Abstract
Isolation of high-quality RNA from coffee is challenging because of high level of polysaccharides, polyphenols and other secondary metabolites. In the present study, a rapid and efficient RNA extraction protocol from different tissues of coffee was optimized. Sufficiently high quality and quantity (225.6–454.8 µg/g) of RNA was obtained by using the optimized protocol. The presence of two distinct bands of 28S rRNA and 18S rRNA in agarose gel proved the intactness of the RNA samples. The average spectrophotometric values of the isolated RNA ranged from 1.96 to 2.02 (A260/280) and 1.95 to 2.14 (A260/230), indicating the high quality of RNA devoid of polyphenols, polysaccharides and protein contamination. In the optimized protocol, addition of PVPP to the extraction buffer and a brief incubation of samples at 65 °C and subsequent purification with potassium acetate resulted in good-quality RNA isolation. The suitability of RNA for downstream processing was confirmed by PCR amplification with cytochrome c oxidase gene-specific primers. The amplification of a single 392 bp fragment using cDNA and 1.5 kb fragment using genomic DNA samples confirmed the absence of DNA contamination. The present protocol is rapid and yielded good quality and quantity of RNA suitable for functional genomics studies.
Keywords: Coffee, RNA isolation, SDS, PCR, Sequencing
Introduction
Coffee is one among the most popular beverages consumed throughout the world. It is cultivated in about 10.2 million ha of land spanning over 80 countries in the tropical and subtropical regions of Africa, Asia and Latin America (Mishra and Slater 2012). Among more than 124 coffee species (Razafinarivo et al. 2013) known so far, only two species, Coffea arabica (Arabica coffee) and Coffea canephora (Robusta coffee), are commercially cultivated and have more economical significance.
Genetic improvement of coffee is of paramount importance for its sustainable cultivation. However, coffee improvement using conventional breeding is a slow and tedious process due to the perennial nature of the plant species. Recent developments in the areas of functional genomic research could complement and hasten the conventional breeding process by enhancing the availability of potential genes. However, one of the important prerequisite for various functional genomics studies is the availability of an efficient and robust RNA isolation protocol from different target tissue. A number of RNA isolation protocols have been standardized across plant species using either guanidinium thiocyanate or phenol/SDS by various workers (Tan and Yiap 2009; Choudhary et al. 2016; Vasanthaiah et al. 2008). However, none of these methods are found suitable for RNA isolation in coffee due to its high polyphenol and secondary metabolite content. In a previous study, de Paula et al. (2012) tried four different methods for extraction of RNA from different tissues of coffee and found the CTAB method to be the best. However, this method requires a large sample volume (2 g) and a lengthy extraction protocol (6–24 h) and therefore not suitable for transcriptomic experiments where sample volume is the major constraint. Recently, Deepa et al. (2014) developed a RNA isolation protocol in Curcuma longa which contains high levels of polyphenols, polysaccharides and alkaloids. However, when this protocol was used for RNA isolation from coffee leaf tissue, low and poor-quality RNA was obtained. Therefore, it was imperative to develop a simple, rapid and efficient RNA extraction protocol from different tissues of coffee.
Materials and methods
Plant material
Fresh young leaves, secondary roots, bark tissue of C. arabica (cultivar Chandragiri) and C. canephora (cultivar CxR) were collected from 3- to 5-year-old plants maintained in the nursery at Tissue Culture and Biotechnology Division Mysore, Karnataka, India. The young growing leaves near the apical bud were collected. The soil adjoining root zone was carefully removed and fresh secondary fibrous roots were collected. The outer dead cork cambial tissue of bark was removed and thin slices from inner soft tissue was collected. All the plants were maintained in healthy conditions and recommended package of practices were followed.
RNA isolation protocol
All the materials used in the RNA extractions were treated with 1.0% diethylpyrocarbonate solution (DEPC) and all the reagents were prepared with 0.5% DEPC-treated distilled water. All the accessories required for gel electrophoresis were treated with 0.5 M EDTA solution for 1 h and washed with DEPC-treated water.
