Abstract
The screening test for alcohol use disorder (AUD) patients has been of subjective nature and could be misleading in particular cases such as a misreporting the actual quantity of alcohol intake. Although the neuroimaging modality such as electroencephalography (EEG) has shown promising research results in achieving objectivity during the screening and diagnosis of AUD patients. However, the translation of these findings for clinical applications has been largely understudied and hence less clear. This study advocates the use of EEG as a diagnostic and screening tool for AUD patients that may help the clinicians during clinical decision making. In this context, a comprehensive review on EEG-based methods is provided including related electrophysiological techniques reported in the literature. More specifically, the EEG abnormalities associated with the conditions of AUD patients are summarized. The aim is to explore the potentials of objective techniques involving quantities/features derived from resting EEG, event-related potentials or event-related oscillations data.
Keywords: Alcohol use disorder, EEG, REEG, ERP, ERO, Alcoholics screening, P300 intensities, Coherence, Phase delay
Introduction
Drinking Problems that become severe are termed as alcohol use disorder (AUD) (APA 2013). Alcohol consumption less than 48 g per day or 144 g per week is categorized as safe (Parsons and Nixon 1998). In contrast, more intake than the mentioned quantity is categorized as unsafe or heavy drinking. Eventually, chronic heavy drinking leads to AUD including both alcohol abuse (AA) and alcohol dependence (AD) (a severe form of AA). According to the Diagnostic and Statistical Manual of Mental Disorders V (DSM V), people with alcohol abuse keep drinking despite social and personal problems (APA 2013). On the other hand, people with alcohol dependence not only fulfill the criteria of alcohol abuse, but also have increasing tolerance and withdrawal symptoms once abandoned drinking, also termed as alcoholics (Moss et al. 2007). The cumulative toxic effects and heavy consumption of alcohol may lead to medical, neurological, psychiatric and social problems.
Although both the screening and assessment of AUD patients are effective policies to reduce associated harmful effects; however, these policies are of subjective nature and heavily dependent on self-assessment questionnaires. The self-reporting renders the screening procedures subjective and may have many challenges such as involving patient’s dishonesty. In addition, most AUD patients are found unable to measure individual drinking quantities and are less candid about their consumption (Popham and Schmidt 1981; Solomon et al. 1980; Watson et al. 1984). It may lead to misjudgments regarding the actual alcohol intake and treatment administration unless verified by objective measures such as reliable markers of alcohol consumption or evidence provided by neuroimaging technologies (Solomon et al. 1980). Hence, to improve the screening and assessment accuracy and ultimately to improve the treatment efficiency, it is necessary to combine self-report tests with these objective techniques.
Neurological data have been analyzed to extract useful information for the successful screening and assessment of alcoholic conditions. For example, the frontal atrophy and white matter damages caused by chronic heavy drinking are discovered by Magnetic Resonance Images (MRI). In addition, Positron Emission Tomography (PET) and Single Photon Emission Computed Tomography (SPECT) have been used to detect the changes at neurotransmitter level (Ritsner 2009). In practice, the modalities can provide in vivo information and encourage investigations of new options for behavioral intervention or pharmacological modification to reduce alcohol craving and the risk of relapse (Heinz et al. 2009). With respect to other modalities, electroencephalography (EEG) has been preferred by researchers for AUD treatment due to its low cost, non-invasiveness, high temporal resolution (~ 1 ms), portability, and convenience. Hence, in this review paper, the EEG-based studies are discussed.
In literature, EEG-based methods have been proposed to analyze AUD related problems such as deficiency in attention (Saletu-Zyhlarz et al. 2004), imbalance of neurotransmitter system (Rangaswamy et al. 2002) and recovery of mental state in individuals prohibited from drinking. Those evidences may be used to facilitate drug discovery, optimization and candidate compound selection. In addition, EEG can help clinicians understand better the alterations in brain activities during AUD as well as medication effects on structural and functional recovery (Parvaz et al. 2011). To summarize, EEG could offer three potential means to improve treatment efficiency (NIAAA 2012):
Combined with other self-report tools (CAGE, MINI) during screening to identify individuals with alcohol-related problems and who are at risk (Miller et al. 2006);
Identifying the subset of abstainers (alcoholics who stop using alcohol after detoxification) at highest risk for relapse (Bauer 2001; Winterer et al. 1998);
Evaluating new medications or behavioral interventions by providing outcome measures to determine if the interventions are having desirable effect (treatment outcome prediction).
In this paper, various EEG-based methods to perform the AUD screening and assessments are reviewed including their key findings and important research gaps. In addition, the related state-of-the art approaches are discussed to explore their potentials for future research in the area including resting-electroencephalography (REEG), event-related potential (ERP) and event-related oscillation (ERO).
The rest of the paper is organized as follows. The “Resting EEG (REEG)” section discusses REEG-based studies that are based on electrophysiological measures such as changes in amplitudes and power of different frequency bands and their behavior after detoxification or long-time treatment. In addition, the paper emphasizes the EEG coherence, phase delay and synchronization likelihood as promising electrophysiological measures for further research involving AUD patients. Moreover, the “Event related potential (ERP)” section discusses the ERP component, i.e., P300. More specifically, the P300-based methods are evaluated and compared with each other to show deficits in cognitive processes of AUD patients. The “Event related osscilations (ERO)” section discusses the findings involving the ERO, for example the P300 can be decomposed into different frequency components which have already shown some progress in the study of alcoholism recovery after long time treatment. In short, the paper discusses the known effects of AUD on EEG signal in each subsection along with related EEG methods. Lastly, in “EEG as utilities for alcoholics treatment” section, the studies discussing AUD identification and relapse prediction are highlighted with an intention to show their potential for clinical practice for AUD treatment. In addition, their limitations are also presented.
Resting EEG (REEG)
Resting EEG (REEG) data involved electrophysiological recordings during a state of rest, e.g., eyes closed (EC) and eye open (EO) conditions without being involved in any experimental activity (e.g., an odd-ball task). The REEG data are of composite nature and may be decomposed into frequency bands such as delta (0.5–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), beta (12–30 Hz) and gamma (> 30 Hz). The frequency bands carry different kind of physiological information and have been associated with the brain activities. For example, the delta and theta bands are manifested during information encoding in order to create new memory (Klimesch 1999); alpha band indicates the information retrieval from memory and attention (Klimesch 1999); the beta and gamma bands are associated with perception (Sedley and Cunningham 2013), learning (de Souza et al. 2013) and attention (Barry et al. 2010). The alpha band may be further divided into two or more sub-bands, e.g., low alpha (8–10 Hz); high alpha (10–12 Hz) (Rangaswamy et al. 2002; Saletu-Zyhlarz et al. 2004; Winterer et al. 1998). As alpha activity expresses normal functioning of the brain, beta band is considered as excitatory while slow bands including delta and theta may be interpreted as inhibitory (Saletu-Zyhlarz et al. 2004). There is a speculation that the gamma band is modulated by theta band and also shows the action of inhibitory interneurons by a mechanism of nesting of gamma rhythms within theta rhythms (Nyhus and Curran 2010).
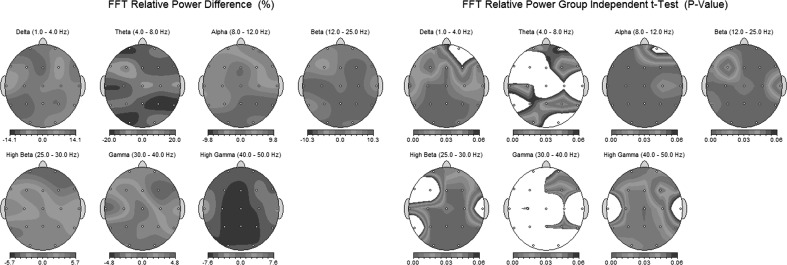
Studies based on REEG data have identified differences of neuronal activities between alcoholics and control subjects involving different brain regions (Campanella et al. 2009; Parvaz et al. 2011; Porjesz et al. 2005). As an example, techniques such as spectral power analysis combined with Statistical Probability Maps (SPM) may illustrate the difference in the power distribution of various brain regions (Saletu-Zyhlarz et al. 2004). As shown in Fig. 1, the SPM are brain topographic maps that describe differences between two sets of EEG variables based on statistical tests. The statistical results are used to generate maps with color codes. Figure 1 shows topographic maps based on absolute (AP) and relative power (RP) computed using the Welch periodogram method (Welch 1947). In this study, the SPMs were constructed according to the procedure mentioned elsewhere (Duffy et al. 1981; Saletu et al. 1987).
Fig. 1.
Differences between AUD patients and controls in EO state. Inter-group differences are depicted in relative power (RP) measures (left) and significant independent t test (p-value) measures (right)
The relative power is the percentage of power computed for one band (e.g., delta) with the total power computed for all EEG frequency bands (e.g., delta, theta, alpha, beta, and gamma). In the figure, SPM highlight not only the differences between mean values of each group but also the variability within each group. The figure shows bird’s eye view of the human scalp, nose at the top and 19 dots corresponding to 19 scalp electrode positions. The differences of activations (quantified by the statistical test) are color coded such as the red and orange colors represent significant increases of EEG power observed in AUD patients as compared with control subjects. The blue and cyan colors represent significant decreases of power found in AUD patients as compared with control subjects. In the absences of significant differences, the white color is used. In Fig. 1 (left side), AUD patients show a decrease in theta and high gamma RP and an increase in the other bands as compared with controls. In Fig. 1 (right side), the changes were not significant overall, but they were mostly distributed in some regions such as occipital or left parietal.
