Abstract
Juvenile onset open-angle glaucoma (JOAG) affects patients before 40 years of age, causing high intraocular pressure and severe optic nerve damage. To expand the mutation spectrum of the causative genes in JOAG, with a view to identify novel disease-causing mutations, we investigated MYOC, OPTN, NTF4, WDR36 and CYP1B1 in a cohort of 67 unrelated Chinese JOAG patients. Whole exome sequencing was used to identify possible pathogenic mutations, which were further excluded in normal controls. After sequencing and the use of a database pipeline, as well as predictive assessment filtering, we identified a total of six mutations in three genes, MYOC, OPTN and CYP1B1. Among them, 2 heterozygous mutations in MYOC (c. 1109C > T, p. (P370L); c. 1150G > C, p. (D384H)), 2 heterozygous mutations in OPTN (c. 985A > G, p.(R329G); c. 1481T > G, p. (L494W)) and 2 homozygous mutations in CYP1B1 (c. 1412T > G, p.(I471S); c. 1169G > A, p.(R390H)) were identified as potentially causative mutations. No mutation was detected in NTF4 or WDR36. Our results enrich the mutation spectra and frequencies of MYOC, OPTN and CYP1B1 in JOAG among the Chinese population. Further studies are needed to address the pathogenicity of each of the mutations detected in this study.
Introduction
Glaucoma, the second leading cause of irreversible blindness worldwide1, is a group of heterogeneous optic neuropathies characterized by retinal nerve fibre layer damage and visual field defects. The disease is progressive and leads to permanent visual impairment and even blindness in some patients2. Age and high intraocular pressure (IOP) are the main risk factors. Primary open-angle glaucoma (POAG) is a common form of glaucoma, which can be further subdivided into juvenile-onset open-angle glaucoma (JOAG) and adult-onset POAG according to the age of onset3. JOAG patients often have higher intraocular pressure (IOP) and suffer from more severe optic nerve damage than adult-onset POAG patients4.
Genetic factors play an important role in the development of glaucoma5. Several genes have been identified to be associated with POAG, primary congenital glaucoma (PCG) and JOAG, including myocilin (MYOC)6; optineurin (OPTN)7; WD repeat domain 36 (WDR36)8; neurotrophin 4 (NTF4)9; and cytochrome P450 family 1, subfamily B(CYP1B1)10. The same candidate gene may lead to different phenotypes of glaucoma11. MYOC is the first candidate gene mapped for POAG and has been confirmed to be associated with both POAG and JOAG12. Mutations of OPTN were found in POAG and amyotrophic lateral sclerosis (ALS)13,14. CYP1B1 is a PCG gene but has also been reported in association with JOAG15,16. To date, the mutations of known genes only account for approximately 5% of patients with POAG17. Compared with adult-onset POAG, JOAG may be more likely to be genetically determined and less likely the consequence of environment16. Investigation of the POAG genes in JOAG might provide a good opportunity for understanding the genetic components and heterogeneity of JOAG.
Whole-exome sequencing (WES) is available in commercial service and has been proved to be useful in mapping disease genes. It is rapid and comparatively more cost-effective than other genomic technologies, especially for complex diseases18. In developmental and congenital glaucoma, WES has led to the identification of novel variants in LTBP2 and PXDN19. It has also been used to identify mutations in known genes in primary glaucoma effectively and quickly20. In the current study, we performed WES on 67 Chinese JOAG patients to detect the full spectra of variants in MYOC, OPTN, NTF4, WDR36 and CYP1B1, with a view to identify novel disease-causing mutations for JOAG.
Results
From the whole exome results of 67 Chinese JOAG patients, totally 79 variants in MYOC, 354 variants in OPTN, 139 variants in WDR36, 45 variants in NTF4 and 199 variants in CYP1B1 were detected. Among them, a total of 6 variants in MYOC, OPTN and CYP1B1 were identified as potential disease-causing mutations in 8 patients (11.94%) after a series of filtering steps (Tables 1 and 2). These variants included 2 variants in MYOC, 2 variants in OPTN and 2 variants in CYP1B1 (Fig. 1). The variants were confirmed by Sanger sequencing. No MYOC or OPTN potential disease causing mutations were detected in 125 controls, while two heterozygous mutations of CYP1B1 (c. 1169G > A p. (R390H)) were found in controls (Table 1).
Table 1.
