Abstract
Salvia leriifolia Benth. (Lamiaceae) is an endangered medicinal plant with hypoglycemic, anti-inflammatory and analgesic properties. Many of the beneficial effects of Salvia spp. are attributed to the phenolic compounds. In the present study, an efficient procedure has been developed for establishment of cell suspension culture of S. leriifolia as a strategy to obtain an in vitro phenolic acids producing cell line for the first time. The effect of growth regulators and various concentrations of sucrose have been analyzed, to optimize biomass growth and phenolic acids production. The callus used for this purpose was obtained from leaves of 15-day-old in vitro seedlings, on Murashige and Skoog (MS) basal medium supplemented with different hormone balances including benzylaminopurine (BAP) and indole butyric acid (IBA); 2,4-dichlorophenoxyacetic acid (2,4-D) and kinetin (KIN); naphthaleneacetic acid (NAA) and BAP. Modified MS medium supplemented with 5 mg/L BAP and 5 mg/L NAA was the optimal condition for callus formation with the highest induction rate (100%), the best callus growth and the highest phenolic acids content. No callus induction was observed in combinations of IBA and BAP. Cell suspension cultures were established by transferring 0.5 g of callus to 30 mL liquid MS medium supplemented with 5 mg/L BAP and 5 mg/L NAA. Dynamics of phenolic acids production has been investigated during the growth cycle of the suspension cultures. The maximum content of caffeic acid and salvianolic acid B were observed on the 15th day of the cultivation cycle while the highest amount of rosmarinic acid was observed on the first day. In response to various sucrose concentrations, cell cultures with 40 g/L sucrose not only produced the highest dry biomass but also the highest induction of caffeic acid and salvianolic acid B. The highest amount of rosmarinic acid was observed in media containing 50 g/L sucrose. These prepared cell suspension cultures provided a useful system for further enhanced production of phenolic acids at a large scale.
Keywords: Salvia leriifolia, Phenolic acids, Growth regulators, Sucrose, Suspension culture
Introduction
Salvia is the largest genus of the family Lamiaceae and includes nearly 900 species throughout the world (Ghorbani and Esmaeilizadeh 2017). Fifty-eight species of the genus Salvia are found in Iran, of which 17 are endemic (Jalili and Jamzad 1999). Salvia leriifolia Benth. is an endemic plant with the local name Nowrozak which is mainly grows in Khorasan Razavi and Semnan provinces of Iran (Rechinger 1982). It has been reported that ethanolic extract of S. leriifolia has several pharmaceutical effects. For instance, its Neuroprotective effects on cerebral ischemia reperfusion injury in mice (Sadeghnia et al. 2003), attenuation of morphine dependence and anti-inflammatory effects (Hosseinzadeh and Yavary 1999) has been shown. Additionally, S. leriifolia has been used as anti-diabetic, anti-cancer and anti-Alzheimer’s agent (Loizzo et al. 2009). Many of the beneficial medicinal effects of Salvia species are attributed to polyphenol compounds including caffeic acid, rosmarinic acid, salvianolic acids and lithospermic acids (Lu and Foo 2002). It has been reported that caffeic acid derivatives have extensive biological activities including antioxidant, antivirus, anti-thrombosis, antihypertension, anticancer (Ren-Wang et al. 2005), anti-inflammatory and antitumor activities (Gamaro et al. 2011). Furthermore, rosmarinic acid has been known to induce antibacterial, antioxidant, antiviral anti-inflammatory effects and used in treatment of neurological disorders such as Alzheimer's disease (Petersen and Simmonds 2003; Shimojo et al. 2010). Salvianolic acids which are derived from S. miltiorrhiza show anticancer and antifibrotic and cardioprotective properties (Yang et al. 2016; Wang et al. 2013). Pharmaceutical properties of S. leriifolia have brought about its demise through being overharvested to the point it has been listed as endangered species in Iran (Jalili and Jamzad 1999). However, physiological impediments like seed dormancy and low germination frequency restrict the propagation of this plant. Therefore, in vitro culture of S. leriifolia would be a reasonable alternative approach to undertake in order to produce large-scale bioactive secondary metabolites (Owis et al. 2016; Khanpour-Ardestani et al. 2015). Manipulation of the culture environment must be effective in enhancement of the product. The induction of secondary metabolites could be altered by chemical factors such as nutritional factors, sugar and growth regulators. The level of sucrose has been shown to affect the productivity of secondary metabolites (Murthy et al. 2014). Higher sucrose concentrations were found to be optimal for rosmarinic acid production in S. officinalis, Coleus blumei and Anchusa officinalis cell cultures (Hippolyte et al. 1992, Martinez and Park, 1993; Su and Humphrey, 1990). Recently, Ali et al. (2016) have reported 50 g/L sucrose as the optimum concentration for maximum accumulation of phenolics and flavonoids in cell suspension cultures of Artemisia absinthium L. In spite of many investigations on in vitro production of phenolic acids in plant species, there are very few reports about production of phenolic acids in cell culture of Salvia species. For example, establishment of cell suspension culture and phenolic acids production has been reported in S. miltiorrhiza (Dong et al. 2010), S. officinalis (Santos-Gomes et al. 2003; Grzegorczyk et al. 2007) and S. fruticose (Karam et al. 2003). Previously, we determined the amounts of rosmarinic acid, salvianolic acid B and caffeic acid in the roots, leaves and calli (induced from shoot apical meristem) of S. leriifolia by HPLC method (Modarres et al. 2014). This research aims: (1) to establish callus (from leaves) and cell suspension culture of S. leriifolia. through application of a combination of growth regulators, (2) to induce production of phenolic acids, and (3) to test whether production of phenolic acids in cell suspension culture under different concentrations of sucrose is enhanced.