EEG spectral power changes
The statistical differences of spectral power between alcoholics and control groups have been linked with different pathologies involving the AUD patients. For example, the higher theta power has been reported in alcoholics when compared with control subjects (Bauer 2001; de Bruin et al. 2004, 2006; Rangaswamy et al. 2003; Winterer et al. 1998). This abnormal increase might reflect the reduction or blocking the ability to encode new information (Klimesch 1999). Similarly, by performing comparison under EC condition, Rangaswamy et al. (2003) found an increase of theta power at all scalp loci, prominent at central and parietal in males and at the parietal for females. In addition, the significant changes in theta power was considered to be associated with cortical atrophy (Coutin-Churchman et al. 2006; Saletu-Zyhlarz et al. 2004).
Alpha band is predominant REEG rhythm observed during relaxed alert state. Decrement in alpha band of alcoholics, especially in the occipital region, is indicative of deficiency in information retrieval from memory and attention (Saletu-Zyhlarz et al. 2004). Alpha band can also be analyzed in time domain and is categorized based on voltage (microvolts squared per octave). For example, low-voltage alpha (LVA) (< 10 µV) is observed more in alcoholics than controls by Ehlers and Phillips (2007; Ehlers et al. 2004). However, the observed difference was not significant.
In the studies of alcoholism, power of beta band is more popular than the power of gamma band. For example, elevated beta band power was observed in AD patients in the whole brain. In addition, it was studied to be not influenced by age and other clinical variables like recentness and quantity of drinking (Coutin-Churchman et al. 2006; Rangaswamy et al. 2002; Saletu-Zyhlarz et al. 2004). However, gender influences beta power, i.e., more manifested in male as compared with female alcoholics. The alteration of beta band power was explained as an electrophysiological index of excitation-inhibition homeostasis in the cortex (Rangaswamy et al. 2002). Beta power was also utilized to perform the relapse prediction for detoxified patients and compared their spectral power with controls (Bauer 2001). The classification and analysis results showed a significant increase in beta power of relapse-prone patients as compared with abstinence-prone patients and controls. However, it was also found that these changes might be related to medication (especially benzodiazepine), sensoperceptual alterations (hallucinations, illusions), clinical seizures and family history of alcoholics. These factors can alter beta power and affect the classification accuracy (Coutin-Churchman et al. 2006).
As compared with alcoholics, the detoxified patients showed similar spectral power changes as summarized in Table 1. The changes are also different between relapse-prone and abstaining patients. By evaluating detoxified patients enrolled in residential treatment programs, Saletu-Zyhlarz et al. (2004) showed an increase in delta and theta power in relapsing patients and found no difference in abstaining patients as compared with controls after 6 months follow-up. In alpha band, the changes are reversed with the decrease of relative power in relapsing patients and increase of relative power in abstaining patients (Bauer 2001; Saletu-Zyhlarz et al. 2004). In beta band, the changes showed consistency between relapsing and abstaining patients with an increase of absolute power and relative power in both groups. It was more significant in relapsing group. However, these changes have not been analyzed or explained clearly if they are the signs of recovery or they are the effects of medication and withdrawal symptoms caused by the detoxification.
Table 1.
Spectral power alterations in alcoholics and detoxified patients as compared with controls
| Frequency band | Subjects | Phenomenon |
|---|---|---|
| Theta and delta | Detoxified patients | Decrease power, especially more significant in delta band |
| Alcoholics | Decrease power in frontal | |
| Alpha | Detoxified patients | Decrease power in relapsing patients and increase power in abstaining patients |
| Alcoholics | Decrease power and show lower voltage but not significant | |
| Beta | Detoxified patients | Increase power in all scalp loci, more significant in relapsing patients and fast-beta |
| Alcoholics | Increase power of slow beta in central and parietal, increase fast beta in frontal | |
| Gamma | No confirmation about the alteration of gamma band in alcoholics | |
EEG coherence, phase delay and synchronization likelihood
The EEG-based quantities/features such as power spectral density provide information regarding a specific brain region. For example, the absolute or relative power shows the brain activity at a EEG sensor location. In contrary, EEG measures such as the inter-hemispheric coherence, phase delay and synchronization likelihood are used to explore functional influences among different brain regions involving more than one EEG sensor locations. The computation of coherence considers relationship of brain electrical activities between two different brain areas and provides their coupling information. While coherence and power spectrum represent relationship of different brain activities due to the changes in volume conduction of EEG signal. The phase delay expresses direction of the connection which is computed by measuring directional flow between 2 EEG electrodes at different scalp locations.
Tcheslavski and Gonen (2012) highlighted significant reduction of EEG power, coherence and phase synchronization in alcoholics as compared with controls. Integration of coherences, phase delays and spectral powers may reveal more about the alterations in brain activities during alcohol addiction. In addition, higher coherence in first degree male relative (parents, full siblings, or children) of alcoholics was found in the frontal and cento-parietal than in controls without a family history of AUD (Michael et al. 1993). This coherence abnormality observed in relatives of alcoholics might have proved the heritable risk of alcohol abuse.
The coherence estimation is more suitable for stationary data with linear relationships. In case of non-stationary REEG data, coherence may not detect rapidly changing interdependencies (Stam and Van Dijk 2002). Improvements such as incorporation of nonlinear dynamical systems have been proposed to analyze synchronization between two interacted chaotic systems. One method is to use synchronization likelihood (SL), which is a measure of generalized synchronization between 2 dynamic systems (de Bruin et al. 2004). The time-based EEG data has to be transformed into a state-space model to compute SL. de Bruin et al. (2004) stated that heavy drinkers exhibit increased theta synchronization as compared with light drinkers during EC condition. The augmented theta synchronization in heavy drinkers resembled an increase in theta coherence in AUD patients versus controls. This similar effect from both heavy drinkers and alcoholics implied pure effects of alcohol intake on synchronization of brain activity, while minimizing confounding influence of genetic factors related to AD. These differences also showed that coherence, phase delay and SL could be potential features for identification of people with AUD.
EEG-based machine learning methods for AUD patients
More recently, various REEG-based machine learning (ML) methods have been proposed to automate the screening and diagnosis of AUD patients (Acharya et al. 2014; Faust et al. 2013a; Guntaka and Tcheslavski 2013; Mumtaz et al. 2016, 2017; Yazdani and Setarehdan 2007). The methods are based on automatic detection of alcoholism-related changes in the EEG signals. In addition, the methods involved combination of signal processing techniques such as wavelet, nonlinear dynamics, and chaos theory and pattern recognition and classification techniques. The advancements in signal processing techniques allowed the researchers to compute EEG-based features that could be utilized for automatic identification of AUD patient’s specific EEG patterns.
Summary
Table 2 summarizes EEG changes commonly reported in the AUD patients. These EEG changes might be useful as a guiding tool for further research studies, e.g., the increases of theta power as indicator for alcoholics, and increase of beta power as an overall assessment for alcoholics and relative problems. Increase of beta power was primary characteristic feature found in alcoholics and high-risk subjects. However, it was associated with benzodiazepines intake (Bauer 2001) that were mainly used for alcohol detoxification. Therefore, beta power needs to be considered when used for alcohol treatment evaluation. In contrary, theta and delta bands were found significantly increased in alcoholics than reported in controls. In addition, these bands were not affected by medication or found in people with family history of alcohol abuse. However, the findings based on alpha and gamma bands are not matured and yet considered as active research areas that warrants further investigations.
Table 2.
