Abstract
Owing to inevitable thermal/moisture instability for organic–inorganic hybrid perovskites, pure inorganic perovskite cesium lead halides with both inherent stability and prominent photovoltaic performance have become research hotspots as a promising candidate for commercial perovskite solar cells. However, it is still a serious challenge to synthesize desired cubic cesium lead iodides (CsPbI3) with superior photovoltaic performance for its thermodynamically metastable characteristics. Herein, polymer poly-vinylpyrrolidone (PVP)-induced surface passivation engineering is reported to synthesize extra-long-term stable cubic CsPbI3. It is revealed that acylamino groups of PVP induce electron cloud density enhancement on the surface of CsPbI3, thus lowering surface energy, conducive to stabilize cubic CsPbI3 even in micrometer scale. The cubic-CsPbI3 PSCs exhibit extra-long carrier diffusion length (over 1.5 μm), highest power conversion efficiency of 10.74% and excellent thermal/moisture stability. This result provides important progress towards understanding of phase stability in realization of large-scale preparations of efficient and stable inorganic PSCs.
Inorganic cesium lead iodide perovskite is inherently more stable than the hybrid perovskites but it undergoes phase transition that degrades the solar cell performance. Here Li et al. stabilize it with poly-vinylpyrrolidone and obtain high efficiency of 10.74% with excellent thermal and moisture stability.
Introduction
Due to suitable direct bandgap, high absorption coefficient, and extra-long carrier diffusion length, excellent optoelectronic property, simple and reproducible solution/vapor-chemistry processing1–3, organic–inorganic hybrid halide perovskite materials (ABX3, A=CH3NH3, B=Pb, X=Br, I) have been deemed as a promising candidate for light harvester for next-generation high-performance solar cells4–8. Despite great progress in photovoltaic performance in the last few years, commercial application of perovskite solar cell (PSC) still suffers from moisture and thermal instability owing to the degradation and volatilization of organic component, which presents the uppermost obstacle in further development and mass production9. For this reason, all inorganic halide perovskite formed by substituting the organic cation with cesium (Cs) is an optimal alternative for its native inorganic structure stability, and has demonstrated equally efficient and more stable compared to organic–inorganic halide perovskites10–13.
Of the various inorganic lead halide perovskites, especially, cesium lead iodide (CsPbI3) in cubic phase (α phase) with a bandgap of around 1.73 eV and a visible-light-absorption spectrum up to 700 nm is the mostly desired light harvester in solar cells14–16. However, cubic CsPbI3 can only keep stable at high temperature of above 300 °C14. As temperature decreasing to ambient temperature, CsPbI3 suffers from thermodynamically phase transition to undesired orthorhombic phase (δ phase) with a wide bandgap of 2.82 eV (Supplementary Figure 1), exhibiting an extremely poor photovoltaic conversion efficiency (PCE) of only 0.09% in PSC17. To overcome this obstacle, composition engineering which pursues a certain amount of bromide (Br) to substitute iodide (I) can be one of efficient methods by balancing the tolerance coefficient between PbX6 octahedron and Cs ions18–20. For example, Sutton et al.18 developed a full set of cesium lead halide films from CsPbBr3 to CsPbI3, demonstrating a stabilized power output of 5.6% and J–V efficiency reaching 9.8% for PSC based on cubic CsPbI2Br, although CsPbI2Br still reverts to δ phase over prolonged exposure in air. Increasing continuously bromide proportion induces more prominent phase stability/moisture-stability, dispiritingly, which brings Br-widened bandgap near or above 2.0 eV compared with the ideal solar spectrum (from 1.1 eV to 1.7 eV)21. Another effectual method to stabilize cubic phase CsPbI3 is synthesizing colloidal quantum dots (CQDs) with well-controlled size via hot injection process, and best-performance CsPbI3 solar cells are achieved by assembling cubic phase CsPbI3 CQDs as photoactive layer22–25. Unfortunately, the undesired α-to-δ phase transition of Cs-based inorganic perovskite has not been inhibited in the solution-chemistry processed film. It is of great and fundamental challenge to develop effective and facile route to synthesize cubic Cs-based inorganic perovskite film for high-performance PSC for potential large-scale industrial application.
Herein, poly-vinylpyrrolidone (PVP)-induced surface passivation strategy is reported to stabilize inorganic perovskite CsPbI3 with cubic crystal structure via a reproducible solution-chemistry reaction process. The surface chemical state of cubic CsPbI3 crystals synthesized in the presence of PVP is investigated via Fourier transformed infrared (FTIR) and nuclear magnetic resonance (NMR) techniques, demonstrating that decreased surface tension can be conducive to stabilize cubic CsPbI3 even in large scale of film with micrometer scale, due to enhanced electron cloud density on the surface of CsPbI3 originated from chemical bonding between acylamino group in PVP and CsPbI3. The obtained cubic CsPbI3 exhibits extra-long carrier lifetime of 338.7 ns and diffusion length of greater than 1.5 μm, up to an order of magnitude compared to the active layer depth. The fabricated PSCs based cubic CsPbI3 achieves the highest power conversion efficiency of 10.74% and excellent thermal/moisture stability.
