Abstract
Germline stem cells (GSCs) play an indispensable role in establishing the fertility of an organism. The isolation and culture of adult female GSCs (FGSCs) have provided a robust foundation to study the development of female germ cells in rodents. However, many problems still need to be identified, such as the origin and location of FGSCs and the specific markers for screening. In this study, we acquired FGSCs that stably expressed Oct4 from Oct4 promoter-GFP transgenic mouse ovarian surface epithelium and cortical layer, and identified the cells possessing the representative features including the expression of GSCs marker genes and the potentiality of differentiation into all three germ layers in vitro. Moreover, rapamycin was confirmed to promote proliferation of mouse FGSCs and inhibit the differentiation capability in vivo. In addition to the reported disinfection function, rapamycin inhibited the activation of primordial follicles, as the inhibitor of mechanistic target of rapamycin pathway. These results will contribute to the study on folliculogenesis or oogenesis mechanism and have important implications on developing new technology and therapeutic approach in medicine for premature ovarian failure, infertility and even ovary remodelling in future.
Electronic supplementary material
The online version of this article (10.1007/s10616-018-0196-6) contains supplementary material, which is available to authorized users.
Keywords: Female germline stem cells, Characterization, Rapamycin, Mouse
Introduction
Germline stem cells (GSCs) are essential for genome delivery during sexual reproduction. In spite of unipotency, GSCs have a unique capability to generate female gametes successively. Oocytes production in women and in most female mammals only occurs in the foetal period and lost after birth, due to reduction in the number of oocytes (Peters 1970; Green and Zuckerman 1951), because GSCs enter meiosis before birth and are blocked in the diplotene of meiotic I phase (Bland et al. 2003; Inoue et al. 2011; Mitchell et al. 2010). However, this classical theory has been challenged by recent studies in which FGSCs with self-renewal and differentiation potential have been discovered in ovaries of many kinds of adult mammals, such as humans, mice and rats (Johnson et al. 2004; White et al. 2011; Zhang et al. 2011; Zhou et al. 2013). Moreover, the balance between self-renewal and differentiation of FGSCs is regulated by a complex network that includes intrinsic and extrinsic factors (Bukovsky 2011). Further research has demonstrated that mouse FGSC lines generated in vitro could undergo oogenesis and give rise to offsprings once transplanted into ovaries of infertile mice (Pan 2014). These findings have important scientific value and significance in preventing and curing premature ovarian, infertility and other ovarian diseases. However, the underlying mechanisms that regulate the identity of FGSCs remain unknown (Zhang et al. 2016). Therefore, more attention is needed to clarify the mechanisms that maintain the unipotent and undifferentiated state of FGSCs, which is pivotal for understanding GSC biology.
Rapamycin, a lipophilic macrolide compound produced by the bacterium Streptomyces hygroscopicus, which was isolated for the first time in the 1970s in a soil sample (Vézina et al. 1975), has an inhibitory effect on cell mitosis by blocking cell signaling (Chung et al. 1992). It is the most potent immunosuppressive agent in the treatment of organ transplant rejection and autoimmune diseases (Harrison et al. 2009). In 2009, a study showed that such drugs can prolong the life span of yeast, nematodes, fruit flies and other invertebrates (Harrison et al. 2009). Moreover, rapamycin was found to have anti-aging properties and could extend the life span of mice up to 10–15% by regulating the activation of primordial follicles and developmental differentiation (Adhikari et al. 2010; Dou et al. 2017). Mechanistic target of rapamycin (mTOR) is common in mammals as an evolutionarily conserved 289-kDa serine/threonine kinase regulating the processes of multiple cellular functions, such as translation, cell growth, proliferation, and metabolism (Fingar and Blenis 2004; Laplante and Sabatini 2009; Sonenberg and Hinnebusch 2011). In mammals, the mTOR kinase interacts with several proteins to form two functionally distinct multiprotein complexes, namely, mTOR Complex 1 (mTORC1) and mTOR Complex 2 (mTORC2) (Ryskalin et al. 2017). Studies have shown that mTORC1 is mainly involved in cell growth and proliferation in response to energetic and nutritional conditions (Haissaguerre et al. 2014). Activation of mTORC1, results in increased protein synthesis and cell survival by direct phosphorylation of its effectors, such as the ribosomal S6K1, and further promotes cell growth, proliferation, and metabolism, and tumorigenesis (Laplante and Sabatini 2009; Wullschleger et al. 2006). Rapamycin is an allosteric inhibitor of mTORC1, which leads to the inhibition of one serine/threonine kinase of the phosphoinositide kinase-related kinase (PIKK) family (Hay and Sonenberg 2004). In 2008, mTORC1 has been shown to regulate the activation and differentiation of primordial follicles. Increasing mTORC1 activity can lead to premature follicular depletion and eventually cause premature ovarian failure in adult mice (Adhikari et al. 2009, 2010). Recent studies have shown that mTOR can also be used to calorie restriction to slow the aging process (Blagosklonny 2010), and the mTOR signal pathway may be involved in the initiation of puberty and the extension of the life cycle (Roa and Tenasempere 2010; Santos et al. 2011). Thus, we can hypothesise that rapamycin is strongly related to proliferation, differentiation, self-renewal and delayed aging of ovarian germ cells.
