Abstract
Filamentous fungi are used for the production of a multitude of highly relevant biotechnological products like citric acid and penicillin. In submerged culture, fungi can either grow in dispersed form or as spherical pellets consisting of aggregated hyphal structures. Pellet morphology, process control and productivity are highly interlinked. On the one hand, process control in a bioreactor usually demands for compact and small pellets due to rheological issues. On the other hand, optimal productivity might be associated with less dense and larger morphology. Over the years, several publications have dealt with aforementioned relations within the confines of specific organisms and products. However, contributions which evaluate such interlinkages across several fungal species are scarce. For this purpose, we are looking into methods to manipulate fungal pellet morphology in relation to individual species and products. This review attempts to address (i) how variability of pellet morphology can be assessed and (ii) how morphology is linked to productivity. Firstly, the mechanism of pellet formation is outlined. Subsequently, the description and analysis of morphological variations are discussed to finally establish interlinkages between productivity, performance and morphology across different fungal species.
Keywords: Fungal pellet morphology, Interlinks between productivity and morphology, Variability and alteration of morphology, Analysis of morphology
Introduction
In submerged culture, filamentous fungi either grow in spherical pellets, consisting of compact hyphal aggregation, or in filamentous form, featuring homogeneously dispersed hyphae (Pirt 1966). A pellet forming cultivation system is necessarily heterogenous and aerobic (Wosten et al. 2013; Amanullah et al. 2001). The morphological state of filamentous fungi has a large impact on process performance in a bioreactor. Morphology, physiology and productivity of filamentous fungi are influenced by process parameters on many levels (Ehgartner 2017). These characteristics are highly interlinked with each other and therefore have to be addressed collectively to understand the interdependencies between them. For example, in free mycelia, high biomass concentrations result in highly viscous fermentation media, resulting in issues with gas−liquid mass transfer, liquid mixing and a generally complex rheology in Aspergillus terreus (Porcel et al. 2005). However, pellet morphology also comes with disadvantages: within Penicillium chrysogenum pellets, problems with internal transport of substrates and products may occur, depending on size and compactness of pellets (Dynesen and Nielsen 2003). Therefore, it is highly important to individually assess each production task.
This review strives to provide a general overview across several pellet forming fungal species. In the following, basic forces that trigger pellet formation are briefly summarised. Subsequently, description and analysis of pellet morphology are outlined. This provides the basis for understanding the interlinks between productivity, performance and morphology, as will be discussed in the final chapter of this review.
Mechanism of pellet formation
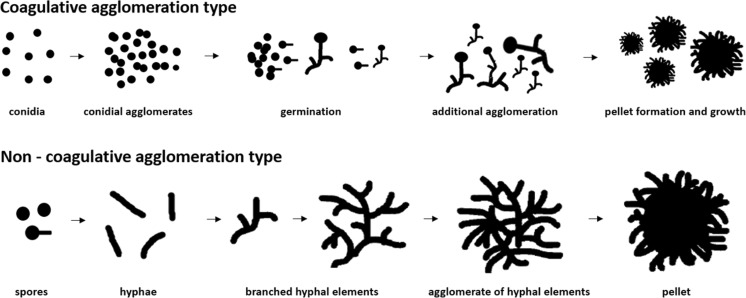
Traditionally, fungal pellets are attributed to either coagulative or non-coagulative types of formation (Nielsen et al. 1995; Pirt 1966). Table 1 provides an overview on pellet classification across several species.
Table 1.
Overview on several fungal species: agglomeration type, variations in pellet morphology and possibilities of morphological alteration; if not mentioned otherwise, preferred morphology refers to cultivation
| Type | Species | Preferred morphology | Alteration of morphology | References |
|---|---|---|---|---|
| Coagulative type |
Aspergillus
A. niger A. nidulans A. oryzae |
For citric acid production: Swollen hyphal branches, compact agglomerates = clumps, pellets featuring thin biomass layers and loose core For production of fructosyl-transferase: Small, spherical |
Strong agitation (filament fragmentation wanted) Aeration using oxygen/air 1:1 mixture, high growth rate low pH (2.0 ± 0.2), Manganese presence Spore inoculum level Pellet dispersion instead of spore inoculum Surfactant: Tween 20 |
Wilkinson 1998 Papagianni 2007 Papagianni and Mattey 2006 Prosser and Tough 1991 Wang et al. 2017 Kurakake et al. 2017 |
| Phanerochaete chrysosporium | Small pellet size (~ 5.5 mm3) for lignin peroxidase production | High shear rate | Zhang and Zhang 2016 Zmak et al. 2006 |
|
| Blakeslea and Choanephora | Compact pellet form | Anionic polymers hinder spore aggregation prior to germination | Prosser and Tough 1991 | |
| C. unicolor | Compact star-shaped pellet form, | Microparticle-enhanced cultivation (Al2O3 particles) | Antecka et al. 2016 | |
| C. fumago | Compact pellet form | Small inoculum volume, carbon source (fructose) | Carmichael and Pickard 1989 | |
| C. sinensis | Small and loose pellets | Surfactant: Tween 80 pH: optimum 6.0 | Liu and Wu 2012 | |
| Non – coagulative type | A. ochraceus | Compact pellet form | Spore inoculum and agitation | Abd-Elsalam 2009 |
| R. oryzae | Loose pellets in lactic acid production | Medium: peptone, dextrose, calcium carbonate | Liao et al. 2007 | |
| Hyphal element agglomerating type | P. chrysogenum | “Fluffy” pellet to ensure largest possible active layer with high quantities of cytosol | Aeration using oxygen/air, controlled growth rate CSL in medium, CO2 concentration Spore inoculum level Physiological process control based on morphological modelling approach |
Wilkinson 1998 Nielsen et al. 1995 Prosser and Tough 1991 Ho and Smith 1986 Posch and Herwig (2014) |
| P. sp. L1 | Large pellet size | Pellet dispersion instead of spore inoculum | Liu et al. 2017 |
Figure 1 depicts the coagulative and non-coagulative model of pellet formation. In the coagulative type, spores aggregate fast and subsequently germinate involving hyphal tip growth (Zhang and Zhang 2016). Finally, a great number of spores of the coagulative type form pellets. On the contrary, spores of the non-coagulative type germinate before pellet formation. Therefore, one pellet theoretically can be formed by one single spore. Non-coagulative pellet formation is interlinked with agitation and aeration (Pazouki and Panda 2000). It should be noted, however, that depending on cultivation factors, fungal species will exhibit different morphological behaviour. Therefore, a final classification of coagulation type is difficult (Zhang and Zhang 2016; Pazouki and Panda 2000). For instance, P. chrysogenum exhibits characteristics of both types, as agglomeration of hyphal elements leads to hyphal clumps which form pellets in the end (Wilkinson 1998; Nielsen et al. 1995). Wilkinson (1998) therefore suggested a new term for species like P. chrysogenum: the hyphal element agglomerating type.
