Abstract
Objective
We aim to explore the connection between Tim-3 expression in both cancerous pancreatic and pericarcinous tissues and the clinicopathological features of pancreatic cancer. We will also preliminarily assess the role and significance of Tim-3 in the diagnosis, treatment, and prognosis of pancreatic cancer.
Methods
Cancerous pancreatic and pericarcinous tissues from 50 patients with pancreatic cancer and six healthy pancreatic tissues were collected from the pathological specimens of traumatic patients to distinguish Tim-3 expression using immunohistochemistry. Tim-3 expression was observed to be correlated with cell invasion, metastasis, and recurrence of pancreatic cancer.
Results
1. For the immunohistochemical method, Tim-3 expression in pancreatic cancer tissues was observed to be elevated and statistically significant (P < .01) compared to pericarcinous and normal pancreatic tissues. No statistically significant difference (P > .05) was observed between Tim-3 expression in pericarcinous and normal pancreatic tissues. 2. While Tim-3 expression was observed to be closely related to the history of smoking, fasting blood glucose, tumor size, TNM stage, it was not observed to be related to gender, age, tumor location, pathological type, and degree of tumor differentiation.
Conclusion
1. Tim-3 expression in pancreatic cancer tissues was high. 2. The high Tim-3 expression in pancreatic cancer tissues may be closely related to cell invasion, metastasis, and the recurrence of pancreatic cancer.
Keywords: Tim-3, Pancreatic cancer, Immunohistochemical method
1. Introduction
Pancreatic cancer is a digestive system cancer that is highly malignant and difficult to diagnose and treat, and the number of new cases is increasing worldwide. T-cell immunoglobulin and mucin domain 3 (Tim-3) expression is observed to be related to gastric, colorectal, and ovarian cancers, as well as renal cell carcinoma (Shen et al., 2016, Yang et al., 2016, Yu et al., 2017, Xu et al., 2017). However, to what extent Tim-3 is related to pancreatic cancer has not yet been reported. The aim of the present study is to compare Tim-3 expression in pancreatic cancer and pericarcinous tissues to determine the relationship of Tim-3 expression with the clinicopathological features of pancreatic cancer. Tim-3 is analyzed to determine the occurrence and development of pancreatic cancer, and to assess its role and clinical value in the diagnosis, treatment, and prognosis of pancreatic cancer.
2. Materials and methods
2.1. Clinical data
Carcinous and pericarcinous pancreatic tissues (5 cm from the lesion and free of cancer confirmed by pathology) were obtained from fifty patients who had undergone pancreatoduodenectomy. Six normal pancreatic tissues were obtained from the pathological specimens of traumatic patients. Both samples were obtained in our hospital between January 2016 and January 2017. All patients had complete medical records and had not undergone any radiochemotherapy before surgery. The subjects included 30 males (60.0%) and 20 females (40.0%), aged 41–72, with a median age of 55.06 ± 7.99. We obtained signed consent of the patients or their family, and the study and was approved by the Ethics Committee. The pathological stage of tumors was based on the American Joint Committee on Cancer (AJCC), TNM Stage of Pancreatic Cancer. The clinical data of patients included: gender, age, history of smoking, HP infection, tumor size, tumor location, TNM stage, pathological type, degree of tumor differentiation, lymphatic metastasis, vascular tumor thrombus, CA199, and so on.
2.2. Experimental reagents
Calretinin TIM-3 antibodies were purchased from Abcam (list location). Goat anti-rabbit secondary antibodies labeled by HRP and DAB immunohistochemistry kits were purchased from Beijing Zhongshan Jinqiao Biological Technology Co., Ltd.
2.3. Immunohistochemical method
Conventional immunohistochemical methods (Chuanping et al., 2001) were as follows: pancreatic cancer and pericarcinous tissues were obtained from 50 cancer patients and six normal pancreatic tissues were prepared for sectioning. Tissues were embedded in paraffin, sliced, and dewaxed. Slices were immersed in PBS and 50 µl 0.3% H2O2 was added for PBS washing; added with serum for closing, and incubated at 37 °C for 20 min; added with primary antibodies (with a 1:300 dilution ratio) respectively, and incubated at 37 °C overnight. After PBS washing, secondary antibody working solutions were added and incubated at 37 °C for 30 min. Color was rendered with DAB and sections were dewatered and dried in turn after being counterstained with hematoxylin and affixed with neutral gum. Scoring was conducted in relation to two variables: staining intensity and the percentage of positive cells. For staining intensity, the following scoring was used: negative if the intensity of staining was 0 point; weakly positive intensity was given 1 point; moderately positive intensity was 2 points; strongly positive staining was 3 points. For the percentage of positive cells, the following scoring system was used: 0 point if there was no positive cell; 1 point if positive cells were <25%; 2 points if positive cells were 25–50%; 3 points if positive cells were >50%; negative (−) if the sum of two scores was 0 point–2 points; weakly positive (+) if the sum of two scores was 3 points–4 points; moderately positive (++) if the sum of two scores was 5 points–6 points; strongly positive (+++) if the sum of two scores was 7 points–9 points.