About 100 mg fresh tissue samples of leaf, secondary roots and bark was collected in three replications from C. arabica and C. canephora plants. All the samples were processed uniformly by grinding them into a fine powder using liquid nitrogen in pre-chilled pestle and mortar with 2% (W:V), PVPP (polyvinylpolypyrrolidone). To the finely powdered sample, 1.5 ml of freshly prepared, pre-warmed extraction buffer (100 mM Tris–HCl, 25 mM EDTA, 2% SDS and 1% β mercaptoethanol of pH 8) was added and transferred to 2 ml micro centrifuge tubes. The samples were incubated in water bath for 15–20 min at 65 °C and cooled to room temperature. To this equal volume of water-saturated phenol (pH 5.6), chloroform (1:1 freshly prepared) was added, vortex mixed for 2 min and allowed to stand for 5 min at room temperature. The tubes were centrifuged at 15000g at 4 °C for 10 min and the supernatant was transferred to fresh 2 ml microcentrifuge tubes, following which 0.3 volume of 5 M potassium acetate (pH 5) and 0.7 volume of water saturated phenol (pH 5.6):chloroform (1:1 freshly prepared) was added. The tubes were mixed vigorously and kept at − 20 °C freezer for 10 min and centrifuged at 15000g at 4 °C for 10 min. The supernatant collected was transferred to fresh tubes and 0.1 volumes each of 3 M potassium acetate (pH 5) and ice cold isopropanol were added and mixed well by inverting the tubes gently. The tubes were incubated at − 20 °C for 30 min and centrifuged at 15000g at 4 °C for 15 min. The supernatant was discarded and the pellet was washed with 70% chilled ethanol by centrifuging at 15000g at 4 °C for 15 min. Further, the supernatant was discarded and RNA pellets were dried using vacuum dessicator. The dried RNA pellets were re-suspended in 50 µl RNase-free distilled water. To remove the DNA contamination, samples were treated with RNase-free rDNase I (Ambion) according to the manufacturer’s instructions and stored at – 20 °C for future use. The purity and concentration of isolated RNA samples were assessed by Nanodrop. The integrity of RNA was analyzed by separating it on 1.0% agarose gel electrophoresis, stained with ethidium bromide and visualized under gel documentation system (BioRad).
cDNA synthesis and PCR analysis
Total RNA extracted from bark tissues of C. arabica and C. canephora was reverse transcribed into cDNA using high-capacity RNA to cDNA conversion kit (Applied Biosystem, USA). The first-strand cDNA synthesis was carried out in 20 µl reaction volume containing 10 µl of 2 × RT buffer, 1.0 µl of 2× enzyme mix, 4.0 µl of RNA sample and 5.0 µl of nuclease-free water. Reverse transcription was performed using thermal cycler (BioRad) at 37 °C for 60 min and 95 °C for 5 min. The product was stored at − 20 °C for further analysis.
PCR was carried out with cytochrome c oxidase gene-specific primers using genomic DNA and cDNA samples of C. arabica and C. canephora. Cytochrome c oxidase gene-specific primers were designed from the sequence information of C. canephora available in the public database. The forward and reverse primer sequences (F- AATGCCGCAAGCAAGCAAA and R- TGCACCGGGCAAAAATTAGG) of cytochrome c oxidase gene primers were designed to amplify a 392 bp gene fragment in cDNA and 1.5 kb fragment containing intronic region in genomic DNA of coffee. The primers were synthesized at Sigma-Aldrich, Bangalore. PCR assay was carried out in 20 µl reaction volume containing 2.0 µl of 10x PCR buffer, 2.0 µl of 25 mM MgCl2, 2.0 µl of 3 µM of each primer, 4.0 µl of 10 ng/µl DNA/1.0 µl cDNA and 1.0 µl of 1 U/µl Taq DNA polymerase. All the components used in PCR were procured from Thermo Fisher Scientific. PCR cycles were programmed as follows: 5 min at 94 °C for initial denaturing followed by 34 cycles of 45 s denaturing at 94 °C, 45 s annealing at 64 °C and 1 min elongation at 72 °C, followed by final elongation at 72 °C for 10 min. The amplified PCR products were separated on 1.2% agarose gel stained with ethidium bromide (10 mg/ml) and visualized under gel documentation system (BioRad).