Overview of REEG studies on alcohol addiction
| Methods | Findings | |
|---|---|---|
| Features | Participants | |
| Spectral power | 191 detoxified patients and 240 controls (Coutin-Churchman et al. 2006) | Significant correlation between decreased power of slow bands and cortical atrophy in detoxified patients |
| Increased power in beta band correlated with medication, clinical seizures and family history of AUD | ||
| 22 detoxified patients and 58 controls (Saletu-Zyhlarz et al. 2004) | Decrease delta and slow alpha power, increase beta power in alcoholics compared with controls | |
| 6 months abstainers showed an increase of slow band and fast beta, a decrease in fast alpha and slow beta | ||
| 307 alcoholics and 307 controls (Rangaswamy et al. 2002, 2003) | Increased theta power at all scalp locations, prominent at the central and parietal in male, and at the parietal in female in alcoholics as compared with controls | |
| Increased low beta (12–20 Hz) power in the whole brain, but most prominent in the central and parietal, increased high beta (20–30 Hz) power in frontal | ||
| The increase of beta power was more prominent in male alcoholics than female | ||
| People with family history of AD including 108 Hispanic and 269 non-Hispanic (Ehlers et al. 2004) | Low voltage alpha in alcoholics | |
| Alpha amplitude may be associated with ethnic variation, but not drinking status, family history of AD, or other disorders | ||
| Female with family history of AD were found to have higher slow alpha (8–9 Hz) and beta power than male | ||
| 61 alcoholics and 176 controls (Ehlers and Phillips 2007) | AD was associated with LVA in occipital areas and in men only | |
| No association between alpha amplitude and family history of AD | ||
| 48 relapsers, 59 abstainers and 22 controls (Bauer 2001) | Achieve accuracy with 61% sensitivity, 85% specificity, 75% positive predictive and 74% negative predictive respectively using logistic regression | |
| Coherence and phase delay | 77 alcoholics and 45 controls (Tcheslavski and Gonen 2012) | Spectral power and coherence of slow bands in alcoholics are lower than in controls |
| Phase synchrony of alcoholics also reduced in α2 (10–12 Hz) and β1 (12–20 Hz) frequency at central region | ||
| Synchronization likelihood (SL) | 11 heavy drinkers and 11 light drinkers (de Bruin et al. 2004) | The increases in theta and gamma synchronization that of heavy drinkers might indicate damages in hippocampal–neocortical connectivity |
| 18 light, 33 moderate and 34 heavy drinkers (de Bruin et al. 2006) | Low synchronization of alpha and slow-beta in left hemisphere in heavy drinkers | |
| Lower synchronization of fast-beta band in moderate and heavy male drinkers | ||
In addition, techniques including chaos theory may reveal more information about alcoholics because of inherent complexity and nonlinearity of REEG data. To improve further understanding, the REEG data analysis can utilize its nonlinear characteristics by integrating chaos analysis with traditional time–frequency methods. This can also pave a way for discovering discriminants between people with AUD and controls. The findings in detoxified patients including relapsing and abstaining patients (Bauer 2001; Saletu-Zyhlarz et al. 2004) also provide utility for predicting patients with high risk of relapse. However, the results need to be verified to observe the effects of medication on beta power of alcoholics. Despite rapid progress on alcohol addiction, few reports and studies focused on alcohol treatment utilizing REEG. Future research can focus on integration of features such as spectral power, coherence, phase delay etc. In addition, the features can be data mined based on novel classification algorithms to achieve better accuracy, sensitivity and specificity in addition to automating the whole process during screening and treatment prediction.
Event related potential (ERP)
Segments of electrophysiological data recorded during a cognitive activity time locked with an experimental stimuli (e.g., sensory, motoric, or cognitive events) are grand averaged, termed as event-related potentials (ERPs). Unlike REEG data, the variations of ERP components in alcoholic’s exhibit reduced P300 (P3) amplitudes and larger latencies as compared with controls. These abnormalities of alcoholics could be analyzed by (1) evaluating and comparing ERP components in various brain regions between alcoholics and controls, and (2) examining the effects of different stimuli types, socio-demographic data, and family history and drinking habits on ERP components.
One method to extract P300 is to average only epochs without eye artifacts and pick the largest positive peak in the latency window from 250 to 450 ms after stimulation (peak detection). Using this method, Suresh et al. (2003) highlighted the finding that lower P300 amplitude observed in alcoholics in both gender for auditory oddball task, but found more significant in male patients. Wan et al. (2010) applied P3 as predictor in discrimination function (a statistical analysis to predict a categorical dependent variable) (Fisher 1936) for those patients who did complete treatment (abstainers) and achieved 63.9% of prediction rate. The result indicated that there might be difference in P3 between relapsers and abstainers.
In Go/No-Go paradigm, the target stimulus occurs more frequently than non-target stimulus. In a study using the same P3 extraction method with Go/No-Go paradigm, Kamarajan et al. (2005) showed lower amplitude of P3 components in alcoholics to both target (Go) stimuli and non-target (NoGo) stimuli as compared with controls, and there was less difference between these two stimuli in alcoholics. P3 has shown its ability for identify the chronic alcoholics. However, way back to 1985, it was suggested that low P3 voltages might not be reversible, or might recover slowly after long abstinent periods (Porjesz and Begleiter 1985). In addition, the results have been confirmed in the study of Alcoholics Anonymous members (fellowship of people who share their experiments and support each other to overcome their alcohol related problems and recover from alcohol addiction). The study showed that alcoholics still manifest low P3 amplitudes after extremely prolonged abstinent (3–10 years) (Porjesz and Begleiter 1985). The finding means that P3 cannot be used as assessment tool for alcohol addiction treatment because there is not much difference in P3 component between relapsers and abstainers even after a long-time treatment (3–10 years). However, it was suggested that the low voltages of P3 in alcoholics could be an indicator for alcohol addiction screening. Kamarajan et al. (2006) also suggested that the amplitude of P3 might be considered as an endophenotype for genetic studies (biochemical markers that reveal about individual inherited risk of alcohol abuse).
Some improvements have been achieved in alcoholic treatment prediction by analyzing P3a. Marinkovic et al. (2001) reported the decrease in both P3a and P3b as compared to baseline following a low dose of alcohol (0.4 g of alcohol per kg of body weight), and also more significant decrease were shown in P3a than P3b. The result suggested P3a as a potential screening tool for alcoholics. On the other hand, Anderson et al. (2011) examined P3a amplitude as a predictor in the discriminant function for treatment success for substance dependence. They claimed that P3a amplitude was a robust predictor of AD treatment, and even more accurate than P3b and other AD measurements.
However, there are significant issues with the artifact rejection process. Firstly, it is fundamental difficult to apply for early components because these components are relatively small as compared with the P300 and easily affected by artifacts. In this case, the artifact rejection is a relatively crude process because it eliminates large number of trials with artifact from the EEG averages. Secondly, it is difficult for alcoholics to control eye blinking and movements, hence resulted into EEG data with more artifacts than usual. In addition, interval of stimulus is around 1500 ms or we have two stimuli for every 3 s. However, the frequency of eye-blink is about 20 times per minute or blinking occurs every 3 s (Iwasaki et al. 2005). In conclusion, the number of stimulus is only twice of the blinks and it means that half of trials will be eliminated and lead a great loss of information and an unrepresentative sample of trials due to bypass artifacted trials. Moreover, some of the experiment models themselves such as visual stimulus required the participants to keep their eyes open for a long time (> 5 min) that will make them tired and blink more frequently, especially for AUD patients. Under these eye-blinking conditions, the artifact correction techniques (techniques that do the subtraction of the voltages due to eye blinking and eye movement from recorded signal to recover original signal) are alternative solution that is more suitable to recover those small components. By using artifact correction to retrieve early components, Marco et al. (2005) reported about the deficit in auditory sensory gating (neurological processes of filtering out redundant or unnecessary auditory stimuli in the brain from all possible auditory stimuli) reflected by P50 component in abstainers. Curtin and Fairchild (2003) also indicated the relationship between alcohol and cognitive function from the N450 reduction and negative slow wave (NSW) associate with behavioral impairment resulted from failure in cognitive control function during alcohol consumption. More investigation about the impairments of early ERP components may help to reveal the deficiency observed in cognitive processing of alcoholics.
The conventional method of averaging and peak detection could lead to misinterpretation of ERP components. To minimize this, single trial analysis methods have been proposed for the identification of ERP components. Pfefferbaum et al. (1991) applied Woody filter techniques to analyze single trial ERP and found that the frequency of P3 generation to startling stimuli was the same in both alcoholics and controls but with smaller amplitude in alcoholics when analyzing the blink responses. This difference was considered to reflect the abnormality of automatic processes in alcoholics. They also found that family history of drinking might be the primary determinant of small P3 amplitude, and more prominent than the time and amount of alcohol exposure.
In another approach, Crego et al. (2009) has proposed principal component analysis (PCA) as a promising technique for acquiring ERP components and reducing their dimensions for multichannel EEG recordings. In their study, N2 component was found to be significantly larger during binge drinking than found in control group. In addition, P3 also exhibited deficits in working memory function during binge drinking in frontal, central and parietal regions.
Summary
Table 3 summarizes key findings commonly reported in the literature involving the discrimination of AUD patients and healthy controls. For example, the findings such as less P300 peak amplitude and longer latency of the ERP components (especially P3a and P3b) are commonly reported. The lower amplitude, in general, is more significant and more consistent than the longer delay of peak latency. However, the changes in P3 components seem to be irreversible after long time abstinent. Thus, the P300 amplitude is suggested to be taken as a screening tool or to provide endophenotype for genetics studies. Other ERP components need more investigation for their ability of classification and prediction in clinical treatment. Novel techniques in artifact correction and component extraction can help reveal more information in small components and the nature of these neurocognitive deficits in alcoholics.
Table 3.