Fig. 1.
Illustration depicting the coagulative and non-coagulative model of pellet formation
Electrostatics, hydrophobicity and interactions between spore wall components are main triggers for pellet formation (Zhang and Zhang 2016). Fungal spores generally exhibit negative surface charges (Douglas et al. 1959) which are affected by pH and ionic strength (Akiba et al. 1994). In a simplified view, higher pH values are considered to cause negative charges which in turn decrease spore aggregation (Zhang and Zhang 2016). However, conflicting observations suggest that electric repulsion is not the only driving factor. For Aspergillus niger, it was proposed that mainly single conidia are affected by surface charge, aggregated conidia might additionally be stabilised through higher electric charges (Grimm et al. 2005b). Wargenau et al. (2011) further found that the electrostatic surface potential of A. niger spores is considerably affected by pH-dependent release of melanin pigment leading to an irreversible reduction of the outermost layer between spores and surrounding solution. In short, they concluded that thickness and accessibility of surface coating, as well as ionic strength of the medium have additional effects on pH-dependent spore repulsion. Furthermore, pH also heavily affects hydrophobicity of proteins (Pascual et al. 2000). Especially hydrophobins strongly influence adhesion forces. For instance, deletion in different hydrophobin encoding genes in A. nidulans mutants leads to a decrease in pellet biomass and size (Dynesen and Nielsen 2003).
Another important contribution to pellet formation are interactions between spore wall components, notably salt bridging between polysaccharides (Zhang and Zhang 2016). Gerin et al. (1993) even stated that aggregation is only dependent on salt bridging between polysaccharides in Phanerochaete chrysosporium. Depending on respective physiological conditions, spores undergo several changes in spore wall components and properties (Zhang and Zhang 2016). In the beginning, water uptake combined with the swelling of spores leads to an increase of metabolic activities that leads to water uptake combined with swelling of spores. This is followed by germination of conidia, which represents the beginning of fungal growth. After germination, hyphal elongation takes place and the fungus can initiate hyphal branching which is critical for the formation of the mycelium (Paul and Thomas 1996). These initial steps in fungal development strongly affect spore aggregation: shortly after incubation, spore aggregation occurs due to electrostatic and hydrophobic interactions. However, as soon as the swelling of spores leads to polysaccharides being exposed, hydrophobic interactions decrease in favour of interactions between cell wall components (Priegnitz et al. 2012; Zhang and Zhang 2016).
Recently, efforts to describe aggregation kinetics via population dynamics modelling were made (Grimm et al. 2004; Lin et al. 2008). Via an in-line particle size analyser, two distinct aggregation steps of coagulating fungi A. niger could be described. In the first step, conidial aggregates are formed from individual conidia. Population dynamics in this first step combine formation and disappearance of particles through aggregation and breakage. The second step considers germination of conidia, thereby hyphal growth greatly increases the surface area necessary for aggregation. This process is closely related to the specific length growth rate and the rate of germination (Grimm et al. 2005b). More recently, Priegnitz et al. (2012) concluded that germination of conidia within the second step is essential for pellet formation in A. niger.
All of these findings signify that specific aggregation triggers vary during the pellet formation process. When summarising influences on electrostatic and hydrophobic interactions, it can be concluded that pH is the driving factor for both. Regarding interactions between spore wall components, no definite conclusions can be drawn from the scientific literature. However, publications suggest that it is mostly driven by the presence of polysaccharides (Zhang and Zhang 2016).
Description and analysis of pellet morphology
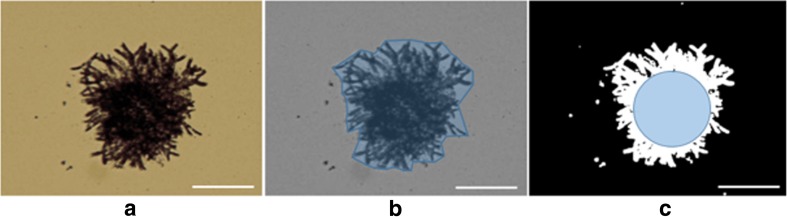
Pellet diameters extend from a few hundred micrometres up to 1 mm. The basic morphology of fungal pellets is characterised by the compact inner core consisting of densely packed hyphae (Cox et al. 1998). The existence of such a core is the distinction between pellets and clumps. The core is surrounded by a less dense hyphal layer, known as the hairy region (Krull et al. 2010). Core region and hairy region can be defined by parameters, mostly derived from microscopic analysis as displayed in Table 2 (Paul and Thomas 1998; Posch et al. 2012; Ehgartner 2017). Figure 2 displays images related to respective parameter estimation.
Table 2.
Parameters for pellet characterisation
| Parameter | Definition | |
|---|---|---|
| Core region | Fullness | Ratio of actual area of the particle to convex area; = 1 for pellets without hairy regions |
| Circularity | Deviation from a true circle, derived from area and perimeter | |
| Core area | Encompasses core equivalent diameter | |
| Hairy region | Roughness | Irregularity of the perimeter of an object, obtained from circularity measurement around an object boundary |
| Equivalent diameter | Diameter of a circle having the same area as the pellet |
Fig. 2.
Light microscopic images of the same P. chrysogenum pellet, white line = 100 μm; parameters for depiction of core region and hairy region. Blue line (b): perimeter for estimation of roughness, blue area (b): convex area for fullness calculation, blue circle (c): core area
The most common method to analyse pellet morphology is microscopy (Cox et al. 1998; Paul and Thomas 1998; Posch et al. 2012). Usually, image recording is combined with some form of automated image analysis software to ensure statistical verification. Thereby most parameters described in Table 2 can be determined. However, sample preparation on microscopy slides is problematic as it affects fungal biomass dimensions due to potential squeezing between cover slide and specimen slide. In this process also, all three dimensional informations are lost.