Sequencing
The PCR amplified products of cDNA and genomic DNA were gel eluted and purified using QIAquick gel extraction kit (Qiagen) by following the manufacturer’s instructions and sequenced directly by Sanger method.
Results and discussion
Assessment of RNA quality
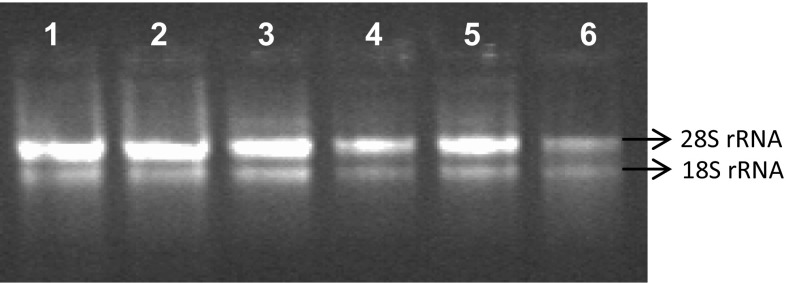
Two intact and clearly separated 28S rRNA and 18S rRNA bands without any shearing were observed in agarose gel (Fig. 1), indicating that the protocol was successful in extracting good-quality RNA from different tissue of both C. arabica and C. canephora. In general, the A260/A230 absorbance ratio indicates potential contamination with polysaccharides and polyphenols, and the A260/A280 ratio indicates protein contamination in nucleic acids (Logemann et al. 1987 and Manning 1991). According to Asif et al. (2006), the spectophotometric values of 260/280 ranging from 1.8 to 2.1 indicate the purity of nucleic acids. The spectrophotometric readings of our samples revealed the presence of good-quality RNA, since the ratio of 260/280 nm and 260/230 nm ranged from 1.96 to 2.02 and 1.95 to 2.14, respectively. The yields of RNA were satisfactory and were in the range of 225.6–454.8 µg/g (Table 1). In the present protocol, smaller sample volume (100 mg) was processed and good amount of RNA was obtained unlike the earlier report by de Paula et al. (2012) in C. arabica, wherein 0.5–2.0 g leaf tissue were processed and comparatively less RNA yield was obtained. Previously Deepa et al. (2014) obtained 102–180 µg/g of RNA from different tissues of Curcuma longa using a modified SDS protocol. However in coffee, RNA isolated using this protocol resulted in low yield (Table 1). The current protocol was more efficient and resulted in higher RNA yields (225.6–454.8 µg/g) of good quality, devoid of any trace of genomic DNA contamination (Fig. 1).
Fig. 1.
Total RNA isolated from different tissues of Arabica and Robusta coffee. Lanes 1–2: Arabica and Robusta leaves. Lanes 3–4: Arabica and Robusta roots. Lanes 5–6: Arabica and Robusta bark tissue
Table 1.
The average spectrophotometric values of RNA isolated from different tissues of C. arabica and C. canephora
| Sample | Quantity of sample used (mg) | Current protocol | Using protocol by Deepa et al. 2014 | Current protocol | Using protocol by Deepa et al. 2014 | ||
|---|---|---|---|---|---|---|---|
| A 260/280 | A 260/230 | A 260/280 | A 260/230 | RNA concentration (µg/g) | RNA concentration (µg/g) | ||
| Arabica Leaf tissue | 100 | 1.99 | 2.05 | 1.79 | 1.91 | 454.8 | 119.0 |
| Robusta Leaf tissue | 100 | 2.02 | 2.14 | 1.82 | 1.97 | 444.8 | 164.5 |
| Arabica Root tissue | 100 | 1.98 | 2.02 | – | – | 424.8 | – |
| Robusta Root tissue | 100 | 2.01 | 1.98 | – | – | 281.3 | – |
| Arabica Bark tissue | 100 | 1.96 | 2.01 | – | – | 347.0 | – |
| Robusta Bark tissue | 100 | 1.98 | 1.95 | – | – | 225.6 | – |
Since availability of plant tissue for RNA isolation at the site of infection for downstream processing is often a limiting factor, the present protocol assumes significance, as high-quality RNA could be extracted from minimum tissue compared to earlier methods in coffee. Further, the current method of RNA isolation is not only efficient, but also takes less time (3–5 h) to complete unlike earlier methods described by Sah et al. (2014), de Paula et al. (2012), Vasanthaiah et al.(2008) and Zarei et al. 2012, which takes more time (6–24 h).