Mutations of MYOC, OPTN and CYP1B1 identified in this study.
| Gene | Mutation | Status | Polyphen2 | SIFT | Mutation taster | Reported or not | Frequency in control |
|---|---|---|---|---|---|---|---|
| MYOC | c.1109C > T p. (P370L) | Het | D (1) | D (0.02) | DC (0.999) | Reported26 | 0/125 |
| MYOC | c.1150G > C p. (D384H) | Het | D (1) | D (0) | DC (0.999) | Novel | 0/125 |
| OPTN | c.1481T > G p. (L494W) | Het | D (0.999) | D (0.03) | P (0.712) | Reported in ALS33 | 0/125 |
| OPTN | c.985A > G p. (R329G) | Het | P (0.856) | T (0.17) | DC (0.519) | Novel | 0/125 |
| CYP1B1 | c.1412T > G p. (I471S) | Hom | D (1) | D (0) | DC (0.999) | Reported21 | 0/125 |
| CYP1B1 | c.1169G > A p. (R390H) | Hom | D (1) | D (0) | DC (0.999) | Reported38 | 2/125 (Het) |
Het, heterozygous mutation; D, damaging; DC, disease causing; P, probably damaging; ALS, Amyotrophic lateral sclerosis; T, tolerated; Hom, homozygous mutation; Reported or not: Mutations with reference citations were reported to be pathogenic.
Table 2.
Clinical data of the eight patients with mutations.
| Case ID | Gene | Mutation | Status | Effect | Age of diagnosis (Y) | Sex | IOP | C/D | VF(MD) |
|---|---|---|---|---|---|---|---|---|---|
| OD OS |
OD OS |
OD OS |
|||||||
| G303 | MYOC | c.1109C > T p. (P370L) |
hetero | Missense | 24 | M | 54 49 |
0.9 1.0 |
−29.36 −34.12 |
| G022 | MYOC | c.1109C > T p. (P370L) |
hetero | Missense | 21 | M | 40 40 |
1.0 1.0 |
NA NA |
| G8–1 | MYOC | c.1109C > T p. (P370L) |
hetero | Missense | 23 | M | 42.3 42.9 |
0.9 0.9 |
−32.28 −32.34 |
| G13–1 | MYOC | c.1150 G > C p. (D384H) |
hetero | Missense | 25 | M | 38 33.6 |
0.9 0.6 |
−20.28 −2.88 |
| G335 | OPTN | c.1481T > G p. (L494W) |
hetero | Missense | 33 | F | 26 19 |
0.9 0.4 |
−29.29 −0.84 |
| G092 | OPTN | c.985A > G p. (R329G) |
hetero | Missense | 27 | M | 31 18 |
1.0 0.6 |
−29.88 −0.77 |
| G398 | CYP1B1 | c.1412T > G p. (I471S) |
homo | Missense | 19 | M | 47 36 |
0.9 0.9 |
−25.78 −32.17 |
| G447 | CYP1B1 | c.1169G > A p. (R390H) |
homo | Missense | 29 | F | 38 17 |
0.9 0.9 |
−33.54 −23.84 |
Note: IOP, intraocular pressure; C/D, cup/disc ratio; VF, visual field; MD, mean defect; hetero, heterozygous mutation; homo, homozygous mutation.
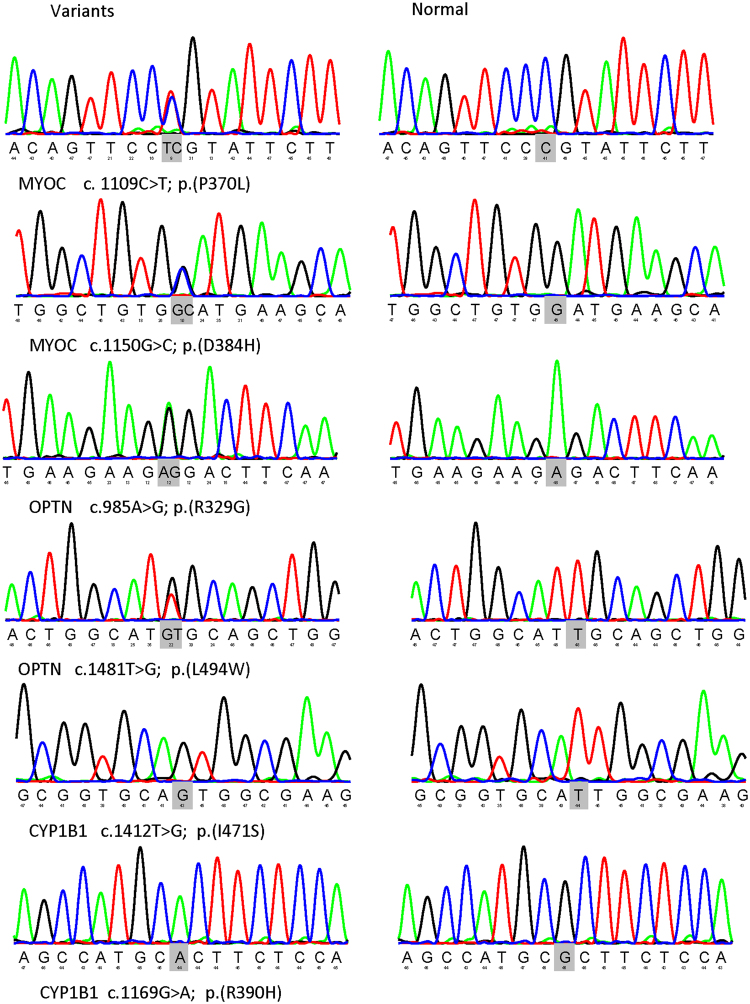
Figure 1.