Materials and methods
Plant material and embryo culture
S. leriifolia seeds were collected from Kohsanghi Park district, Mashhad (36.26 N, 59.61 E), Iran, kept cool at 4 °C for 3 weeks and were stratified afterwards. Because of low rates of seed germination, embryonic culture was applied in order to produce sterile seedlings. After the seed coats were aseptically removed, the embryos were placed on MS medium containing 3% (w/v) sucrose, 0.7% (w/v) agar, glycine (2 mg/L), active charcoal (2 mg/L), pH 5.8 and were incubated in darkness for 7 days. Afterwards, the cultures were maintained at 24 ± 1 °C temperature and 16 h light (40–45 μmol−2 S−1)/8 h dark condition (Modarres et al. 2014).
Callus induction
Leaf pieces (1 cm x1cm) of aseptic 15 days old seedling were used as primary explants for establishing callus cultures. The explants were cultured on MS basal medium supplemented with 38 hormone combinations of different compositions including [Benzylaminopurine (BAP) (0, 0.5, 1, 2, 3 mg/L) and indole butyric acid (IBA) (0, 0.5, 1, 2 mg/L); 2,4-dichlorophenoxyacetic acid (2,4-D) (0, 1, 2, 3, 4, 5 mg/L) and kinetin (KIN) (0. 0.5, 1 mg/L); naphthaleneacetic acid (NAA) and BAP (0, 1, 2, 3, 4, 5, 6 mg/L)] (Table 1). Then, 20 cc of each hormonal MS medium was transferred to vials (10 × 3 cm) and autoclaved at 121 °C for 20 min. The cultures were incubated in dark and 25 °C. The calli were subcultured 4 times with interval of 30 days on MS medium with the same hormonal conditions. At the end of the fourth subculture, data were recorded including callus induction rate, callus fresh weight, dry weight and callus status. Optimum callus culture media were determined according to callus induction rate, growth and phenolic acids content. All plant growth regulators were purchased from Sigma-Aldrich Co (Gillingham, Dorset, UK).
Table 1.
Composition of plant growth regulators used for callus induction
| Treatment | NAA (mg/L) | BAP (mg/L) |
|---|---|---|
| 1 | 0 | 0 |
| 2 | 0.5 | 1 |
| 3 | 1 | 0.5 |
| 4 | 1 | 1 |
| 5 | 1 | 2 |
| 6 | 2 | 1 |
| 7 | 2 | 2 |
| 8 | 2 | 3 |
| 9 | 3 | 2 |
| 10 | 3 | 3 |
| 11 | 3 | 4 |
| 12 | 4 | 3 |
| 13 | 4 | 4 |
| 14 | 4 | 5 |
| 15 | 5 | 4 |
| 16 | 5 | 5 |
| 17 | 5 | 6 |
| 18 | 6 | 5 |
| 19 | 6 | 6 |
| Treatment | 2,4-D (mg/L) | KIN (mg/L) |
|---|---|---|
| 1 | 0 | 0 |
| 2 | 1 | 0.3 |
| 3 | 1 | 1 |
| 4 | 2 | 1 |
| 5 | 3 | 1 |
| 6 | 4 | 1 |
| Treatment | BAP (mg/L) | IBA (mg/L) |
|---|---|---|
| 1 | 0 | 0 |
| 2 | 0.5 | 0.5 |
| 3 | 0.5 | 1 |
| 4 | 0.5 | 2 |
| 5 | 1 | 0.5 |
| 6 | 1 | 1 |
| 7 | 1 | 2 |
| 8 | 2 | 0.5 |
| 9 | 2 | 1 |
| 10 | 2 | 2 |
| 11 | 3 | 0.5 |
| 12 | 3 | 1 |
| 13 | 3 | 2 |
Cell suspension culture
Cell suspension culture was established by transferring 0.5 g of callus into 100 mL Erlenmeyer flasks containing 30 mL of fresh MS liquid medium supplemented with 5 mg/L NAA + 5 mg/L BAP and sucrose (30 g/L), pH 5.8. The suspension cultures were shaken at 125 rpm and maintained at 16 h light (40–45 μmol−2 S−1)/8 h dark condition. The suspension cultures were subcultured in the MS liquid medium with interval of 12 days by transferring 10 mL of culture containing freely dispersed cells and aggregates into 100 mL Erlenmeyer flasks containing 20 mL of fresh medium with the same composition. For determination of cell suspension growth curve, cell dry weight, phenolic acids content was determined with sets of Erlenmeyer flasks harvested at 2 day-intervals in a period of 18 days. Readings were taken from three Erlenmeyer flasks for each parameter. Cells were filtered using nylon mesh and freeze-dried.