Suitability of RNA for downstream processing
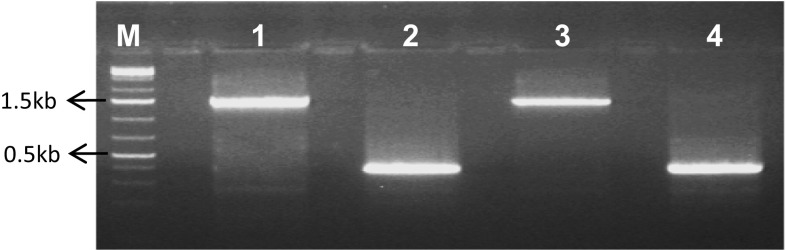
The suitability of RNA isolated by the current method was tested by amplifying the cytochrome c oxidase gene fragment. A single full-length gene fragment of 1.5 kb was amplified using genomic DNA as template, and a single 392 bp fragment alone was amplified using cDNA as template (Fig. 2). This differential amplification of fragment in genomic and cDNA samples not only proved the integrity of isolated RNA samples without any DNA contamination, but also confirmed its amenability for downstream processing without any ambiguity. Further, the gene fragments amplified using DNA and cDNA templates of both C. arabica and C. canephora were sequenced and the presence of intron in gene amplified from genomic DNA was confirmed (Data not shown).
Fig. 2.
Gel picture depicting differential PCR amplification of cytochrome oxidase gene from genomic DNA and cDNA samples of C. arabica and C. canephora. Lane M: 1 Kb plus DNA ladder (Thermo scientific). Lane 1: Arabica genomic DNA. Lane 2: Arabica cDNA. Lane 3: Robusta genomic DNA. Lane 4: Robusta cDNA
Optimized RNA extraction protocol
Extraction of good-quality RNA from coffee is challenging because of high polysaccharide and polyphenol content. These polyphenols easily oxidize to quinone compounds that bind irreversibly to RNA molecule and makes RNA isolation difficult (Graham 1993). Deepa et al. (2014) have used PVP in the RNA extraction buffer to prevent polyphenol oxidation. However, in the current study, high concentration of PVPP was used instead of PVP, because PVPP is insoluble unlike PVP and can be easily removed by centrifugation. A further high concentration of PVPP helps in dissociation of complexes of polysaccharide, phenols and other compounds. It also helps in dissolving the SDS–RNA complex so that more SDS and polysaccharides are removed in phenol:chloroform extraction (Ainsworth, 1994). In addition to the above, incubation of samples at 65 °C for 15–20 min might have facilitated cell lysis and thereby resulted in higher RNA yields compared to the RNA yields obtained by Deepa et al. (2014), wherein samples were not incubated. In the present protocol, 5 M potassium acetate (pH-5.0) was used to remove the traces of SDS and phenol:chloroform from the sample. RNA was precipitated with ice cold isopropanol along with 3 M potassium acetate (pH 5.0). The potassium acetate helps to maintain the pH of the denatured cell lysate during acid extraction and provides the salt necessary for RNA precipitation.
Conclusion
A modified SDS-based protocol of RNA extraction from different coffee tissues rich in polyphenols and secondary metabolites was developed. The protocol is rapid, efficient and yields sufficient quantity of high-quality RNA irrespective of source tissue. Thus, this protocol might be useful for other plant species with high levels of polyphenols and secondary metabolites for rapid and large-scale RNA extraction.
Acknowledgements
The authors thank Dr. Y. Raghuramulu, Director of Research, Central Coffee Research Institute, India, for providing laboratory facilities and encouragement. Funding support from Coffee Board, Govt. of India, is gratefully acknowledged.