Materials and methods
Animals and animal experimental protocol
All experiments were performed on healthy adult female (1 month old) Oct4-GFP transgenic (OG2) mice or ICR mice weighing 25 ± 1 g. The mice were obtained, respectively, from the National Laboratory Animal Center of Nanjing University and the Fourth Military Medical University. The mice were housed in plastic cages at 25 °C, 12 h light–dark cycle and free feed. All procedures were performed under the supervision of the Chinese Association for Laboratory Animal Science, and were approved by the Shaanxi Centre of Stem Cells Engineering and Technology, Northwest A&F University. For the in vivo experiment, fifty age-matched ICR mice were randomly separated into two groups (25 per group): Control group received vehicle injection (DMSO), the experimental group received rapamycin. Specifically, rapamycin, diluted in DMSO to 1 mg/ml, was injected intraperitoneally at 5 mg/kg body weight of mice every 2 days. DMSO used in this step was diluted in physiological saline to the concentration of 2%. After 1 month, all mice were sacrificed by vertebra dislocation after 4 mg/g averdin abdominal injection to collect their ovaries from rapamycin-treated group and control group and their body and ovarian weight were recorded.
Isolation and enrichment of mouse FGSCs
FGSCs were obtained from OG2 mouse ovaries via one-step digestion. Concretely, ovarian tissue was placed in a Petri dish with CDD liquid containing 2 mg/ml collagenase type IV, 2 µg/ml DNase I and 2 mg/ml trypsin (Invitrogen, Carlsbad, CA, USA), and incubated at 37 °C, 5% CO2 and saturated humidity for 50 min and pipetted every10 min to facilitate digestion. 100 μl fetal bovine serum (FBS; Hyclone, Logan, UT, USA) was added to the tube for ending digestion, and the rest of the ovarian tissues were picked out. Then a single-cell suspension was obtained through gently washing with α- Dulbecco’s Modified Eagle’s Medium (α-DMEM, Gibco, Grand Island, NY, USA) and was plated on a culture dish containing irradiated mouse embryonic fibroblasts (MEFs) that were obtained from ICR fetuses (13.5 days postcoitum) .These cells were called FGSCs. Concurrently, the remaining tissues were cut into pieces, digested with 0.25% trypsin (W/V) and inoculated to the culture dish using the same method. These tissues were marked as Remains. The fresh medium was replaced after 1 day, the cells were cultured for 7 days, and then the colonies were detected and collected through 1 mg/ml collagenase type IV (Sigma-Aldrich, St. Louis, MO, USA). Then, FGSCs were replated into 24-well culture plate containing MEFs and passaged at about 1 week at a ratio of 1:3.
FGSCs were cultured with α-MEM complemented with 10% FBS (Hyclone), 2 mM l-glutamine (Merck), 1% non-essential amino acids (Invitrogen), 0.1 mM 2-mercaptoethanol (Sigma-Aldrich), 10 ng/ml of mouse epidermal growth factor (EGF; Invitrogen), 1 ng/ml of human basic fibroblast growth factor (bFGF; Tebu-bio, Shanghai, PRC), 40 ng/ml of human glial cell line–derived neurotrophic factor (GDNF, Peprotech Inc. Rocky Hill, NJ, USA), 5 µg/ml of insulin, 20 µg/ml transferrin factor, 1 mM sodium selenite, 60 µM putrescine and 10 ng/ml LIF (Peprotech Inc.), 100 U/ml penicillin and 100 mg/ml streptomycin (Invitrogen).
With the same method, FGSCs were isolated, respectively, from the ovaries of ICR mouse injected with rapamycin or DMSO.
In vitro differentiation of FGSCs
FGSCs were suspended in DMEM (Invitrogen) supplemented with 20% FBS, 2 mM l-glutamine, 0.1 mM nonessential amino acids, 0.1 mM 2-mercaptoethanol, 1 mM sodium pyruvate (Invitrogen), 100 U/ml penicillin and 100 mg/ml streptomycin. Cell clusters formed after 3 days, and then were transferred to 0.1% gelatin-coated (Invitrogen) Petri dishes for 5–7 days to further spontaneous differentiation into adipocyte, osteoblast and cartilage. After differentiation, the formed EBs were collected according to the previously reported method (Hua and Zhu et al. 2011; Wang et al. 2014), and were detected the marker genes of three germ layers were detected via immunofluorescence staining, using the antibodies against NSE, PDX1 and Islet1 (Abcam, Shanghai, China) and the corresponding secondary antidodies (anti-rabbit IgG, 1:1000, Boster, Wuhan, China).
Immunofluorescence staining
The cells were fixed in 4% formaldehyde solution for 10–15 min, and treated with 0.1% Triton X-100 for 10 min, then blocked with 1% BSA for 30 min at RT. The cells were incubated in primary antibodies against PCNA (1:100, rabbit IgG, Chemicon, Temecula, CA, USA), VASA (1:200, rabbit IgG, Abcam), Oct4 (1:500, rabbit IgG, Sangon Biotech, Shanghai, PRC), c-Kit (1:200, rabbit IgG, Sangon Biotech), NES (1:100, rabbit IgG, Abcam), Desmin (1:200, rabbit IgG, Abcam) and Pdx1 (1:100, rabbit IgG, Sangon Biotech), overnight at 4 °C. After washing three times with PBS, the cells were incubated with the corresponding secondary antibodies (anti-rabbit IgG, 1:1000, Boster) at RT for 1 h. Then, the cells were added Hoechst 33342 (1 µg/ml, Sigma-Aldrich) and incubated at RT for 5 min. Negative controls were added with conjugated secondary antibodies alone for staining. Images were captured with Evosf1 fluorescence microscope (AMG, Millcreek, Washington, USA).
Tissues were fixed in 4% (w/v) paraformaldehyde solution for 10 min, then washed in PBS, dehydrated in ethanol (70, 90, and 100%) and embedded in paraffin wax (Shanghai Specimen and model Factory, Shanghai, China). Ovary sections (5 µm) were rehydrated (xylene for 5 min; ethanol 100, 95 and 70%, each time for 5 min, washed in distilled water, and then subjected to immunofluorescence staining according to the method described above).
The processes of immunofluorescence and HE staining were performed following the instruction as described in our previous studies (Hua and Zhu et al. 2011).