Results
PVP-induced cubic phase stability studies
The specific cubic-phase CsPbI3 films were prepared via a simple and reproducible one-pot solution spin-coating process using a mixture of CsI, PbI2, and PVP as a precursor. X-ray diffraction (XRD) patterns of the CsPbI3 films coated on the F-doped SnO2 (FTO) substrates present the difference in the presence and absence of PVP. In the one-pot solution process without PVP, the CsPbI3 film exhibits a prompt transition from cubic phase to orthorhombic phase when prolonging anneal time or cooling to room temperature, as shown in the XRD pattern (black line) in Fig. 1a and the photograph in Fig. 1b. After adding PVP and gradually increasing the concentration to 10 wt%, the CsPbI3 can keep stable cubic phase both at high and room temperature, even stable at exceeding 80 days (Fig. 1a, b; Supplementary Fig. 2). Similarly, the prominent phase stability is demonstrated achievable in full series of inorganic perovskite compositions from CsPbI3 to CsPbBr3 shown in Supplementary Figures 3 and 4.
Fig. 1.
Structure and morphology of CsPbI3 films and CsPbI3 perovskite solar cell. a X-ray diffraction (XRD) spectra of CsPbI3 with orthorhombic phase (δ, black line), cubic phase (α, red line) and stable cubic phase aging 80 days (blue line). The reference powder pattern for CsPbI3 (cubic and orthorhombic phase) is from Swarnkar et al.25 b Images of prepared orthorhombic and cubic CsPbI3 films aging for different times. Scale bar, 1 cm. c, d Scanning electron microscope (SEM) images of the overlayers for orthorhombic and cubic CsPbI3 films deposited on the meso-TiO2 annealing for 5 min at 300 °C. e The typical cross-section SEM image of fabricated inorganic perovskite CsPbI3 solar cell
We further fabricate CsPbI3 film on mesoporous TiO2 via one-step solution spin-coating process with different PVP concentrations. As shown in SEM images of Fig. 1c, d, both orthorhombic and cubic CsPbI3 exhibit high-surface coverage. Compared with irregular grain size distribution of orthorhombic ones, the obtained PVP-induced cubic CsPbI3 film presents a dense grained uniform morphology with comparatively small grain size of around 100 nm. The cross-section morphology of the fabricated solar device architecture is shown in Fig. 1e, consisting mainly of two uniform layers containing a 400 nm mesoporous TiO2/CsPbI3 nanocomposite film and a 100 nm pure CsPbI3 perovskite overlayer. It is shown that the inorganic perovskite materials are fully permeated into TiO2 mesoporous layer, forming a very uniform overlayer with 100% coverage. Intriguingly, tuning anneal time range, the spin-coating obtained CsPbI3 exhibits crystalline size of over 1 μm and high-crystalline with cubic phase structure (Supplementary Figs. 5, 6 and 7), which is different from the previous reports involving of phase transition of perovskite materials in large grain size22.
In order to gain insight into the PVP stabilization mechanism on cubic CsPbI3, we examine the infrared transmittance spectra of CsPbI3 films (Fig. 2a) for pure PVP, CsPbI3 film synthesized in the presence of PVP, and the CsPbI3 film after removing PVP washed with isopropanol (IPA). The IR spectrum of pure PVP shows absorption bands in the region of 1668, 1421, and 1297 cm−1, which are attributed to typically functional groups of C=O, C–H, and C–N stretching vibration in acylamino of PVP, respectively26,27. For the IR spectrum of the CsPbI3 film synthesized in the presence of PVP, these characteristic vibrations are still persisted, but only blue-shifting to 1652 cm−1 for the C=O stretching, and 1282 cm−1 for the C–N stretching, respectively, indicating an interaction effect between functional groups of PVP and precursor ions of CsPbI3. For the CsPbI3 film washed with IPA, characteristic bands for C=O, C–N, and C–H stretching decreases considerably in terms of intensity, while it remains at the same location. A similar binding energy variation of CsPbI3 surface elements can be found in X-ray photoelectron spectroscopy (XPS) measurements in Supplementary Figure 9. The variation tendency demonstrates that PVP is not only absorbed on the surface of CsPbI3 physically, but also functions chemically in formation and stabilization of cubic CsPbI3 through N–C=O bond of acylamino group27.
Fig. 2.