Overview about ERP studies on alcohol addiction
| Methods | Findings | |
|---|---|---|
| ERP extraction | Participants | |
| Averaging and peak- detection | 393 alcoholics and 170 controls (Costa et al. 2000) | Significant decrement of P300 amplitude in alcoholics and antisocial personality disorder patients (APSD) at anterior region (significant among subjects age < 30 for APSD) |
| 27 alcoholics and 27 controls (Hada et al. 2000) | Smaller P3a amplitudes in anterior region correspondent to the rare non-targets in alcoholics without any difference in peak latency | |
| 71 abstainers and 159 controls (Suresh et al. 2003) | Significant decrease of P3 amplitudes in the whole brain of abstainers | |
| Smaller P3 amplitudes in women with family history of AD | ||
| 12 controls, 12 alcoholics, 12 depressed and 12 alcoholic-depressed (Maurage et al. 2008) | Alcoholics showed more delayed P3b (longer reaction time) than controls for the Emotional facial expression (EFE) discrimination tasks | |
| 17 detoxified patients and 17 controls (Marco et al. 2005) | Deficit in P50 auditory in abstainers which related to deficit in sensory gating | |
| Partial P50 recovered after abstinence period | ||
| 51 abstainers and 24 controls (Glenn et al. 1993) | Predicting relapsers/abstainer status using N2L, P3A and N1A indicated low accuracy (63%) | |
| 44 detoxified patients (Anderson et al. 2011) | Significantly reduced P3 amplitude in patients who did not complete the treatment could provide 63.9% accuracy classified for alcoholics | |
| 48 alcohol user and placebo (Curtin and Fairchild 2003) | Reduced N450 and NSW associate with failure in cognitive control function and lead to behavioral impairments | |
| 30 alcoholics and 30 controls (Kamarajan et al. 2005) | Significantly lower P3 amplitudes in alcoholics. Less difference between the NoGo and Go conditions in alcoholics. Less anteriorization of CSD polarity in alcoholics during NoGo processing | |
| 78 abstainers and 58 controls (Pandey et al. 2012) | Significantly reduced N2 peak amplitudes and current density in alcoholics. Controls showed significantly larger NoGo than Go N2 amplitudes | |
| Woody filter | 27 alcoholics and 32 male controls (Pfefferbaum et al. 1991) | Alcoholics generated a P3 as often as controls but with smaller amplitude. Family history of drinking, rather than the time and amount of alcohol exposure appears to be the primary determinant of small P3 amplitude |
| PCA | 42 heavy drinkers and 53 controls (Crego et al. 2009) | In matching conditions, the N2 component was significantly larger in the heavy drinker than in controls in central and parietal regions. P3 also exhibited the deficit in working memory function in binge drinking in the frontal, central and parietal regions |
There are several limitations with the conventional method of averaging and peak detection that can lead to misinterpretation of ERP components. To minimize the misinterpretation by using averaging method and understand deeper about cognitive mechanism of alcoholics, many single trial analysis methods have been proposed for the identification of ERP components, e.g., Woody Filter technique, PCA and ICA.
Event related osscilations (ERO)
Event related oscillations (ERO) are electrophysiological data recorded during performance of a cognitive activity. Both ERO and REEG data share similar frequency bands; however, their interpretations are different. For example, faster frequencies (alpha, beta and gamma) observed during ERO recordings show synchronization of local neurons, whereas slower frequencies (delta and theta) correspond to long distance synchronizations. EROs are categorized into either evoked (phase-locked to the stimulus during trial) or induced oscillations (non-phase-locked to the stimulus during trial). Quantified in time–frequency domain, the induced activities can reflect either decrease or an increase in power (relative to the pre-stimulus period) termed as event-related desynchronization (ERD) or event-related synchronization (ERS), respectively (Andrew and Fein 2010b). Like REEG analyses, studies involving EROs also analyze alterations in the brain activities based on power of various frequency bands located in different brain regions with three terms: event-related evoked power EROEVK, induced or non-phase locked power ERONPL (including ERS power and ERD power) and event-related total power EROTOT (sum of EROEVK and ERONPL). All these powers are analyzed using time–frequency presentation such as spectrogram to obtain the differences between alcoholics and controls which will be discussed in the next subsection.
Studies involving ERO P300 components
Based on power of different frequency bands, many studies have found that the P300 components primarily involved the oscillations of theta and delta bands (Andrew and Fein 2010b; Jones et al. 2006; Kamarajan et al. 2004, 2006; Krause et al. 2002; Rangaswamy and Porjesz 2008). Jones et al. (2006) indicated that frontally focused theta band activity (4–5 Hz) and posterior distributed delta band activity constitute the P300 component. They also found that alcoholics showed significant deficits in evoked delta and total theta power which contributed the most power to P300 waveform. The findings provided the reason of lower P300 amplitude by the deficits in ERO power. According to these studies, the theta oscillations formed N200 and early part of the P300 component, while delta oscillations formed main part of the P300 component. Moreover, these powers were also found significantly lessened among alcoholics as compared with controls when responding to the visual stimuli (Gilmore and Fein 2012; Rangaswamy and Porjesz 2008) and Go/NoGo paradigm (Kamarajan et al. 2004, 2006). EROTOT and EROEVK provide an alternative representation of the group difference but less effective than P3b amplitude (Andrew and Fein 2010a). In addition, Andrew and Fein (2010a) suggested that theta ERONPL might provide discriminatory information for alcoholics.
The recovery from alcoholic condition is a slow process and usually takes approximately 3 months (Addolorato et al. 1998). Therefore, process involving EEG can be scheduled into 2 stages to compare with the results from laboratory test (Andrew and Fein 2010a, b; Gilmore and Fein 2012): (1) Short-Term Abstinent Alcoholic (STAA) is defined as 3–6 months abstinent patients; and (2) Long-Term Abstinent Alcoholic (LTAA) is defined as 6–12 months abstinent patients. By studying ERONPL during the recovery of alcoholics, it was found that the LTAA showed a larger theta ERS power to the target stimulus than controls. The same finding was achieved in both STAA and LTAA (Gilmore and Fein 2012). In addition, the magnitude of enhancement in STAA was greater than in LTAA. Therefore, the induced theta ERS may be an indicator of brain function recovery for alcohol addiction treatment. Besides that, delta EROEVK, delta and theta EROTOT were also found to be significantly lower in LTAA as compared with controls. This finding could provide an alternative and comparable representation of P3b amplitude reduction for assessing recovery progress or relapse prediction. The changes in ERO power of slow bands were reported not only in alcoholics but also in those who are at high risk of AUD. Rangaswamy et al. (2007) found a decrease of theta EROTOT and delta EROEVK for visual target stimuli in the offspring of high risk alcohol addiction. This finding might be used for detecting the inherited risk of alcohol abuse.
Regarding the induced theta ERS power, it was found to be associated with working memory and attention processes (Krause et al. 2000). Hence, greater theta ERS in STAA and LTAA suggested that alcoholics had to pay more attention and greater working memory to perform target detection during oddball task. The greater theta ERS in STAA relative to LTAA may indicate compensatory mechanisms in alcoholics to overcome working memory and attention deficits. However, there may be a limitation for an increase of induced theta ERS power that can cause breakdown of compensatory mechanism as task demands increase. Therefore, induced theta ERS of alcoholics need more investigation under heavy task (condition that requires heavy memory load) to examine how the task demands could affect the differences between alcoholics and controls.
Other ERO studies
Alcohol consumption was also observed to affect slow oscillations. By examining the effects of alcohol on EEG during an auditory memory task, Krause et al. (2002) have discovered significant effects in theta and alpha frequency bands. The administration of alcohol caused a decrease of early-appearance of ERS responses during auditory encoding and increased the later-appearing ERD responses during retrieval. This indicated that alcohol had disorganizing effects on brain electric oscillatory systems in both theta and lower alpha frequency range during cognitive processing. However, the study did not investigate more about the correlation between long-time effect of alcohol consumption and alteration of slow oscillations. By studying alcohol dependence related alterations, Tcheslavski and Gonen (2012) concluded that a decrease of average EEG power in alcoholics were significantly observed in left central and right cento-parietal regions for delta oscillation; in left frontal-central, central, parietal, right temporal, and right temporal-parietal regions for theta oscillation; in left frontal-central and right parietal and occipital regions for slow alpha oscillation; and in right occipital region for fast alpha and slow beta oscillations.
Besides delta and theta oscillation, gamma ERO was also found to be affected under visual stimuli in alcoholics. Rangaswamy et al. (2007) and Padmanabhapillai et al. (2006) highlighted gamma ERO as a strong marker for AD screening. In their studies, the responding suppression of gamma band activity to target stimuli observed in the frontal region of alcoholics were associated with cognitive processes (Padmanabhapillai et al. 2006). Moreover, evoked gamma oscillation during visual perception and alteration in gamma power could be used as screening tool for alcoholics. However, this application required more investigation (Andrew and Fein 2010a).
Summary
In conclusion, most of the ERO studies applied the traditional trials averaging methods to acquire ERP and ERO waveform components, also described in Table 4. These methods have similar limitations of overlapping between different trials as discussed in ERP section (“Event related potential (ERP)”). To overcome it, Jones et al. (2006) suggested to use dynamic time–frequency windows defined during single trial at single electrode per study participant. It helped to minimize misinterpretation of averaging method.
Table 4.