Confocal laser scanning microscopy (CLSM) in combination with staining is a powerful method for visualisation of metabolically active regions in pellets. Furthermore, biomass segregation, density and growth could by monitored via this technique (Villena et al. 2010). Wargenau and Kwade (2010) were able to directly measure A. niger spore adhesion forces at different pH and ionic strength values by further employing atomic force microscopy (AFM). Information on hydrophobic and hydrophilic domains can be obtained via adhesion force mapping across spore surfaces. This technique also provides information on the role of hydrophobins on adhesion (Krull et al. 2013). Via scanning electron microscopy (SEM), Villena and Gutierrez-Correa (2007) were able to differentiate highly intertwined superficial hyphae and densely packed deep mycelium in A. niger pellets.
In recent years, flow cytometry in combination with fluorescent staining (Golabgir et al. 2015) was also used to depict morphological distributions in filamentous fungi samples. Limitations of this technique are also related to large pellet dimensions; consequently, flow cytometers must be adapted for large particle size ranges. Flow cytometry is very fast and statistically robust as a large number of particles can be measured in low time spans. When compared with microscopic image analysis, Ehgartner et al. were able to obtain similar size parameters as well as enhanced characterisation of fungal pellet compactness and hyphal region (Ehgartner et al. 2017). Potential issues include size exclusion effects at the sampling tube. Rather large pellet dimensions might lead to overrepresentation of smaller particles. In addition, large particles could cause saturation effects on certain detector signals (Ehgartner 2017). Thereby important information about various morphological features (e.g. hairiness/fluffiness) is lost in relation to pellet diameters.
Other methods successfully used for macroscopic analysis of A. niger include focused beam reflectance measurement (FBRM), a size-determining optical method based upon backscattering laser light (Krull et al. 2013). Grimm et al. (2004) characterised conidial inocula to determine effects on the conidial aggregation process. Lin et al. (2010) analysed pellet slices of several pellet regions via microscopy in order to determine regularity/circularity and surface structures. Furthermore, the impact of pellet surface structure on sedimentation behaviour in water was described. Although optical methods involving microscopy and flow analysis can be used to determine macro-morphological features, characterisation of pellet morphology and physiology remains challenging. This is mainly due to the fact that common macro-morphological parameters do not cover all important pellet characteristics (Hille et al. 2006). Regarding physiology, diffusion of substrates into pellets needs to be considered dependent on varying degrees of compactness and roughness, which cause variable oxygen uptake rates (Krull et al. 2010). If homogeneous biomass distribution is assumed, oxygen concentration gradients are the same for pellets of equal size. However, while mean biomass density may be similar for pellets from different cultivations, their inner structures could differ considerably. Hille et al. (2006) describe variations in biomass density between A. niger pellets related to inoculum conditions. The authors used CLSM to measure fluctuating density distributions in the outer layers. Dense outer pellet sections strongly impact oxygen delivery and consumption in two ways: (i) oxygen consumption is increased and (ii) substrate diffusion is restricted. Furthermore, hydrodynamics significantly affect substrate diffusion and internal oxygen concentration profiles. In their periphery, loosely structured pellets exhibit convective tendencies in substrate transport, whereas in tightly packed core regions, diffusion is predominant. Assuming that the number of hyphal tips is the essential factor for oxygen conversion, dense pellets are favoured despite restricted oxygen transport (Hille et al. 2005). Related oxygen concentration profiles in fungal pellets obtained via microelectrodes were successfully correlated with hyphal distribution in outer pellet regions (Grimm et al. 2005b).
Consequently, a major task when characterising morphology is to come up with novel or expanded parameters also depicting micro-morphological and physiological characteristics. Such parameters must come from robust analytical tools and have to consider culture conditions as well, thereby deepening understanding of fungal morphology and physiology.
Effects of pellet morphology on productivity
There have been several attempts to specify definite links between morphology and productivity (Walisko et al. 2015), but no simple relationship that would favour a specific morphology has been identified. Grimm et al. (2005a) identified the large amount of process parameters affecting morphology as an important factor. Consequently, any reported co-dependencies are limited to individual processes with specific organisms and products. When comparing these various results, it becomes clear that there are no generally applicable rules. In fact, different morphologies are in favour of different products, conflicting reports are available even for the same species. Consequently, comprehensive process design must align morphology with metabolism and productivity (Grimm et al. 2005a). For fungal pellets, the following relationship to metabolism has been proposed (Grimm et al. 2005a): biomass density is inhomogeneous and inversely proportional to pellet porosity, which in turn leads to limited accessibility for nutrients. Such complex hyphal networks hinder substrate uptake thereby directly affecting metabolism. Within this context, oxygen has been identified as the prime limiting substrate.
Interlinkages between pelletised morphology and productivity were studied intensively for A. niger (Papagianni 2007). While it is not entirely clear if pelletised or filamentous morphology is more appropriate for citric acid production, it has been proven that productivity is linked to short, swollen hyphal branches that may have swollen tips. Available literature (Papagianni 2007; Papagianni and Mattey 2006; Zhang and Zhang 2016) mainly favours compact agglomerates and pellets (< 0.5 mm), but conflicting reports also find pellet growth disadvantageous. More detailed studies (Papagianni et al. 1998) have suggested that the so-called clump form would be most suitable. Such clumps are stable agglomerates of filaments, but do not exhibit a compact core (Papagianni 2007) as opposed to pellets. Driouch et al. (2012) were able to stir A. niger pellet morphology towards a reduced thickness of biomass layer via smaller pellets as well as altered core shell structure to enhance productivity. Such a trend was also established for A. terreus as smaller pellets are deemed more compact, hence more stable (Porcel et al. 2005). However, for A. oryzae, filamentous growth achieved higher α-amylase productivity (Carlsen et al. 1996).
For P. chrysogenum, Paul and Thomas (1998) discriminated between different parts of hyphae: actively growing regions, non-growing cytoplasm, vacuolised hyphae and inactive regions (Justen et al. 1998). Penicillin production is happening in the non-growing cytoplasm (equivalent to subapical hyphal cells). This differentiation can be expanded to larger pellet structures: also pellets feature distinct active regions (Baumgartl et al. 1984). Such an active region is found in the outer layer of the pellet, which contains high quantities of cytoplasm. Cytoplasm content is decreasing in the inner regions. The pellet’s centre exhibits hyphal degradation (Ehgartner 2017). Therefore, the largest possible active layer would characterise the optimal pellet form for Penicillin production in bioreactors.