Quantitative RT-PCR analysis
Total RNA was extracted from the 1st passage and 7th passage FGSCs, remains and ovaries using the TRIzol reagent (Thermo Fisher Scientific, Foster City, CA, USA) according to the manufacturer’s program, and then was reverse transcribed using RevertAid Frist Strand cDNA Synthesis Kit (Thermo Fisher Scientific). Real time-PCR analysis was performed under the ABI StepOnePlus PCR system (Thermo Fisher Scientific) using SYBR Premix ExTaq II (TaKaRa Bio, Otsu, Japan). The beta-actin expression was used as the house-keeping control. The expression levels of all target genes were normalized to β-actin. The comparative CT method was used to measure the relative gene expression. Primer sequences for qRT-PCR were listed in Table 1.
Table 1.
Primers used in real time-PCR
| Primers | Sense primer | Anti-sense primer |
|---|---|---|
| Nanog | TTCTTGCTTACAAGGGTCTGC | AGAGGAAGGGCGAGGAGA |
| Oct4 | GGCGTTCTCTTTGGAAAGGTGTTC | CTCGAACCACATCCTTCTCT |
| Sox2 | CATGAGAGCAAGTACTGGCAAG | CCAACGATATCAACCTGCATGG |
| SSEA1 | CTGGTGGGCGAGATCATCA | CACTGCCATGAATGATGTTCC |
| Vasa | TATGTGCCTCCCAGCTTCAGTA | CTGGATTGGGAGCTTGTGAAGA |
| C-kit | CGCCTGCCGAAATGTATG | TCAGCGTCCCAGCAAGTC |
| CyclinD1 | TGAACTACCTGGACCGCT | CAGGTTCCACTTGAGYTTGT |
| c-Myc | CCTAGTGCTGCATGAGGAGAC | TCTTCCTCATCTTCTTGCTCTTC |
| Klf4 | TGGTGCTTGGTGAGTTGTGG | GCTCCCCCGTTTGGTACCTT |
| CyclinA | TGGCTGTGAACTACATTGA | ACAAACTCTGCTACTTCTGG |
| Zp3 | GAGCTTTTCGGCATTTCAAG | AGCTTATCGGGGATCTGGTT |
Western blot analysis
Proteins were extracted from ovarian tissues of mouse that were administrated with rapamycin for 1 month, and the BCA Protein Quantification Kit (Vazyme, Piscataway, NJ, USA) was then used to detect protein concentration. The protein samples were denatured in 5% SDS–PAGE loading buffer, and separated by SDS-PAGE and transferred to PVDF membranes. Then, the samples were detected with β-ACTIN (1:2000; mouse IgG, Abcam), PCNA (1:1000, rabbit IgG, Chemicon), VASA (1:200, rabbit IgG, Abcam), mTOR (1:1000, rabbit IgG, Cell Signaling Technlogy, Danvers, MA, USA), p-mTOR (1:1000, rabbit IgG, Cell Signaling Technlogy). The secondary antibody was rabbit/anti-mouse IgG (1:1000; Boster). Protein blots were probed with the indicated primary antibodies and the appropriate secondary antibodies. The protein bands were visualized using the Thermo Scientific Pierce ECL Western blot substrate. The results were analyzed using a Bio-Rad imaging system 203 (Bio-Rad, Hercules, CA, USA) and quantified using Image J (V1.48d).
Statistical analysis
Statistical comparisons were performed through one-way ANOVA with Newman–Keuls multiple range test, and evaluated by analysis of Student’s t test. Data were presented as mean ± SEM and the standard errors of the mean in this study were carried out in triplicates. P < 0.05 was considered statistically significant.
Results
Isolation and enrichment of mouse FGSCs
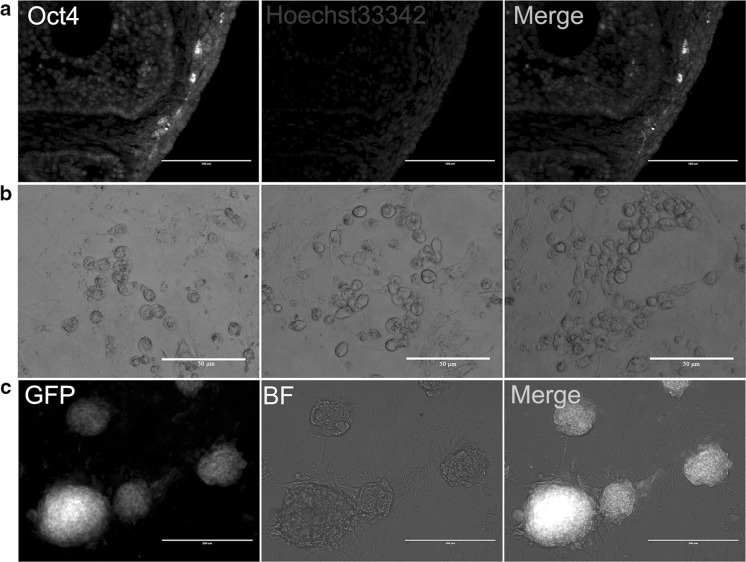
In this study, we chose Oct4-GFP transgenic mice as the experimental animal, using Oct4 as specific expression marker of FGSCs. By observing the localization of GFP, FGSCs were found to exist mainly in the surface epithelium of ovarian and cortical layer (Fig. 1a). Further, the whole ovary was digested with CDD liquid. And the surface cells, which proliferated with a bead-like structure, were obtained (Fig. 1b). By selecting the single clone, Oct4-positive cells that stably expressed GFP were obtained and purified. These cells possessed proliferation capability and grew in clusters that resembled embryonic stem cell (ESC) colony (Fig. 1c). In particular, the cells were seeded at a concentration of 105 cells/well in 35 mm cell culture dishes, and medium was changed every other day. After 4–5 days, the number of cells could be used for passage to enrich. In addition, cells could continue to grow normally after cryopreservation and recovery. This results suggested that mouse FGSCs were successfully isolated and enriched.