Fourier transform infrared and nuclear magnetic resonance spectra of CsPbI3. a Fourier transform infrared (FTIR) spectroscopy of pure PVP, cubic phase CsPbI3 films synthesized in the presence of PVP, and cubic CsPbI3 films after IPA treatment. b, c 1H and 13C liquid-state nuclear magnetic resonance (NMR) spectra of PVP solution and CsPbI3 perovskite solution in the presence of PVP dissolved with DMSO-d6
On the basis of the above IR information, it is indicated that acylamino group of PVP plays a dominant role on the nucleation and growth of cubic CsPbI3 perovskite film. For further understanding this specific effect of acylamino group of PVP, the liquid-state 1H/13C NMR measurement is conducted on pure PVP samples and CsPbI3 perovskite synthesized in the presence of PVP in deuterated DMSO-d6 solution, as shown in Fig. 2b, c. In 1H NMR spectra (Fig. 2b) of neat PVP sample, resonance signals attributed to the acylamino group appear at δ = 2.5 and 3.35 ppm, which are characteristic of CH2 attached to C=O group and N atom, respectively28. The interaction of unique groups in PVP with precursor ions of CsPbI3 induces a downfield chemical shift of ∆δ ≈ 0.5 ppm for CH2 adjoining with acylamino group. Reversely, almost no variation for the resonances of hydrogen in backbone chain appears. This can be rationally explained in terms of strengthening effects of resonance for organic constituents through the interaction between cesium cations of perovskite and atoms in organic molecules of PVP, reflecting on the shift of chemical resonances29. Moreover, the result indicates that the N and O atoms in acylamino group are jointly responsible for the chemical shift and can be as two possible centers for coordination with cesium ions. Furthermore, 13C NMR spectroscopy in Fig. 2c show that resonance signal of δ = 175 ppm arising from C=O group undergoes a significant downfield shift of Δδ ≈ 2 ppm on interaction of PVP with CsPbI3, which is indicative of the coordination-bonding interaction between oxygen atoms of acylamino group and cesium ions in perovskite30. In contrast, the resonances at δ = 41 and 43 ppm for C(1)H2 and C(α)H attached to nitrogen atoms exhibit slight chemical shift. Such variation of PVP molecule structure further confirms that there exist two potential centers, i.e. the nitrogen and the carbonyl oxygen, interacting with Cs+ ions of perovskite exposed31. Moreover, the oxygen in acylamino group occupies a dominate position in the formation of C=O···Cs bonds, since nitrogen in planar conformation of internal amide can only have relatively weak influence.
A conceivable PVP-induced surface tension-driven mechanism for the formation of stable cubic phase CsPbI3 is proposed based on the above experiment facts. It is known that the acylamino group in N-vinylpyrrolidone molecule of PVP has donated lone pairs related to oxygen and nitrogen atoms, which offer a large number of coordination centers. As shown in Fig. 3a, the coordination modus indicates the polymer molecules coordinate onto the surface of CsPbI3 through the oxygen atoms, to a lesser extent, via the nitrogen of N–C=O groups, resulting in a weakening of the C=O bonding and an increasing of N–C bond. At the initial stage (Fig. 3b), PVP molecules initiate to attract cations of CsPbI3 precursors due to long backbone chain and electronegative acylamino group structure. The positive and negative ions of CsPbI3 tend to assemble and bond to form cubic CsPbI3 a metastable state around the N–C=O coordination centers of PVP. With time increasing, more nuclei of CsPbI3 are promptly launched with PVP attached. The PVP molecules, in the meantime, stabilize the CsPbI3 nanocrystals from aggregation owing to the intermolecular rejection effect, as shown in Fig. 3c. For the grown CsPbI3 nanocrystals, long-chain PVP molecules with a large number of acylamino groups anchored at the surface of CsPbI3 provide more coordination polymer units for interactions between oxygen, nitrogen in acylamino and cesium ions of inorganic perovskite. With the growth of CsPbI3 stabilized with PVP, the interactions between N–C=O of acylamino and Cs+ of inorganic perovskite exposed at the surface are enhanced, contributing increasing negative field in Cs+-PVP complex on the surface of CsPbI3 (Fig. 3d), which results in the enhancement of the electron cloud for Cs+ of CsPbI326,32. According to study by Grätzel’s group that the surface free energy is a function of the surface tension33. While the surface tension is related to charge density34. An increase in charge density decreases the surface tension. Therefore, in the CsPbI3-PVP complex, the increase in the electron cloud density may result in low surface tension, thus greatly reduces the surface energy of CsPbI3. As a result, the cubic CsPbI3 can be maintained at ambient temperature. Furthermore, cubic structure of CsPbI3 can even be still maintained after 80 days for the PVP chemically functionalized CsPbI3. Owing to the increase of surface charge originated from the interaction between PVP and CsPbI3, the surface tension of CsPbI3 grains reduced significantly, which plays an essential role in the stabilization of cubic phase CsPbI3.
Fig. 3.