Pellet formation greatly enhances lactic acid production and fumaric acid when compared to clump-like morphological structures for R. oryzae (Liao et al. 2007). This is especially interesting as less compact morphology usually displays more productivity as viability is apparently not hindered by diffusion through dense structures. Fu et al. (2014) also found that low-density pellets exhibiting a hollow core greatly decreased lactic acid production. However, pellet densities over 60 kg/m3 were identified to be disadvantageous as this led to a generally limited mass transfer.
Kim and Song (2009) found that pellet size of white rot fungus Pleuratus ostreatus affects its biodegrading capacity. The overall biodegradation rates were closely related to laccase and esterase activity. Small-pelleted cultures were determined as favourable morphology for optimal activity of both degradative enzymes.
In general, any enhanced productivity of mycelia and clumps is due to easier supply of oxygen and substrates. Consequently, the ideal pellet is a large agglomeration of productive sections that have open access to substrates and oxygen. As mentioned, such a loose structure is associated with highly viscous fermentation media, resulting in issues with gas−liquid mass transfer, liquid mixing and complex rheology. Therefore, morphological optimisation has to consider the following: rheological requirements for the bioprocess on the one hand and ideal pellet compactness for enhanced productivity on the other hand. Possible options to favourably alter pellet morphology are discussed below.
Alteration of pellet morphology
In this section, we take a closer look at factors, which affect pellet formation and morphology. All these potential factors are interdependent. Therefore, any form of morphological control strategy is highly complex.
Agitation
The general rule states that strong agitation results in smaller pellets. If pellet formation is proceeded by spore aggregation, strong agitation could decrease pellet growth (Prosser and Tough 1991). If pellets have already formed, they are affected by agitation in two ways (Tanaka et al. 1975): (1) hyphal elements on the pellet surface can be shaved off and (2) there is also the possibility of total pellet rupture. In general, the shaving of hyphal elements is preferred, as total pellet rupture would lead to impurity release.
For several Aspergillus species, strong agitation forces facilitate a morphology of short, thick and highly branched filaments advantageous for citric acid production (Papagianni 2007). Fragmentation of filaments can also occur, but is mainly limited to old and heavily vacuolated parts. Therefore, there is a leeway for beneficial breakage of filaments through agitation: a balance between new growth and fragmentation of inactive sections (Papagianni 2007).
P. chrysogenum also displays clear relations between morphology and agitation. Agglomeration of hyphal elements and thereby pellet formation is negatively affected by strong agitation (Walisko et al. 2015). Nielsen et al. (1995) have reported that a shift from pellet morphology to disperse mycelia could be achieved for fed-batch cultivations.
Pellet size of P. ostreatus is predominantly controlled via agitation; Kim and Song (2009) were able to obtain either large-pelleted or small-pelleted morphology at respective agitation speeds. Tinoco-Valencia et al. (2014) further studied agitation and aeration effects on P. ostreatus growth. They observed predominantly pellet morphology. High agitation in combination with high aeration flow rates led to increased oxygen mass transfer and decreased pellet size, respectively (Serrano-Carreon et al. 2015). Consequently, growth rate and maximum biomass concentration were increased.
To recapitulate, agitation considerably alters morphology, especially during agglomeration. If pellets have already formed, strong agitation could lead to adverse effects like pellet rupture. Several production processes favour thin biomass layers and loose core structures in pellets due to strong agitation, for example citric acid production in A. niger or lignin peroxidase production in Phanerochaete chrysosporium.
Broth viscosity, medium composition and pH
In general, an inverse relation between pellet size and broth viscosity has been reported. For example, Prosser and Tough (1991) state that adjustment of broth viscosity via carbohydrates is advantageous for cultivations of Blakeslea and Choanephora. The use of anionic polymers has been shown to hinder spore aggregation prior to germination. Thereby organisms that usually favour pellet growth can be stirred towards dispersed growth and vice versa. Trinci (1983) reported this effect for Aspergillus and some basidiomycetes. These findings might also apply to naturally formed polysaccharides as has been speculated for P. chrysogenum (Prosser and Tough 1991).
Kisser et al. (1980) studied the effect of manganese sufficient or deficient cultivation on A. niger morphology and cell wall composition in citric acid production. Omission of manganese from the nutrient medium results in “abnormal morphological development which is characterised by increased spore swelling and bulbous hyphae”. Only compact pellets produce citric acid; consequently, manganese deficiency is to be avoided. The same effects of manganese deficiency have also been confirmed by Papagianni et al. (1998). Sensitivity to the presence of trace metals equals that there is also sensitivity for metal complexing ions like EDTA (Prosser and Tough 1991).
Wucherpfennig et al. (2011) studied the effect of osmolality on A. niger morphology and productivity. Culture broth osmolality was increased by the addition of sodium chloride. It was found that pellet size declined with osmolality. However, it was determined that the culture was also becoming more homogenous.
Investigations into optimal medium composition for R. oryzae implied that peptone had a positive effect on pellet formation. The addition of metal ions as well as interaction of metal ions and peptone impeded pellet formation (Liao et al. 2007). Through the concentrations of potato dextrose broth, soybean peptone and calcium carbonate in the medium pellet size were controlled. Fu et al. (2014) additionally state that pellet density is positively affected by addition of peptone. Under low peptone concentrations, low-density pellets with hollow structures were observed.
For P. chrysogenum, addition of corn steep liquor (CSL) to the culture medium is known to have positive effects on pellet formation at an early stage and penicillin production (Sajjad-Ur-Rahman et al. 2012). There is still ongoing research if the state-of-the-art penicillin fermentation medium (composed of CSL, glucose, lactose, minerals, oil, nitrogen source and precursor) can be modified and improved. Cultivation in buffered medium had positive effects on biomass growth for several Penicillium species: pellets displayed greater diameters as well as smaller hyphae on the surface (Walisko et al. 2015). Studies using SEM indicate that the morphology of P. chrysogenum is affected by CO2 presence in the medium: at low CO2 concentrations (up to 8%), the filamentous form was predominant, higher concentrations led to the formation of swollen and stunted hyphae affecting pellet morphology (Ho and Smith 1986).
