Abstract
Melilotus indicus, is a traditional medicine used as analgesic and emollient. Although Melilotus indicus extract (MIE) has recently been shown to suppress growth of several tumor cell lines, information regarding its antitumor mechanism is completely unknown. Here, we report the mechanism underlying the effects of MIE on human hepatocellular carcinoma cells, specifically HepG2, and SNU-182 cells. Methanolic MIE impaired the proliferation, and induced cell death in both HepG2 and SNU-182 cells but not in normal hepatic L-02 cells. Mechanistically, flow cytometric analysis revealed that MIE induces apoptosis in HepG2, and SNU-182 cells. However, MIE-induced apoptosis were not affected by a pan caspase inhibitor z-VAD-fmk as well as MIE did not stimulate caspase activation. Furthermore we found that MIE-induced apoptosis could be attributed to a mechanism involving mitochondria-mediated pathways evidenced by decrease in the mitochondrial membrane potential (ΔΨm), increase in the Bax/Bcl-2 ratio, and translocation of apoptosis inducing factor (AIF) from the mitochondria to the nucleus. Suppression in AIF expression by siRNA reduced MIE-induced apoptosis which suggested the dependency of MIE on AIF to induce apoptosis in hepatocellular carcinoma cells. To the best of our knowledge this is the first report elucidating the anticancer mechanism of MIE. Our findings suggested that MIE might be a good extract for developing anticancer drugs for human hepatocellular carcinoma treatment.
Electronic supplementary material
The online version of this article (10.1007/s10616-018-0195-7) contains supplementary material, which is available to authorized users.
Keywords: Melilotus indicus, Cancer, Apoptosis, AIF
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common cancer worldwide and the third most common cause of cancer death (Dai et al. 2014). Apoptosis is known as a mechanism against carcinogenesis by eliminating damaged cells due to many chemical agent inductions (Hengartner 2000). It is regulated by two mechanisms, extrinsic “death-receptor-mediated” and intrinsic “mitochondrial-mediated” (Elmore 2007). The extrinsic pathway includes activation of caspase-8 while the intrinsic pathway, includes activation of caspase-9 (Parrish et al. 2013). The two pathways are leading to caspase-3 activation and eventually causing apoptosis (Fulda and Debatin 2006). Another important mitochondrial pathway could be involved in apoptosis, which is independent of caspases activation (Tait and Green 2008). In this case, apoptosis-inducing factor (AIF), a mitochondrial oxidoreductase, is showing an important role in caspase-independent apoptosis (Joza et al. 2001; Susin et al. 1999). It has been established that many chemotherapeutic agents are working by induction of apoptosis, which has become a main mechanism for effective antitumor therapy (Horinaka et al. 2005; Kundu et al. 2005; Pistritto et al. 2016). However, most therapeutics remain in the preclinical stages, and many recent clinical trials have failed by disappointing responses mandating us for searching new apoptosis-inducer agents for cancer treatment.
In this context, medicinal herbs are considered rich source for anticancer drugs. Melilotus species (family Fabeaceae) are distributed in many parts of the world and are very common in different habitats in Egypt (Al Sherif 2009; El-Ghani and El-Sawaf 2004; Rogers et al. 2008). These plants are grown as a contaminant of clover eaten by animals and occasionally of wheat consumed by human (Rizk 1990). Melilotus species is known for their medicinal efficacies such as antibacterial, anticoagulant, anti-inflammatory, antioxidant, laxative and narcotic effects (Hussain et al. 2008; Stefanović et al. 2015; Zhao et al. 2007). An in vivo study attributed the anti-inflammatory effects of Melilotus extract to its ability to decrease the citrulline production (Pleşca‐Manea et al. 2002). Moreover, Melilotus extract tablets have been widely used for clinical anti-inflammatory purposes (Shun-jiu and Li 2007; Xu et al. 2008). On the other hand, Melilotus species are considered as poisonous plants at high doses (Quattrocchi 2012; Rizk 1990), however, the anti-cancer usefulness at low doses has been reported. Through simple screening, researchers showed that Melilotus indicus extract (MIE) has a potent cytotoxic effect toward liver, colon, cervix, and breast cancer cells (Abu-Dahab and Afifi 2007; Ahmed and Al-Refai 2014). Furthermore, Karakaş et al. (2012) showed that Melilotus officinalis and Melilotus alba extracts have great tumor inhibition activities. From a mechanistic point of view, however the anti-inflammatory effect of Melilotus extract could be attributed to the inhibition of TNF-α mRNA, COX-2 mRNA, and NF-kB (Zhang et al. 2007), such candidates are implicated in the apoptotic pathways in cancer cells (Dong et al. 2015), the underlying anticancer mechanisms of Melilotus extracts have remained completely unknown. Therefore, we aimed through studying the effect of M. indicus extract on cell proliferation of human hepatocellular carcinoma cell lines to elucidate its anticancer mechanism.
Materials and methods
Plant material and methanolic extract preparation
Melilotus indicus were collected from Shusha in El-Minia Governorate, Egypt. A botanist, Prof. Magdy Hussein Abd El-Twab, identified the collected plants by using the artificial keys from the taxonomy books and through comparisons with herbarium specimens. Plants preserved in the herbarium of Botany and Microbiology Department, Faculty of Science, Minia University (MIN) under the number AM4044.
The aerial parts of the plant were dried and thoroughly grounded into powder. The powdered form (80 g) was extracted using methanol (200 ml) for 72 h by a soxhlet apparatus. Then filtration was performed using Whatman filter paper no. 1 and the extract was evaporated in a rotar vapour at 40 °C to obtain the dried form. The dried powder was transferred to sterile screw caps and stored at − 20 °C. At use, the frozen dried extract was dissolved in dimethyl sulfoxide (DMSO; 100 mg/ml).
Cell cultures and reagents
Two human hepatocellular carcinoma cell lines, HepG2 and SNU-182, and one normal hepatic cell line, L-02, were obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA), and were cultured in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum (FBS; Sigma-Aldrich, St. Louis, MO, USA) in a humidified atmosphere with 5% CO2 at 37 °C. Staurosporine (STS) was purchased from Sigma-Aldrich. Carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP, Chuo-ku, Tokyo, Japan) was purchased from abcam. The chemicals used in this study were of high purity or cell-culture grade.