Effect of sucrose concentrations
The effect of sucrose was evaluated by transferring 0.5 g fresh weight of callus into 100 mL Erlenmeyer flasks containing 30 mL of MS liquid medium supplemented with 5 mg/L NAA + 5 mg/L BAP and concentrations of sucrose (30 g/L as control (normally used in MS medium), 40 and 50 g/L as treatment). Cultures were incubated under 16 h light (40–45 μmol−2 S−1)/8 h dark condition on a 125 rpm shaker. Dry weight (DW) and accumulation of selected phenolic acids were evaluated after 15 days.
Determination of phenolic acids content
Phenolic acids were extracted using 0.5 g of callus and cells by 20 mL of ethanol (60% w/w) and then sonicated for 30 min. The solvent was removed by a rotary evaporator. Then distilled water (20 mL) was added to the remaining residue and the pH of the solution was adjusted at 2.0. Then, ethyl acetate was added to the solution in five separate stages and then separated from the aqueous phase and removed on a rotary evaporator. The remaining residue was dissolved in 10 mL of methanol and filtered through a 0.25 μm filter. The isolated phenolic acids were analyzed by HPLC (Knauer, Berlin, Germany) with a 2800 photodiode array detector (Knauer smartline) and a C-18 column (5 µm, 4.6 × 150 mm, Macherey–Nagel, Düren, Germany). The mobile phase consisted of phosphoric acid 1% and methanol (min, water: methanol; 0, 60:40; 17, 50:50; 18, 40:60; 25–30, 60:40). The flow rate was 1 mL/min. Components were identified at 333 nm using a UV detector. The presence of phenolic acids in the samples was verified by comparison of retention times and UV spectral peaks of the sample with authentic standard (Modarres et al. 2014). Caffeic acid, rosmarinic acid and salvianolic acid B standards were purchased from Sigma-Aldrich Co.
Statistical analysis
All data were analyzed using JMP and MSTAT-C statistical softwares in a completely randomized design with three replicates. Statistical significance of differences in treatments were determined by a one-way analysis of variance (ANOVA) followed by a Duncan’s test. All errors are expressed as standard errors (SE). P ≤ 0.05 was considered to be a significant difference.
Results
Callus induction
In the present study, to determine the optimum level of plant growth regulators in S. leriifolia, different concentrations of IBA + BAP, 2,4-D + KIN and NAA + BAP (mg/L) were used in MS medium for callus induction. Results in Tables 2 and 3 showed no response on callus induction in control specimens (no growth regulators) and in combinations of IBA and BAP. The explants did not induce callus in most of the 2,4-D + KIN combinations except in combination of 3 mg/L 2,4-D + 1 mg/L KIN and 4 mg/L 2,4-D + 1 mg/L KIN. Combination of 3 mg/L 2,4-D + 1 mg/L KIN showed the greatest effect on callus induction rate (76.27%) and callus dry weight (Table 2 and Fig. 1b). The highest percentage of callus induction rate (100%) and callus dry weight (0.38 g) was observed in combination of 5 mg/L NAA + 5 mg/L BAP (Table 3, Fig. 1a).
Table 2.
Effect of different levels of 2,4-D and KIN on S. leriifolia leaf explants in MS medium
| 2,4-D (mg/L) | KIN (mg/L) | Callus induction rate (%) | Fresh weight (g) | Dry weight (g) | Callus status |
|---|---|---|---|---|---|
| 0 | 0 | 0 | 0 | 0 | – |
| 1 | 0.3 | 0 | 0 | 0 | – |
| 1 | 1 | 0 | 0 | 0 | – |
| 2 | 1 | 0 | 0 | 0 | – |
| 3 | 1 | 76.27a | 0.698a | 0.078a | Compact and opaque |
| 4 | 1 | 75.82a | 0.572b | 0.061b | Compact and opaque |
Data represent means (n = 3) for each different level of 2,4-D and KIN as a treatment. Means sharing the same letter in each column do not differ significantly at P ≤ 0.05 (Duncan’s test)
Table 3.