Fig. 1.
Derivation and cultivation of mouse FGSCs. a The expression and localization of Oct4 in ovarian surface epithelium and cortical surface in OG2 mice were measured via immunohistochemistry. Scale bars, 100 μm. b Isolated cells proliferated with a beaded-like structure in vitro, from left to right, day 2, day 3, day 4. c Despite several passages, the clones still stably expressed the GFP protein under the control of the Oct4 promoter. BF, Bright Field.
Characterization of mouse FGSCs
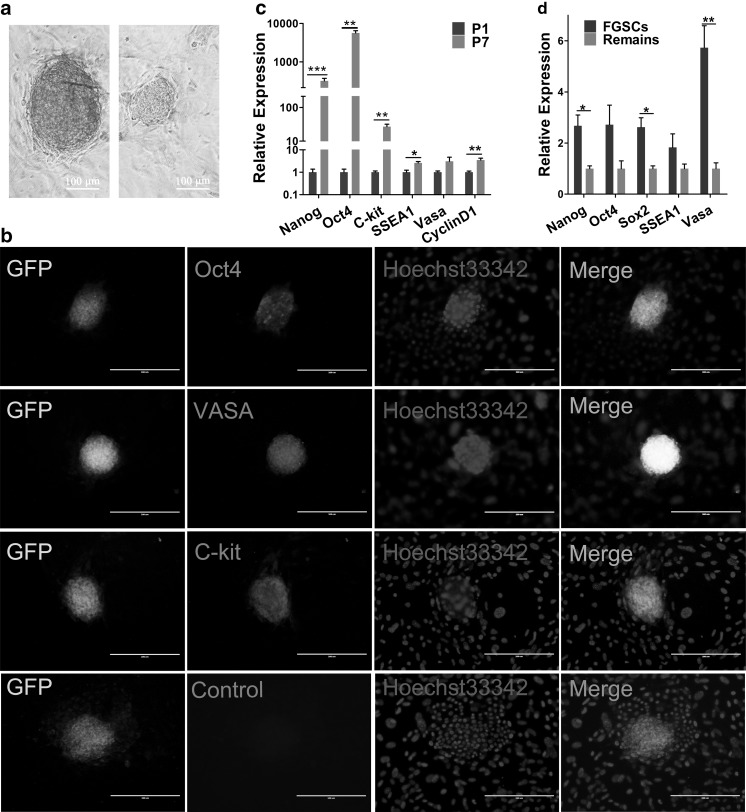
To determine the biological characteristics of the obtained FGSCs, the expression of a series of molecules was detected through qRT-PCR and immunofluorescence staining (Fig. 2). Most FGSCs stained positively for alkaline phosphatase (AP) (Fig. 2a). These characteristics were similar to that of ESCs (Llinas et al. 2005). The result of immunofluorescence staining showed that FGSCs which expressed pluripotency markers Oct4, germ cell marker Vasa and differentiation marker C-kit (Fig. 2b). At the mRNA level, isolated FGSCs expressed pluripotent genes, such as Nanog, Oct4, Sox2, SSEA1 and differentiation gene VASA, proliferation-related marker CyclinD1 (Fig. 2c, d). Moreover, compared with the remains of ovarian tissues, these genes were highly expressed in FGSCs (Fig. 2d). Further, to detect whether the cell characteristics were stable after several passages, related-gene expression in the first and seventh passage of FGSCs was tested via qRT-PCR. These results indicated that the expression levels of genes mentioned above were significantly increased except Vasa (Fig. 2c). Therefore, we concluded that FGSCs could maintain their characteristics well after several passages. The seventh passage of FGSCs had higher pluripotency and reproductive specificity than the primary generation after six generations of purification.
Fig. 2.
Detection of the markers of mouse FGSCs. a AP staining of isolated mouse FGSCs. The clone on the left represented positive AP staining, and the right one was negative control. Scale bars, 100 μm. b Immunofluorescence staining for Oct4, C-kit, VASA of mouse FGSCs. c, d Comparison of the mRNA expression for pluripotency gene Oct4, Nanog and SSEA1, proliferation related marker gene CyclinD1, differentiation markers Vasa and C-kit in mouse FGSCs both at P1 and P7 (c), mouse FGSCs and remaining ovary (d) via qRT-PCR. All experiments were done at least three times. Data are presented as mean ± SD (n = 3). Statistical significance was expressed as follows: *P < 0.05; **P < 0.01; ***P < 0.001
Differentiation potential of FGSCs in vitro
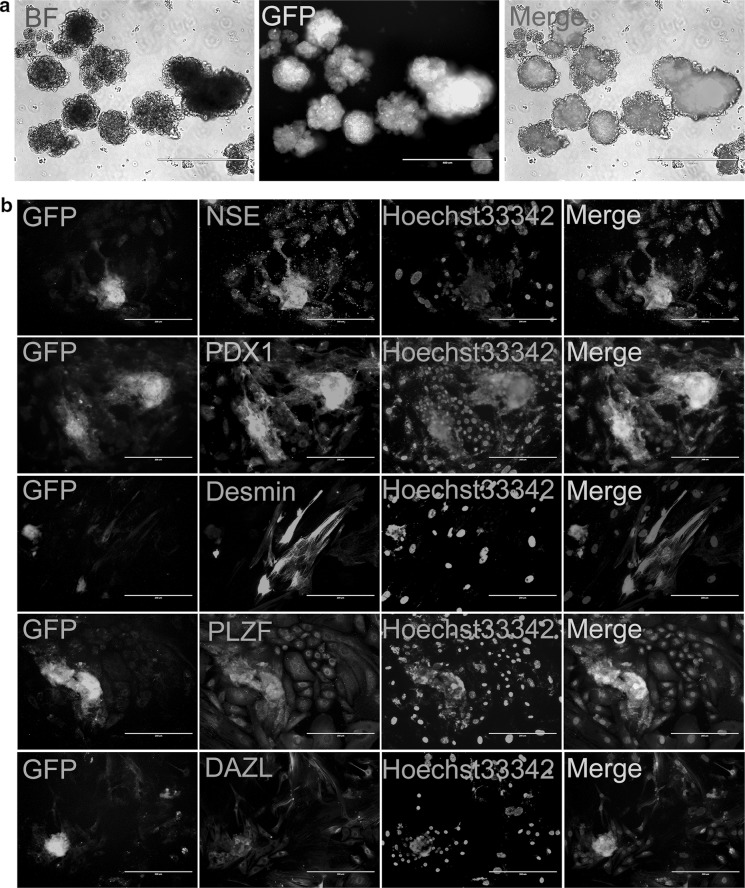
The differentiation potential of FGSCs was analyzed in vitro by mean of embryoid bodies (EBs) formation. FGSCs were suspended and cultured to form EBs, which then were plated on gelatin-coated dishes after 3 days (Fig. 3a). Then the EBs were cultured for 5–7 days to differentiate spontaneously. Positive immunostaining for NES, Desmin and PDX1 demonstrated ectodermal, mesodermal and endodermal differentiation, respectively (Fig. 3b). Moreover, the cells were also positive for DAZL and PLZF (germ cell markers) (Fig. 3b). All these results suggested that the enriched mouse FGSCs have similar capabilities with that of spermatogonial stem cells (SSCs), embryonic stem cells (ESCs), and FGSCs were found to maintain the capability to differentiate into the three germ layers in vitro.