As noted previously, pH is a driving factor on electrostatic and hydrophobic interactions. For citric acid production, pH of culture medium is preferably low and strongly affects production, simply because of the pH sensitivity of enzymes in the TCA cycle (Papagianni 2007). Morphological development of small pellet aggregates and short filaments is best sustained at pH values of 2.0 ± 0.2. Liu and Wu (2012) also found pH-related effects on the morphology of C. sinensis. The mycelial pellets became less uniform at lower pH (< 6.0). Filamentous growth was observed at higher pH (8–9). The growth of ascomycete fungus Neurospora intermedia in uniform pellet form can be achieved using a pH range of 3.0–4.0 (Nair et al. 2016).
In recent years, the effect of surfactants has been tested. Liu and Wu (2012) found that Tween exhibited a promoting effect on production of exopolysaccharide in the fungus Cordyceps sinensis. Improved productivity was combined with medium inhibited pellet formation leading to small and loose pellets. More recently, Kurukake et al. (2017) observed that A. oryzae pellets became mall and spherical on addition of Tween surfactant. Furthermore, production of fructosyl-transferase could be enhanced. Addition of Tween also led to an increased specific surface area of Pleurotus eryngii pellets (Wu et al. 2016). Antecka et al. (2016) successfully applied a morphological engineering technique to a laccase production process in basidiomycetes. The authors found that the addition of Al2O3 microparticles led to a decrease of pellet size, shape and structure in Cerrena unicolor and Pleurotus sapidus. Similar morphological engineering techniques—namely the use of magnesium silicate microparticles—were also successful for oleaginous fungus Mortierella isabellina (Gao et al. 2014).
To summarise, effects of medium composition on morphology and productivity are highly diversified and entirely dependent on species and process conditions. According to the available literature, we can only assume that pH has considerable impact across several species. Nevertheless, a selection of preferred medium compositions is available for several fungal pellet processes.
Spore inoculum level and other inoculation strategies
Generally, an inverse relationship between spore number and pellet size has been identified for several Aspergillus species (Prosser and Tough 1991). Citric acid production in A. niger is particularly affected by spore inoculum. Papagianni and Mattey (2006) found studied spore development and morphology in a bioreactor in relation to spore inoculum concentrations. They classified four morphological classes: globular pellets, elongated pellets, clumps and free mycelia. Glucosamine formation and release was clearly related to spore inoculum level. At higher inoculum levels (10^8–10^9 spores/mL), lower dissolved oxygen levels were measured which led to mycelium developed in dispersed morphologies. For Caldariomyces fumago, aforementioned relationships were also found. Additionally, pellet density was reported to be inversely related to pellet size (Carmichael and Pickard 1989).
For P. chrysogenum, it was reported that with low spore inoculum concentrations, only few agglomerations of hyphal elements happen which in turn leads to pellets with small diameters (Nielsen et al. 1995). At very high concentrations (> 10^5 spores/mL), the hyphal element size is small and agglomeration, respectively, pellet formation does not occur in the first place. For a spore inoculum concentration of 3.7 * 10^4 spores/mL maximum, pellet concentration was measured which amounts to roughly 1.5 * 10^4 pellets/mL. In a different study, Posch and Herwig (2014) describe a positive effect of spore inoculum concentration on penicillin formation during early production phases, which feature excess substrate, biomass growth and overflow metabolism. Simultaneously, a negative effect on pellet morphology is observed: pellets develop larger structures at reduced spore inocula; consequently, pellet breakage resulting in dispersed morphology is more likely.
In recent years, the so-called pellet-dispersion strategy has brought promising results for seed cultivation of A. niger niger (Wang et al. 2017). Thereby pellets were used to substitute spores during inoculation. Traditionally, long time spans are needed for spore preparation of seed culture. This drawback was avoided through the development of a novel seed-recycling process. The morphological structure displayed “densely intertwined hyphae” and pellet compactness increased considerably. A 48-h pellet inoculum was also advantageous for growth of a newly isolated nitrifying fungus defined as Penicillium sp. L1. When compared to inoculation with spore suspension, pellet size could be increased significantly by using a pellet inoculum (Liu et al. 2017).
Other factors—aeration and growth rate
A. niger generally features an indirect relation between pellet compactness and aeration rate. However, using a 1:1 oxygen/air mixture leads to dense growth and increased hyphal branching (Prosser and Tough 1991). Krull et al. (2013) state that A. niger pellets derived from high aeration rates have overall a much larger structure and have unstructured and irregular outer sections. Low aeration results in rather compact peripheral structures. By comparison, it was found that agitation effects are less severe.
Some Aspergillus species are associated with filamentous morphology at low growth rates and reversibly produce pellets at higher rates (Prosser and Tough 1991). High growth rates in P. chrysogenum cultivations lead to an increase in hyphal branching (Nielsen et al. 1995). Decreasing the specific growth rate was followed by pellet breakage. In addition, hyphal elements were torn away from the surface. Nielsen et al. assume that such pellet breakage happens due to cell lysis within the pellet, which leads to a loss in stability. Posch and Herwig (2014) were able to describe parameter effects on pellet morphology through morphological and physiological bioprocess modelling approaches. In the beginning, high growth rates yield large pellet fractions. If pellet growth reaches a critical level, a transition phase leads to dispersed growth due to increasing pellet erosion and breakage.
Summarising, factors such as agitation and media pH have universal impact on pellet morphology. However, the effect of most factors described in this section only applies to specific species and processes. In general, definite guidelines for morphological alteration or control cannot be given.
Conclusions
Any preference in fungal pellet morphology depends on species and specific task at hand. To timely assess current process morphology, novel techniques like flow cytometry combined with fluorescent staining are convenient. Once desired relations between pellet form and productivity have been established, one can draw from a multitude of options to favourably alter and optimise morphology. Some of these possibilities are summarised in Table 1: in this table, pellet forming species are divided into coagulative or non-coagulative type. Based upon this distinction, preferred pellet morphologies are cited, extended by possible techniques to favourably alter morphology.
From our perspective, modelling approaches involving raw data from morphological classification seem promising to increase process understanding. We envision a combination of timely morphological assessment with specific morphological alteration in order to favourably stir fungal pellet processes towards increased productivity.