Proliferation and viability assays
Cell proliferation was quantified as BrdU incorporation using a colorimetric Cell Proliferation ELISA, BrdU Kit (Roche Diagnostics, Indianapolis, IN, USA) according to the manufacturer’s instructions. HepG2, SNU-182 or L-02 cells were seeded at 5 × 103 cells/well and cultured overnight in a 96-well plate. The cells were treated with 1, 5, 10, 25, 50 or 100 μg/ml MIE, or either 250 ng/ml STS as a positive control or DMSO (vehicle) as a negative control for 24 h. BrdU was added to a final concentration of 10 μM and incubated for the last 2 h. BrdU incorporation was quantified by OD450 according to the manufacturer’s instructions.
Cell viability was determined by using trypan blue exclusion assay after treatment with MIE or STS. HepG2, SNU-182 or L-02 cells (5 × 103 cells/well) were cultured in 6-well plates and treated with 1, 5, 10, 25, 50 or 100 μg/ml MIE, or either 250 ng/ml STS or DMSO for 24 h. The cells were harvested, re-suspended in 100 μl DMEM, and mixed with an equal volume of 0.4% trypan blue staining solution (Gibco, Grand Island, NY, USA). Numbers of viable and dead cells were counted using a hemocytometer and the cell viability percentage was determined by dividing the number of viable cells by the number of total cells (viable + dead) and multiplying by 100. The selectivity index (SI) was calculated by dividing the IC50 value for the non-cancer cell line L-02 by the value of the IC50 for cancer cell lines (HepG2 or SNU-182). This value indicated the specificity of the extract to cancer cells. A value of two or more indicated high specificity.
Apoptosis analysis
Induction of apoptosis of HepG2 or SNU-182 cells was analyzed by a FACS Calibur flow cytometer (BD Biosciences, San Jose, CA, USA) using the annexin V/propidium iodide (PI) staining kit (BioLegend, San Diego, CA, USA), according to the manufacturer’s instructions. HepG2 or SNU-182 cells were treated with 5, 10 or 20 μg/ml MIE, 20 μg/ml MIE combined 30 µM Pan-caspase inhibitor (z-VAD-fmk) or either 250 ng/ml STS or DMSO for 24 h. FACS data was analyzed with FlowJo software (FlowJo, Ashland, OR, USA).
Mitochondrial membrane potential
Alteration of mitochondrial membrane potential (ΔΨm) was measured using a fluorescent probe JC-1 dye (tetraethylbenzimidazolylcarbocyanine iodide) (Rockville, GeneCopoeia, MD, USA). The HepG2 and SNU-182 cells were seeded in 6-well plates at a density of 1 × 106 and incubated for 24 h. Cells were treated with 5, 10 or 20 μg/ml MIE, or either 1 μg/ml FCCP as a positive control or DMSO as a negative control for 24 h and incubated in a CO2 incubator at 37 °C. Later, the cells were stained with 1× JC-1 reagent (100 µl) for 30 min at 37 °C. Mitochondria with normal functions (normal ΔΨm) gives red fluorescence whereas the depolarized mitochondria gives green fluorescence. The red fluorescence (excitation 550 nm, emission at 600 nm) and green fluorescence intensity was measured (excitation 485 nm, emission at 535 nm) using fluorescence plate reader. The ratio of fluorescent intensity of j-aggregates (red fluorescence) to fluorescent intensity of j-monomers (green fluorescence) was used as indicator for the loss of ΔΨm. The lower the value of this ratio is, the more is the loss of the mitochondrial membrane potential.
Western blotting analysis
HepG2 or SNU-182 cells were harvested after 24 h incubation with MIE, STS, or DMSO (control), washed with PBS, and centrifuged for 10 min at 1000×g. Then, the cell pellets were suspended in ice–cold lysis buffer containing 15 mmol/l Tris, pH 7.6, 1 mmol/l MgCl2, 2.5 mmol/l EDTA, 250 mmol/l sucrose, 1 mmol/l dithiothreitol, 1 mmol/l ethylene glycol–bis (β–aminoethyl ether) tetraacetic acid, 1.25 mg/ml pepstatin A, 2.5 mg/ml aprotinin, 10 mg/ml leupeptin, 1.0 mmol/l PMSF, 50 mmol/l NaF, 0.1 mmol/l Na3VO4, and 2 mmol/l Na4P2O7 (Sigma-Aldrich) and homogenized with a glass Pyrex microhomogenizer (20 strokes). Then, the homogenates centrifuged at 800×g for 10 min at 4 °C to get the nuclear fraction pellet. The supernatants were then centrifuged at 15,000×g at 4 °C for 10 min to get the supernatant containing the cytosolic fraction and the pellet containing the mitochondria. All pellets were re-suspended in lysis buffer. The protein content of each cellular fraction was determined using the Bradford protein assay kit (Bio-Rad Laboratories, Hercules, CA, USA) according to the manufacturer’s instructions. For Western blotting analysis, 30 μg protein weight of cell lysate was loaded onto a 15% SDS-PAGE gel under reduced condition. Proteins separated on the gel were transferred onto a PVDF membrane. The PVDF membrane was incubated in blocking buffer containing 3% nonfat milk powder, 1% bovine serum albumin (Sigma-Aldrich) and 0.5% Tween-20 in PBS for 1 h. Subsequently, the PVDF membrane was incubated with the suitable primary antibody overnight, followed by horseradish peroxidase (HRP)-conjugated IgG (Cell Signaling Technology, Danvers, MA, USA) for 1 h with agitation at room temperature. Finally, the binding process was detected using X-ray film (Konica Minolta Medical Imaging, Wayne, NJ, USA) with ECL prime (GE Healthcare, Little Chalfont, UK).
Knockdown of AIF by siRNA
Cultured HepG2 or SNU-182 cells (2 × 105 cells/well) in a 6-well plate were transfected with AIF siRNA or negative control siRNA (Santa Cruz Biotechnology, Santa Cruz, CA, USA) with Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA). After 48 h incubation, 1.5 ml fresh DMEM medium was added and then the cells were cultured with 20 μg/ml MIE or DMSO for an additional 24 h for Western blotting analysis and apoptosis assay.