Effect of different levels of NAA and BAP on S. leriifolia leaf explants in MS medium
| NAA (mg/L) | BAP (mg/L) | Callus induction rate (%) | Fresh weight (g) | Dry weight (g) | Callus status |
|---|---|---|---|---|---|
| 0 | 0 | 0 | 0 | 0 | – |
| 0.5 | 1 | 0 | 0 | 0 | – |
| 1 | 0.5 | 0 | 0 | 0 | – |
| 1 | 1 | 0 | 0 | 0 | – |
| 1 | 2 | 0 | 0 | 0 | – |
| 2 | 1 | 0 | 0 | 0 | – |
| 2 | 2 | 0 | 0 | 0 | – |
| 2 | 3 | 0 | 0 | 0 | – |
| 3 | 2 | 0 | 0 | 0 | – |
| 3 | 3 | 0 | 0 | 0 | – |
| 3 | 4 | 0 | 0 | 0 | – |
| 4 | 3 | 0 | 0 | 0 | – |
| 4 | 4 | 0 | 0 | 0 | – |
| 4 | 5 | 0 | 0 | 0 | – |
| 5 | 4 | 0 | 0 | 0 | – |
| 5 | 5 | 100a | 4.356a | 0.379a | Yellowish |
| 5 | 6 | 89.5c | 2.42b | 0.220b | Yellowish |
| 6 | 5 | 95b | 2. 95b | 0.197b | Yellowish |
| 6 | 6 | 100a | 2.36b | 0.193b | Yellowish |
Data represent means (n = 3) for each different level of NAA and BAP as a treatment. Means sharing the same letter in each column do not differ significantly at P ≤ 0.05 (Duncan’s test)
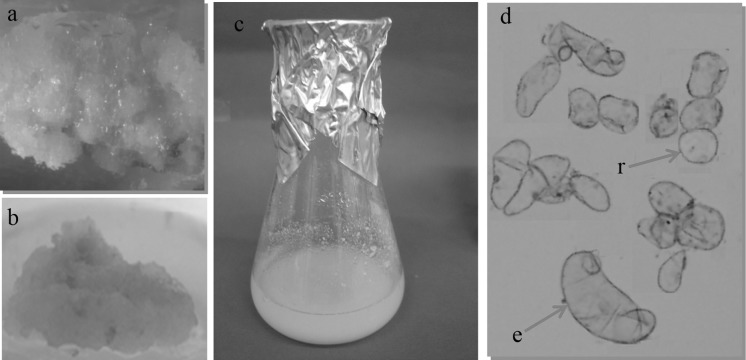
Fig. 1.
Establishment of S. leriifolia callus and cell suspension culture. a callus from leaf explants on MS medium with 5 mg/L BAP and 5 mg/L NAA, b callus from leaf on MS medium with 3 mg/L 2,4-D and 1 mg/L KIN, c S. leriifolia suspension culture grown in a flask, d cell suspension culture showing photomicrograph of round (r) and elongated (e) shaped cells (× 40)
Phenolic acids content of calli
Preliminary screening results showed two combinations of 5 mg/L NAA + 5 mg/L BAP (from different concentrations of NAA and BAP) and 3 mg/L 2,4-D + 1 mg/L KIN (from different concentrations of 2,4-D and KIN) produced the greatest callus induction and dry weight. Therefore, in the next step, these combinations were supplemented into MS medium for determining phenolic acids production. Results showed supplemented MS medium with 5 mg/L NAA and 5 mg/L BAP induced 16.72 mg/g DW rosmarinic acid which was 4 times greater than in MS medium supplemented with 3 mg/L 2,4-D and 1 mg/L KIN (Table 4). There were no significant differences between caffeic acid and salvianolic acid B contents in treatments with these media.
Table 4.
Caffeic acid, rosmarinic acid and salvianolic acid B contents in extracts of S. leriifolia callus
| Compound content (mg/g DW) | |||
|---|---|---|---|
| Callus | Caffeic acid | Rosmarinic acid | Salvianolic acid B |
| 2,4-D 3 mg/L + KIN 1 mg/L | 0.32a | 3.57b | 0.48a |
| NAA 5 mg/L + BAP 5 mg/L | 0.33a | 16.72a | 0.41a |
Data represent means (n = 3). Means sharing the same letter in each column do not differ significantly at P ≤ 0.05 (Duncan’s test)
Cell suspension culture
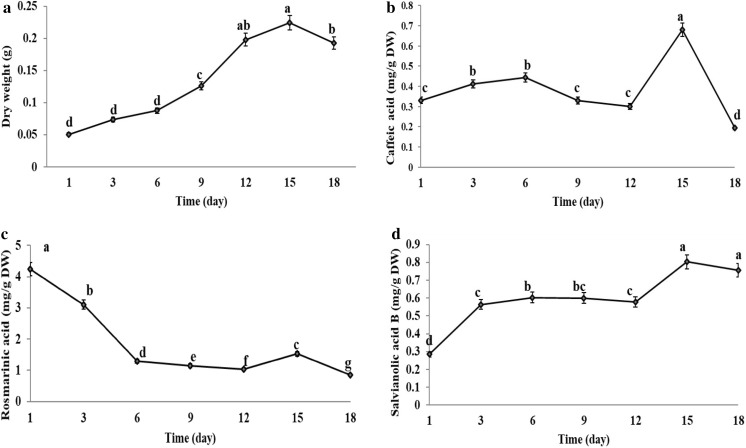
Calli grown on 5 mg/L NAA + 5 mg/L BAP were used for establishment of cell suspension cultures (Fig. 1c, d). Cell growth was determined by recording the dry weight of the cells every 2 days (Fig. 2a). The growth curve of suspension cultures showed a slow growth till day 8 followed by a sharp increase till day 14. The greatest dry weight was reached on day 15. Then the growth curve revealed a decreased growth rate till day 18.
Fig. 2.
Growth curve of suspension-cultured S. leriifolis cells during 18 days of incubation in MS medium containing 5 mg/L NAA and 5 mg/L BAP and phenolic acids content during different growth stages. a cell dry weight, b caffeic acid, c rosmarinic acid and d salvianilic acid B. Data represent mean ± SE, n = 3; Means sharing the same letter do not differ significantly at P ≤ 0.05 (Duncan’s test)
Dynamics of phenolic acids production in suspension culture
Dynamics of caffeic acid production in S. leriifolia cell suspension culture are shown in Fig. 2b. Caffeic acid content in the culture increased during the first 6 days, decreased afterwards to the lowest concentration on day 12, increased to the peak on day 15, and decreased at day 18. The content of rosmarinic acid reached its highest level on the first day after culture, decreased steadily until day 12, hiked again till day 15 and decreased again till day 18 (Fig. 2c). Salvianolic acid B content increased markedly during the first 6 days of cell suspension culturing and reached its highest level on day 15 (Fig. 2d).