Mechanism of PVP-induced cubic phase stability. a Schematic diagram of the chemical bonding between CsPbI3 and PVP molecules. PVP molecule contains of long-chain alkyls and acylaminos. The unbounded lone pairs for N/O atoms in acylaminos offer excess electrons and interact with Cs ions in CsPbI3. Mechanism and scheme for the formation of cubic phase CsPbI3 with the assistant of PVP in three stages. b PbI2 and Cs ions in DMF/DMSO solvent assemble and interact with PVP molecules spontaneously, and maintain a metastable state. c CsPbI3 nanocrystals formed attached on PVP molecules, and remain relatively independent and stable under the effect of PVP molecules. d PVP anchored at the surface of CsPbI3 crystals via the combination between N/O and Cs. The negative state in CsPbI3 crystals surface reduces surface tension significantly and stabilizes cubic phase
Optical and photovoltaic performance
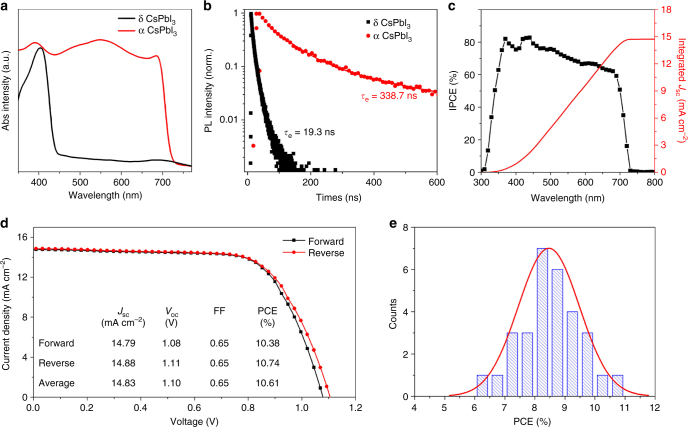
Weighing the phase stability and power conversion efficiency (Supplementary Figs. 8, 14, 15 and 16), the optimal synthetic condition (10 wt% of PVP, 5 min annealing, and 30 min IPA treatment) was selected and applied for the following optical, electrical and photovoltaic investigation. Figure 4a presents the ultraviolet–visible absorption spectra of the obtained cubic and orthorhombic phase CsPbI3 films. The orthorhombic CsPbI3 exhibits limited visible-light-absorption range less than 450 nm, demonstrating that it is unsatisfactory as an optical active material for solar devices. Oppositely, the cubic CsPbI3 shows a desired absorption width to 700 nm, nearly covering full visible-light region. Furthermore, we investigate the effect of anneal time (crystalline grains) on the optical properties and carry out the PL measurement of cubic CsPbI3 perovskite films. The result shows that, tuning size of cubic CsPbI3 grains, the emission peaks red-shift gradually until to a constant value of around 710 nm (Supplementary Fig. 10).
Fig. 4.
Optical and photovoltaic performance of cubic CsPbI3. a The ultraviolet–visible (UV) absorption spectra of orthorhombic and cubic CsPbI3 films. b Time-resolved photoluminescence (TRPL) spectra of orthorhombic and cubic CsPbI3 films deposited on glass substrates. The excitation wavelength was fixed at 300 nm, the emission wavelengths were set at 412 and 704 nm for orthorhombic and cubic, respectively. c The incident photon-to-current efficiency (IPCE) spectrum and corresponding integrated Jsc for the best-performance cubic CsPbI3 solar cell. d The J–V curves for the best cubic CsPbI3 cell measured by forward and reverse scans. The average photovoltaic performance values form the two J–V curves are summarized (inset). e Histogram of average efficiencies for 30 devices of cubic CsPbI3
The time-resolved photoluminescence (TRPL) measurement (Fig. 4b; Supplementary Figure 18) is conducted to investigate the carrier lifetime of cubic and orthorhombic CsPbI3 films. To eliminate the effect of quenching treatment, the CsPbI3 films are deposited on glass slides under the same solution-method and the same thickness. The corresponding steady-state PL spectra of cubic and orthorhombic CsPbI3 films are shown in Supplementary Figure 11. The PL decay for neat orthorhombic CsPbI3 film exhibits a time-constant of τe = 19.3 ns. In contrast, cubic CsPbI3 film shows an extra-long carrier lifetime of τe = 338.7 ns. To simulate the carrier diffusion length in perovskite films, only electron/hole extraction layers and inorganic perovskite layer (i.e., TiO2/CsPbI3 and CsPbI3/spiro-OMeTAD) are fabricated via same solution-chemistry processing and the same thickness with the fabricated cell, the PL decay curves with electron/hole extraction layers are shown in Supplementary Figures 12 and 13, the PL decay dynamics are modeled via accounting the excitations number and distributions based on the one-dimensional diffusion equation1.
| 1 |
in which n(x,t) is the number of excitations within a certain thickness of perovskite film, k(t) is the PL decay rate without quenching layer, and D is the diffusion coefficient. Table 1 shows the carrier diffusion length for both orthorhombic and cubic CsPbI3 films, which depends on electron or hole quenching layer used, and it is assumed that all photogenerated carriers reach the quenching interface. It is clear the diffusion length of both electron and hole in orthorhombic CsPbI3 film is around 120 nm. However, for cubic CsPbI3 film, the carriers exhibit a diffusion length for electrons over 1 μm, and even over 1.5 μm. As reported (Supplementary Table 1), the average carrier diffusion length in organic–inorganic hybrid perovskites MAPbI3 and FAPbI3 is 129 and 813 nm, respectively1,8. In addition, in pure/mixed Br based inorganic perovskite, the carrier diffusion length is less than 200 nm12. The ultra-long carrier diffusion length not only originates from the excellent carrier transport capability of cubic CsPbI3, but also from the inhibition of defect recombination via the surface passivation effect, which provides the feasibility in planar-structure PSCs or even thicker light-absorption layers.