Statistical analysis
The data presented in this study are represented as mean ± SD. Student’s t test was performed to determine the statistical significance compared to a corresponding negative control. Statistical significance was defined as p < 0.05 or p < 0.005. The IC50 of MIE for HepG2, SNU-182 and L-02 was calculated by Graph Pad Prism 5 (Version 5.01, GraphPad Software, San Diego, CA, USA) from the trypan blue exclusion results. The data shown in the figures are representative data for three independent experiments.
Results
MIE induces selective anti-proliferative and cytotoxic activities against hepatocellular carcinoma cells
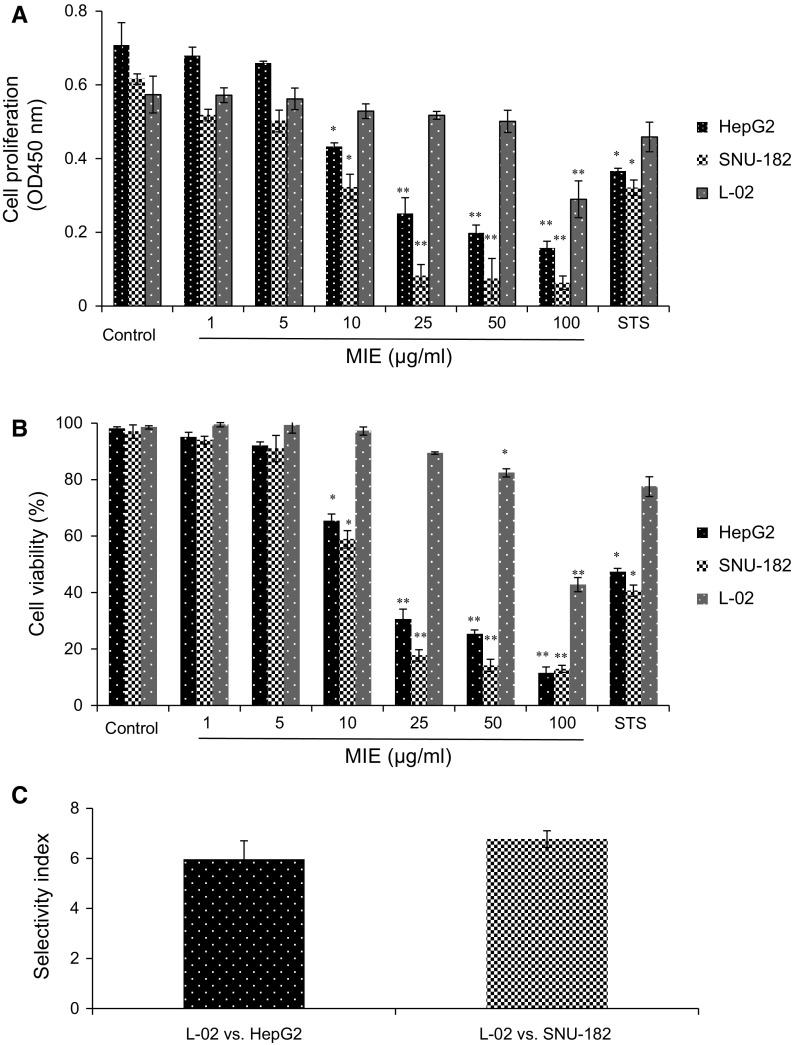
The effects of Melilotus indicus extract (MIE) on the proliferation and viability of HepG2, SNU-182 hepatocellular carcinoma cells and L-02 normal hepatic cells were determined via BrdU incorporation and trypan blue exclusion assays, respectively. BrdU incorporation assay revealed that treatment with MIE (10 µg/ml and above) significantly impairs cell proliferation in a dose-dependent manner in HepG2 and SNU-182 cancer cells but not in L-02 normal cells, however, SNU-182 cells showed a more elevated susceptibility to the treatments (Fig. 1a). Meanwhile, trypan blue exclusion assay revealed that treatment with MIE decreases the viability of HepG2 and SNU-182 cancer cells but not of L-02 normal cells in a dose-dependent manner upon incubation for 24 h (Fig. 1b). The IC50 of MIE was estimated approximately to be 16.6 μg/ml for HepG2 cells, 13.21 μg/ml for SNU-182 cells and 90.91 μg/ml for L-02 cells from the experimental results of cell viability assay. The 10 μg/ml MIE treatment has anti-proliferative and cytotoxic effects comparable to those of 250 ng/ml staurosporine (STS) as a positive control. Furthermore, results in Fig. 1c showed that MIE has selective cytotoxicity toward the hepatocellular carcinoma cells evidenced by the selectivity index (SI) values of the MIE for HepG2 and SNU-182 (5.9 and 6.7, respectively). These data suggested that MIE has the capacity to inhibit cell proliferation and induce cell death in hepatocellular carcinoma cells but not in normal hepatic cells.
Fig. 1.
Cell proliferation and viability of HepG2, SNU-182, and L-02 cells after MIE-stimulation. a Effect of MIE on the proliferation of HepG2, SNU-182, and L-02 cells were determined by BrdU incorporation assay. b The cytotoxic effect of MIE on HepG2, SNU-182, and L-02 cells was determined by trypan blue exclusion assay. c The selectivity index (SI) was calculated by dividing the IC50 value for the non-cancer cell line L-02 by the value of the IC50 for cancer cell lines (HepG2 and SNU-182). Representative data of three independent experiments are shown as mean ± SD. *p < 0.05 and **p < 0.005 indicate significant differences compared with control by Student’s t-test. (Color figure online)
MIE induces apoptosis in hepatocellular carcinoma cells independently of caspases
To clarify whether MIE-induced cell death in both HepG2 and SNU-182 cells was associated with apoptosis, MIE-induced apoptosis was analyzed by flow cytometry using a combination of annexin V and propidium iodide (PI) staining (Fig. 2a–c). The results showed that after the MIE treatment, early apoptosis or cell death rate and the late apoptosis rate were increased in a dose dependent manner in both HepG2 and SNU-182 cells. Furthermore, the pancaspase inhibitor z-VAD-fmk has no effect on MIE-induced apoptosis in both cell lines (Fig. 2a–c), suggesting that MIE induced caspase-independent apoptosis. To confirm, we evaluated the effect of MIE on the caspases proteins activation using Western blotting analysis (Fig. 2d). The results revealed that MIE has no effect on cleaved caspases-8 and 9 protein levels. Moreover, the relative protein expression levels of cleaved caspase-3 normalized with those of pro-caspase-3 revealed that MIE has no effect on cleaved caspase-3 expression in both HepG2 and SNU-182 cells (Fig. 2d and Supplementary Figure 1A and B). These results confirm that MIE induces apoptosis via caspase-independent pathway.