2.4. Statistical treatment
Statistical analysis of the experimental data was carried out using SPSS 20 statistical software, and the counting data were tested with χ2. The survival curves were displayed and tested using a Kaplan-Meier method and Log-Rank test, respectively. The independent features affecting patient prognosis with pancreatic cancer were screened using a Cox proportional hazard regression model. (P < .05) indicated a difference considered statistically significant.
3. Results
3.1. TIM-3 expression in pancreatic cancer and pericarcinous tissues
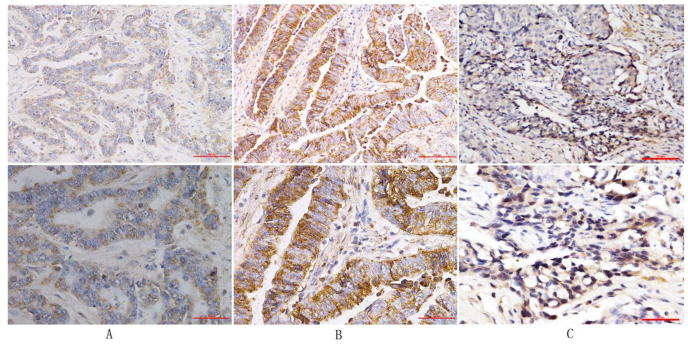
Immunohistochemistry showed that positive Tim-3 expression occurred mostly in the cytoplasm, less in the nucleus, and was almost nonexistent in the cell membrane. Tim-3 expression in pancreatic cancer tissues was strongly positive: 10% (5/50); moderately positive: 50% (25/50); weakly positive: 12% (6/50); negative: 28% (14/50). In contrast, Tim-3 expression in pericarcinous tissues was strongly positive: 16% (8/50), moderately positive: 8% (4/50); weakly positive: 4% (2/50); negative: 72% (36/50). These differences were observed to be statistically significant (P < .01) (Fig. 1). These results were analyzed using the Mann-Whitney test (Table 1).
Fig. 1.
Tim-3 Expression in three kinds of tissues, ×20, ×40. A: Normal pancreatic tissues; B: Pancreatic cancer tissues; C: Pericarcinous tissues.
Table 1.
Expression of Tim-3 in pancreatic cancer tissues and pericarcinous tissues.
| Tim-3 expression | (+++) | (++) | (+) | (−) | Z | P |
|---|---|---|---|---|---|---|
| Pancreatic cancer tissues | 5 | 25 | 6 | 14 | −3.399 | .001 |
| Pericarcinous tissues | 8 | 4 | 2 | 36 | ||
| Normal pancreatic tissues | 2 | 4 | – | – | ||
| Total | 14 | 29 | 10 | 54 | ||
3.2. Relationship between Tim-3 expression and the clinicopathological features of pancreatic cancer
The relationship between Tim-3 expression and the clinicopathological features of pancreatic cancer is shown in Table 2, According to the Chi-square test, Tim-3 expression was observed to be closely related to a history of smoking, fasting, blood glucose, tumor size, TNM stage, and CA199. However, it was not observed to be related to gender, age, tumor location, pathological type, or degree of tumor differentiation.
Table 2.
Relationship between the expression of Tim-3 and the clinicopathological features of pancreatic cancer.