Author contributions statement
The corresponding author designed the experiment and wrote the manuscript. The first and second authors carried out the experiments and participated in manuscript preparation. All the authors read and approved the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest in the publication.
Ethical standards
The experiment complied with the ethical standards.
References
- Ainsworth C. Isolation of RNA from floral tissue of Rumex acetosa (Sorrel) Plant Mol Biol Rep. 1994;12(3):198–203. doi: 10.1007/BF02668741. [DOI] [Google Scholar]
- Asif M, Trivedi P, Solomos T, Tucker M. Isolation of high-quality RNA from apple (Malus domestica) fruit. J Agric Food Chem. 2006;54:5227–5229. doi: 10.1021/jf053137n. [DOI] [PubMed] [Google Scholar]
- Choudhary SB, Kumar M, Chowdhury I, Singh RK, Pandey SP, Sharma HK, Karmakar PG. An efficient and cost effective method of RNA extraction from mucilage, phenol and secondary metabolite rich bark tissue of tossa jute (C. olitorius) actively developing phloem fiber. 3 Biotech. 2016;6(1):100. doi: 10.1007/s13205-016-0415-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Paula MFB, Sagio SA, Lazzari F, Barreto HG, Paiva LV, Antonio C., Jr . Efficiency of RNA extraction protocols in different types of coffee plant tissues. Lavras: Coffee Sci; 2012. pp. 284–293. [Google Scholar]
- Deepa K, Sheeja TK, Santhi R, Sasikumar B, Cyriac A, Deepesh PV, Prasath D. A simple and efficient protocol for isolation of high quality functional RNA from different tissues of turmeric (Curcuma longa L) Physiol Mol Biol Plants. 2014;20(2):263–271. doi: 10.1007/s12298-013-0218-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham GC. A method for extraction of total RNA from Pinus radiata and other conifers. Plant Mol Biol Rep. 1993;11(1):32–37. doi: 10.1007/BF02670557. [DOI] [Google Scholar]
- Logemann J, Schell J, Willmitzer L. Improved method for the isolation of RNA from plant tissues. Anal Biochem. 1987;163:16–20. doi: 10.1016/0003-2697(87)90086-8. [DOI] [PubMed] [Google Scholar]
- Manning K. Isolation of nucleic acids from plants by differential solvent precipitation. Anal Biochem. 1991;195(1):45–50. doi: 10.1016/0003-2697(91)90292-2. [DOI] [PubMed] [Google Scholar]
- Mishra MK, Slater A. Recent advances in the genetic transformation of coffee. Hindawi Publishing Corporation. Biotechnol Res Int. 2012 doi: 10.1155/2012/580857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razafinarivo NJ, Guyol R, Davis AP, Couturon E, Hamon S, Crouzillat D, Rigoreau M, Tranchant CD, Poncet V, Kochko ADE, Rakotomalala JJ, Hamon P. Genetic structure and diversity of coffee (Coffea) across Africa and the Indian Ocean islands revealed using microsatellites. Ann Bot. 2013;111:229–248. doi: 10.1093/aob/mcs283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah SK, Kaur G, Kaur A. Rapid and reliable method of high-quality RNA extraction from diverse plants. Am J Plant Sci. 2014;5:3129–3139. doi: 10.4236/ajps.2014.521329. [DOI] [Google Scholar]
- Tan SC, Yiap BC. DNA, RNA, and protein extraction: the past and the present. J Biomed Biotechnol. 2009 doi: 10.1155/2009/574398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasanthaiah HKN, Katam R, Sheikh MB. Efficient protocol for isolation of functional RNA from different grape tissue rich in polyphenols and polysaccharides for gene expression studies. Electron J Biotechnol. 2008;11(3):1–8. doi: 10.2225/vol11-issue3-fulltext-5. [DOI] [Google Scholar]
- Zarei A, Zamani Z, Mousavi A, Fatahi R, Alavijeh MK, Dehsara B, Salami SA. An effective protocol for isolation of high-quality RNA from pomegranate seeds. Asian Aust J Plant Sci Biotechnol. 2012;6:32–37. [Google Scholar]