Fig. 2.
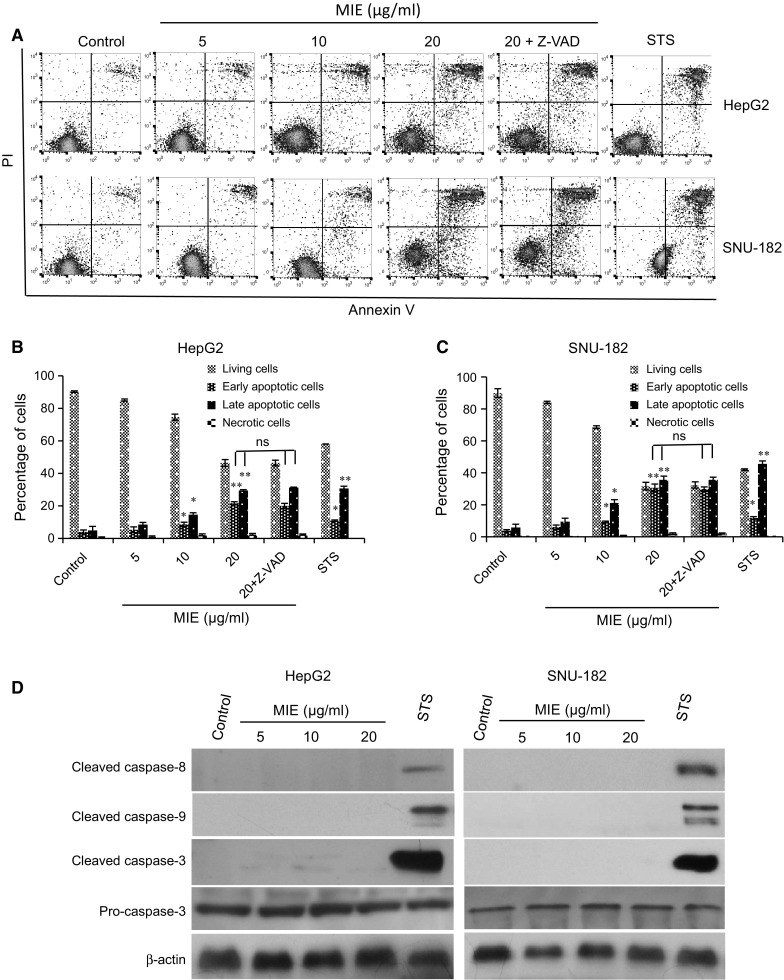
MIE induces apoptosis in HepG2 and SNU-182 cells independently of caspases. a Representative cytograms of flow cytometric analysis of the apoptotic cells in HepG2 and SNU-182 cells. The lower-right (annexin V+PI− cells) and the upper-right (annexin V+PI+ cells) quadrants show early and late apoptotic cells, and the lower-left (annexin V−PI− cells) and the upper-left (annexin V−PI− cells) quadrants represent viable and necrotic cells, respectively. b–c Percentage of HepG2 and SNU-182 cells. d The levels of cleaved caspases-8, 9, and 3, and pro-caspase-3 were determined by Western blotting analysis. β-actin served as a loading control. The values are the mean ± SD of three different experiments. *p < 0.05 and **p < 0.005 indicate significant difference compared with control. ns. there is no significant differences in the apoptotic cells percentage after adding z-VAD-fmk
MIE induces apoptosis via decrease in mitochondrial membrane potential (ΔΨm), increase in Bax/Bcl-2 ratio, and translocation of AIF from the mitochondria to the nucleus
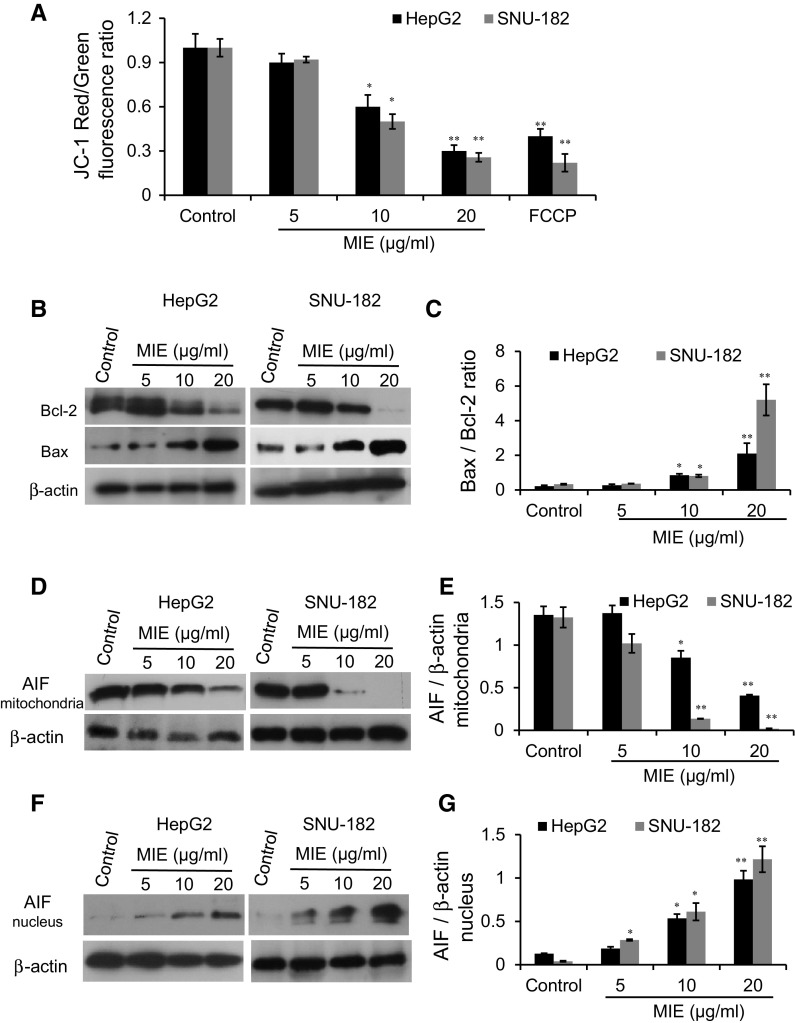
Mitochondrial changes, including loss of ΔΨm, are considered as key events that occur due chemotherapeutic agents induced caspase-independent-mitochondrial apoptosis pathway. Thus, the effect of MIE on mitochondria, especially the changes in ΔΨm, was examined using the lipophilic dye JC-1. The results as shown in Fig. 3a indicating that MIE decreases ΔΨm in a dose-dependent manner in HepG2 and SNU-182 cells. To further investigate whether MIE induced apoptosis by triggering the caspase-independent-mitochondrial apoptosis pathway, we examined the expression of the Bcl-2 family proteins, including Bcl-2 and Bax, in response to MIE using Western blotting analysis (Fig. 3b, c). We observed a decrease in Bcl-2 expression and an increase in the expression of Bax when cells were treated with MIE, which led to an increase in the proapoptotic/antiapoptotic (Bax/Bcl-2) ratio, in a dose-dependent manner, in HepG2 and SNU-182 cells (Fig. 3b, c). Since the translocation of AIF from the mitochondria to the nucleus is implicated in the caspase-independent-mitochondrial apoptosis, we asked whether MIE induces the nuclear translocation of AIF in both cell lines. Indeed, using Western blotting analysis, MIE treatment led to the translocation of AIF from the mitochondria to the nucleus, as evidenced by a decrease in the mitochondrial AIF protein levels and an increase in the nuclear AIF protein levels in a dose dependent manner in both HepG2 and SNU-182 cells (Fig. 3d–g). These results indicate that MIE induces the dysfunction of mitochondria via AIF mitochondrial export leading to apoptosis.