Six variants of MYOC, OPTN and CYP1B1 identified in this JOAG cohort. Sequence changes detected in the patients with JOAG are presented in the left column, whereas sequences from healthy individuals appear in the right column.
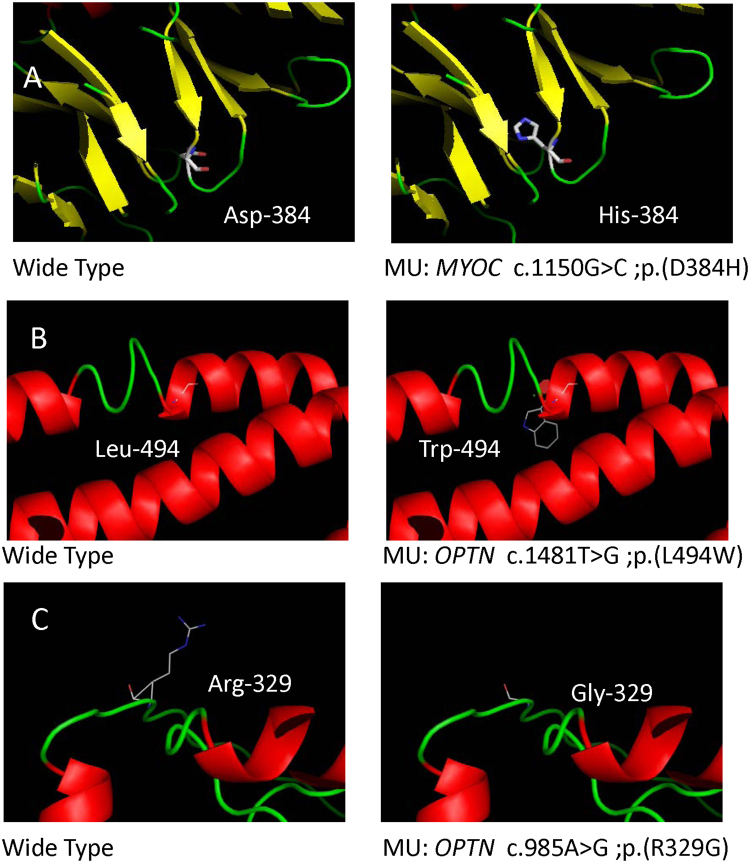
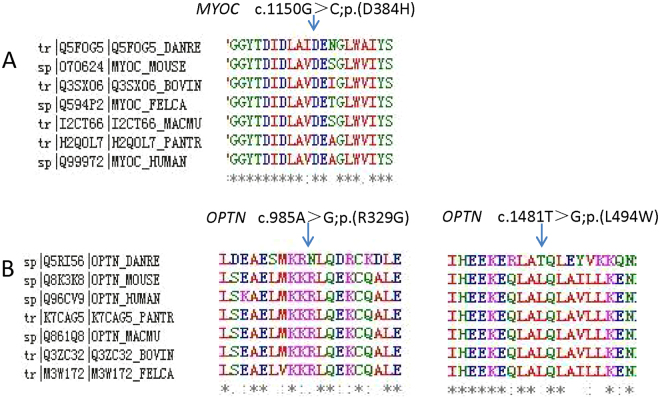
In MYOC, there were two heterozygous mutations (c. 1109C > T, p. (P370L); c. 1150G > C, p. (D384H)) detected from two cases in this study, among which p.D384H was novel (Fig. 2A). Substitution of p.D384H was predicted to affect protein function by Polyphen-2, SIFT and Mutation Taster. Additionally, the mutated amino acid is highly conserved among all the tested species (Fig. 3A). p. (P370L) was a reported mutation associated with POAG.
Figure 2.
Prediction of the three-dimensional structure of proteins of MYOC and OPTN. Predicted crystal structures of wild-type (left) and mutant (right) proteins (A–C).