Effect of sucrose on growth and phenolic acids production in suspension culture
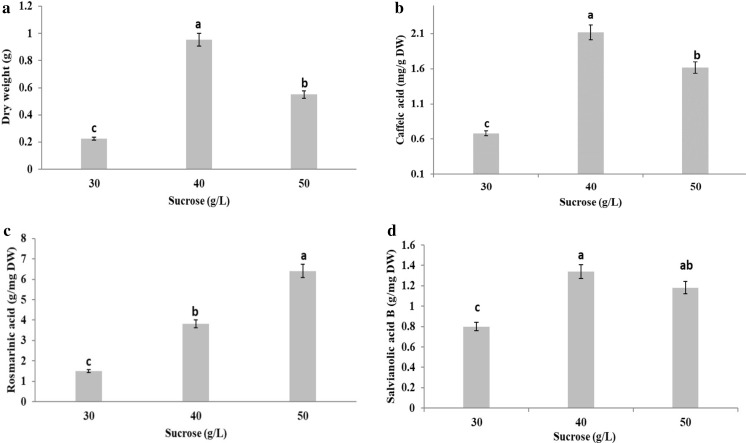
The growth of cell suspension cultures and biosynthesis of selected phenolic acids was affected by the amount of sucrose added to the nutrient medium. The results show that the dry weight of the cells grown on medium containing 40 g/L sucrose increased and the highest contents of caffeic acid and salvianolic acid B in the medium recorded (Fig. 3a, b, d). The highest amount of rosmarinic acid was observed in medium containing 50 g/L sucrose (Fig. 3c) compared with the control.
Fig. 3.
Effect of different concentrations of sucrose on cell biomass (a), caffeic acid (b), rosmarinic acid (c) and salvianilic acid B (d) content of S. leriifolia cell suspension culture. Data represent mean ± SE, n = 3; Means sharing the same letter do not differ significantly at P ≤ 0.05 (Duncan’s test)
Discussion
The present study provides a strategy for production of valuable phenolic acids of the endangered medicinal plant species, S. leriifolia, through the application of in vitro culture techniques. Callus cultures were successfully induced from S. leriifolia leaves on solid MS medium with optimized concentrations of growth regulators. Maximum induction of callus from S. leriifolia leaves was obtained from a combination of 5 mg/L NAA and 5 mg/L BAP. Kintzios et al. (1999) also achieved the best callus induction with NAA/BAP combination for S. officinalis. Liu et al. (2012) reported highest callus induction of S. splendens under combination of 2, 4-D and BAP. In our study, no callus induction was observed in combination of IBA and BAP. This could be because of the variation in the cell sensitivity of S. leriifolia towards the growth regulators concentrations and their specific receptors present in the different plant tissues (Moore 1989; Rahayui et al. 2016). In the present study, rosmarinic acid, caffeic acid and salvianolic acid B were detected, respectively, in greater concentrations in the callus cultures. The highest content of rosmarinic acid (16.72 mg/g DW) was obtained from media supplemented with 5 mg/L NAA and 5 mg/L BAP which was about fourfolds higher than for calli grown in 3 mg/L 2, 4-D and 1 mg/L KIN media. It was also about fourfold higher than rosmarinic acid content we previously reported from leaves of S. leriifolia (Modarres et al. 2014). The type and concentration of auxin or cytokinin alters both the growth and production of secondary metabolites (Rao and Ravishankar 2002). Huang et al. (2000) reported that 2, 4-D decreased the rosmarinic acid production in S. miltiorrhiza while BAP increased it. Although, different responses of plants to growth regulators were studied in the past, we still know few about the regulatory mechanism of induced secondary metabolites by the same growth regulators. Some investigators have reported different amounts of phenolic acids production in Salvia species. However, their results are different from the results obtained in this study. In previous studies, rosmarinic acid production has been reported in other Salvia species (Grzegorczyk et al. 2005; Wu et al. 2016). However, contents of rosmarinic acid were lower than those obtained in the present study. There are few reports on caffeic acid production in plant callus cultures. In the present study, callus culture yielded 0.6-0.7 mg/g DW of caffeic acid which was higher than in the leaves of S. leriifolia (Modarres et al. 2014). Santos-Gomes et al. (2003) reported production of caffeic acid (0.008 mg/g) in callus culture of S. officinalis which was much lower than we obtained in S. leriifolia callus culture. Our results showed that the yield of salvianolic acid B in callus cultures was 0.41–0.48 mg/g DW which was considerably higher than what we have found previously in S. leriifolia leaves (Modarres et al. 2014). The phenomenon seen in our investigation is in agreement with data of Wu et al. (2016) showing greater production of salvinolic acid in callus culture than in leaves in S. miltiorrhiza. The rapid suspension culture system for S. leriifolia was established in MS medium supplemented with 5 mg/L NAA + 5 mg/L BAP for the first time. In agreement with us, cell suspension culture of S. officinalis has been established on MS media supplemented with NAA and BAP (Bolta et al. 2000; Grzegorczyk et al. 2005). There are some reports on the use of other hormones for induction of cell suspensions culture from Salvia species. For example, Karam et al. (2003) used thidiazuron (TDZ) and IAA for S. officinalis. However, Dong et al. (2010) induced cell suspension culture from S. miltiorrhiza in the absence of any hormone. Production of phenolic acids has been reported in Salvia cell suspension cultures (Santos-Gomes et al. 2003; Grzegorczyk et al. 2005).