Fig. 3.
MIE decreases the mitochondrial membrane potential (Δψm), increases the Bax/Bcl-2 ratio, and induces nuclear translocation of AIF in HepG2 and SNU-182 cells. a The average JC-1 red/green fluorescence ratio in HepG2 and SNU-182 B, protein levels of Bcl-2 and Bax were determined by Western blotting analysis. β-actin was immunostained as a loading control. c Bax/Bcl-2 ratio in HepG2 and SNU-182 cells d, f Protein levels of mitochondrial and nuclear AIF in HepG2 and SNU-182 were determined by Western blotting analysis. β-actin was immunostained as a loading control. e, g The relative protein expression levels of mitochondrial and nuclear AIF normalized with β-actin were quantified by the Image J software. The values are the mean ± SD of three different experiments. *p < 0.05 and **p < 0.005 indicate a significant difference compared with negative control. (Color figure online)
MIE induces apoptosis via AIF-dependent pathway
Furthermore, HepG2 and SNU-182 cells were transiently transfected with AIF siRNA to identify whether AIF is responsible for the MIE-induced apoptosis. A Western blotting analysis demonstrated that AIF siRNA potently down-regulates the expression level of AIF (Fig. 4a, b). As shown in Fig. 4c, d, silencing AIF attenuates MIE-induced apoptosis compared with the cells transfected with negative control siRNA. These findings suggest that AIF plays an important role in MIE-induced apoptosis in HepG2 and SNU-182 cells.
Fig. 4.
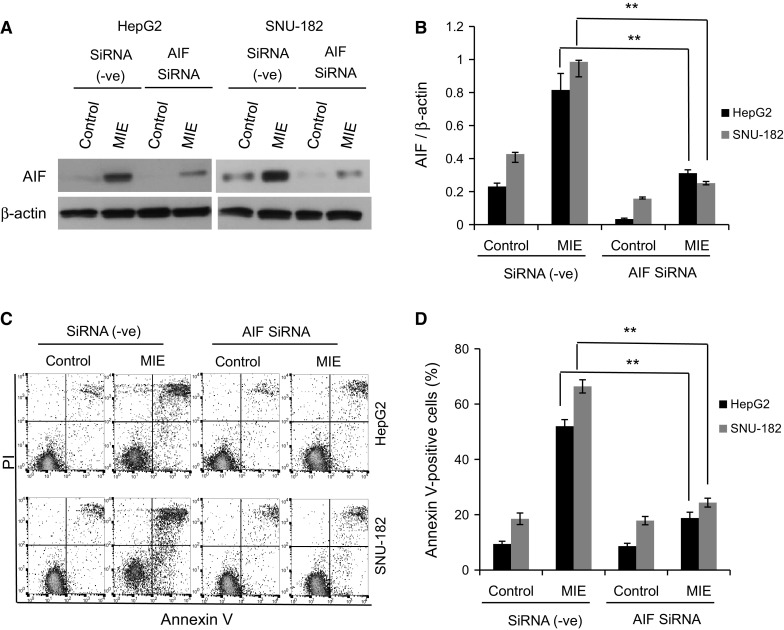
Effect of AIF knockdown on MIE-induced apoptosis in HepG2 and SNU-182 cells. a AIF knockdown by siRNA specific to AIF decreases the nuclear AIF protein expression in MIE-treated cells determined by Western blotting. β-actin served as a loading control. b The relative protein expression levels of AIF normalized by those of β-actin were quantified by Image J. c, d MIE-induced apoptosis is substantially abrogated upon siRNA knockdown of AIF. Apoptotic cells in AIF-silenced and MIE-stimulated HepG2 or SNU-182 cells were analyzed by flow cytometry, and total annexin V-positive cells were quantified. The values are the mean ± SD of three different experiments. *p < 0.05 and **p < 0.005 indicate a significant difference compared with negative siRNA transfectants
Discussion
Medicinal plants play an important role in the cancer prevention and treatment. Melilotus indicus extract (MIE) has recently been shown to suppress growth of several tumor cells including liver, breast, colon and cervix (Abu-Dahab and Afifi 2007; Ahmed and Al-Refai 2014). However, the mechanisms by which MIE initiates its anticancer activity are completely unknown. To our knowledge, this is the first report showing the mechanism underlying the effects of MIE on regulating the fate of human hepatocellular carcinoma (HCC) cells. We noted that the methanolic extract of M. indicus has anti-proliferative and cytotoxic activities against HCC cells (Fig. 1a, b). However, many methanolic plant extracts were screened for their anti-cancer activities against HCC cells, including, Aristolochia macroura (IC50 = 513 µg/ml), Lithraea molleoides (IC50 = 244 µg/ml), Achyrocline satureioides (IC50 = 237 µg/ml), and Schinus molle (IC50 = 50 µg/ml) (Ruffa et al. 2002), but our results indicates that MIE has superior anti-cancer activity (IC50 range = 13–16 μg/ml) against HCC cells. These results indicate the usefulness of MIE in the fight against HCC. This assumption is supported by the good selectivity index (SI = 5.9 and 6.7) of the tested extract, which is compatible with their possible use in cancer chemotherapy. Phytochemical analysis has shown the plant to contain flavonoid glycosides, coumarins, terpenoids, and steroids (Ahmed et al. 2012; Yadava and Jain 2005). The attribution of the anticancer activity of MIE to a specific chemical constituent is a critical point that needs to be clarified in future studies.