Figure 3.
Conservation analysis revealed evolutionary conservation of the mutations by using Clustal Omega. Multiple alignments of the amino acids from different species are shown. The arrow indicates the position of the mutations (A–C).
In OPTN, 2 heterozygous mutations (c. 985A > G, p. (R329G); c.1481T > G, p. (L494W)) were detected in two unrelated individual. Among them, p. (R329G) was novel and p. (L494W) was reported in amyotrophic lateral sclerosis (ALS) (Fig. 2B,C). p. (R329G) was predicted to be disease causing by Mutation taster and probably damaging by Polyphen-2. Furthermore, the p. (R329G) mutation occurred at a remarkably conserved region in all the tested species apart from danio (Fig. 3B).
In CYP1B1, 2 homozygous mutations (c.1412T > G, p. (I471S); c.1169G > A, p. (R390H)) were detected in two patients. Both mutations were predicted to be a pathogenic mutation in all three pathogenicity prediction tools used in this study. The I471S mutation was reported in a Chinese primary congenital glaucoma (PCG) study21.
Discussion
In this study, the results of exome sequencing data from 67 JOAG patients identified 6 mutations in the MYOC, OPTN and CYP1B1 genes in 8 unrelated patients. Among them, D384H in MYOC and R329G and L494W in OPTN were novel. These mutations are predicted to affect protein function and were absent in 125 control individuals without glaucoma.
MYOC is the first identified glaucoma-causing gene22. Over 20 mutations in MYOC have been reported, and the frequency of mutation ranged from 10% to 30%6,23,24 in study cohorts with the familial trait. In the present study, 5.97% (4/67) of JOAG patients carry a heterozygous mutation in MYOC. Three patients have mutant p. (P370L) in the third exon in MYOC. It has been reported that the turnover rate of mutant p. (P370L) in MYOC fusion proteins was much prolonged compared with wild type25. The ubiquitin-proteasome function is compromised and autophagy is induced with mutant p. (P370L) in MYOC25. Further study showed a causal association between this p. (P370L) mutation of MYOC and juvenile glaucoma with goniodysgenesis26. Of the two mutations detected in the present study, p. (P370L) has been previously reported26,27. p. (D384H) is a novel mutation in the third exon of MYOC, near the position of p. (P370L). Further studies are needed to investigate the biological functions of these two mutations.
The reported role of OPTN in JOAG remains inconsistent. Previous studies have identified several mutations in OPTN associated with POAG7, including c.C160G28 and p.(Lys322Glu)29. On the other hand, several studies have shown an absence of OPTN mutations in POAG or JOAG30–32. L494W has been reported in a Chinese amyotrophic lateral sclerosis study but not in any glaucoma study33. The patient who carries p. (L494W) is 33 years old. After a detailed systemic review, we confirmed that she has no manifestation f ALS. The mean age at onset of ALS range from 52.9 to 59.9 years in several reports33,34. Therefore, it is necessary to follow that patient at regular intervals. p. (R329G) is a novel mutation in OPTN in our study. Further studies are needed to understand the role of mutations in OPTN in JOAG.
CYP1B1 is associated with PCG, characterized by an autosomal recessive model. CYP1B1 is also involved in the development of JOAG. CYP1B1 (G61E, R368H, R390H, E229K, and 4340delG) may be associated with severe or moderate angle abnormalities and plays an important role in PCG35. Suri, F., R, et al. first reported that mutations in CYP1B1 were implicated in POAG among Iranians, notably in the juvenile-onset form36. Suri, F., R, et al. further reported that PCG nonpenetrant individuals harbouring CYP1B1 mutations may develop JOAG or POAG to varying degrees37. The c.1169 G > A, p. (Arg390His) mutation of CYP1B1 may be a risk factor for the development of JOAG38. In the current study, a homozygous mutation of R390H in the third exon of CYP1B1 was found in this Chinese JOAG group, consistent with previous reports38. A homozygous mutation of I471S in the third exon of CYP1B1 was identified. The locations of Arg390 and I470 at CYP1B1 are in the K helix and L helix, respectively. Both helixes are conserved regions for CYP1B1 and are expected to be involved in proper folding of the molecule. p. (I471S) was first reported to be associated with PCG in a Chinese PCG study21, indicating that same genotype may have a different degree of phenotypic expression in Chinese PCG and JOAG. López-Garrido et al. have found that the onset of glaucoma in mutant CYP1B1 genotypes may vary even when CYP1B1 activity is completely absent. Residual CYP1B1 activity levels can influence the phenotypic outcome of both homozygous and compound heterozygous carriers, leading to either congenital or non-dominant juvenile glaucoma39. These findings help explain why the two homozygous mutations of CYP1B1 found in our study led to the development of JOAG but not PCG. Further studies are needed to elucidate the role of mutations in CYP1B1 in the development of JOAG in Chinese patients.