Results of this investigation also showed that dynamics of phenolic acids accumulation in S. leriifolia cell suspension culture is time dependent for different products (Fig. 2). For example, the highest content of caffeic acid and salvianolic acid B was observed on the 15th day of the cultivation cycle, while the greatest content of rosmarinic acid was observed on the first day of the cultivation cycle. These results are in agreement with findings of Santos-Gomes et al. (2003), that reported rosmarinic acid production decreased parallel to cell growth in S. officinalis cell culture. Weremczuk-Jezyna et al. (2017) also showed similar patterns of rosmarinic acid accumulation in Dracocephalum moldavica L. cell cultures. As rosmarinic acid is a substrate for salvialonic acid B (Petersen and Simmonds 2003), it seems that probably under special hormonal conditions, rosmarinic acid is used for the production of salvianolic acid B. The level of sucrose has been shown to affect the productivity of secondary metabolite-accumulating cultures. In this study, we report for the first time on the increase of selected phenolic acids contents in S. leriifolia under treatment with different sucrose concentrations. In response to various sucrose concentrations from 30 to 50 g/L, cultures with 40 g/L sucrose not only produced the highest dry biomass but also showed the highest induction of caffeic acid and salvianolic acid B compared with the control. Maximum accumulation of rosmarinic acid (6. 409 mg/g DW) was observed in cultures supplemented with 50 g/L sucrose. It was fourfold greater than in 30 g/L (control) sucrose supplemented cells. Similar results were reported by Hippolyte et al. (1992) on S. officinalis cell cultures. They showed the highst rosmarinic acid accumulation at 50 g/L sucrose. Bauer et al. (2004) has reported increased sucrose concentrations in MS medium diminished the growth of cells of Coleus blumei in suspension culture and media with 40 and 50 g/L of sucrose stimulated rosmarinic acid accumulation more than MS medium with 30 g/L sucrose. Moreover, the synthesis of rosmarinic acid has been shown to be upregulated by elevation of sucrose levels from 10 to 50 g/L (Gertlowski and Petersen, 1993; Petersen 1991). These increased levels of phenolic acids production could possibly be due to changes in the surrounding environment, such as osmolarity and disturbed cellular metabolism (Yang et al. 2012). It has long been known that sucrose provides energy, serves as a structural unit and generates physiological signals that regulate the expression of genes involved in both primary and secondary metabolism (Ali et al. 2016). It seems that lower sucrose concentration cannot provide adequate energy and, therefore, may not be able to act as a building block, a finding consistent with the results of this study.
Conclusion
In conclusion, for the first time, we successfully established callus and cell suspension cultures of S. leriifolia for the phenolic acids biotechnological production. The best callus growth and the highest phenolic acids production were obtained under cultivation in basal MS medium containing 5 mg/L NAA and 5 mg/L BAP. Prepared cell suspension cultures provide an efficient alternative method for the production of valuable phenolic acids. In addition, sucrose-treated suspension cultures showed improved cell biomass generation and phenolic acids accumulation. Additional sucrose concentration above the normally used level (30 g/L) enhanced accumulation of rosmarinic and caffeic acids in the culture medium. Furthermore, the application of growth regulators and sucrose in bioreactors could give rise to the commercial production of valuable secondary metabolites, such as phenolic acids. Further commercially focused studies are required to investigate the potential of Salvia plant species for the enhanced production of phenolic acids through elicitation, precursor feeding and metabolic engineering which is of potential research and development value in the field of secondary metabolites.
Acknowledgements
The authors thank the Ferdowsi University of Mashhad for providing equipments and facilities.