Acknowledgements
Open access funding provided by TU Wien (TUW).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
References
- Abd-Elsalam, IS (2009) Role of the biomass and pelleted forms of Aspergillus ochraceus ATCC 3150 on the 11 α-hydroxylation of progesterone. Malaysian Journal of Microbiology 5(2):119–122
- Akiba T, Nishi A, Takaoki M, Nagaoka S, Tomita F. Electrophoretic free mobility and viability of microbial cells: a preliminary study in preparation for space experiments. Appl Theor Electrophor. 1994;4(2):65–69. [PubMed] [Google Scholar]
- Amanullah A, Leonildi E, Nienow AW, Thomas CR. Dynamics of mycelial aggregation in cultures of Aspergillus oryzae. Bioprocess Biosyst Eng. 2001;24(2):101–107. doi: 10.1007/s004490100235. [DOI] [Google Scholar]
- Antecka A, Bizukojc M, Ledakowicz S (2016) Modern morphological engineering techniques for improving productivity of filamentous fungi in submerged cultures. World J Microbiol Biotechnol 32(12):193. 10.1007/s11274-016-2148-7 [DOI] [PMC free article] [PubMed]
- Baumgartl H, Wittler R, Lubbers DW, Schugerl K. Oxygen profiles and structure of Penicillium chrysogenum pellets. Adv Exp Med Biol. 1984;169:793–799. doi: 10.1007/978-1-4684-1188-1_72. [DOI] [PubMed] [Google Scholar]
- Carlsen M, Spohr AB, Nielsen J, Villadsen J. Morphology and physiology of an alpha-amylase producing strain of Aspergillus oryzae during batch cultivations. Biotechnol Bioeng. 1996;49(3):266–276. doi: 10.1002/(SICI)1097-0290(19960205)49:3<266::AID-BIT4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Carmichael RD, Pickard MA. Continuous and batch production of Chloroperoxidase by mycelial pellets of Caldariomyces fumago in an airlift Fermentor. Appl Environ Microbiol. 1989;55(1):17–20. doi: 10.1128/aem.55.1.17-20.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox PW, Paul GC, Thomas CR. Image analysis of the morphology of filamentous micro-organisms. Microbiology. 1998;144(Pt 4):817–827. doi: 10.1099/00221287-144-4-817. [DOI] [PubMed] [Google Scholar]
- Douglas HW, Collins AE, Parkinson D. Electric charge and other surface properties of some fungal spores. Biochim Biophys Acta. 1959;33(2):535–538. doi: 10.1016/0006-3002(59)90145-3. [DOI] [PubMed] [Google Scholar]
- Driouch H, Hansch R, Wucherpfennig T, Krull R, Wittmann C. Improved enzyme production by bio-pellets of Aspergillus niger: targeted morphology engineering using titanate microparticles. Biotechnol Bioeng. 2012;109(2):462–471. doi: 10.1002/bit.23313. [DOI] [PubMed] [Google Scholar]
- Dynesen J, Nielsen J. Surface hydrophobicity of Aspergillus nidulans conidiospores and its role in pellet formation. Biotechnol Prog. 2003;19(3):1049–1052. doi: 10.1021/bp0340032. [DOI] [PubMed] [Google Scholar]
- Ehgartner D. A comprehensive analytical and process-technological toolbox for improved penicillin production. Vienna: Vienna University of Technology; 2017. [Google Scholar]
- Ehgartner D, Herwig C, Fricke J. Morphological analysis of the filamentous fungus Penicillium chrysogenum using flow cytometry—the fast alternative to microscopic image analysis. Appl Microbiol Biotechnol. 2017;101(20):1–14. doi: 10.1007/s00253-017-8475-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu YQ, Yin LF, Zhu HY, Jiang R, Li S, Xu Q. Effects of pellet characteristics on L-lactic acid fermentation by R. oryzae: pellet morphology, diameter, density, and interior structure. Appl Biochem Biotechnol. 2014;174(6):2019–2030. doi: 10.1007/s12010-014-1146-1. [DOI] [PubMed] [Google Scholar]
- Gao D, Zeng J, Yu X, Dong T, Chen S. Improved lipid accumulation by morphology engineering of oleaginous fungus Mortierella isabellina. Biotechnol Bioeng. 2014;111(9):1758–1766. doi: 10.1002/bit.25242. [DOI] [PubMed] [Google Scholar]
- Gerin PA, Dufrene Y, Bellon-Fontaine MN, Asther M, Rouxhet PG. Surface properties of the conidiospores of Phanerochaete chrysosporium and their relevance to pellet formation. J Bacteriol. 1993;175(16):5135–5144. doi: 10.1128/jb.175.16.5135-5144.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golabgir A, Ehgartner D, Neutsch L, Posch AE, Sagmeister P, Herwig C. Imaging flow cytometry and high-throughput microscopy for automated macroscopic morphological analysis of filamentous fungi. Fung Biol-Us. 2015;2:201–210. doi: 10.1007/978-3-319-10503-1_17. [DOI] [Google Scholar]
- Grimm LH, Kelly S, Hengstler J, Gobel A, Krull R, Hempel DC. Kinetic studies on the aggregation of Aspergillus niger conidia. Biotechnol Bioeng. 2004;87(2):213–218. doi: 10.1002/bit.20130. [DOI] [PubMed] [Google Scholar]
- Grimm LH, Kelly S, Krull R, Hempel DC. Morphology and productivity of filamentous fungi. Appl Microbiol Biotechnol. 2005;69(4):375–384. doi: 10.1007/s00253-005-0213-5. [DOI] [PubMed] [Google Scholar]
- Grimm LH, Kelly S, Volkerding II, Krull R, Hempel DC. Influence of mechanical stress and surface interaction on the aggregation of Aspergillus niger conidia. Biotechnol Bioeng. 2005;92(7):879–888. doi: 10.1002/bit.20666. [DOI] [PubMed] [Google Scholar]
- Hille A, Neu TR, Hempel DC, Horn H. Oxygen profiles and biomass distribution in biopellets of Aspergillus niger. Biotechnol Bioeng. 2005;92(5):614–623. doi: 10.1002/bit.20628. [DOI] [PubMed] [Google Scholar]
- Hille A, Neu TR, Hempel DC, Horn H. Effect of morphology on transport of matter and conversion in Aspergillus niger-pellets. Chem Ing Tech. 2006;78(5):627–632. doi: 10.1002/cite.200600018. [DOI] [Google Scholar]