Previous studies have reported that natural compounds decrease cell proliferation and induce cell death via induction of apoptosis (Abd El-Hafeez et al. 2017a, b; Diederich and Noworyta 2012; Mukhtar et al. 2012). Similarly, MIE induces cell apoptosis significantly in HCC cells (Fig. 2). Apoptosis may be activated by two different pathways: extrinsic or intrinsic “mitochondrial” pathway (Elmore 2007). These pathways activate caspases family proteins (Parrish et al. 2013). Apoptosis can also occur through another mitochondrial pathway, which is independent on caspases activation (Tait and Green 2008). Interestingly, our results showed that pancaspase inhibitor, z-VAD-fmk, failed to suppress MIE-induced apoptosis (Fig. 2a–c). Furthermore, from a molecular point of view, MIE treatment did not result in activation of caspase-3, 8, and 9 (Fig. 2d). These results indicate that MIE induces caspase-independent apoptosis in hepatocellular carcinoma cells. Changes in the mitochondrial membrane potential (ΔΨm) have been considered to be early, obligate events in the apoptosis process (Cohen 1997; Zamzami et al. 1995). Early loss of ΔΨm may occur independently of caspase activation. For example, resveratrol, a natural flavonoid, induced ΔΨm loss prior to any detectable caspase activation (Mahyar-Roemer et al. 2001; Marzo et al. 2001). Similarly, in our study we noticed that MIE induced loss of ΔΨm without activation of caspases, which supports the ability of the phytochemicals to induce apoptosis via mitochondrial dysfunction independently of caspases.
On the other hand, from mechanistic point of view, the loss of ΔΨm has been partially attributed to the Bcl-2 family proteins (Basañez et al. 2012; Bleicken et al. 2013; García-Sáez et al. 2010). Some pro-apoptotic members of Bcl-2 family like Bax and Bak can form membrane channels or pores, resulting in the loss of Δψm (Bleicken et al. 2013). On the contrary, the anti-apoptotic molecules such as Bcl-2 and Bcl-xL can prevent the conformational change and oligomerization of Bax and Bak (Cabon et al. 2012; Martinou and Green 2001). Furthermore, induction of apoptosis is well correlated with the increase in the ratio of proapoptotic/antiapoptotic Bax/Bcl-2 (Abd El-Hafeez and Rakha 2017). Our data revealed that MIE increases the proapoptotic/antiapoptotic (Bax/Bcl-2) ratio which could be the possible mechanism by which MIE conductes loss in the Δψm leading to apoptosis.
Moreover, Mitochondria contain several potentially apoptogenic factors, including apoptosis-inducing factor (AIF) (Susin et al. 1999). It has been shown that when apoptosis is induced, AIF translocates from the mitochondria to the cytosol and the nucleus (Lipton and Bossy-Wetzel 2002; Susin et al. 1999). Furthermore, it was demonstrated that loss of Δψm correlates with AIF translocation to the cytosol and nuclear alterations. Mitochondrion AIF is thought to be feeble, as far as apoptosis modulation is concerned. In contrast, nuclear AIF triggers chromatin condensation and DNA fragmentation (Nagata et al. 2003) leading to apoptosis (Loeffler et al. 2001). AIF is believed to induce caspase-independent apoptosis as inhibition of caspase activation or caspase activity does not eliminate the proapoptotic action of AIF (Susin et al. 1999). We measured the translocation of AIF from the mitochondria to the nucleus by Western blotting. As expected, the expression of mitochondrial AIF decreased while the expression of nuclear AIF increased, in a dose-dependent manner, (Fig. 3d, f, g). Taken together, the translocation of AIF from the mitochondria to the nucleus due to MIE-treatment without activation of caspases indicates that MIE induced apoptosis via the caspase-independent-mitochondrial pathway. We also found that AIF knockdown attenuates MIE-induced apoptosis in HepG2 and SNU-182 cells. Although our data clearly show that AIF plays an important role in MIE-induced apoptosis, detailed molecular mechanism underlying the AIF-driven apoptosis, especially its effect on chromatin condensation and DNA fragmentation is still unknown and needs to be addressed. In addition, further analysis is needed to elucidate the upstream molecular events by which MIE activates AIF. Kim et al. (2012) reported that AIF-induced apoptosis mediated by activation of ERK and p38 kinases. So, the effect of MIE on the upstream molecules, in particular MAP Kinases (ERK and p38), and its relation to the apoptotic induction needs to be clarified in future studies. On the other hand, however M. indicus is considered as a poisonous plant at high doses (Quattrocchi 2012; Rizk 1990), our in vitro results showed its anti-tumor usefulness at low doses. However, few studies demonstrated the efficacy of the in vitro doses in the in vivo models (Abd El-Hafeez et al. 2017a, b; Wang et al. 2006), but the dose that is effective in in vitro studies is not often the same as the dose that would be effective in animals or humans, so further in vivo and clinical studies, including toxicological aspects, should be done in the future to determine the optimal effective and safe dose of MIE for the prevention and treatment of HCC.
In conclusion, to our knowledge, this study is the first to report the antitumor mechanism of action of MIE. The treatment with MIE impaired cell proliferation and decreased cell viability via induction of caspase-independent-mitochondrial apoptosis in hepatocellular carcinoma cells through the activation of the AIF pathway. This might be the main mechanism of MIE that suppressed growth of hepatocellular carcinoma cells. MIE may be a good extract for developing anticancer drugs for human hepatocellular carcinoma prevention and treatment.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Compliance with ethical standards
Conflict of interest
All authors declare that they have no conflicts of interest.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s10616-018-0195-7) contains supplementary material, which is available to authorized users.