Author contribution
This research paper was accomplished with the collaboration of all authors. Masoomeh Modarres contributed to the design of research, performed the experiments and wrote the manuscript; Sedighe Esmaeilzadeh Bahabadi supervised the study, wrote the manuscript and Mohammd Ehsan Taghavizadeh performed the experiments.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Ali M, Abbasi BH, Ahmad N, Ali SS, Ali S, Ali GS. Sucrose-enhanced biosynthesis of medicinally important antioxidant secondary metabolites in cell suspension cultures of Artemisia absinthium L. Bioprocess Biosyst Eng. 2016;39:1945–1954. doi: 10.1007/s00449-016-1668-8. [DOI] [PubMed] [Google Scholar]
- Bauer N, Leljak-Levanic D, Jelaska S. Rosmarinic acid synthesis in transformed callus culture of Coleus blumei Benth. Zeitschrift für Naturforschung C. 2004;59:554–560. doi: 10.1515/znc-2004-7-819. [DOI] [PubMed] [Google Scholar]
- Bolta Z, Baricevic D, Bohanec B, Andrensek s. A preliminary investigation of ursolic acid in cell suspension culture of Salvia officinalis. Plant Cell, Tissue Organ Cult. 2000;62:57–63. doi: 10.1023/A:1006498431099. [DOI] [Google Scholar]
- Dong J, Wan G, Liang Z. Accumulation of salicylic acid-induced phenolic compounds and raised activities of secondary metabolic and antioxidative enzymes in Salvia miltiorrhiza cell culture. J Biotechnol. 2010;148:99–104. doi: 10.1016/j.jbiotec.2010.05.009. [DOI] [PubMed] [Google Scholar]
- Gamaro GD, Suyenaga E, Borsoi M, Lermen J, Pereira P, Ardenghi P. Effect of rosmarinic and caffeic acids on inflammatory and nociception process in rats. ISRN Pharmacol. 2011;10:1–6. doi: 10.5402/2011/451682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gertlowski C, Petersen M. Influence of the carbon source on growth and rosmarinic acid production in suspension cultures of Coleus blumei. Plant Cell, Tissue Organ Cult. 1993;34:183–190. doi: 10.1007/BF00036100. [DOI] [Google Scholar]
- Ghorbani A, Esmaeilizadeh M. Pharmacological properties of Salvia officinalis and its components. J Tradit Complement Med. 2017;7:433–440. doi: 10.1016/j.jtcme.2016.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzegorczyk I, Bilichowski I, Mikiciuk-Olasik E, Wysokinska H. In vitro cultures of Salvia officinalis L. as a source of antioxidant compounds. Acta Soc Bot Pol. 2005;74:17–21. doi: 10.5586/asbp.2005.003. [DOI] [Google Scholar]
- Grzegorczyk I, Matkowski A, Wysokińska H. Antioxidant activity of extracts from in vitro cultures of Salvia officinalis L. Food Chem. 2007;104:536–541. doi: 10.1016/j.foodchem.2006.12.003. [DOI] [Google Scholar]
- Hippolyte I, Marin B, Baccou J, Jonard R. Growth and rosmarinic acid production in cell suspension cultures of Salvia officinalis L. Plant Cell Rep. 1992;11:109–112. doi: 10.1007/BF00232160. [DOI] [PubMed] [Google Scholar]
- Hosseinzadeh H, Yavary M. Anti-inflammatory effect of Salvia leriifolia Benth. leaf extract in mice and rats. Harm Pharmacol Lett. 1999;9:60–62. [Google Scholar]
- Huang L, Liu D, Hu Z. Effects of phytohormones on growth and content of depsides in Salvia miltiorrhiza suspension cells. Zhong Yao Cai. 2000;2:1–4. [PubMed] [Google Scholar]
- Jalili A, Jamzad Z. Red data book of Iran. A preliminary survey of endemic, rare and endangered plants species in Iran. Tehran: RIFR Publication; 1999. [Google Scholar]
- Karam NS, Jawad FM, Arikat NA, Shibl RA. Growth and rosmarinic acid accumulation in callus, cell suspension, and root cultures of wild Salvia fruticosa. Plant Cell, Tissue Organ Cult. 2003;73:117–121. doi: 10.1023/A:1022806420209. [DOI] [Google Scholar]
- Khanpour-Ardestani N, Sharifi M, Behmanesh M. Establishment of callus and cell suspension culture of Scrophularia striata Boiss.: an in vitro approach for acteoside production. Cytotechnology. 2015;67:475–485. doi: 10.1007/s10616-014-9705-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kintzios S, Nikolaou A, Skoula M. Somatic embryogenesis and in vitro rosmarinic acid accumulation in Salvia officinalis and S. fruticosa leaf callus cultures. Plant Cell Rep. 1999;18:462–466. doi: 10.1007/s002990050604. [DOI] [Google Scholar]
- Liu H, Guoping Z, Guozheng S, Songlin R, Qiaojuan F. Callus induction and plant regeneration from mature seeds of Salvia splendens. Int J Agric Biol. 2012;14:445–452. [Google Scholar]
- Loizzo MR, Menichini F, Tundis R, Bonesi M, Conforti F, Nadjafi F, Menichini F. In vitro biological activity of Salvia leriifolia Benth essential oil relevant to the treatment of Alzheimer’s disease. J Oleo Sci. 2009;58:443–446. doi: 10.5650/jos.58.443. [DOI] [PubMed] [Google Scholar]
- Lu Y, Foo LY. Polyphenolics of Salvia. Phytochem. 2002;59:117–140. doi: 10.1016/S0031-9422(01)00415-0. [DOI] [PubMed] [Google Scholar]