| Parameter | Group | No. | Tim-3 |
χ2 | P value | |
|---|---|---|---|---|---|---|
| (+) | (−) | |||||
| Gender | Male | 30 | 21 | 9 | 0.149 | .700 |
| Female | 20 | 15 | 5 | |||
| Age | ≥50 | 40 | 30 | 10 | 0.304 | .581 |
| <50 | 10 | 6 | 4 | |||
| History of smoking | Yes | 34 | 28 | 6 | 4.158 | .041 |
| No | 16 | 8 | 8 | |||
| Fasting blood glucose (mmol/L) | <6.1 | 38 | 31 | 7 | 5.363 | .021 |
| ≥6.1 | 12 | 5 | 7 | |||
| Tumor size (cm) | >2 | 23 | 21 | 2 | 7.873 | .005 |
| ≤2 | 27 | 15 | 12 | |||
| Tumor location | Head of pancreas | 35 | 27 | 8 | 0.304 | .581 |
| Non-head of pancreas | 15 | 9 | 6 | |||
| TNM stage | III-IV | 12 | 5 | 9 | 10.322 | .001 |
| I-II | 38 | 31 | 5 | |||
| Pathological type | Ductal adenocarcinoma other | 46 | 35 | 11 | 2.567 | .109 |
| 4 | 1 | 3 | ||||
| Degree of tumor differentiation | Moderately and poorly differentiated | 45 | 34 | 11 | 1.334 | .248 |
| Well differentiated | 5 | 2 | 3 | |||
| CA199 (μ/ml) | >37 | 40 | 33 | 7 | 8.488 | .004 |
| <37 | 10 | 3 | 7 | |||
3.3. Relationship between Tim-3 expression in pancreatic cancer tissues and prognosis
The overall median survival time of the 50 cancer patients was 10.3 months. Among the 36 patients that showed positive Tim-3 expression, 8.2 months was revealed as the median survival time. The 14 patients that showed a negative Tim-3 expression were observed to have a median survival time of 15.5 months. The differences between them was observed to be statistically significant (Log-rank test, χ2 = 13.869, P = .000) (Fig. 2).
Fig. 2.
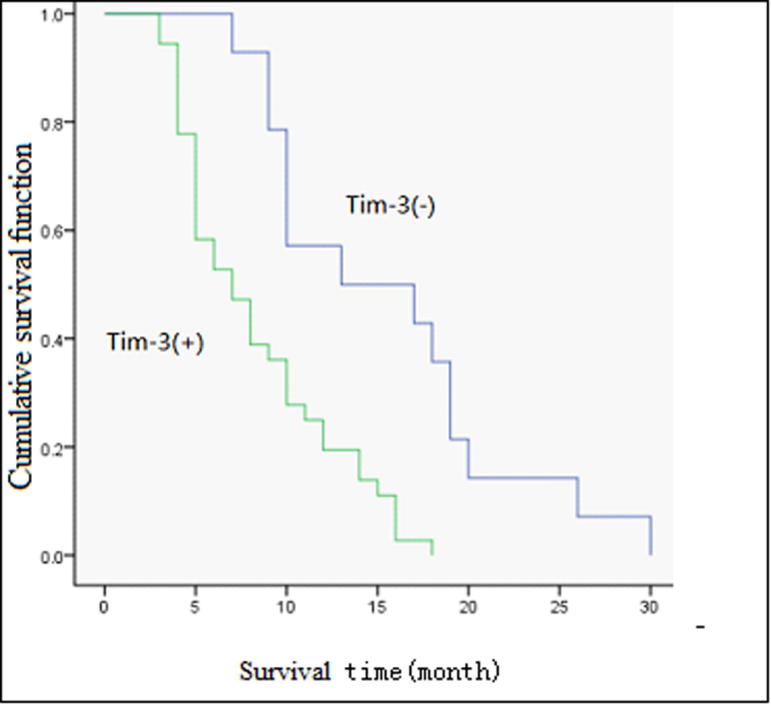
Survival curves of 50 pancreatic cancer patients with different TIM-3 levels are shown. Kaplan-Meier survival curves for positive expression of TIM-3 group were significantly different (log-rank test, χ2 = 13.869, P < .001) from the negative expression group.
The independent features affecting patient prognosis with pancreatic cancer, such as Tim-3 and TNM stage, were screened by a Cox proportional hazard model (χ2 = 58.235, P = .000). While Tim-3 expression (P = .000), TNM stage (P = .000), and tumor size (P = .008) were demonstrated by the multivariate regression analysis to be independent factors affecting the prognosis of patients with pancreatic cancer, a history of smoking (P = .052), fasting blood glucose (P = .502), and CA199 (P = .093) were not considered independent factors (see Table 3).
Table 3.