Table 1.
The carrier diffusion constant (D) and diffusion length (LD) simulated form PL decays using the diffusion model
| Phase | Species | D (cm2 s−1) | LD (nm) |
|---|---|---|---|
| Cubic | Electrons | 0.061 ± 0.016 | 1566 ± 254 |
| Holes | 0.057 ± 0.013 | 1427 ± 238 | |
| Orthorhombic | Electrons | 0.014 ± 0.009 | 121 ± 51 |
| Holes | 0.011 ± 0.007 | 117 ± 35 |
The errors arise predominantly from perovskite film thickness variations, which is ±50 nm for both orthorhombic and cubic CsPbI3 films
On the basis of optical and carrier transport properties, we conduct the photovoltaic measurements of the cubic CsPbI3 PSCs fabricated with mesoporous TiO2 scaffold. Of the solar devices acquired, Fig. 4d depicts the current–voltage (J–V) curves measured via forward and reverse bias sweep for the best-performance PSCs. The corresponding photovoltaic parameters under the optimized conditions with an active area of 0.09 cm2, including of short-circuit current density (Jsc), open-circuit voltage (Voc), fill factor (FF), and PCE values are summarized in the insert of Fig. 4d. The Jsc, Voc, and FF for forward sweep of the device are 14.79 mA cm−2, 1.08 V, and 65%, respectively, corresponding to a PCE of 10.38% under standard AM 1.5 G condition. With faint hysteresis, the solar device for reverse sweep exhibits a Jsc of 14.88 mA cm−2, a Voc of 1.11 V and a PCE of 10.74%, which are higher than those of previous reports on CsPbBr3 and CsPbBr3−xIx (Supplementary Table 2)18,19,35. Moreover, the stability of Jsc and PCE for both devices is shown in Supplementary Figure 19. The cubic CsPbI3 device shows a stable output with a Jsc of 13.1 mA cm−2 and a PCE of 10.0%. The corresponding incident photo-to-current efficiency (IPCE) spectrum in Fig. 4c for the best cell exhibits a broad plateau of over 60% between 350 and 700 nm. The integrated Jsc of 14.7 mA cm−2 is in good agreement with the current density acquired from the current–voltage measurement. Intriguingly, compared with other inorganic PSCs18,19,35, the CsPbI3 based solar cell exhibits a much higher Jsc, which can be attributed to the extended visible-light-absorption range and extra-long carrier diffusion length for CsPbI3, beneficial to more photoelectrons/hole generations and captures by corresponding transport layers. Moreover, the PVP covered on CsPbI3 grains decreases surface defects and suppresses nonradiative recombination, significantly (Supplementary Figure 17). Figure 4e shows a histogram of average PCEs from all of the cubic CsPbI3 PSCs fabricated under the same condition for the repeatability purpose. Over 70% of the devices exhibit over 8% PCE, and the average PCE summarized shows 8.50%, which is better than most current stable and efficient CsPbI2Br perovskite cells (Supplementary Figure 20).
Moisture and thermal stability
The excellent stability of PSCs is an essential factor for the reproducibility and commercial application. To investigate the moisture and thermal stability under different conditions, the performance of inorganic cubic perovskite (CsPbI3) PSCs with average PCE is comparatively measured with that of solar cells based on typical organic–inorganic hybrid perovskite (MAPbI3). The ambient-humidity-stability test was conducted under ambient condition for 500 h without encapsulation (average humidity of 45–55% with temperature of 26 °C fixed). Fig. 5a shows the device moisture-stability as a function of aging time in terms of normalized power conversion efficiency (PCE). During 500 h, the cell of MAPbI3 shows a dramatic drop with 70% efficiency loss with respect to the fresh solar cell. Comparatively, cubic CsPbI3 based device exhibits a better moisture-stability with 75% retention after 500 h. Figure 5b shows the thermal-stability measurement of PSCs with cubic CsPbI3 and MAPbI3, which was conducted at different temperature ranging from 20 to 100 °C. It is clear that, with the increasing of temperature, the inorganic cubic perovskite exhibits more prominent thermal stability, showing over 90% efficiency retention even at 80 °C. It is worth noting that, as increasing the temperature to 100 °C, the devices of both CsPbI3 and MAPbI3 show obvious decay in PCE, which might result from the failure of the organic hole transport material. For further investigating the long-term thermal stability of the inorganic perovskite CsPbI3 solar cell, we measured the device performance as stored at high temperature (60 °C) under normal sunlight exposure, which is shown in Fig. 5c. During 500 h measurement, the cubic CsPbI3 based PSC shows a slight efficiency decay of only around 10%, demonstrating an outstanding superiority in thermal stability compared to MAPbI3 based solar cell (70% efficiency loss). Notably, the thermal-stability efficiency test for inorganic perovskite exhibits a slower decay rate than the humidity stability. The result demonstrates that the CsPbI3 inorganic perovskite possesses more outstanding stability, especially in thermal stability.