- Ho CS, Smith MD. Morphological alterations of Penicillium-Chrysogenum caused by carbon-dioxide. J Gen Microbiol. 1986;132:3479–3484. [Google Scholar]
- Justen P, Paul GC, Nienow AW, Thomas CR. Dependence of Penicillium chrysogenum growth, morphology, vacuolation, and productivity in fed-batch fermentations on impeller type and agitation intensity. Biotechnol Bioeng. 1998;59(6):762–775. doi: 10.1002/(SICI)1097-0290(19980920)59:6<762::AID-BIT13>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Kim YM, Song HG. Effect of fungal pellet morphology on enzyme activities involved in phthalate degradation. J Microbiol. 2009;47(4):420–424. doi: 10.1007/s12275-009-0051-8. [DOI] [PubMed] [Google Scholar]
- Kisser M, Kubicek CP, Rohr M. Influence of manganese on morphology and cell wall composition of Aspergillus niger during citric acid fermentation. Arch Microbiol. 1980;128(1):26–33. doi: 10.1007/BF00422301. [DOI] [PubMed] [Google Scholar]
- Krull R, Cordes C, Horn H, Kampen I, Kwade A, Neu TR, Nortemann B. Morphology of filamentous fungi: linking cellular biology to process engineering using Aspergillus niger. Adv Biochem Eng Biotechnol. 2010;121:1–21. doi: 10.1007/10_2009_60. [DOI] [PubMed] [Google Scholar]
- Krull R, Wucherpfennig T, Esfandabadi ME, Walisko R, Melzer G, Hempel DC, Kampen I, Kwade A, Wittmann C. Characterization and control of fungal morphology for improved production performance in biotechnology. J Biotechnol. 2013;163(2):112–123. doi: 10.1016/j.jbiotec.2012.06.024. [DOI] [PubMed] [Google Scholar]
- Kurakake M, Hirotsu S, Shibata M, Takenaka Y, Kamioka T, Sakamoto T. Effects of nonionic surfactants on pellet formation and the production of beta-fructofuranosidases from Aspergillus oryzae KB. Food Chem. 2017;224:139–143. doi: 10.1016/j.foodchem.2016.12.054. [DOI] [PubMed] [Google Scholar]
- Liao W, Liu Y, Chen S. Studying pellet formation of a filamentous fungus Rhizopus oryzae to enhance organic acid production. Appl Biochem Biotechnol. 2007;137-140(1–12):689–701. doi: 10.1007/s12010-007-9089-4. [DOI] [PubMed] [Google Scholar]
- Lin PJ, Grimm LH, Wulkow M, Hempel DC, Krull R. Population balance modeling of the conidial aggregation of Aspergillus niger. Biotechnol Bioeng. 2008;99(2):341–350. doi: 10.1002/bit.21569. [DOI] [PubMed] [Google Scholar]
- Lin PJ, Scholz A, Krull R. Effect of volumetric power input by aeration and agitation on pellet morphology and product formation of Aspergillus niger. Biochem Eng J. 2010;49(2):213–220. doi: 10.1016/j.bej.2009.12.016. [DOI] [Google Scholar]
- Liu Y, Hu T, Zhao J, Lv Y, Ren R. Simultaneous removal of carbon and nitrogen by mycelial pellets of a heterotrophic nitrifying fungus-Penicillium sp. L1. J Biosci Bioeng. 2017;123(2):223–229. doi: 10.1016/j.jbiosc.2016.08.009. [DOI] [PubMed] [Google Scholar]
- Liu YS, Wu JY. Effects of tween 80 and pH on mycelial pellets and exopolysaccharide production in liquid culture of a medicinal fungus. J Ind Microbiol Biotechnol. 2012;39(4):623–628. doi: 10.1007/s10295-011-1066-9. [DOI] [PubMed] [Google Scholar]
- Nair RB, Lennartsson PR, Taherzadeh MJ. Mycelial pellet formation by edible ascomycete filamentous fungi, Neurospora intermedia. AMB Express. 2016;6(1):31. doi: 10.1186/s13568-016-0203-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J, Johansen CL, Jacobsen M, Krabben P, Villadsen J. Pellet formation and fragmentation in submerged cultures of Penicillium chrysogenum and its relation to penicillin production. Biotechnol Prog. 1995;11(1):93–98. doi: 10.1021/bp00031a013. [DOI] [PubMed] [Google Scholar]
- Papagianni M. Advances in citric acid fermentation by Aspergillus niger: biochemical aspects, membrane transport and modeling. Biotechnol Adv. 2007;25(3):244–263. doi: 10.1016/j.biotechadv.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Papagianni M, Mattey M. Morphological development of Aspergillus niger in submerged citric acid fermentation as a function of the spore inoculum level. Application of neural network and cluster analysis for characterization of mycelial morphology. Microb cell fact. 2006;5:3. doi: 10.1186/1475-2859-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papagianni M, Mattey M, Kristiansen B. Citric acid production and morphology of Aspergillus niger as functions of the mixing intensity in a stirred tank and a tubular loop bioreactor. Biochem Eng J. 1998;2(3):197–205. doi: 10.1016/S1369-703X(98)00032-1. [DOI] [Google Scholar]
- Pascual S, De Cal A, Magan N, Melgarejo P. Surface hydrophobicity, viability and efficacy in biological control of Penicillium oxalicum spores produced in aerial and submerged culture. J Appl Microbiol. 2000;89(5):847–853. doi: 10.1046/j.1365-2672.2000.01189.x. [DOI] [PubMed] [Google Scholar]
- Paul GC, Thomas CR. A structured model for hyphal differentiation and penicillin production using Penicillium chrysogenum. Biotechnol Bioeng. 1996;51(5):558–572. doi: 10.1002/(SICI)1097-0290(19960905)51:5<558::AID-BIT8>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Paul GC, Thomas CR. Characterisation of mycelial morphology using image analysis. Adv Biochem Eng Biotechnol. 1998;60:1–59. doi: 10.1007/BFb0102278. [DOI] [PubMed] [Google Scholar]
- Pazouki M, Panda T. Understanding the morphology of fungi. Bioprocess Eng. 2000;22(2):127–143. doi: 10.1007/s004490050022. [DOI] [Google Scholar]
- Pirt SJ. A theory of the mode of growth of fungi in the form of pellets in submerged culture. Proc R Soc Lond B Biol Sci. 1966;166(1004):369–373. doi: 10.1098/rspb.1966.0105. [DOI] [PubMed] [Google Scholar]