References
- Abd El-Hafeez AA, Rakha OM. Paederus alfieri extract induces apoptosis in human myeloid leukemia K562 cells. Asian J Pharm Clin Res. 2017;10:1–4. [Google Scholar]
- Abd El-Hafeez AA, Fujimura T, Kamei R, Hirakawa N, Baba K, Ono K, Kawamoto S. A methoxyflavanone derivative from the Asian medicinal herb (Perilla frutescens) induces p53-mediated G2/M cell cycle arrest and apoptosis in A549 human lung adenocarcinoma. Cytotechnology. 2017 doi: 10.1007/s10616-017-0116-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Hafeez AA, Fujimura T, Kamei R, Hirakawa N, Baba K, Ono K, Kawamoto S. Synergistic tumor suppression by a Perilla frutescens-derived methoxyflavanone and anti-cancer tyrosine kinase inhibitors in A549 human lung adenocarcinoma. Cytotechnology. 2017 doi: 10.1007/s10616-017-0124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Dahab R, Afifi F. Antiproliferative activity of selected medicinal plants of Jordan against a breast adenocarcinoma cell line (MCF7) Sci Pharm. 2007;75:121–146. doi: 10.3797/scipharm.2007.75.121. [DOI] [Google Scholar]
- Ahmed SAK, Al-Refai M. Chemical constituents and cytotoxic activities of the extracts of Melilotus indicus. Eur J Chem. 2014;5:503–506. doi: 10.5155/eurjchem.5.3.503-506.1070. [DOI] [Google Scholar]
- Ahmed D, Baig H, Zara S. Seasonal variation of phenolics, flavonoids, antioxidant and lipid peroxidation inhibitory activity of methanolic extract of Melilotus indicus and its sub-fractions in different solvents. Int J Phytomed. 2012;4:326. [Google Scholar]
- Al Sherif EA. Melilotus indicus (L.) All., a salt-tolerant wild leguminous herb with high potential for use as a forage crop in salt-affected soils. Flora Morphol Distrib Funct Ecol Plants. 2009;204:737–746. doi: 10.1016/j.flora.2008.10.004. [DOI] [Google Scholar]
- Basañez G, Soane L, Hardwick JM. A new view of the lethal apoptotic pore. PLoS Biol. 2012;10:e1001399. doi: 10.1371/journal.pbio.1001399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleicken S, Landeta O, Landajuela A, Basañez G, García-Sáez AJ. Proapoptotic Bax and Bak proteins form stable protein-permeable pores of tunable size. J Biol Chem. 2013;288:33241–33252. doi: 10.1074/jbc.M113.512087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabon L, et al. BID regulates AIF-mediated caspase-independent necroptosis by promoting BAX activation. Cell Death Differ. 2012;19:245–256. doi: 10.1038/cdd.2011.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen GM. Caspases: the executioners of apoptosis. Biochem J. 1997;326:1–16. doi: 10.1042/bj3260001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai L, Ren P, Liu M, Imai H, Tan EM, Zhang J-Y. Using immunomic approach to enhance tumor-associated autoantibody detection in diagnosis of hepatocellular carcinoma. Clin Immunol. 2014;152:127–139. doi: 10.1016/j.clim.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diederich M, Noworyta K. Natural compounds as inducers of cell death. Berlin: Springer; 2012. [Google Scholar]
- Dong QM, Ling C, Chen X, Zhao L. Inhibition of tumor necrosis factor-α enhances apoptosis induced by nuclear factor-κB inhibition in leukemia cells. Oncol Lett. 2015;10:3793–3798. doi: 10.3892/ol.2015.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Ghani MMA, El-Sawaf N. Diversity and distribution of plant species in agro-ecosystems of Egypt. Syst Geogr Plants. 2004;74:319–336. [Google Scholar]
- Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulda S, Debatin K. Extrinsic versus intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene. 2006;25:4798–4811. doi: 10.1038/sj.onc.1209608. [DOI] [PubMed] [Google Scholar]
- García-Sáez AJ, Fuertes G, Suckale J, Salgado J. Permeabilization of the outer mitochondrial membrane by Bcl-2 proteins. In: Anderluh G, Lakey J, editors. Proteins membrane binding and pore formation. Advances in experimental medicine and biology. NY: New York; 2010. pp. 91–105. [DOI] [PubMed] [Google Scholar]
- Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- Horinaka M, Yoshida T, Shiraishi T, Nakata S, Wakada M, Nakanishi R, Nishino H, Matsui H, Sakai T. Luteolin induces apoptosis via death receptor 5 upregulation in human malignant tumor cells. Oncogene. 2005;24:7180–7189. doi: 10.1038/sj.onc.1208874. [DOI] [PubMed] [Google Scholar]
- Hussain K, Shahazad A, Zia-ul-Hussnain S. An ethnobotanical survey of important wild medicinal plants of Hattar district Haripur, Pakistan. Ethnobot Leafl. 2008;2008:5. [Google Scholar]
- Joza N, Susin SA, Daugas E, Stanford WL. Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature. 2001;410:549–554. doi: 10.1038/35069004. [DOI] [PubMed] [Google Scholar]
- Karakaş FP, Yildirim A, Türker A. Biological screening of various medicinal plant extracts for antibacterial and antitumor activities . Turk J Biol. 2012;36:641–652. [Google Scholar]
- Kim MJ, Woo JS, Kwon CH, Kim JH, Kim YK, Kim KH. Luteolin induces apoptotic cell death through AIF nuclear translocation mediated by activation of ERK and p38 in human breast cancer cell lines. Cell Biol Int. 2012;36:339–344. doi: 10.1042/CBI20110394. [DOI] [PubMed] [Google Scholar]
- Kundu T, Dey S, Roy M, Siddiqi M, Bhattacharya R. Induction of apoptosis in human leukemia cells by black tea and its polyphenol theaflavin. Cancer Lett. 2005;230:111–121. doi: 10.1016/j.canlet.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Lipton SA, Bossy-Wetzel E. Dueling activities of AIF in cell death versus survival: DNA binding and redox activity. Cell. 2002;111:147–150. doi: 10.1016/S0092-8674(02)01046-2. [DOI] [PubMed] [Google Scholar]