- Martinez B, Park C. Characteristics of batch suspension cultures of preconditioned Coleus blumei cells: sucrose effect. Biotechnol Prog. 1993;9:97–100. doi: 10.1021/bp00019a014. [DOI] [Google Scholar]
- Modarres M, Asili J, Lahouti M, Iranshahi M, Sahebkar A. Simultaneous determination of rosmarinic acid, salvianolic acid B and caffeic acid in Salvia leriifolia Benth. root, leaf and callus extracts using a high-performance liquid chromatography with diode-array detection, technique. J Liq Chromatogr Relat Technol. 2014;37:1721–1730. doi: 10.1080/10826076.2013.807466. [DOI] [Google Scholar]
- Moore TC. Biochemistry and physiology of plant hormones. Berlin: Springer; 1989. pp. 234–242. [Google Scholar]
- Murthy HN, Lee EJ, Peak KY. Production of secondary metabolites from cell and organ cultures: strategies and approaches for biomass improvement and metabolite accumulation. Plant Cell, Tissue Organ Cult. 2014;118:1–16. doi: 10.1007/s11240-014-0467-7. [DOI] [Google Scholar]
- Owis AI, Abdelwahab NS, Abul-Soad AA. Elicitation of phenolics from the micropropagated endangered medicinal plant Calligonum polygonoides L. (Polygonoaceae) Pharmacogn Mag. 2016;12:465–470. doi: 10.4103/0973-1296.191458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen MS. Characterization of rosmarinic acid synthase from cell cultures of Coleus blumei. Phytochem. 1991;30:2877–2881. doi: 10.1016/S0031-9422(00)98217-7. [DOI] [Google Scholar]
- Petersen M, Simmonds MS. Rosmarinic acid. Phytochem. 2003;62:121–125. doi: 10.1016/S0031-9422(02)00513-7. [DOI] [PubMed] [Google Scholar]
- Rahayui S, Roostikai I, Bermawie N. The effect of types and concentrations of auxins on callus induction of Centella asiatica. Nusantara Biosci. 2016;8:283–287. doi: 10.13057/nusbiosci/n080224. [DOI] [Google Scholar]
- Rao SR, Ravishankar GA. Plant cell cultures: chemical factories of secondary metabolites. Biotechnol Adv. 2002;20:101–153. doi: 10.1016/S0734-9750(02)00007-1. [DOI] [PubMed] [Google Scholar]
- Rechinger KH. Flora Iranica. Gratz: Academishe Druk.u.Verlag sustalt; 1982. [Google Scholar]
- Ren-Wang J, Kit-Man L, Po-Ming H, Thomas CW, Mak KS, Kwok-Pui F. Chemistry and biological activities of caffeic acid derivatives from Salvia miltiorrhiza. Curr Med Chem. 2005;12:237–246. doi: 10.2174/0929867053363397. [DOI] [PubMed] [Google Scholar]
- Sadeghnia H, Nassiri Asl M, Haddad Khodaparast M, Hosseinzadeh H. The effect of Salvia leriifolia Benth. Root extracts on lipid peroxidation level during global ischemic-reperfusion in rats. J Med Plant. 2003;3:19–28. [Google Scholar]
- Santos-Gomes PC, Seabra RM, Andrade PB, Fernandes-Ferreira M. Determination of phenolic antioxidant compounds produced by calli and cell suspensions of sage (Salvia officinalis L.) J Plant Physiol. 2003;160:1025–1032. doi: 10.1078/0176-1617-00831. [DOI] [PubMed] [Google Scholar]
- Shimojo Y, Kosaka K, Noda Y, Shimizu T, Shirasawa T. Effect of rosmarinic acid in motor dysfunction and life span in a mouse model of familial amyotrophic lateral sclerosis. J Neurosci Res. 2010;88:896–904. doi: 10.1002/jnr.22242. [DOI] [PubMed] [Google Scholar]
- Su W, Humphrey AE. Production of rosmarinic acid in high density perfusion cultures of Anchusa officinalis using a high sugar medium. Biotechnol Lett. 1990;12:793–798. doi: 10.1007/BF01022597. [DOI] [Google Scholar]
- Wang J, Xiong X, Feng B. Cardiovascular effects of salvianolic acid B. Corporation evidence-based complementary and alternative medicine. Cairo: Hindawi Publishing; 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weremczuk-Jezyna I, Grzegorczyk-Karolak I, Frydrych B, Hnatuszko-Konka K, Gerszberg A, Wysokinska H. Rosmarinic acid accumulation and antioxidant potential of Dracocephalum moldavica L. cell suspension culture. Not Bot Horti Agrobot. 2017;45:215–219. doi: 10.15835/nbha45110728. [DOI] [Google Scholar]
- Wu CF, Karioti A, Rohr D, Bilia AR, Efferth T. Production of rosmarinic acid and salvianolic acid B from callus culture of Salvia miltiorrhiza with cytotoxicity towards acutelymphoblastic leukemia cells. Food Chem. 2016;201:292–297. doi: 10.1016/j.foodchem.2016.01.054. [DOI] [PubMed] [Google Scholar]
- Yang SY, Hong C, Lee H, Park S, Park B, Lee K. Protective effect of extracts of Perilla frutescens treated with sucrose on tert-butyl hydroperoxide-induced oxidative hepatotoxicity in vitro and in vivo. Food Chem. 2012;133:337–343. doi: 10.1016/j.foodchem.2012.01.037. [DOI] [PubMed] [Google Scholar]
- Yang D, Huang Z, Xing B, Jin W, Yan X, Guo Z, Liang Z. Regulation of folic acid on phenolic acids production in Salvia miltiorrhiza hairy roots. Plant Cell, Tissue Organ Cult. 2016;127:175–185. doi: 10.1007/s11240-016-1040-3. [DOI] [Google Scholar]