No mutations were detected in NTF4 orWDR36. The role of NTF4 in POAG remains controversial. NTF4 is not found to be candidate gene for glaucoma in several studies40,41. However, the results of Chen et al., Pasutto et al. and Vithana et al. supported its implication in POAG42–44. The mutation frequency of NTF4 is known to be low in the Chinese population42,44. Thus, it is not surprising that no mutation was detected in our small cohort. Mutations of WDR36 have been detected in different JOAG populations, including German and Chinese45,46. The frequency of mutation of WDR36 is unclear in the Chinese population, and further studies are needed to estimate the WDR36 mutation frequency in this population.
This study is limited by the lack of parents’ samples so that we are not able to perform segregation analysis. The mutations carriers’ parents were reported to be free of glaucoma. Therefore, whether the mutations detected are de novo mutations cannot be confirmed as for now. Also, there could be other factors that might have affected the penetrance of the mutations so that the patients, even carried the mutations, might not have developed the disease. JOAG may belongs to heterogeneity with different variants in numerous genes, which makes it some familial but most occurring sporadically.
In summary, six variants of MYOC, OPTN and CYP1B1 were found in 11.94% of this Chinese JOAG cohort, including two heterozygous mutations in MYOC in four patients, two heterozygous mutations in OPTN in two patients, and two homozygous mutations in CYP1B1 in two patients. No mutation of NTF4 or WDR36 was detected in this cohort. Although the sample size of this study is small and we have not conducted segregation analysis or functional analyses of the novel mutations, our results provide additional evidence of the mutation spectra and frequencies in Chinese JOAG.
Materials and Methods
Patient recruitment
We recruited unrelated Chinese JOAG patients at the Shantou University/Chinese University of Hong Kong Joint Shantou International Eye Center, Shantou, Guangdong Province, China. Written informed consent was obtained from the participants or their guardians. The study was conducted following the tenets of the Declaration of Helsinki and was approved by the institutional review board and ethics committee of Joint Shantou International Eye Center.
The inclusion criteria for patients with JOAG are based on an age of onset between 3 years of age and early adulthood and the manifestation of highly elevated intraocular pressures without angle abnormalities3. Briefly, the recruited patients have an onset of open-angle glaucoma between 3 to 40 years old, an intraocular pressure elevated greater than 22 mmHg, characteristic optic disc damage and/or visual field damage, and open angles under gonioscopy. Patients recruited in this study are sporadic JOAG. Blood samples from patients’ parents or siblings were not available. The control subjects were recruited from patients with mild cataracts and age 60 years or above who attended an ophthalmic check-up. The control subjects did not have ophthalmic or systemic diseases.
Mutational screening
Total genomic DNA was extracted from peripheral blood using a DNA Extraction Kit (QIAGEN, QIAamp® DNA Blood Mini Kit) according to the manufacturer’s instructions. DNA was quantified with Nanodrop 1000 (ND-1000 3.1.0, NanoDrop Spectrophotometer).
WES was performed with an Agilent Sure Select All Human Exon v5.0 kit (Santa Clara, US.). DNA fragments were sequenced using an Illumina HisSeq. 4000 system (Illumina, San Diego, CA). The average sequencing depth was 100-fold. The results were mapped against UCSC hg19 by Burrows-Wheeler Aligner.
Exome sequencing results were filtered with the following steps: (1) Noncoding variants without altering splicing sites predicted by the Berkeley Drosophila Genome Project (available in the public domain at http://www.fruitfly.org/seq_tools/splice.html) were excluded; (2) The synonymous variants without altering splicing sites were removed; (3) SNPs with minor allele frequency (MAF) greater than or equal to 1% in the 1000Genome database were excluded; (4) Missense variants predicted to be benign on protein function consistently by Polyphen-2 (http://genetics.bwh.harvard.edu/pph/), SIFT (availablehttp://sift.jcvi.org)and Mutation Taster (http://www.mutationtaster.org/) were removed. Detected variants affecting coding residues in MYOC, OPTN, NTF4, WDR36 and CYP1B1 were selected for further validation and analysis.
Sanger sequencing was used to confirm the candidate variants after filtering. Primers were designed using the Primer3 online tool (Table 3). The methods used to perform Sanger sequencing, including amplification, sequencing, and analysis of the target fragments, have been previously described47. Sequence alignment and analysis of variations were performed by using the NovoSNP program48.