Cox multivariate regression analysis of the independent factors that may affect the prognosis of patients.
| B | SE | Wald | df | Sig. | Exp(B) | 95.0% CI was used for Exp(B) |
||
|---|---|---|---|---|---|---|---|---|
| Top | Bottom | |||||||
| Tim-3 | −3.155 | 0.747 | 17.848 | 1 | 0.000 | 0.043 | 0.010 | 0.184 |
| TNM stage | −2.635 | 0.687 | 14.694 | 1 | 0.000 | 0.072 | 0.019 | 0.276 |
| Tumor size | −1.329 | 0.503 | 6.971 | 1 | 0.008 | 0.265 | 0.099 | 0.710 |
| History of smoking | −0.932 | 0.479 | 3.777 | 1 | 0.052 | 0.394 | 0.154 | 1.008 |
| Fasting blood glucose | −0.327 | 0.487 | 0.450 | 1 | 0.502 | 0.721 | 0.278 | 1.872 |
| CA199 | −0.733 | 0.436 | 2.830 | 1 | 0.093 | 0.480 | 0.204 | 1.129 |
4. Discussion
The incidence of pancreatic cancer has increased in China in recent years, and is widely considered a top ten cause of cancer related mortality (Chen et al., 2016). Specifically, ductal adenocarcinoma is considered among the top five most common causes of cancer deaths worldwide. It is predicted that, by 2030, pancreatic cancer in the USA will become the second leading cause of cancer related mortality (Rahib et al., 2014). With its low early diagnosis, high operation mortality, and low cure rates, compounded by poor prognosis, pancreatic cancer is observed to have a 5-year survival rate of only about 6% (Siegel, 2015).
T-cell immunoglobulin mucin (Tim) is mainly expressed on the surface of immune cells, and has a potential impact on tumor immune surveillance and evasion. Tim-3 is one of many transmembrane proteins that form the immunoglobulin family (Sakuishi et al., 2011, Zhu et al., 2011). Several studies have shown Tim-3 to be a putative antitumor negative mediating factor because it can preferentially express on the exterior of activated Th1 cells (Anderson, 2012). A high Tim-3 expression in gastric, colorectal, liver, and other gastrointestinal cancers is observed to be closely related to tumor invasion, clinical prognosis, and TNM stage (Shen et al., 2016, Yang et al., 2016, Dong, 2014). The function of Tim-3 in the study of autoimmune, inflammatory, and allergic diseases has received widespread attention. However, to what extent it is related to pancreatic cancer in tumor immunity remains to be elucidated.
In this study, Tim-3 expression observed in pancreatic cancer, pericarcinous, and normal pancreatic tissues was detected using immunohistochemistry. Tim-3 expression was observed to occur mostly within the cytoplasm of both pancreatic cancer and normal pancreatic tissues. Tim-3 expression in pancreatic cancer tissues was observed to be significantly higher (P < .01) than in pericarcinous and normal pancreatic tissues. The differences between Tim-3 expression in pericarcinous and normal pancreatic tissues was not observed to be statistically significant (P > .05). Tim-3 expression in pancreatic cancer and pericarcinous tissues, as well as clinicopathological parameters, were analyzed statistically. The immunohistochemical analysis revealed a significant difference (P < .01) between Tim-3 positive expression rate in pancreatic cancer and pericarcinous tissues at 72.0% (36/50) and 28.0% (14/50), respectively. Therefore, high Tim-3 expression in pancreatic cancer tissue is suggestive of its importance as a marker of the invasion and metastasis of pancreatic cancer tissue.
Pancreatic cancer is observed to be complex, often involving changes and that lead to progressive accumulation of multiple factors, stages, and genes. While epidemiological investigations have confirmed the incidence of pancreatic cancer to be related to heredity, environment, and living habits, the specific molecular mechanisms remain unclear. The inactivation of oncogenes, tumor suppressor, and apoptotic genes has been observed to lead to the uncontrolled proliferation of pancreatic cells, which can lead to the formation of pancreatic tumor entities. Interestingly, the site mutation of the 12th codon of K-ras genes is observed to accompany 90% of pancreatic cancers (Ryan et al., 2014). The IL-27/NFIL-3 (nuclear factor in interleukin-3) pathway can promote the expression of Tim-3 on the cell membrane, and IL-27 can promote NFIL3 expression. Additionally, the interaction between NFIL3 and T-bet can promote the generation of Tim-3 and IL-10, which can indirectly lead to Tim-3 + T cell failure and tumor cell proliferation (Zhu et al., 2015). Tim-3 can also activate (IL-6)-signal transduction and signal transducers and activators of the transcription (STAT)-3 pathway to promote the growth of tumors and participate in metastasis (Yun et al., 2012). Tim-3 can not only inhibit tumor infiltrating T cells, but also negatively regulate the innate immune cells in a tumor microenvironment (Wenjiang, 2014). Tim-3 was also observed to regulate various immune-mediated diseases (Kuchroo et al., 2008). While Tim-3 expression in the present study was closely correlated to the history of smoking, fasting blood glucose, tumor size, TNM stage, and CA199, it was not observed to be related to gender, age, tumor location, pathological type, and degree of tumor differentiation. The Cox multivariate regression analysis indicated that Tim-3 expression, TNM stage, and tumor size were independent factors observed to affect the prognosis of patients with pancreatic cancer. The survival curves further indicated that the median survival time of patients with Tim-3 positive expression was significantly shorter than those patients with a negative expression. The patients with high Tim-3 expression were observed to have a lower overall survival rate and worse prognosis than those patients with a low Tim-3 expression.