- Loeffler M, et al. Dominant cell death induction by extramitochondrially targeted apoptosis-inducing factor. FASEB J. 2001;15:758–767. doi: 10.1096/fj.00-0388com. [DOI] [PubMed] [Google Scholar]
- Mahyar-Roemer M, Katsen A, Mestres P, Roemer K. Resveratrol induces colon tumor cell apoptosis independently of p53 and precede by epithelial differentiation, mitochondrial proliferation and membrane potential collapse. Int J Cancer. 2001;94:615–622. doi: 10.1002/ijc.1516. [DOI] [PubMed] [Google Scholar]
- Martinou J-C, Green DR. Opinion: breaking the mitochondrial barrier. Nat Rev Mol Cell Biol. 2001;2:63. doi: 10.1038/35048069. [DOI] [PubMed] [Google Scholar]
- Marzo I, Perez-Galan P, Giraldo P, Rubio-Felix D, Alberto A, Naval J. Cladribine induces apoptosis in human leukaemia cells by caspase-dependent and-independent pathways acting on mitochondria. Biochem J. 2001;359:537–546. doi: 10.1042/bj3590537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhtar E, Mustafa Adhami V, Khan N, Mukhtar H. Apoptosis and autophagy induction as mechanism of cancer prevention by naturally occurring dietary agents. Curr Drug Targets. 2012;13:1831–1841. doi: 10.2174/138945012804545489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata S, Nagase H, Kawane K, Mukae N, Fukuyama H. Degradation of chromosomal DNA during apoptosis. Cell Death Differ. 2003;10:108–116. doi: 10.1038/sj.cdd.4401161. [DOI] [PubMed] [Google Scholar]
- Parrish AB, Freel CD, Kornbluth S. Cellular mechanisms controlling caspase activation and function. Cold Spring Harb Perspect Biol. 2013;5:a008672. doi: 10.1101/cshperspect.a008672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistritto G, Trisciuoglio D, Ceci C, Garufi A, D’Orazi G. Apoptosis as anticancer mechanism: function and dysfunction of its modulators and targeted therapeutic strategies. Aging. 2016;8:603. doi: 10.18632/aging.100934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleşca-Manea L, Pârvu AE, Pârvu M, Taaˇmaş M, Buia R, Puia M. Effects of Melilotus officinalis on acute inflammation. Phytother Res. 2002;16:316–319. doi: 10.1002/ptr.875. [DOI] [PubMed] [Google Scholar]
- Quattrocchi U. CRC world dictionary of medicinal and poisonous plants: common names, scientific names, eponyms, synonyms, and etymology (5 volume set) Boca Raton: CRC Press; 2012. [Google Scholar]
- Rizk A-F. Poisonous plant contamination of edible plants. Boca Raton: CRC Press; 1990. [Google Scholar]
- Rogers ME, Colmer TD, Frost K, Henry D, Cornwall D, Hulm E, Deretic J, Hughes SR, Craig AD. Diversity in the genus Melilotus for tolerance to salinity and waterlogging. Plant Soil. 2008;304:89–101. doi: 10.1007/s11104-007-9523-y. [DOI] [Google Scholar]
- Ruffa M, Ferraro G, Wagner M, Calcagno M, Campos R, Cavallaro L. Cytotoxic effect of Argentine medicinal plant extracts on human hepatocellular carcinoma cell line. J Ethnopharmacol. 2002;79:335–339. doi: 10.1016/S0378-8741(01)00400-7. [DOI] [PubMed] [Google Scholar]
- Shun-jiu ZJ-wZ, Li QS-hL. Treatment of deep venous thrombosis of lower extremities by low molecular weight heparin sodium and melilotus extract tablet. Chin J Clin Pharm. 2007;3:018. [Google Scholar]
- Stefanović OD, Tešić JD, Čomić LR. Melilotus albus and Dorycnium herbaceum extracts as source of phenolic compounds and their antimicrobial, antibiofilm, and antioxidant potentials. J Food Drug Anal. 2015;23:417–424. doi: 10.1016/j.jfda.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susin SA, Lorenzo HK, Zamzami N, Marzo I. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397:441–446. doi: 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- Tait SW, Green DR. Caspase-independent cell death: leaving the set without the final cut. Oncogene. 2008;27:6452–6461. doi: 10.1038/onc.2008.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Yuan S, Wang J, Lin P, Liu G, Lu Y, Zhang J, Wang W, Wei Y. Anticancer activity of litchi fruit pericarp extract against human breast cancer in vitro and in vivo. Toxicol Appl Pharmacol. 2006;215:168–178. doi: 10.1016/j.taap.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Xu F, Zeng W, Mao X, Fan G-K. The efficacy of melilotus extract in the management of postoperative ecchymosis and edema after simultaneous rhinoplasty and blepharoplasty. Aesthet Plast Surg. 2008;32:599–603. doi: 10.1007/s00266-008-9149-3. [DOI] [PubMed] [Google Scholar]
- Yadava R, Jain S. A new bioactive flavone glycoside from the seeds of Melilotus indica All. J Asian Nat Prod Res. 2005;7:595–599. doi: 10.1080/10286020310001608949. [DOI] [PubMed] [Google Scholar]
- Zamzami N, Marchetti P, Castedo M, Zanin C, Vayssiere J-L, Petit PX, Kroemer G. Reduction in mitochondrial potential constitutes an early irreversible step of programmed lymphocyte death in vivo. J Exp Med. 1995;181:1661–1672. doi: 10.1084/jem.181.5.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XY, Tao JY, Zhao L, Huang ZJ, Xiong FL, Zhang SL, Li CM, Xiao F. In vitro anti-inflammatory effects of different solution fractions of ethanol extract from Melilotus suaveolens Ledeb. Chin Med J. 2007;120:1992. [PubMed] [Google Scholar]
- Zhao L, Tao J-Y, Zhang S-L, Pang R, Jin F, Dong J-H, Guo Y-J. Inner anti-inflammatory mechanisms of petroleum ether extract from Melilotus suaveolens Ledeb. Inflammation. 2007;30:213–223. doi: 10.1007/s10753-007-9039-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.