Fig. 5.
Moisture and thermal stability investigation of perovskite solar cells based cubic CsPbI3. a Efficiency evolution of the devices exposed in ambient air under relative humidity of 45–55% without any sealing. The measurements were carried every 50 h during 500 h. b Efficiency variation as a function of temperature from 20 to 100 °C. The PCEs were measured under nitrogen atmosphere after an equilibration time of 30 min at each temperature setting. c Efficiency evolution of the cells in a nitrogen atmosphere at 60 °C during 500 h
Discussion
In summary, we developed a surface passivation engineering for preparing long-term stable cubic phase CsPbI3 films via a reproducible solution-chemistry process with the assistant of PVP. We proposed a plausible mechanism for the formation of stable cubic CsPbI3 by investigating the surface chemical states of the perovskite crystals. The decreased surface tension can be obtained to stabilize CsPbI3 grains in cubic phase even in micrometer scale, due to electron cloud density enhancement on the surface of CsPbI3 originated from chemical bonding between acylamino in PVP and CsPbI3. Furthermore, we found the obtained cubic CsPbI3 exhibits prominent photoelectronic properties with extra-long carrier lifetime of 338.7 ns and diffusion length of greater than 1.5 μm, up to an order of magnitude compared to the absorption depth. Based on this strategy, we have achieved the highest PCE of 10.74%, as well as excellent thermal/moisture stability in the fabricated inorganic PSCs. This result provides important progress towards the understanding of phase stability in the realization of large-scale preparations of efficient and stable inorganic PSCs.
Methods
Inorganic perovskite film preparation
A certain amount of PVP (Aladdin, K13-18) was first dissolved in mixed solvent with DMF (Aladdin, 99.9%) and DMSO (Aladdin, anhydrous) (1:1) and stirred for 30 min at room temperature. The CsPbI3 precursor solution (0.8 M) was synthesized by dissolving stoichiometric CsI (Aladdin, 99.9%) and PbI2 (Aladdin, 99.9%) in above solution, then, was stirred at 90 °C for 1 h on hot plate. The perovskite precursor solution was spin-coated on glass substrate or mesoporous TiO2 film at 2500 rpm for 45 s and sintered at 300 °C for 5 min to form CsPbI3 film. The other series of cesium lead halide perovskite films with different iodide/bromide proportions were synthesized by changing the percentage of PbI2 and PbBr2, and keeping other methods fixed.
Device fabrication
A compact TiO2 layer (about 50 nm) was deposited on the FTO substrates (OPV Tech) which were ultrasonically washed and underwent oxygen plasma treatment by spin-coating a mildly acidic solution of titanium isopropoxide in ethanol (2000 rpm, 30 s), and annealed at 500 °C for 30 min. A 400–500 nm thick mesoporous TiO2 film was coated on the compact layer via TiO2 paste (2500 rpm, 45 s), next, heated for 30 min at 500 °C. Then, the CsPbI3 active layer was deposited on mesoporous TiO2 scaffold with 300 °C annealing treatment for 5 min. To increase the surface coverage of the inorganic perovskite, the substrates with TiO2 coated were preheated at 150 °C. After cooling to room temperature, the substrates were immersed into IPA for 30 min to remove redundant PVP. Finally, an around 200 nm hole transport layer of spiro-OMeTAD (OPV Tech, 99.5%) was spin-coated at 2500 rpm for 30 s and a 50 nm gold counter electrode was prepared by thermal evaporation. The optical active layer and hole transport layer were fabricated in glove box.
Characterization
The XRD spectra of inorganic perovskite films were measured by Phillips Rigaku D/Max-kA X-ray diffractometer. The surface-section/cross-section morphologies of the perovskite films were characterized using field-emission scanning electron microscopy (FESEM, SU-70). The high-resolution transition electron microscope (HRTEM, Phillips, Tecnai 20U-Twin) was applied to analyze the structures and morphologies of the perovskite crystalline grains. The ultraviolet–visible absorption spectra of the perovskite films were recorded on the TU-1901 spectro-photometer. The FTIR spectra (NEXUS 670) were used to measure the surface functional group of the films. The liquid-state NMR were conducted by VNMRS 600. The XPS measurement was carried out using the Escalab 250Xi electron spectrometer via monochromatized Al Kαradiation (hν = 1486.7 eV). The TRPL measurement were carried by FLS920 all functional fluorescence spectrometer (Edinburgh) with an excitation wavelength of 400 nm. The photovoltaic performances (J–V curves) were analyzed by a solar simulator (Newport, Class 3 A, 94023 A) set an AM 1.5 G simulated sunlight (100 mW cm−2) equipped with a Keithley 2420, and the solar cells were measured using a metal aperture to define the active area to be around 0.09 cm2. The IPCE was characterized using a power source (Newport 300 W xenon lamp, 66920) equipped with a monochromator (Newport Cornerstone 260) and a multimeter (Keithley 2400) at 100 mW cm−2, AM 1.5 G illumination, and was corrected by a calibrated Si-reference cell (NREL).