A high Tim-3 expression in pancreatic cancer tissues is suggestive of the importance of Tim-3 in the infiltration, development, invasion, and metastasis of pancreatic cancer. Tim-3 was observed to be related to the occurrence, development, invasion, and metastasis of pancreatic cancer; thus, it can be used as a prognostic index for clinical diagnosis and prognosis evaluation of pancreatic cancer. Tim-3 provides a new approach for the study of the pathogenesis, clinicopathological correlation, and prognosis of pancreatic cancer, as well as a new basis and therapeutic target for pancreatic cancer gene therapy. Therefore, studying the relationship between Tim-3 and pancreatic cancer has been demonstrated to have important theoretical value and potential applications.
Footnotes
Peer review under responsibility of King Saud University.
References
- Shen P., Yue R., Tang J. Preferential Tim-3 expression on Treg and CD8(+) T cells, supported by tumor-associated macrophages, is associated with worse prognosis in gastric cancer. Am. J. Transl. Res. 2016;8(8):3419. [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Cai P., Lei L. Co-expression of TIM-3 and CEACAM1 promotes T cell exhaustion in colorectal cancer patients. Int. Immunopharmacol. 2016;43:210. doi: 10.1016/j.intimp.2016.12.024. [DOI] [PubMed] [Google Scholar]
- Yu M., Lu B., Liu Y. Interference with Tim-3 protein expression attenuates the invasion of clear cell renal cell carcinoma and aggravates anoikis. Mol. Med. Rep. 2017 doi: 10.3892/mmr.2017.6136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Y., Zhang, H., Huang, Y., et al., 2017. Role of TIM-3 in ovarian cancer. [DOI] [PubMed]
- Chuanping Xing, Bin Liu, Liang Dong. Method for judging the immunohistochemical marker results. Chinese J. Pathol. 2001;30(4) 318 318. [Google Scholar]
- Chen W., Zheng R., Baade P.D. Cancer statistics in China, 2015. CA Cancer J. Clin. 2016;66(2):115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- Rahib L., Smith B., Aizenberg R. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Can. Res. 2014;74(11):2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2015. CA Cancer J. Clin. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- Sakuishi K., Jayaraman P., Behar S.M., Anderson A.C., Kuchroo V.K. Emerging TIM-3 functions in antimicrobial and tumor immunity. Trends Immunol. 2011;32:9–345. doi: 10.1016/j.it.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C., Anderson A.C., Kuchroo V.K. TIM-3 and its regulatory role in immune responses. Curr. Top Microbiol. Immunol. 2011;350:1–15. doi: 10.1007/82_2010_84. [DOI] [PubMed] [Google Scholar]
- Anderson A.C. Tim·3, a negative regulator of anti—tumor immunity. Curr. Opin. Immunol. 2012;24(2):213–216. doi: 10.1016/j.coi.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Dong Wei. The Third Military Medical University; 2014. Expression of Tim-3 in hepatocellular carcinoma and its significance. [Google Scholar]
- Ryan D.P., Hong T.S., Bardeesy N. Pancreatic adenocarcinoma (PDF) N. Engl. J. Med. 2014;371(11):1039–1049. doi: 10.1056/NEJMra1404198. [DOI] [PubMed] [Google Scholar]
- Zhu C., Sakuishi K., Xiao S. An IL-27/NFIL 3signaling axis drives TIM-3 and IL-10 expression and T-celldysfunction. Nat. Commun. 2015;6(10):4839–4847. [Google Scholar]
- Yun U.J., Park S.E., Jo Y.S. DNA damage induces the IL-6/STAT3 signaling pathway, which has anti-senescence and growth-promoting functions in human tumors. Cancer Lett. 2012;323(2):155–160. doi: 10.1016/j.canlet.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Wenjiang Yan. Shandong University; 2014. Study on the role and mechanism of Tim-3 in tumor-associated macrophage polarization and development of hepatocellular carcinoma. [Google Scholar]
- Kuchroo V.K., Dardalhon V., Xiao S. New roles for TIM family members in immune regulation. Nat. Rev. Immunol. 2008;8(8):577–580. doi: 10.1038/nri2366. [DOI] [PubMed] [Google Scholar]