Fig. 3.
In vitro differentiation potential determination for mouse FGSCs. a In vitro, mouse FGSCs could form EBs and then differentiate in appropriate conditions. BF, Bright Field. Scale bars, 200 μm. b The cultured cells were positive for NSE, PDX1, Desmin, PLZF, and DAZL analyzed by immunofluorescence staining. Scale bars, 400 μm
Effect of rapamycin on proliferation and differentiation of mouse FGSCs
As the inhibitor of mTOR pathway, rapamycin could restrain the activation of primordial follicles in the mouse ovary (Adhikari et al. 2009, 2010). Thus, we attempted to explore the effect of rapamycin on the characteristics of mouse ovaries and FGSCs.
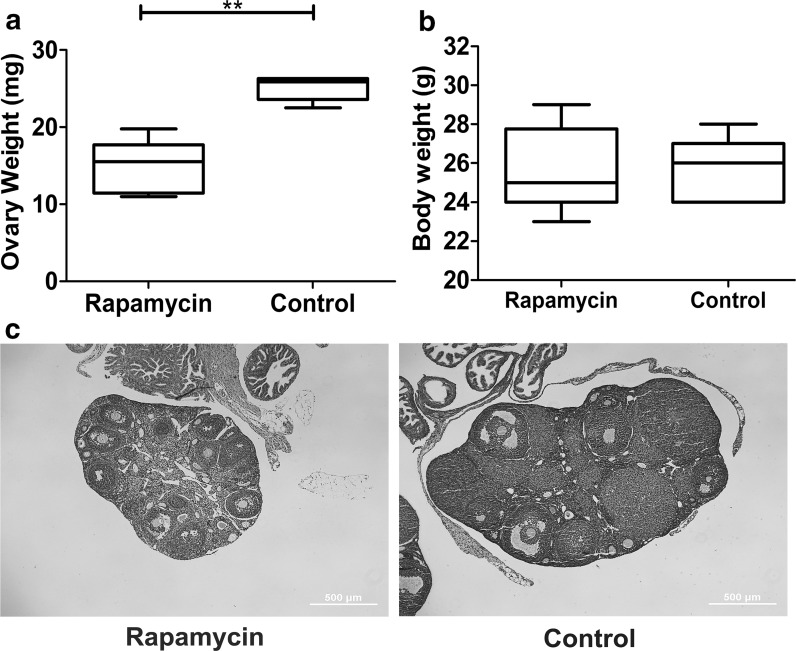
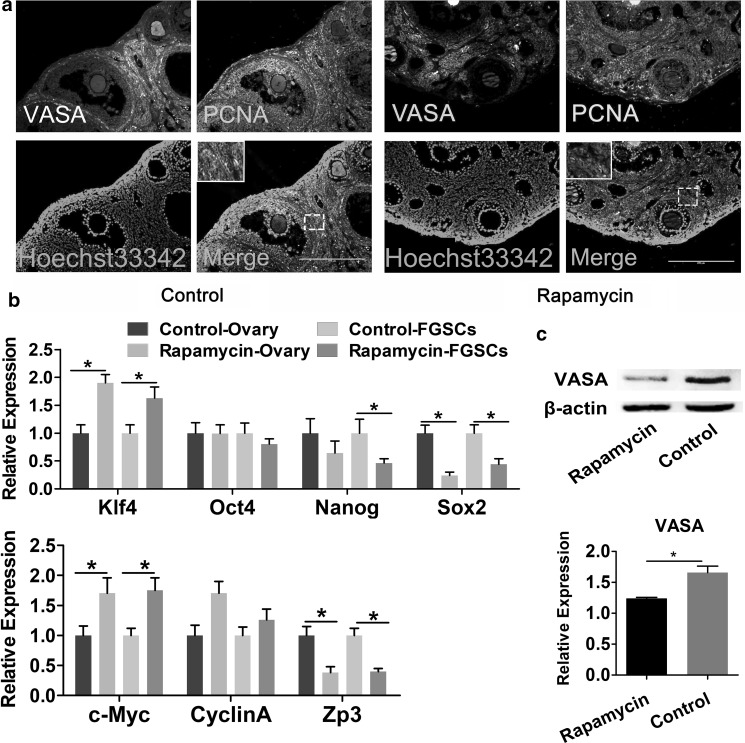
First, to explore the effect of rapamycin on the ovaries, mouse ovaries tissue was extracted after 30-day rapamycin treatment. The weight and volume of the ovaries decreased in rapamycin-treated group compared with control group (Fig. 4a, b). Further, aggregation of a large number of primordial follicles was also observed in rapamycin group (Fig. 4c), suggesting that rapamycin inhibited primordial follicle development and was consistent with the results of previous studies (Zhang et al. 2013). Then, the differences in the expression level related to proliferation and differentiation genes were examined. qRT-PCR results indicated that, in ovaries of rapamycin-treated group and FGSCs isolated from them the expression level of proliferation-associated gene CyclinA and c-Myc were increased, whereas that of differentiation marker Zp3 were significantly decreased in the obtained ovaries, compared with control group (Fig. 5b). Immunofluorescence staining of tissue sections showed that VASA and PCNA co-located in the cortex in the control group, whereas the cells were PCNA negative in follicular granulosa in the rapamycin group. No significant change was noted on VASA and PCNA expression in cortex, as shown in Fig. 5a. Western blot analysis results further showed that after 30-day treatment, with the inhibition of mTOR signaling pathways, rapamycin downregulated the expression of germ specific gene VASA in the mouse ovaries compared with the control group (Fig. 5c, and Supplementary Fig. 1).