Data availability
All data used in this study are available from the corresponding authors upon reasonable request.
Electronic supplementary material
Acknowledgements
We acknowledge support from the project supported by the State Key Program of National Natural Science of China (No.: 51532005), the National Nature Science Foundation of China (No.: 51472148, 51272137), and the Tai Shan Scholar Foundation of Shandong Province.
Author contributions
L.Y. initiated and directed the study. B.L. conceived the original research idea. B.L. and Y.Z. conducted most of the device fabrication and measurements. S.Z. contributed to the deposition of electron extraction layer. T.Y. contributed to the deposition of hole extraction layer. L.F. contributed to the structural characteristics. L.Z. provided the mechanism idea. The manuscript was co-written by L.Y. and B.L. All authors contributed to the discussion and revising of this manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary Information accompanies this paper at 10.1038/s41467-018-03169-0.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Stranks SD, et al. Electron-hole diffusion lengths exceeding 1 micrometer in an organometal trihalide perovskite absorber. Science. 2013;342:341–344. doi: 10.1126/science.1243982. [DOI] [PubMed] [Google Scholar]
- 2.Lin Q, et al. Electro-optics of perovskite solar cells. Nat. Photons. 2015;9:106–112. doi: 10.1038/nphoton.2014.284. [DOI] [Google Scholar]
- 3.Nie. W, et al. High-efficiency solution-processed perovskite solar cells with millimeter-scale grains. Science. 2015;347:522–525. doi: 10.1126/science.aaa0472. [DOI] [PubMed] [Google Scholar]
- 4.Kim HS, et al. Lead iodide perovskite sensitized all-solid-state submicron thin film mesoscopic solar cell with efficiency exceeding 9% Sci. Rep. 2012;2:591. doi: 10.1038/srep00591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee MM, et al. Efficient hybrid solar cells based on meso-superstructured organometal halide perovskites. Science. 2012;338:643–647. doi: 10.1126/science.1228604. [DOI] [PubMed] [Google Scholar]
- 6.Burschka J, et al. Sequential deposition as a route to high-performance perovskite-sensitized solar cells. Nature. 2013;499:316–319. doi: 10.1038/nature12340. [DOI] [PubMed] [Google Scholar]
- 7.Jeon NJ, et al. Solvent engineering for high-performance inorganic–organic hybrid perovskite solar cells. Nat. Mater. 2014;13:897–903. doi: 10.1038/nmat4014. [DOI] [PubMed] [Google Scholar]
- 8.Jeon NJ, et al. Compositional engineering of perovskite materialsfor high-performance solar cells. Nature. 2015;517:476–480. doi: 10.1038/nature14133. [DOI] [PubMed] [Google Scholar]
- 9.Wang Z, et al. Stability of perovskite solar cells: a prospective on the substitution of the A cation and X anion. Angew. Chem. Int. Ed. 2017;56:1190–1212. doi: 10.1002/anie.201603694. [DOI] [PubMed] [Google Scholar]
- 10.Kulbak M, Cahen D, Hodes G. How important is the organic part of lead halide perovskite photovoltaic cells? Efficient CsPbBr3 cells. J. Phys. Chem. Lett. 2015;6:2452–2456. doi: 10.1021/acs.jpclett.5b00968. [DOI] [PubMed] [Google Scholar]
- 11.Liang J, et al. All-inorganic perovskite solar cells. J. Am. Chem. Soc. 2016;138:15829–15832. doi: 10.1021/jacs.6b10227. [DOI] [PubMed] [Google Scholar]
- 12.Li B, et al. PbCl2-tuned inorganic cubic CsPbBr3(Cl) perovskite solar cells with enhanced electron lifetime, diffusion length and photovoltaic performance. J. Power Sources. 2017;360:11–20. doi: 10.1016/j.jpowsour.2017.05.050. [DOI] [Google Scholar]
- 13.Kulbak M, et al. Cesium enhances long-term stability of lead bromide perovskite-based solar cells. J. Phys. Chem. Lett. 2016;7:167–172. doi: 10.1021/acs.jpclett.5b02597. [DOI] [PubMed] [Google Scholar]
- 14.Eperon GE, et al. Inorganic caesium lead iodide perovskite solar cells. J. Mater. Chem. A. 2015;3:19688–19695. doi: 10.1039/C5TA06398A. [DOI] [Google Scholar]
- 15.Zhang T, et al. Bication lead iodide 2D perovskite component to stabilize inorganic α-CsPbI3 perovskite phase for high-efficiency solar cells. Sci. Adv. 2017;3:e1700841. doi: 10.1126/sciadv.1700841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu Y, et al. Bismuth incorporation stabilized α-CsPbI3 for fully inorganic perovskite solar cells. ACS Energy Lett. 2017;2:2219–2227. doi: 10.1021/acsenergylett.7b00508. [DOI] [Google Scholar]