- Porcel EMR, Lopez JLC, Perez JAS, Sevilla JMF, Chisti Y. Effects of pellet morphology on broth rheology in fermentations of Aspergillus terreus. Biochem Eng J. 2005;26(2–3):139–144. doi: 10.1016/j.bej.2005.04.011. [DOI] [Google Scholar]
- Posch AE, Herwig C. Physiological description of multivariate interdependencies between process parameters, morphology and physiology during fed-batch penicillin production. Biotechnol Prog. 2014;30(3):689–699. doi: 10.1002/btpr.1901. [DOI] [PubMed] [Google Scholar]
- Posch AE, Spadiut O, Herwig C. A novel method for fast and statistically verified morphological characterization of filamentous fungi. Fungal Genet Biol. 2012;49(7):499–510. doi: 10.1016/j.fgb.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Priegnitz BE, Wargenau A, Brandt U, Rohde M, Dietrich S, Kwade A, Krull R, Fleissner A. The role of initial spore adhesion in pellet and biofilm formation in Aspergillus niger. Fungal Genet Biol. 2012;49(1):30–38. doi: 10.1016/j.fgb.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Prosser JI, Tough AJ. Growth mechanisms and growth kinetics of filamentous microorganisms. Crit Rev Biotechnol. 1991;10(4):253–274. doi: 10.3109/07388559109038211. [DOI] [PubMed] [Google Scholar]
- Sajjad-Ur-Rahman, Rasool MH, Rafi M. Penicillin production by wild isolates of Penicillium chrysogenum in Pakistan. Braz J Microbiol. 2012;43(2):476–481. doi: 10.1590/S1517-83822012000200007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano-Carreon L, Galindo E, Rocha-Valadez JA, Holguin-Salas A, Corkidi G. Hydrodynamics, fungal physiology, and morphology. Adv Biochem Eng Biotechnol. 2015;149:55–90. doi: 10.1007/10_2015_304. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Mizuguchi T, Ueda K. Studies on Effect of Agitation on Mycelia in Submerged Mold Culture .5. Index Representing Mycelial Strength to Maintain Physiological-Activity on Mechanical Agitation. J Ferment Technol. 1975;53(1):35–43. [Google Scholar]
- Tinoco-Valencia R, Gomez-Cruz C, Galindo E, Serrano-Carreon L. Toward an understanding of the effects of agitation and aeration on growth and laccases production by Pleurotus ostreatus. J Biotechnol. 2014;177:67–73. doi: 10.1016/j.jbiotec.2014.02.013. [DOI] [PubMed] [Google Scholar]
- Trinci APJ. Effect of Junlon on morphology of Aspergillus niger and its use in making turbidity measurements of fungal growth. Trans Br Mycol Soc. 1983;81:408–412. doi: 10.1016/S0007-1536(83)80098-9. [DOI] [Google Scholar]
- Villena GK, Fujikawa T, Tsuyumu S, Gutierrez-Correa M. Structural analysis of biofilms and pellets of Aspergillus niger by confocal laser scanning microscopy and cryo scanning electron microscopy. Bioresour Technol. 2010;101(6):1920–1926. doi: 10.1016/j.biortech.2009.10.036. [DOI] [PubMed] [Google Scholar]
- Villena GK, Gutierrez-Correa M. Morphological patterns of Aspergillus niger biofilms and pellets related to lignocellulolytic enzyme productivities. Lett Appl Microbiol. 2007;45(3):231–237. doi: 10.1111/j.1472-765X.2007.02183.x. [DOI] [PubMed] [Google Scholar]
- Walisko R, Moench-Tegeder J, Blotenberg J, Wucherpfennig T, Krull R. The taming of the shrew--controlling the morphology of filamentous eukaryotic and prokaryotic microorganisms. Adv Biochem Eng Biotechnol. 2015;149:1–27. doi: 10.1007/10_2015_322. [DOI] [PubMed] [Google Scholar]
- Wang B, Chen J, Li H, Sun F, Li Y, Shi G. Pellet-dispersion strategy to simplify the seed cultivation of Aspergillus niger and optimize citric acid production. Bioprocess Biosyst Eng. 2017;40(1):45–53. doi: 10.1007/s00449-016-1673-y. [DOI] [PubMed] [Google Scholar]
- Wargenau A, Fleissner A, Bolten CJ, Rohde M, Kampen I, Kwade A. On the origin of the electrostatic surface potential of Aspergillus niger spores in acidic environments. Res Microbiol. 2011;162(10):1011–1017. doi: 10.1016/j.resmic.2011.07.006. [DOI] [PubMed] [Google Scholar]
- Wargenau A, Kwade A. Determination of adhesion between single Aspergillus niger spores in aqueous solutions using an atomic force microscope. Langmuir. 2010;26(13):11071–11076. doi: 10.1021/la100653c. [DOI] [PubMed] [Google Scholar]
- Wilkinson MHFH. Digital image analysis of microbes: imaging, morphometry, Fluorometry, and motility techniques and applications 1st. New York: Wiley; 1998. [Google Scholar]
- Wosten HAB, van Veluw GJ, de Bekker C, Krijgsheld P. Heterogeneity in the mycelium: implications for the use of fungi as cell factories. Biotechnol Lett. 2013;35(8):1155–1164. doi: 10.1007/s10529-013-1210-x. [DOI] [PubMed] [Google Scholar]
- Wu M, Xu Y, Ding W, Li Y, Xu H. Mycoremediation of manganese and phenanthrene by Pleurotus eryngii mycelium enhanced by tween 80 and saponin. Appl Microbiol Biotechnol. 2016;100(16):7249–7261. doi: 10.1007/s00253-016-7551-3. [DOI] [PubMed] [Google Scholar]
- Wucherpfennig T, Hestler T, Krull R. Morphology engineering - osmolality and its effect on Aspergillus niger morphology and productivity. Microb Cell Factories. 2011;10(1):58. doi: 10.1186/1475-2859-10-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhang J. The filamentous fungal pellet and forces driving its formation. Crit Rev Biotechnol. 2016;36(6):1066–1077. doi: 10.3109/07388551.2015.1084262. [DOI] [PubMed] [Google Scholar]
- Zmak PM, Podgornik A, Podgornik H, Koloini T. Impact of pellet size on growth and lignin peroxidase activity of Phanerochaete chrysosporium. World J Microbiol Biotechnol. 2006;22(12):1243–1249. doi: 10.1007/s11274-006-9168-7. [DOI] [Google Scholar]