Fig. 4.
Effect of rapamycin on the mouse ovaries. a–c The comparison of mouse ovary weight (a), body weight (b) and HE staining (c) after treatment with rapamycin for 30 days. Date are presented as mean ± SD (n=20). Statistical significance was expressed as follows: **P < 0.01
Fig. 5.
Effect of rapamycin on proliferation and pluripotency of mouse FGSCs. a Immunofluorescence staining for VASA and PCNA in mouse ovaries after rapamycin treatment. Boxed regions of interest were enlarged and shown to the bottom line. Scale bar, 200 µm. b Comparison of the mRNA expression of proliferation marker genes (CyclinA and c-Myc), pluripotency marker genes (Klf4, Oct4, Sox2 and Nanog), and differentiation marker Zp3 in mouse FGSCs, and the whole ovaries. c The expression of VASA was detected by Western blot and quantified via Image J gradation analysis in rapamycin and control group. All experiments are repeated at least three times. Data were presented as mean ± SD (n = 3), and statistical significance was expressed as follows: *P < 0.05
These results suggested that rapamycin could promote proliferation, inhibit differentiation of mouse FGSCs.
Discussion
In this study, FGSCs with self-renewal and multilineage differentiation potential were isolated and enriched via Oct4-GFP tracing. Further, the effects of rapamycin on the proliferation and differentiation of mouse FGSCs were investigated.
Previous studies have shown that mouse FGSCs have the capabilities to proliferate normally and express germ-specific genes (Zou et al. 2009). Presently, the separation of mouse FGSCs can be achieved by several methods, including marking the cell surface protein Vasa and Fragilis, immunomagnetic separation and flow cytometry sorting. However, Vasa is not only expressed in FGSCs, but also in oocytes (Pennetier et al. 2004), so other cell types might exist in the acquired FGSCs. In addition, Fragilis2 was also expressed in nerve cells. Thus, whether Fragilis could be a marker for identifying FGSCs was yet to be determined (Lange et al. 2003). Therefore, stem cell marker gene Oct4 was selected. FGSCs were obtained successfully, and by digesting Oct4 promoter-GFP transgenic mouse OSE and cortical layer with CDD liquid, the characteristics of these cells were found to be still similar to those reported FGSCs (Zou et al. 2009). The obtained FGSCs possessed for the potential of pluripotency, proliferation and reproductive specificity. They expressed germ cell marker Vasa, and could be differentiated into all three germ layers in vitro. In addition, with the purification of FGSCs, the cells expressed higher pluripotency and reproductive specificity than the first generation. However, our results still lack data after long-time culture. Thus, further investigation is needed.
Current studies of rapamycin focus on inhibiting the activation of primordial follicles, while little is known about its effects on mouse ovarian and FGSCs (Tong et al. 2013). In this study, mouse ovarian volume was significantly smaller and a large number of primordial follicles aggregated after rapamycin treatment, suggesting that rapamycin can inhibit primordial follicle development, which was in line with the results of a previous study (Zhang et al. 2013). The results of quantitative PCR inferred that the expression of proliferation-associated genes c-Myc, and CyclinA increased in ovarian surface epithelium and cortex cells. By contrast, the expression of differentiation related gene Zp3 was downregulated in the whole ovary, implying that rapamycin could promote proliferation, inhibit differentiation in mouse ovary tissues and FGSCs. Based on the previous work, c-Myc is closely related to cell senescence by controlling the downstream target gene hTERT (Yamashita et al. 2014). The upregulation of c-Myc indicated that rapamycin could inhibit the aging of mouse ovary, and that it could be found in the surface epithelium and superficial layer of mouse ovary. Klf4, a transcription factor, plays an important role in maintaining stem cell pluripotency. CyclinA could promote DNA replication and Zp3 is one of oocyte markers (Li et al. 2012; Yamashita et al. 2014; Khosravi-Farsani et al. 2015). In addition, Oct4 and Sox2 often combine with the Nanog promoter and form a regulatory network (Rodda et al. 2005). In this experiment, the expression of Sox2 and Nanog was lower in the rapamycin-treated group than in the control group, indicating that rapamycin might play a negative regulation role in the network mentioned above.