Overview Of ERO studies on alcohol addiction
| Methods | Findings | |
|---|---|---|
| ERO extraction | Participants | |
| Trial averaging | 20 alcohol use (Krause et al. 2002) | Significantly decreased EEG theta ERS (centre and anterior) during encoding and decreased lower alpha ERD during retrieval (centre and posterior) |
| 58 alcoholics and 29 controls (Kamarajan et al. 2004) | Significant reduction in delta and theta power during NoGo trials in frontal → deficient inhibitory control and information-processing mechanism | |
| 50 people with family history of alcoholics and 50 controls (Kamarajan et al. 2006) | Significant reduction in delta and theta and alpha 1 (8–9 Hz) power during No-Go trials | |
| This reduction was prominent at the frontal region | ||
| 77 detoxified patients and 45 controls (Tcheslavski and Gonen 2012) | Reduce power in frontal region for low EEG frequency, and more occipital in higher frequency | |
| 122 alcoholics and 77 controls (Padmanabhapillai et al. 2006) | Significant reduction of the early evoked gamma band response in the frontal region during target stimulus processing | |
| 100 alcoholics and 100 controls (Jones et al. 2006) | Significant lower P300 (compared with N100, N200, P200) amplitudes in alcoholics (compared with controls) | |
| Alcoholic were significantly less ERO power in the posterior delta EROEVK (forming P3) and frontally theta EROTOT (early P3) bands but not in the alpha band | ||
| 48 low risk and 98 high risk subjects (Rangaswamy et al. 2007) | The theta and delta post-stimulus oscillations are remarkably reduced in adolescent offspring of alcoholics | |
| The P300 amplitudes are reduced not as strong as seen for the oscillations | ||
| 48 LTAAs and 48 NACs (Andrew and Fein 2010a) | The LTAA showed significantly lower evoked Delta ERO power and total delta and theta ERO power. It provided an alternative and comparable representation of the reduced P3b amplitude in LTAA | |
| 48 LTAAs and 48 NACs (Andrew and Fein 2010b) | Significantly larger theta ERS to the target stimulus in LTAAs. Induce theta power measures are more powerful and independent group discriminators than the P3b amplitude | |
| 43 LTAAs, 31 STAAs and 72 controls (Gilmore and Fein 2012) | Theta ERS was larger in both STAA and LTAA compared with controls and the magnitude of this enhancement was greater in STAA than in LTAA | |
Alcohol addiction studies based on ERO involving time–frequency analysis revealed new findings about cognitive processes of alcoholics. These findings might provide better understanding on the deficits in alcoholics. The studies in ERO were mainly focusing on evoked delta, gamma induced and total theta power. Evoked delta and theta powers were suggested to contribute the most power in P300 signal. The significant decrease of evoked delta and theta power proved to be more significant than P300 to reflect the deficits observed during decision processes in alcoholics in response to stimulus. The finding might help to improve clinical screening and diagnose for alcohol addiction treatment. Future research can concentrate more on the use of dynamic time–frequency and may analyse other frequency bands to achieve better accuracy and understand more about the cognitive processes of alcohol addiction patients.
EEG as utilities for alcoholics treatment
EEG may be utilized for screening AUD patients, relapse prediction and evaluation of medication effects. As summarized in Table 5, despite the rapid development in physiological studies of alcoholic brains, few reports discuss about application of EEG for early relapse detection and medication evaluation. However, their accuracy is not enough to be applied during clinical practice. Besides that, in the review articles and materials about clinical and neuropsychiatric application for alcohol addiction treatment (Ritsner 2009; Tavakoli et al. 2011), EEG was not mentioned in primary health care. In addition, few studies have applied EEG for AUD treatment.
Table 5.
Related studies about EEG application and their limitations
| Objective | Features | Algorithm | References | Results (%) |
|---|---|---|---|---|
| Predicting relapse | Spectral power | Logistic Regression | Bauer (2001) | 75 |
| Spectral power with Hjorth’s features | Discriminant Analysis and ANN | Winterer et al. (1998) | 83–85 | |
| P300 | Discriminant Function Analysis | Wan et al. (2010) | 63.9 | |
| Screening alcoholics | Spectral power and coherence | Locally Weight Regression | Guntaka and Tcheslavski (2013) | 66.45 |
| ERP’s components | ANN | Lopes et al. (2004) | 71 | |
| ERP’s components | Learning Vector Quantization | Lopes et al. (2005) | 80 | |
| Gamma Visual Evoked Potential (VEP) power | Least square Support Vector Machine (SVM) | Shooshtari and Setarehdan (2010) | 82.98 | |
| Raw EEG in F4 and P8 | Hidden Markov Model | Zhong and Ghosh (2002) | 90.50 | |
| Gamma VEP | MLP – BP with Elliptic filter | Kanna et al. (2005) | 91 | |
| Approximate Entropy (ApEn), Sample Entropy (SampEn), Largest Lyapunov Exponent (LLE), (high order spectra) HOS | SVM | Acharya et al. (2012) | 91.70 | |
| HOS | Fuzzy Sugeno Classifier | Faust et al. (2013b) | 92.40 | |
| ERP’s components | Random Forest | Kuncheva and Rodríguez (2013) | 94.50 | |
| Multi gamma band VEP | MLP | Rangaswamy et al. (2007) | 94.55 | |
| Yule Walker coefficient | Artificial NN | Ek et al. (2013) | 95.00 | |
| Wavelet Relative Power | K-nearest Neighbor | Faust et al. (2013a, b) | 95.80 | |
| Horizontal Visibility Graph Entropy | K-nearest Neighbor | Zhu et al. (2014) | 95.80 | |
| Gamma VEP | PCA | Ong et al. (2005) | 95.83 | |
| Gamma VEP | MLP | Palaniappan et al. (2002) | 96.10 | |
| Gamma VEP | LDA | Palaniappan (2005) | 97.40 | |
| Gamma VEP | KNN | Palaniappan (2006) | 98.71 | |
| Spectral power using Haar wavelet | Multilayer Perceptron Network (MLP) | Kousarrizi et al. (2009) | 98.83 | |
| Spectral Entropy | Probabilistic Neural Network | Padmanabhapillai et al. (2006) | 99.00 | |
| VEP energy in occipital | KNN OR Support Vector Data Description | Zúquete et al. (2010) | 99.20 | |
| Mean and variance of signals | Bayes with KNN and PCA (claim to classify AA) | Yazdani and Setarehdan (2007) | 100 | |
| Classify epileptic and alcoholic | Recurrence Quantification Analysis (RQA) | Gaussian mixture model (GMM) | Ng et al. (2012) | 98.6 |
For relapse prediction, there are few studies using spectral power and nonlinear features, e.g., extracting Hjorth features from REEG. Unfortunately, their accuracy is not efficient enough for clinical practice because of either low sensitivity (Bauer 2001) or low specificity (Winterer et al. 1998). ERP techniques have not shown any evidence about the recovery of detoxified patients. One of ERP components, P300 is not suitable for relapse prediction because it was confirmed to not recover after long time treatment. Wan et al. (2010) have tried to apply P300 for relapse prediction and the result has shown no discrimination between relapsing and abstaining patients with accuracy of 63.9%. On the other hands, derivatives of P300 using ERO techniques such as evoke delta, induce theta ERS and evoke gamma power might act as alternative marker for AUD detection.
Regarding AUD screening, various studies have utilized different electrophysiological features and classification algorithms with high accuracy (> 90%) for the classification between alcoholics and controls. These results once more confirm about the difference between AUD and controls, and provide evidence about EEG capability for AUD screening in clinical practice. However, there is no discussion or analysis about the features and algorithms used in most of those studies.
Organic changes in the brain were observed during alcoholism which leads to seizure like epileptic cases. Ng et al. (2012) has implemented an automatic method to differentiate epileptic, controls and alcoholics using EEG with accuracy of 98.6%. However, the validity of data is doubtful because the study has utilized datasets from different sources with different experiment designs (oddball visual stimulus vs. eye closed resting), equipment (64 channels vs. 128 channels) and references systems (Cz vs. common average reference) without any indication about the synchronization between two datasets. Moreover, the dataset of alcoholism in the study contained not only alcoholics but also control subjects (Zhang et al. 1997). In general, there is also a lack of standard procedures to categorize study populations during clinical evaluation. In addition, factors such as sociodemographic characteristics and family drinking history need to be considered and controlled.
Discussion and conclusion
In this paper, a comprehensive review of the contributions made by EEG based techniques for AUD and its related challenges are discussed. The aim is to highlight key findings that could guide future research in this context. Studies based on neuroimaging techniques have proved their efficiency for AUD screening including alcohol consumption and its related problems. However, these techniques are not popular in clinical practice because physicians have little knowledge of current researches as applied to clinical practice (Miller et al. 2004). Furthermore, the existing EEG methods receive little attention as most of the EEG studies are not efficient enough and robust to be considered clinical effective. This is because of the methodological differences among different studies. In addition, the small sample sizes employed by these methods pose limitation to generalize their findings.
About EEG methodology, there are numerous methodological differences in AUD studies. The differences are based on various experimental paradigms, extracted features, and data processing methods used across studies. Despite of these differences, common points are focused on the neurophysiological variations in alcoholics. The deficit in frontal lobe activities of the brain has been well documented including the decrease of slow band power in REEG (Coutin-Churchman et al. 2006; de Bruin et al. 2006; Rangaswamy et al. 2003; Saletu-Zyhlarz et al. 2004), the smaller amplitude and more latency P300 responses (Costa et al. 2000; Marinkovic et al. 2001; Suresh et al. 2003) and decrease power of slow band oscillation (Jones et al. 2006; Kamarajan et al. 2006; Krause et al. 2002; Tcheslavski and Gonen 2012).
The EEG-based spectral power and coherence show their ability in analyzing and discriminating AUD patients from controls. Spectral power shows overall differences between AUD patients and controls but not significant at every EEG electrodes. Using spectral power would help to explain the alteration of brain activities of AUD patients. In comparison with absolute power, the relative power shows more discrimination between the two groups. Especially, theta power has proved its potential by outperform the other frequency bands with a remarkable power decrease in AUD. Despite few inconsistent results such as (Rangaswamy et al. 2003), more recently, studies have proved the decrease in theta power observed in alcoholics while comparing with control subjects. The changes of theta power in alcoholics were also reported in literature as a consistent indication for alcoholics screening. In addition, power of gamma band shows significant differences between AUD patients and controls. However, its potential has not received adequate attention. On the other hand, the coherence displays the associations between different brain regions and their variation under the effect of alcohol in AUD people. The changes in coherence also synchronized with changes in spectral power, or more specifically in relative power with the decrease in theta bands and the increase in higher frequency bands. However, like the discussion in “EEG coherence, phase delay and synchronization likelihood” section, the changes seemed to be more prominent with the decrease in theta band. Besides that, the diminished EEG coherence is more significant in long range connections.