- 17.Choi H, et al. Cesium-doped methylammonium lead iodide perovskite light absorber for hybrid solar cells. Nano Energy. 2014;7:80–85. doi: 10.1016/j.nanoen.2014.04.017. [DOI] [Google Scholar]
- 18.Sutton RJ, et al. Bandgap-tunable cesium lead halide perovskites with high thermal stability for efficient solar cells. Adv. Energy Mater. 2016;6:1502458. doi: 10.1002/aenm.201502458. [DOI] [Google Scholar]
- 19.Ma Q, et al. Hole transport layer free inorganic CsPbIBr2 perovskite solar cell by dual source thermal evaporation. Adv. Energy Mater. 2016;6:1502202. doi: 10.1002/aenm.201502202. [DOI] [Google Scholar]
- 20.Chen CY, et al. All-vacuum-deposited stoichiometrically balanced inorganic cesium lead halide perovskite solar cells with stabilized efficiency exceeding 11% Adv. Mater. 2017;29:1605290. doi: 10.1002/adma.201605290. [DOI] [PubMed] [Google Scholar]
- 21.Beal RE, et al. Cesium lead halide perovskites with improved stability for tandem solar cells. J. Phys. Chem. Lett. 2016;7:746–751. doi: 10.1021/acs.jpclett.6b00002. [DOI] [PubMed] [Google Scholar]
- 22.Li X, et al. CsPbX3 quantum dots for lighting and displays: room-temperature synthesis, photoluminescence superiorities, underlying origins and white light-emitting diodes. Adv. Funct. Mater. 2016;26:2435–2445. doi: 10.1002/adfm.201600109. [DOI] [Google Scholar]
- 23.Protesescu, L. et al. Nanocrystals of cesium lead halide perovskites (CsPbX3, X=Cl, Br, and I): novel optoelectronic materials showing bright emission with wide color gamut. Nano Lett.15, 3692–3696 (2015). [DOI] [PMC free article] [PubMed]
- 24.Li, B. et al. Graded heterojunction engineering for hole-conductor-free perovskite solar cells with high hole extraction efficiency and conductivity. Adv. Mater. 29, 1701221 (2017). [DOI] [PubMed]
- 25.Swarnkar A, et al. Quantum dot–induced phase stabilization of α-CsPbI3 perovskite for high-efficiency photovoltaics. Science. 2016;354:92–95. doi: 10.1126/science.aag2700. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Z, Zhao B, Hu L. PVP protective mechanism of ultrafine silver powder synthesized by chemical reduction processes. J. Solid State Chem. 1996;121:105–110. doi: 10.1006/jssc.1996.0015. [DOI] [Google Scholar]
- 27.Wang H, et al. Mechanisms of PVP in the preparation of silver nanoparticles. Mater. Chem. Phys. 2005;94:449–453. doi: 10.1016/j.matchemphys.2005.05.005. [DOI] [Google Scholar]
- 28.Lau C, Mi Y. A study of blending and complexation of poly(acrylic acid)/poly(vinyl pyrrolidone) Polymer. 2002;43:823–829. doi: 10.1016/S0032-3861(01)00641-3. [DOI] [Google Scholar]
- 29.Li X, et al. Improved performance and stability of perovskite solar cells by crystal crosslinking with alkyphosphonic acid ω–ammonium chlorides. Nat. Chem. 2015;7:703–711. doi: 10.1038/nchem.2324. [DOI] [PubMed] [Google Scholar]
- 30.Sesta B, et al. 1H NMR, surface tension, viscosity, and volume evidence of micelle-polymer hydrophobic interactions: LiPFN-PVP system. J. Phys. Chem. B. 1997;101:198–204. doi: 10.1021/jp961363h. [DOI] [Google Scholar]
- 31.Zhao Y, et al. A polymer scaffold for self-healing perovskite solar cells. Nat. Commun. 2016;7:10028. doi: 10.1038/ncomms10228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yin Q, et al. Micellization and aggregation properties of sodium perfluoropolyether carboxylate in aqueous solution. J. Ind. Eng. Chem. 2016;42:63–68. doi: 10.1016/j.jiec.2016.07.026. [DOI] [Google Scholar]
- 33.Ummadisingu A, et al. The effect of illumination on the formation of metal halide perovskite films. Nature. 2017;545:208–212. doi: 10.1038/nature22072. [DOI] [PubMed] [Google Scholar]
- 34.Kramer D. Dependence of surface stress, surface energy and surface tension on potential and charge. Phys. Chem. Chem. Phys. 2008;10:168–177. doi: 10.1039/B710065E. [DOI] [PubMed] [Google Scholar]
- 35.Nam JK, et al. Potassium incorporation for enhanced performance and stability of fully inorganic cesium lead halide perovskite solar cells. Nano Lett. 2017;17:2028–2033. doi: 10.1021/acs.nanolett.7b00050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used in this study are available from the corresponding authors upon reasonable request.