Based on these results, we summarized that rapamycin could promote FGSCs proliferation, inhibit their differentiation. Moreover, in our next study, several issues should also be discussed including whether FGSCs can repair damaged ovaries by constructing the model of ovarian injury, as well as the mechanism of rapamycin on mouse ovaries and FGSCs, which will bring a new prospect in delaying ovarian aging.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This study was supported by Basal Research Fund of Northwest A&F University (2452015034) and Agricultural Science and Technology Innovation and Key Project of Shaanxi Province (2016NY-094).
Footnotes
Hong Yang and Xi Yao have contributed equally to this manuscript.
Electronic supplementary material
The online version of this article (10.1007/s10616-018-0196-6) contains supplementary material, which is available to authorized users.
Contributor Information
Hong Yang, Email: yanghongchn@126.com.
Xi Yao, Email: yaoxi65@126.com.
Furong Tang, Email: queen499808@126.com.
Yudong Wei, Email: luckyyd@nwsuaf.edu.cn.
Jinlian Hua, Email: jlhua2003@126.com.
Sha Peng, Phone: +86-029-87080069, Email: pengshacxh@163.com.
References
- Adhikari D, Flohr G, Gorre N, Shen Y, Yang H, Lundin E, Lan Z, Gambello MJ, Liu K. Disruption of Tsc2 in oocytes leads to overactivation of the entire pool of primordial follicles. Mol Hum Reprod. 2009;15:765–770. doi: 10.1093/molehr/gap092. [DOI] [PubMed] [Google Scholar]
- Adhikari D, Zheng W, Shen Y, Gorre N, Hämäläinen T, Cooney AJ, Huhtaniemi I, Lan ZJ, Liu K. Tsc/mTORC1 signaling in oocytes governs the quiescence and activation of primordial follicles. Hum Mol Genet. 2010;19:397. doi: 10.1093/hmg/ddp483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagosklonny MV. Rapamycin and quasi-programmed aging: 4 years later. Cell Cycle. 2010;9:1859–1862. doi: 10.4161/cc.9.10.11872. [DOI] [PubMed] [Google Scholar]
- Bland ML, Desclozeaux M, Ingraham HA. Tissue growth and remodeling of the embryonic and adult adrenal gland. Ann N Y Acad Sci. 2003;995:59–72. doi: 10.1111/j.1749-6632.2003.tb03210.x. [DOI] [PubMed] [Google Scholar]
- Bukovsky A. Ovarian stem cell niche and follicular renewal in mammals. Anat Rec Adv Integr Anat Evolut Biol. 2011;294:1284–1306. doi: 10.1002/ar.21422. [DOI] [PubMed] [Google Scholar]
- Chung J, Kuo CJ, Crabtree GR, Blenis J. Rapamycin-FKBP specifically blocks growth-dependent activation of, and signaling by, the 70 Kd S6 kinase. Cell. 1992;69:1227–1236. doi: 10.1016/0092-8674(92)90643-Q. [DOI] [PubMed] [Google Scholar]
- Dou X, Sun Y, Li J, Zhang J, Hao D, Liu W, Wu R, Kong F, Peng X, Li J. Short-term rapamycin treatment increases ovarian lifespan in young and middle-aged female mice. Aging Cell. 2017;16:825–836. doi: 10.1111/acel.12617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingar DC, Blenis J. Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene. 2004;23:3151–3171. doi: 10.1038/sj.onc.1207542. [DOI] [PubMed] [Google Scholar]
- Green SH, Zuckerman S. The number of oocytes in the mature ovary. Recent Prog Horm Res. 1951;7:194–202. doi: 10.1677/joe.0.0070194. [DOI] [PubMed] [Google Scholar]
- Haissaguerre M, Saucisse N, Cota D. Influence of mTOR in energy and metabolic homeostasis. Mol Cell Endocrinol. 2014;397:67–77. doi: 10.1016/j.mce.2014.07.015. [DOI] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA. Rapamycin fed late in life extends lifespan in genetically heterogenous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- Hua J, Zhu H, Pan S, Liu C, Sun J, Ma X, Dong W, Liu W, Li W. Pluripotent male germline stem cells from goat fetal testis and their survival in mouse testis. Cell Reprogram. 2011;13:133–144. doi: 10.1089/cell.2010.0047. [DOI] [PubMed] [Google Scholar]
- Inoue N, Matsuda F, Goto Y, Manabe N. Role of cell-death ligand-receptor system of granulosa cells in selective follicular atresia in porcine ovary. J Reprod Dev. 2011;57:169–175. doi: 10.1262/jrd.10-198E. [DOI] [PubMed] [Google Scholar]
- Johnson J, Canning J, Kaneko T, Pru JK, Tilly JL. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature. 2004;428:145–150. doi: 10.1038/nature02316. [DOI] [PubMed] [Google Scholar]
- Khosravi-Farsani S, Amidi F, Habibi Roudkenar M, Sobhani A. Isolation and enrichment of mouse female germ line stem cells. Cell J. 2015;16:406–415. doi: 10.22074/cellj.2015.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange UC, Saitou M, Western PS, Barton SC, Surani MA. The fragilis interferon-inducible gene family of transmembrane proteins is associated with germ cell specification in mice. BMC Dev Biol. 2003;3:1. doi: 10.1186/1471-213X-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. An emerging role of mTOR in lipid biosynthesis. Curr Biol. 2009;19:R1046. doi: 10.1016/j.cub.2009.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Zheng H, Yu F, Yu T, Liu C, Huang S, Wang TC, Ai W. Deficiency of the Kruppel-like factor KLF4 correlates with increased cell proliferation and enhanced skin tumorigenesis. Carcinogenesis. 2012;33:1239–1246. doi: 10.1093/carcin/bgs143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinas P, Stura EA, Ménez A, Kiss Z, Stigbrand T, Millán JL, Le Du MH. Structural studies of human placental alkaline phosphatase in complex with functional ligands. J Mol Biol. 2005;350:441–451. doi: 10.1016/j.jmb.2005.04.068. [DOI] [PubMed] [Google Scholar]