Table 3.
Primers used to amplify the sequences harbouring the variants in this study.
| Primer Name | Forward | Reverse |
|---|---|---|
| CYP1B1_3 | AGTCATGCAAGGCCTATTACAG | CCACTACTCATGAAGAACCGC |
| MYOC_3 | ATTTGTCTCCAGGGCTGTCA | GGTGCCACAGATGATGAAGG |
| OPTN_8 | GGATTGATTCACCAGCCAGTC | AAGTTCTCCAGTCCCCAACC |
| OPTN_13 | CAGCTTGTATCTGCTATCGGA | AGCTCCCACAAGTCTCTGTC |
Note: PCR conditions: 35 cycles of amplification. Each cycle consists of 30 s denaturation at 94 °C, 60 s annealing ranging from 59.5 °C to 60.9 °C and 1 min extension at 72 °C, with a final extension at 72 °C for 5 min.
Bioinformatics analysis
Clustal Omega (http://www.ebi.ac.uk/Tools/msa/clustalo/) was used to acquire multiple-sequence alignment of MYOC and OPTN in different species, including Homo sapiens, Pan troglodytes, Macaca mulatta, Bos taurus, Felis catus, Mus musculus, Gallus gallus and Danio rerio. Crystal structures of mutant and wild-type proteins were evaluated by Phyre248 (http://www.sbg.bio.ic.ac.uk/phyre2/html/page.cgi? id = index)49 and further visualized using Pymol Molecular Graphics System (Pymol).
Acknowledgements
The authors thank all of the patients and controls for their participation in this study. This study was supported by the National Natural Science Foundation of China (81470636).
Author Contributions
Conceived and designed the experiments: M.Z., C.P. Performed the experiments: C.H., Y.C., Y.Z. Analysed the data: C.H., Y.C. Contributed reagents/materials/analysis tools: L.X., Z.W., Y.Z. Wrote the paper: C.H., C.P.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Quigley HA. Glaucoma. Lancet. 2011;377:1367–1377. doi: 10.1016/S0140-6736(10)61423-7. [DOI] [PubMed] [Google Scholar]
- 2.Turalba AV, Chen TC. Clinical and genetic characteristics of primary juvenile-onset open-angle glaucoma (JOAG) Seminars in ophthalmology. 2008;23:19–25. doi: 10.1080/08820530701745199. [DOI] [PubMed] [Google Scholar]
- 3.Goldwyn R, Waltman SR, Becker B. Primary open-angle glaucoma in adolescents and young adults. Arch Ophthalmol. 1970;84:579–582. doi: 10.1001/archopht.1970.00990040581004. [DOI] [PubMed] [Google Scholar]
- 4.Foster PJ, Buhrmann R, Quigley HA, Johnson GJ. The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol. 2002;86:238–242. doi: 10.1136/bjo.86.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abu-Amero K, Kondkar AA, Chalam KV. An Updated Review on the Genetics of Primary Open Angle Glaucoma. Int J Mol Sci. 2015;16:28886–28911. doi: 10.3390/ijms161226135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stoilova D, et al. Novel TIGR/MYOC mutations in families with juvenile onset primary open angle glaucoma. J Med Genet. 1998;35:989–992. doi: 10.1136/jmg.35.12.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forsman E, et al. The role of TIGR and OPTN in Finnish glaucoma families: a clinical and molecular genetic study. Mol Vis. 2003;9:217–222. [PubMed] [Google Scholar]
- 8.Hauser MA, et al. Distribution of WDR36 DNA sequence variants in patients with primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2006;47:2542–2546. doi: 10.1167/iovs.05-1476. [DOI] [PubMed] [Google Scholar]
- 9.Pasutto F, et al. Heterozygous NTF4 mutations impairing neurotrophin-4 signaling in patients with primary open-angle glaucoma. Am J Hum Genet. 2009;85:447–456. doi: 10.1016/j.ajhg.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Acharya M, et al. Primary role of CYP1B1 in Indian juvenile-onset POAG patients. Mol Vis. 2006;12:399–404. [PubMed] [Google Scholar]
- 11.Wiggs JL. Genetic etiologies of glaucoma. Arch Ophthalmol. 2007;125:30–37. doi: 10.1001/archopht.125.1.30. [DOI] [PubMed] [Google Scholar]