Besides the novelty as compared with self-report tests, the unpopularity of other AUD screening tools such as EEG also included the challenge in translating preclinical or discovery phase into clinically useful biomarkers. In addition, studies involving biomedical research are expensive, time-consuming, confounded with requisite human samples and lack of analytical standardization (Freeman and Vrana 2010). On the contrary, self-reports have been developed earlier than the other methods and they are also easier, cheaper and faster to apply (only use questionnaire). This might explain why there are many studies using the existence dataset instead of designing and conducting their own experiments.
However, there are few limitations associated with the studies about alcoholics screening described in Table 5:
The studies listed in the Table 5 used common dataset from the study of Zhang et al. (1997). Due to common datasets, between studies comparisons were easier and saved useful time. On the other hand, it will reduce the variety of dataset and the generalization of the results. Ehlers et al. (2004) have mentioned in their study (Ehlers et al. 2004) about the presence of ethnic stratification that different ethnics might exhibit in different ways under the effects of alcohol. More specifically, the association between EEG distribution and alcohol dependence varies, and it also depends on individual’s ethnic heritage. Therefore, it is necessary to study usefulness of EEG in screening for alcoholics on different dataset from different kinds of participants.
The dataset might contain only detoxified alcohol dependent patients. The dataset was described in the study of Zhang et al. (1997) to do classification for participants using SSAGA (Semi-Structured Assessment for the Genetics of Alcoholism) which was indicated to be high reliability for alcohol dependence but not for alcohol abuse (Bucholz et al. 1994). Another proof was that the participants were hospitalized for 30 days of detoxification. However, there are few studies mistaken the participants in dataset with alcohol abusers and social drinkers. In conclusion, no such study exists about AUD screening using EEG and further studies including screening for people with alcohol abuse and high risk of alcohol abuse are needed.
The progress in AUD study using EEG has provided new methods for studying the recovery of AUD patients. The new EEG phenomenon (evoked theta, evoked delta, induced theta ERS, gamma oscillation) of EROs may be a candidate for assessing treatment progress. So far, there is still no study about the association between a deficit in gamma band and the duration of abstinence and its ability for discriminating between relapsers and abstainers.
It appears that REEG and ERP components provide potential mechanism for classifying AUD patients and identifying people with high risk of AUD. Now classification algorithms and combination of various features are recommended that can discriminate between AUD patients and controls. The EEG analysis methods using nonlinear characteristics and non-stationary of REEG and new methods of extracting and analyzing ERP component may provide more features for studying alcohol addiction.
In the future, EEG study on alcohol addiction may focus on the recovery of alcoholics after detoxification. It will help to develop biomarkers for evaluating the treatment efficiency and to understand more alternations of the brain while recovering. EEG signals can also be analysed using novel features to capture various aspects of brain activities in people with AUD. Therefore, better understanding and results may be achieved.
In conclusion, the studies presented in this review have shown their potentials only as research tools. There is a need to put effort to translate their key findings into clinical practice. It needs further combined efforts from researchers from clinical and technology side.
Acknowledgements
This research work is supported by the HICoE grant for CISIR (0153CA-005), Ministry of Education (MOE), Malaysia.
References
- Acharya UR, Sree SV, Chattopadhyay S, Suri JS. Automated diagnosis of normal and alcoholic EEG signals. Int J Neural Syst. 2012;22(03):1250011. doi: 10.1142/S0129065712500116. [DOI] [PubMed] [Google Scholar]
- Acharya UR, Bhat S, Adeli H, Adeli A. Computer-aided diagnosis of alcoholism-related EEG signals. Epilepsy Behav. 2014;41:257–263. doi: 10.1016/j.yebeh.2014.10.001. [DOI] [PubMed] [Google Scholar]
- Addolorato G, Capristo E, Greco A, Caputo F, Stefanini G, Gasbarrini G. Three months of abstinence from alcohol normalizes energy expenditure and substrate oxidation in alcoholics: a longitudinal study. Am J Gastroenterol. 1998;93(12):2476–2481. doi: 10.1111/j.1572-0241.1998.00707.x. [DOI] [PubMed] [Google Scholar]
- Anderson NE, Baldridge RM, Stanford MS. P3a amplitude predicts successful treatment program completion in substance-dependent individuals. Subst Use Misuse. 2011;46(5):669–677. doi: 10.3109/10826084.2010.528123. [DOI] [PubMed] [Google Scholar]
- Andrew C, Fein G. Event-related oscillations versus event-related potentials in a P300 task as biomarkers for alcoholism. Alcohol Clin Exp Res. 2010;34(4):669–680. doi: 10.1111/j.1530-0277.2009.01136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrew C, Fein G. Induced theta oscillations as biomarkers for alcoholism. Clin Neurophysiol. 2010;121(3):350–358. doi: 10.1016/j.clinph.2009.11.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APA . Diagnostic and statistical manual of mental disorders (DSM-5®) Arlington: APA; 2013. [DOI] [PubMed] [Google Scholar]
- Barry RJ, Clarke AR, Hajos M, McCarthy R, Selikowitz M, Dupuy FE. Resting-state EEG gamma activity in children with attention-deficit/hyperactivity disorder. Clin Neurophysiol. 2010;121(11):1871–1877. doi: 10.1016/j.clinph.2010.04.022. [DOI] [PubMed] [Google Scholar]
- Bauer LO. Predicting relapse to alcohol and drug abuse via quantitative electroencephalography. Neuropsychopharmacology. 2001;25(3):332–340. doi: 10.1016/S0893-133X(01)00236-6. [DOI] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock V, Nurnberger J, Jr, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol. 1994;55(2):149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Campanella S, Petit G, Maurage P, Kornreich C, Verbanck P, Noël X. Chronic alcoholism: insights from neurophysiology. Neurophysiol Clin. 2009;39(4):191–207. doi: 10.1016/j.neucli.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Costa L, Bauer L, Kuperman S, Porjesz B, O’Connor S, Hesselbrock V, Begleiter H. Frontal P300 decrements, alcohol dependence, and antisocial personality disorder. Biol Psychiatry. 2000;47(12):1064–1071. doi: 10.1016/S0006-3223(99)00317-0. [DOI] [PubMed] [Google Scholar]
- Coutin-Churchman P, Moreno R, Añez Y, Vergara F. Clinical correlates of quantitative EEG alterations in alcoholic patients. Clin Neurophysiol. 2006;117(4):740–751. doi: 10.1016/j.clinph.2005.12.021. [DOI] [PubMed] [Google Scholar]
- Crego A, Holguín SR, Parada M, Mota N, Corral M, Cadaveira F. Binge drinking affects attentional and visual working memory processing in young university students. Alcohol Clin Exp Res. 2009;33(11):1870–1879. doi: 10.1111/j.1530-0277.2009.01025.x. [DOI] [PubMed] [Google Scholar]
- Curtin JJ, Fairchild BA. Alcohol and cognitive control: implications for regulation of behavior during response conflict. J Abnorm Psychol. 2003;112(3):424. doi: 10.1037/0021-843X.112.3.424. [DOI] [PubMed] [Google Scholar]
- de Bruin EA, Bijl S, Stam CJ, Böcker KB, Kenemans JL, Verbaten MN. Abnormal EEG synchronisation in heavily drinking students. Clin Neurophysiol. 2004;115(9):2048–2055. doi: 10.1016/j.clinph.2004.04.010. [DOI] [PubMed] [Google Scholar]
- de Bruin EA, Stam CJ, Bijl S, Verbaten MN, Kenemans JL. Moderate-to-heavy alcohol intake is associated with differences in synchronization of brain activity during rest and mental rehearsal. Int J Psychophysiol. 2006;60(3):304–314. doi: 10.1016/j.ijpsycho.2005.07.007. [DOI] [PubMed] [Google Scholar]