- Mitchell RT, Saunders PT, Childs AJ, Cassidy-Kojima C, Anderson RA, Wallace WH, Kelnar CJ, Sharpe RM. Xenografting of human fetal testis tissue: a new approach to study fetal testis development and germ cell differentiation. Hum Reprod. 2010;25:2405–2414. doi: 10.1093/humrep/deq183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y. A new tool to generate transgenic rats using female germline stem cells from post-natal ovaries. Mol Hum Reprod. 2014;20:283–285. doi: 10.1093/molehr/gau017. [DOI] [PubMed] [Google Scholar]
- Pennetier S, Uzbekova S, Perreau C, Papillier P, Mermillod P, Dalbiès-Tran R. Spatio-temporal expression of the germ cell marker genes MATER, ZAR1, GDF9, BMP15, and VASA in adult bovine tissues, oocytes, and preimplantation embryos. Biol Reprod. 2004;71:1359–1366. doi: 10.1095/biolreprod.104.030288. [DOI] [PubMed] [Google Scholar]
- Peters H. Migration of gonocytes into the mammalian gonad and their differentiation. Philos Trans R Soc B Biol Sci. 1970;259:91–101. doi: 10.1098/rstb.1970.0048. [DOI] [PubMed] [Google Scholar]
- Roa J, Tenasempere M. Energy balance and puberty onset: emerging role of central mTOR signaling. Trends Endocrinol Metabol. 2010;21:519–528. doi: 10.1016/j.tem.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Rodda DJ, Chew JL, Lim LH, Loh YH, Wang B, Ng HH, Robson P. Transcriptional regulation of nanog by OCT4 and SOX2. J Biol Chem. 2005;280:24731–24737. doi: 10.1074/jbc.M502573200. [DOI] [PubMed] [Google Scholar]
- Ryskalin L, Lazzeri G, Flaibani M, Biagioni F, Gambardella S, Frati A, Fornai F. mTOR-dependent cell proliferation in the brain. Biomed Res Int. 2017;2017:7082696. doi: 10.1155/2017/7082696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos RX, Correia SC, Cardoso S, Carvalho C, Santos MS, Moreira PI. Effects of rapamycin and TOR on aging and memory: implications for Alzheimer’s disease. J Neurochem. 2011;117:927. doi: 10.1111/j.1471-4159.2011.07262.x. [DOI] [PubMed] [Google Scholar]
- Sonenberg N, Hinnebusch AG. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell. 2011;136:731–745. doi: 10.1016/j.cell.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Y, Li F, Lu Y, Cao Y, Gao J, Liu J. Rapamycin-sensitive mTORC1 signaling is involved in physiological primordial follicle activation in mouse ovary. Mol Reprod Dev. 2013;80:1018–1034. doi: 10.1002/mrd.22267. [DOI] [PubMed] [Google Scholar]
- Vézina C, Kudelski A, Sehgal SN. Rapamycin (AY-22,989), a new antifungal antibiotic. I. Taxonomy of the producing streptomycete and isolation of the active principle. Jpn J Antibiot. 1975;28:721–726. doi: 10.7164/antibiotics.28.721. [DOI] [PubMed] [Google Scholar]
- Wang H, Jiang M, Bi H, Chen X, He L, Li X, Wu J. Conversion of female germline stem cells from neonatal and prepubertal mice into pluripotent stem cells. J Mol Cell Biol. 2014;6:164–171. doi: 10.1093/jmcb/mju004. [DOI] [PubMed] [Google Scholar]
- White YAR, Woods DC, Yasushi T, Osamu I, Hiroyuki S, Tilly JL. Oocyte formation by mitotically-active germ cells purified from ovaries of reproductive age women. Nat Med. 2011;18:413–421. doi: 10.1038/nm.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Yamashita S, Ogawa K, Ikei T, Fujiki T, Katakura Y. FOXO3a potentiates hTERT gene expression by activating c-MYC and extends the replicative life-span of human fibroblast. PLoS ONE. 2014;9:e101864. doi: 10.1371/journal.pone.0101864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yang Z, Yang Y, Wang S, Shi L, Xie W, Sun K, Zou K, Wang L, Xiong J, Xiang J, Wu J. Production of transgenic mice by random recombination of targeted genes in female germline stem cells. J Mol Cell Biol. 2011;3:132–141. doi: 10.1093/jmcb/mjq043. [DOI] [PubMed] [Google Scholar]
- Zhang XM, Li L, Xu JJ, Wang N, Liu WJ, Lin XH, Fu YC, Luo LL. Rapamycin preserves the follicle pool reserve and prolongs the ovarian lifespan of female rats via modulating mTOR activation and sirtuin expression. Gene. 2013;523:82–87. doi: 10.1016/j.gene.2013.03.039. [DOI] [PubMed] [Google Scholar]
- Zhang XL, Wu J, Wang J, Shen T, Li H, Lu J, Gu Y, Kang Y, Wong CH, Ngan CY, Shao Z, Wu J, Zhao X. Integrative epigenomic analysis reveals unique epigenetic signatures involved in unipotency of mouse female germline stem cells. Genome Biol. 2016;17:162. doi: 10.1186/s13059-016-1023-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Wang L, Kang JX, Xie W, Li X, Wu C, Xu B, Wu J. Production of fat-1 transgenic rats using a post-natal female germline stem cell line. Mol Hum Reprod. 2013;20:271–281. doi: 10.1093/molehr/gat081. [DOI] [PubMed] [Google Scholar]
- Zou K, Yuan Z, Yang Z, Luo H, Sun K, Zhou L, Xiang J, Shi L, Yu Q, Zhang Y, Hou R, Wu J. Production of offspring from a germline stem cell line derived from neonatal ovaries. Nat Cell Biol. 2009;11:631–636. doi: 10.1038/ncb1869. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.