol. 2015;1(2):73–82. [Google Scholar]
- Gu Guowei. Comparison of Ticagrelor vs clopidogrel in therapeutic effect of acute coronary syndrome patients undergoing percutaneous coronary intervention. Chin. J. Med. Guide. 2016;18(02) 181–182+185. [Google Scholar]
- Marin, Hu Suling, Li Ailing, Yan Juan. Antiplatelet therapeutic effect of Ticagrelor in clopidogrel resistance acute myocardial infarction patients undergoing percutaneous coronary intervention. Hebei Med. 2016;22(03):402–405. [Google Scholar]
- Mellotte G., Maher V., Devitt P.G., Shin V.Y., Leung C.P. Minimally invasive surgical oncology: state of the art. Asian Pac. J. Surg. Oncol. 2015;1(2):101–112. [Google Scholar]
- Wei Xuemei, Zhu Qinghua, Gu Shikui, Zhang Junying, Li Yang. Antiplatelet therapeutic effect of Ticagrelor in clopidogrel resistance acute myocardial infarction patients undergoing percutaneous coronary intervention. Shandong Med. J. 2015;55(15):46–48. [Google Scholar]
- Yang Qiling. Antiplatelet therapeutic effect of Ticagrelor in clopidogrel resistance acute myocardial infarction patients undergoing percutaneous coronary intervention. Cardiovasc. Disease J. Integr. Tradit. Chin. West. Med. 2015;3(17) 7–8+10. [Google Scholar]
- Yung K.W., Yung T.T., Chung C.Y., Tong G.T., Liu Y., Henderson J., Welbeck D., Oseni S. Principles of cancer staging. Asian Pac. J. Surg. Oncol. 2015;1(1):1–16. [Google Scholar]
- Zhang Mingliang, Chen Yuhua, Wang Huanyu, Yin Shi, Li Dapeng, Zhang Guixia. Effect of Ticagrelor on platelet aggregation rate, CRP and major adverse cardiovascular events of Acute ST Segment elevation myocardial infarction patients undergoing percutaneous coronary intervention. J. Clin. Emerg. Call. 2016;17(03) 189–192+197. [Google Scholar]