- de Souza ACS, Yehia HC, Sato M-A, Callan D. Brain activity underlying auditory perceptual learning during short period training: simultaneous fMRI and EEG recording. BMC Neurosci. 2013;14(1):1. doi: 10.1186/1471-2202-14-S1-P1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy FH, Bartels PH, Burchfiel JL. Significance probability mapping: an aid in the topographic analysis of brain electrical activity. Electroencephalogr Clin Neurophysiol. 1981;51(5):455–462. doi: 10.1016/0013-4694(81)90221-2. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Phillips E. Association of EEG alpha variants and alpha power with alcohol dependence in Mexican American young adults. Alcohol. 2007;41(1):13–20. doi: 10.1016/j.alcohol.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Phillips E, Schuckit MA. EEG alpha variants and alpha power in Hispanic American and white non-Hispanic American young adults with a family history of alcohol dependence. Alcohol. 2004;33(2):99–106. doi: 10.1016/S0741-8329(04)00098-9. [DOI] [PubMed] [Google Scholar]
- Ek Z, Akg A, Bozkurt MR. The classificaton of EEG signals recorded in drunk and non-drunk people. Int J Comput Appl. 2013;68(10):40–44. [Google Scholar]
- Faust O, Yanti R, Yu W. Automated detection of alcohol related changes in electroencephalograph signals. J Med Imaging Health Inform. 2013;3(2):333–339. doi: 10.1166/jmihi.2013.1170. [DOI] [Google Scholar]
- Faust O, Yu W, Kadri NA. Computer-based identification of normal and alcoholic eeg signals using wavelet packets and energy measures. J Mech Med Biol. 2013;13(03):1350033. doi: 10.1142/S0219519413500334. [DOI] [Google Scholar]
- Fisher RA. The use of multiple measurements in taxonomic problems. Ann Eugen. 1936;7(2):179–188. doi: 10.1111/j.1469-1809.1936.tb02137.x. [DOI] [Google Scholar]
- Freeman WM, Vrana KE. future prospects for biomarkers of alcohol consumption and alcohol-induced disorders. Alcohol Clin Exp Res. 2010;34(6):946–954. doi: 10.1111/j.1530-0277.2010.01169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore CS, Fein G. Theta event-related synchronization is a biomarker for a morbid effect of alcoholism on the brain that may partially resolve with extended abstinence. Brain Behav. 2012;2(6):796–805. doi: 10.1002/brb3.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn SW, Sinha R, Parsons OA. Electrophysiological indices predict resumption of drinking in sober alcoholics. Alcohol. 1993;10(2):89–95. doi: 10.1016/0741-8329(93)90086-4. [DOI] [PubMed] [Google Scholar]
- Guntaka R, Tcheslavski GV. On the EEG-based automated detection of alcohol dependence. Int J Bioautom. 2013;17:167–176. [Google Scholar]
- Hada M, Porjesz B, Begleiter H, Polich J. Auditory P3a assessment of male alcoholics. Biol Psychiatry. 2000;48(4):276–286. doi: 10.1016/S0006-3223(00)00236-5. [DOI] [PubMed] [Google Scholar]
- Heinz A, Beck A, Grüsser SM, Grace AA, Wrase J. Identifying the neural circuitry of alcohol craving and relapse vulnerability. Addict Biol. 2009;14(1):108–118. doi: 10.1111/j.1369-1600.2008.00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki M, Kellinghaus C, Alexopoulos AV, Burgess RC, Kumar AN, Han YH, Leigh RJ. Effects of eyelid closure, blinks, and eye movements on the electroencephalogram. Clin Neurophysiol. 2005;116(4):878–885. doi: 10.1016/j.clinph.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Jones KA, Porjesz B, Chorlian D, Rangaswamy M, Kamarajan C, Padmanabhapillai A, Begleiter H. S-transform time-frequency analysis of P300 reveals deficits in individuals diagnosed with alcoholism. Clin Neurophysiol. 2006;117(10):2128–2143. doi: 10.1016/j.clinph.2006.02.028. [DOI] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B, Jones KA, Choi K, Chorlian DB, Padmanabhapillai A, Begleiter H. The role of brain oscillations as functional correlates of cognitive systems: a study of frontal inhibitory control in alcoholism. Int J Psychophysiol. 2004;51(2):155–180. doi: 10.1016/j.ijpsycho.2003.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B, Jones KA, Choi K, Chorlian DB, Padmanabhapillai A, Begleiter H. Alcoholism is a disinhibitory disorder: neurophysiological evidence from a Go/No-Go task. Biol Psychol. 2005;69(3):353–373. doi: 10.1016/j.biopsycho.2004.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B, Jones K, Chorlian D, Padmanabhapillai A, Rangaswamy M, Begleiter H. Event-related oscillations in offspring of alcoholics: neurocognitive disinhibition as a risk for alcoholism. Biol Psychiatry. 2006;59(7):625–634. doi: 10.1016/j.biopsych.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanna PS, Palaniappan R, Ravi K (2005) Classification of alcohol abusers: an intelligent approach. Paper presented at the third international conference on information technology and applications, 2005. ICITA 2005
- Klimesch W. EEG alpha and theta oscillations reflect cognitive and memory performance: a review and analysis. Brain Res Rev. 1999;29(2):169–195. doi: 10.1016/S0165-0173(98)00056-3. [DOI] [PubMed] [Google Scholar]
- Kousarrizi MN, Ghanbari AA, Gharaviri A, Teshnehlab M, Aliyari M (2009) Classification of alcoholics and non-alcoholics via EEG using SVM and neural networks. Paper presented at the 3rd international conference on bioinformatics and biomedical engineering, 2009. ICBBE 2009
- Krause CM, Sillanmäki L, Koivisto M, Saarela C, Häggqvist A, Laine M, Hämäläinen H. The effects of memory load on event-related EEG desynchronization and synchronization. Clin Neurophysiol. 2000;111(11):2071–2078. doi: 10.1016/S1388-2457(00)00429-6. [DOI] [PubMed] [Google Scholar]
- Krause CM, Aromäki A, Sillanmäki L, Åström T, Alanko K, Salonen E, Peltola O. Alcohol-induced alterations in ERD/ERS during an auditory memory task. Alcohol. 2002;26(3):145–153. doi: 10.1016/S0741-8329(01)00204-X. [DOI] [PubMed] [Google Scholar]
- Kuncheva LI, Rodríguez JJ. Interval feature extraction for classification of event-related potentials (ERP) in EEG data analysis. Prog Artif Intell. 2013;2(1):65–72. doi: 10.1007/s13748-012-0037-3. [DOI] [Google Scholar]
- Lopes CD, Mainardi JO, Zaro MA, Susin AA (2004) Classification of event-related potentials in individuals at risk for alcoholism using wavelet transform and artificial neural network. Paper presented at the proceedings of the 2004 IEEE symposium on computational intelligence in bioinformatics and computational biology, 2004. CIBCB’04
- Lopes CD, Schuler E, Engel P, Susin AA (2005). ERP signal identification of Individuals at risk for alcoholism using learning vector quantization network. Paper presented at the proceedings of the 2005 IEEE symposium on computational intelligence in bioinformatics and computational biology, 2005. CIBCB’05
- Marco J, Fuentemilla L, Grau C. Auditory sensory gating deficit in abstinent chronic alcoholics. Neurosci Lett. 2005;375(3):174–177. doi: 10.1016/j.neulet.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Marinkovic K, Halgren E, Maltzman I. Arousal-related P3a to novel auditory stimuli is abolished by a moderately low alcohol dose. Alcohol Alcohol. 2001;36(6):529–539. doi: 10.1093/alcalc/36.6.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurage P, Campanella S, Philippot P, de Timary P, Constant E, Gauthier S, Noël X. Alcoholism leads to early perceptive alterations, independently of comorbid depressed state: an ERP study. Neurophysiol Clin. 2008;38(2):83–97. doi: 10.1016/j.neucli.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Michael A, Mirza K, Mukundan C, Channabasavanna S. Interhemispheric electroencephalographic coherence as a biological marker in alcoholism. Acta Psychiatr Scand. 1993;87(3):213–217. doi: 10.1111/j.1600-0447.1993.tb03358.x. [DOI] [PubMed] [Google Scholar]
- Miller PM, Ornstein SM, Nietert PJ, Anton RF. Self-report and biomarker alcohol screening by primary care physicians: the need to translate research into guidelines and practice. Alcohol Alcohol. 2004;39(4):325–328. doi: 10.1093/alcalc/agh070. [DOI] [PubMed] [Google Scholar]
- Miller PM, Spies C, Neumann T, Javors MA, Hoyumpa A, Roache J, Anton RF. Alcohol biomarker screening in medical and surgical settings. Alcohol Clin Exp Res. 2006;30(2):185–193. doi: 10.1111/j.1530-0277.2006.00029.x. [DOI] [PubMed] [Google Scholar]
- Moss HB, Chen CM, Yi H-Y. Subtypes of alcohol dependence in a nationally representative sample. Drug Alcohol Depend. 2007;91(2):149–158. doi: 10.1016/j.drugalcdep.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumtaz W, Vuong PL, Xia L, Malik AS, Rashid RBA. Automatic diagnosis of alcohol use disorder using EEG features. Knowl Based Syst. 2016;105:48–59. doi: 10.1016/j.knosys.2016.04.026. [DOI] [Google Scholar]
- Mumtaz W, Vuong PL, Xia L, Malik AS, Rashid RBA. An EEG-based machine learning method to screen alcohol use disorder. Cogn Neurodyn. 2017;11(2):161–171. doi: 10.1007/s11571-016-9416-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng EP, Lim T-C, Chattopadhyay S, Bairy M. Automated identification of epileptic and alcoholic EEG signals using recurrence quantification analysis. J Mech Med Biol. 2012;12(05):1240028. doi: 10.1142/S0219519412400283. [DOI] [Google Scholar]