- 12.Souzeau E, et al. A novel de novo Myocilin variant in a patient with sporadic juvenile open angle glaucoma. BMC Med Genet. 2016;17:30. doi: 10.1186/s12881-016-0291-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldstein O, et al. OPTN 691_692insAG is a founder mutation causing recessive ALS and increased risk in heterozygotes. Neurology. 2016;86:446–453. doi: 10.1212/WNL.0000000000002334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiao Z, et al. A novel optineurin genetic mutation associated with open-angle glaucoma in a Chinese family. Mol Vis. 2009;15:1649–1654. [PMC free article] [PubMed] [Google Scholar]
- 15.Souzeau E, et al. Occurrence of CYP1B1 Mutations in Juvenile Open-Angle Glaucoma With Advanced Visual Field Loss. JAMA Ophthalmol. 2015;133:826–833. doi: 10.1001/jamaophthalmol.2015.0980. [DOI] [PubMed] [Google Scholar]
- 16.Abu-Amero KK, Morales J, Aljasim LA, Edward DP. CYP1B1 Mutations are a Major Contributor to Juvenile-Onset Open Angle Glaucoma in Saudi Arabia. Ophthalmic Genet. 2015;36:184–187. doi: 10.3109/13816810.2013.841961. [DOI] [PubMed] [Google Scholar]
- 17.Fingert JH. Primary open-angle glaucoma genes. Eye (Lond) 2011;25:587–595. doi: 10.1038/eye.2011.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reis, L. M. et al. Whole exome sequencing identifies multiple diagnoses in congenital glaucoma with systemic anomalies. Clin Genet, 10.1111/cge.12816 (2016). [DOI] [PMC free article] [PubMed]
- 19.Micheal S, et al. Identification of Novel Variants in LTBP2 and PXDN Using Whole-Exome Sequencing in Developmental and Congenital Glaucoma. PLoS One. 2016;11:e0159259. doi: 10.1371/journal.pone.0159259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang XB, et al. Mutation Analysis of Seven Known Glaucoma-Associated Genes in Chinese Patients With Glaucoma. Invest Ophth Vis Sci. 2014;55:3594–3602. doi: 10.1167/iovs.14-13927. [DOI] [PubMed] [Google Scholar]
- 21.Yang M, et al. Investigation of CYP1B1 mutations in Chinese patients with primary congenital glaucoma. Mol Vis. 2009;15:432–437. [PMC free article] [PubMed] [Google Scholar]
- 22.Kubota R, et al. Genomic organization of the human myocilin gene (MYOC) responsible for primary open angle glaucoma (GLC1A) Biochem Biophys Res Commun. 1998;242:396–400. doi: 10.1006/bbrc.1997.7972. [DOI] [PubMed] [Google Scholar]
- 23.Taniguchi F, Suzuki Y, Shirato S, Ohta S. Clinical phenotype of a Japanese family with primary open angle glaucoma caused by a Pro370Leu mutation in the MYOC/TIGR gene. Jpn J Ophthalmol. 1999;43:80–84. doi: 10.1016/S0021-5155(98)00074-4. [DOI] [PubMed] [Google Scholar]
- 24.Yoon SJ, Kim HS, Moon JI, Lim JM, Joo CK. Mutations of the TIGR/MYOC gene in primary open-angle glaucoma in Korea. Am J Hum Genet. 1999;64:1775–1778. doi: 10.1086/302407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qiu Y, Shen X, Shyam R, Yue BY, Ying H. Cellular processing of myocilin. PLoS One. 2014;9:e92845. doi: 10.1371/journal.pone.0092845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen X, et al. Sequence analysis of MYOC and CYP1B1 in a Chinese pedigree of juvenile glaucoma with goniodysgenesis. Mol Vis. 2009;15:1530–1536. [PMC free article] [PubMed] [Google Scholar]
- 27.Campos-Mollo E, et al. MYOC gene mutations in Spanish patients with autosomal dominant primary open-angle glaucoma: a founder effect in southeast Spain. Molecular vision. 2007;13:1666–1673. [PubMed] [Google Scholar]
- 28.Huang X, et al. Mutation analysis of seven known glaucoma-associated genes in Chinese patients with glaucoma. Invest Ophthalmol Vis Sci. 2014;55:3594–3602. doi: 10.1167/iovs.14-13927. [DOI] [PubMed] [Google Scholar]
- 29.Yuan HP, et al. Study of novel mutation of OPTN gene in two primary open angle glaucoma families in northeast China. Zhonghua Yan Ke Za Zhi. 2008;44:147–151. [PubMed] [Google Scholar]
- 30.Yen YC, Yang JJ, Chou MC, Li SY. Absence of optineurin (OPTN) gene mutations in Taiwanese patients with juvenile-onset open-angle glaucoma. Mol Vis. 2008;14:487–494. [PMC free article] [PubMed] [Google Scholar]
- 31.Corcia P, et al. Absence of the OPTN mutation in a patient with ALS and familial primary open angle glaucoma. J Neurol Sci. 2011;309:16–17. doi: 10.1016/j.jns.2011.07.044. [DOI] [PubMed] [Google Scholar]