- NIAAA (2012) Alcohol use disorder. Retrieved from http://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/alcohol-use-disorders. Accessed 19 May 2016
- Nyhus E, Curran T. Functional role of gamma and theta oscillations in episodic memory. Neurosci Biobehav Rev. 2010;34(7):1023–1035. doi: 10.1016/j.neubiorev.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong K-M, Thung K-H, Wee C-Y, Paramesranle R (2005) Selection of a subset of EEG channels using PCA to classify alcoholics and non-alcoholics. Paper presented at the proceedings of the 2005 IEEE engineering in medicine and biology 27th annual conference (Shanghai, China, 2005) [DOI] [PubMed]
- Padmanabhapillai A, Porjesz B, Ranganathan M, Jones KA, Chorlian DB, Tang Y, Begleiter H. Suppression of early evoked gamma band response in male alcoholics during a visual oddball task. Int J Psychophysiol. 2006;60(1):15–26. doi: 10.1016/j.ijpsycho.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Palaniappan R (2005) Discrimination of alcoholic subjects using second order autoregressive modelling of brain signals evoked during visual stimulus perception. Paper presented at the IEC (Prague)
- Palaniappan R. Improved automated classification of alcoholics and non-alcoholics. Int J Inf Technol. 2006;2:182–186. [Google Scholar]
- Palaniappan R, Raveendran P, Omatu S. VEP optimal channel selection using genetic algorithm for neural network classification of alcoholics. IEEE Trans Neural Netw. 2002;13(2):486–491. doi: 10.1109/72.991435. [DOI] [PubMed] [Google Scholar]
- Pandey A, Kamarajan C, Tang Y, Chorlian D, Roopesh B, Manz N, Porjesz B. Neurocognitive deficits in male alcoholics: an ERP/sLORETA analysis of the N2 component in an equal probability Go/NoGo task. Biol Psychol. 2012;89(1):170–182. doi: 10.1016/j.biopsycho.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons OA, Nixon SJ. Cognitive functioning in sober social drinkers: a review of the research since 1986. J Stud Alcohol. 1998;59(2):180–190. doi: 10.15288/jsa.1998.59.180. [DOI] [PubMed] [Google Scholar]
- Parvaz MA, Alia-Klein N, Woicik PA, Volkow ND, Goldstein RZ. Neuroimaging for drug addiction and related behaviors. Rev Neurosci. 2011;22(6):609–624. doi: 10.1515/RNS.2011.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Ford JM, White PM, Mathalon D. Event-Related Potentials in Alcoholic Men: P3 Amplitude Reflects Family History But Not Alcohol Consumption. Alcohol Clin Exp Res. 1991;15(5):839–850. doi: 10.1111/j.1530-0277.1991.tb00611.x. [DOI] [PubMed] [Google Scholar]
- Popham RE, Schmidt W. Words and deeds: the validity of self-report data on alcohol consumption. J Stud Alcohol. 1981;42(3):355–358. doi: 10.15288/jsa.1981.42.355. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H. Human brain electrophysiology and alcoholism. In: Tarter RE, van Thiel DH, editors. Alcohol and the brain. Berlin: Springer; 1985. pp. 139–182. [Google Scholar]
- Porjesz B, Rangaswamy M, Kamarajan C, Jones KA, Padmanabhapillai A, Begleiter H. The utility of neurophysiological markers in the study of alcoholism. Clin Neurophysiol. 2005;116(5):993–1018. doi: 10.1016/j.clinph.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Rangaswamy M, Porjesz B. From event-related potential to oscillations: genetic diathesis in brain (dys) function and alcohol dependence. Alcohol Res Health. 2008;31(3):238–243. [PMC free article] [PubMed] [Google Scholar]
- Rangaswamy M, Porjesz B, Chorlian DB, Wang K, Jones KA, Bauer LO, Reich T. Beta power in the EEG of alcoholics. Biol Psychiatry. 2002;52(8):831–842. doi: 10.1016/S0006-3223(02)01362-8. [DOI] [PubMed] [Google Scholar]
- Rangaswamy M, Porjesz B, Chorlian DB, Choi K, Jones KA, Wang K, Reich T. Theta power in the EEG of alcoholics. Alcohol Clin Exp Res. 2003;27(4):607–615. doi: 10.1111/j.1530-0277.2003.tb04397.x. [DOI] [PubMed] [Google Scholar]
- Rangaswamy M, Jones KA, Porjesz B, Chorlian DB, Padmanabhapillai A, Kamarajan C, Bauer LO. Delta and theta oscillations as risk markers in adolescent offspring of alcoholics. Int J Psychophysiol. 2007;63(1):3–15. doi: 10.1016/j.ijpsycho.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritsner MS. The handbook of neuropsychiatric biomarkers, endophenotypes and genes: volume I: neuropsychological endophenotypes and biomarkers. Berlin: Springer; 2009. [Google Scholar]
- Saletu B, Anderer P, Kinsperger K, Grünberger J. Topographic brain mapping of EEG in neuropsychopharmacology—part II. Clinical applications (pharmaco EEG imaging) Methods Find Exp Clin Pharmacol. 1987;9(6):385–408. [PubMed] [Google Scholar]
- Saletu-Zyhlarz GM, Arnold O, Anderer P, Oberndorfer S, Walter H, Lesch OM, Saletu B. Differences in brain function between relapsing and abstaining alcohol-dependent patients, evaluated by EEG mapping. Alcohol Alcohol. 2004;39(3):233–240. doi: 10.1093/alcalc/agh041. [DOI] [PubMed] [Google Scholar]
- Sedley W, Cunningham MO (2013) Do cortical gamma oscillations promote or suppress perception? An under-asked question with an over-assumed answer. Front Hum Neurosci 7:595. 10.3389/fnhum.2013.00595 [DOI] [PMC free article] [PubMed]
- Shooshtari MA, Setarehdan SK (2010) Selection of optimal EEG channels for classification of signals correlated with alcohol abusers. Paper presented at the 2010 IEEE 10th international conference on signal processing (ICSP)
- Solomon J, Vanga N, Morgan J, Joseph P. Emergency-room physicians’: recognition of alcohol misuse. J Stud Alcohol. 1980;41(5):583–586. doi: 10.15288/jsa.1980.41.583. [DOI] [PubMed] [Google Scholar]
- Stam C, Van Dijk B. Synchronization likelihood: an unbiased measure of generalized synchronization in multivariate data sets. Phys D. 2002;163(3):236–251. doi: 10.1016/S0167-2789(01)00386-4. [DOI] [Google Scholar]
- Suresh S, Porjesz B, Chorlian DB, Choi K, Jones KA, Wang K, Begleiter H. Auditory P3 in female alcoholics. Alcohol Clin Exp Res. 2003;27(7):1064–1074. doi: 10.1097/01.ALC.0000075549.49800.A0. [DOI] [PubMed] [Google Scholar]
- Tavakoli HR, Hull M, Michael Okasinski L. Review of current clinical biomarkers for the detection of alcohol dependence. Innov Clin Neurosci. 2011;8(3):26–33. [PMC free article] [PubMed] [Google Scholar]
- Tcheslavski GV, Gonen FF. Alcoholism-related alterations in spectrum, coherence, and phase synchrony of topical electroencephalogram. Comput Biol Med. 2012;42(4):394–401. doi: 10.1016/j.compbiomed.2011.12.006. [DOI] [PubMed] [Google Scholar]
- Wan L, Baldridge RM, Colby AM, Stanford MS. Association of P3 amplitude to treatment completion in substance dependent individuals. Psychiatry Res. 2010;177(1):223–227. doi: 10.1016/j.psychres.2009.01.033. [DOI] [PubMed] [Google Scholar]
- Watson CG, Tilleskjor C, Hoodecheck-Schow E, Pucel J, Jacobs L. Do alcoholics give valid self-reports? J Stud Alcohol. 1984;45(4):344–348. doi: 10.15288/jsa.1984.45.344. [DOI] [PubMed] [Google Scholar]
- Welch BL. The generalization ofstudent’s’ problem when several different population variances are involved. Biometrika. 1947;34(1/2):28–35. doi: 10.2307/2332510. [DOI] [PubMed] [Google Scholar]
- Winterer G, Klöppel B, Heinz A, Ziller M, Dufeu P, Schmidt LG, Herrmann WM. Quantitative EEG (QEEG) predicts relapse in patients with chronic alcoholism and points to a frontally pronounced cerebral disturbance. Psychiatry Res. 1998;78(1):101–113. doi: 10.1016/S0165-1781(97)00148-0. [DOI] [PubMed] [Google Scholar]
- Yazdani A, Setarehdan SK (2007) Classification of EEG signals correlated with alcohol abusers. Paper presented at the 9th international symposium on signal processing and its applications, 2007. ISSPA 2007
- Zhang XL, Begleiter H, Porjesz B, Litke A. Electrophysiological evidence of memory impairment in alcoholic patients. Biol Psychiatry. 1997;42(12):1157–1171. doi: 10.1016/S0006-3223(96)00552-5. [DOI] [PubMed] [Google Scholar]
- Zhong S, Ghosh J (2002) HMMs and coupled HMMs for multi-channel EEG classification. Paper presented at the proceedings of the IEEE international joint conference on neural networks
- Zhu G, Li Y, Wen PP, Wang S. Analysis of alcoholic EEG signals based on horizontal visibility graph entropy. Brain Inform. 2014;1(1–4):19–25. doi: 10.1007/s40708-014-0003-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zúquete A, Quintela B, Cunha JPS. Biometric authentication using electroencephalograms: a practical study using visual evoked potentials. Electrón Telecomun. 2010;5(2):185–194. [Google Scholar]