- 32.Lopez-Martinez F, et al. Role of MYOC and OPTN sequence variations in Spanish patients with primary open-angle glaucoma. Mol Vis. 2007;13:862–872. [PMC free article] [PubMed] [Google Scholar]
- 33.Soong BW, et al. Extensive molecular genetic survey of Taiwanese patients with amyotrophic lateral sclerosis. Neurobiol Aging. 2014;35(2423):e2421–2426. doi: 10.1016/j.neurobiolaging.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 34.Maruyama H, et al. Mutations of optineurin in amyotrophic lateral sclerosis. Nature. 2010;465:223–U109. doi: 10.1038/nature08971. [DOI] [PubMed] [Google Scholar]
- 35.Hollander DA, et al. Genotype and phenotype correlations in congenital glaucoma: CYP1B1 mutations, goniodysgenesis, and clinical characteristics. Am J Ophthalmol. 2006;142:993–1004. doi: 10.1016/j.ajo.2006.07.054. [DOI] [PubMed] [Google Scholar]
- 36.Suri F, et al. Screening of common CYP1B1 mutations in Iranian POAG patients using a microarray-based PrASE protocol. Molecular vision. 2008;14:2349–2356. [PMC free article] [PubMed] [Google Scholar]
- 37.Suri F, et al. Variable expressivity and high penetrance of CYP1B1 mutations associated with primary congenital glaucoma. Ophthalmology. 2009;116:2101–2109. doi: 10.1016/j.ophtha.2009.04.045. [DOI] [PubMed] [Google Scholar]
- 38.Su CC, Liu YF, Li SY, Yang JJ, Yen YC. Mutations in the CYP1B1 gene may contribute to juvenile-onset open-angle glaucoma. Eye (London, England) 2012;26:1369–1377. doi: 10.1038/eye.2012.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lopez-Garrido MP, et al. Null CYP1B1 genotypes in primary congenital and nondominant juvenile glaucoma. Ophthalmology. 2013;120:716–723. doi: 10.1016/j.ophtha.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 40.Liu YT, et al. No Evidence of Association of Heterozygous NTF4 Mutations in Patients with Primary Open-Angle Glaucoma. Am J Hum Genet. 2010;86:498–499. doi: 10.1016/j.ajhg.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rao KN, et al. Variations in NTF4, VAV2, and VAV3 Genes Are Not Involved with Primary Open-Angle and Primary Angle-Closure Glaucomas in an Indian Population. Invest. Ophthalmol. Vis. Sci. 2010;51:5. doi: 10.1167/iovs.10-5553. [DOI] [PubMed] [Google Scholar]
- 42.Chen LJ, et al. Evaluation of NTF4 as a causative gene for primary open-angle glaucoma. Molecular vision. 2012;18:1763–1772. [PMC free article] [PubMed] [Google Scholar]
- 43.Pasutto F, et al. Heterozygous NTF4 Mutations Impairing Neurotrophin-4 Signaling in Patients with Primary Open-Angle Glaucoma. Am J Hum Genet. 2009;85:447–456. doi: 10.1016/j.ajhg.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vithana EN, et al. Identification of a novel mutation in the NTF4 gene that causes primary open-angle glaucoma in a Chinese population. Molecular vision. 2010;16:1640–1645. [PMC free article] [PubMed] [Google Scholar]
- 45.Pasutto F, et al. Profiling of WDR36 missense variants in German patients with glaucoma. Invest Ophthalmol Vis Sci. 2008;49:270–274. doi: 10.1167/iovs.07-0500. [DOI] [PubMed] [Google Scholar]
- 46.Fan BJ, et al. Different WDR36 mutation pattern in Chinese patients with primary open-angle glaucoma. Mol Vis. 2009;15:646–653. [PMC free article] [PubMed] [Google Scholar]
- 47.Chen JH, Qiu J, Chen H, Pang CP, Zhang M. Rapid and cost-effective molecular diagnosis using exome sequencing of one proband with autosomal dominant congenital cataract. Eye. 2014;28:1511–1516. doi: 10.1038/eye.2014.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weckx S, et al. novoSNP, a novel computational tool for sequence variation discovery. Genome Res. 2005;15:436–442. doi: 10.1101/gr.2754005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJE. The Phyre2 web portal for protein modeling, prediction and analysis. Nat Protoc. 2015;10:845–858. doi: 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]