Abstract
Key message
GWAS analysis for leaf blade area (LA) revealed intriguing genomic regions associated with putatively novel QTL and known plant stature-related phytohormone and sugar-related genes.
Abstract
Despite long-standing studies in the morpho-physiological characters of leaf blade area (LA) in cereal crops, advanced genetic studies to explore its natural variation are lacking. The importance of modifying LA in improving cereal grain yield and the genes controlling leaf traits have been well studied in rice but not in temperate cereals. To better understand the natural genetic variation of LA at four developmental stages, main culm LA was measured from 215 worldwide spring barleys including 92 photoperiod-sensitive accessions [PHOTOPERIOD RESPONSE LOCUS 1 (Ppd-H1)] and 123 accessions with reduced photoperiod sensitivity (ppd-H1) locus under controlled greenhouse conditions (long-day; 16/8 h; ~ 20/~ 16 °C day/night). The LA of Ppd-H1-carrying accessions was always smaller than in ppd-H1-carrying accessions. We found that nine SNPs from the Ppd-H1 gene were present in the collection of which marker 9 (M9; G/T in the CCT-domain) showed the most significant and consistent effect on LA at all studied developmental stages. Genome-wide association scans (GWAS) showed that the accessions carrying the ppd-H1 allele T/M9 (late heading) possessed more genetic variation in LA than the Ppd-H1 group carrying G/M9 (early heading). Several QTL with major effects on LA variation were found close to plant stature-related heading time, phytohormone- and sugar-related genes. The results provide evidence that natural variation of LA is an important source for improving grain yield, adaptation and canopy architecture of temperate cereals.
Electronic supplementary material
The online version of this article (10.1007/s00122-018-3053-2) contains supplementary material, which is available to authorized users.
Introduction
Leaf blade area (LA) is considered as one of the major drivers of plant architecture that in turn influences the adaptations to environmental cues and grain yield. Donald (1968) and Jennings (1964) proposed a model for enhancing grain yield potential by modifying traits such as LA in cereals using an “ideotype” breeding. LA has a direct impact on crop stature, growth and yield through photosynthesis (Chen et al. 1995) which can be maximized by expanding photosynthetic-LA in rice, sorghum and wheat (Driever et al. 2014; Jiang et al. 2015; Kebrom and Mullet 2015). Factually, the leaf is the major photosynthetic organ that supplies assimilates for regulating plant stature, e.g. photosynthetic-LA is tightly linked with sugar production that in turn regulates shoot branching in sorghum (Evers 2015; Kebrom and Mullet 2015). The position of individual leaves affects their contribution to grain yield, especially the flag leaf, which is considered as the main source of carbohydrate synthesis in barley (Tao 1999). The relationship between flag-LA and yield traits was intensively studied in rice (Wang et al. 2011) and barley (Tao 1999). Moreover, the genetic analysis of flag-LA during grain filling had been studied in rice (Zhang et al. 2015) and barley (Yang and Lu 1991). Alqudah and Schnurbusch (2015) identified a high contribution of main culm LA on single plant yield in barley and showed the quantitative inheritance of LA at pre-anthesis developmental stages under different environments. Greenhouse (GH) conditions and single plant analysis were found to be suitable for studying leaf developmental traits because they maximized phenotypic/genetic variation among accessions (Alqudah and Schnurbusch 2015). Therefore, to study LA progression during pre-anthesis development is important to better understand the genetic link between LA and phase duration.
The embryo of the barley grain has up to four leaf primordia covering the young shoot apical meristem (SAM), whereas further leaf primordia can be produced after germination under non-inductive conditions (Kirby and Appleyard 1987). The newly induced leaf primordia arise as a result of periclinal cell division in the dermatogen cells of the SAM, which is followed by periclinal and anticlinal cell divisions and expansions in the dermatogen and the hypodermal cells at the leaf primordia (Sharman 1945). Subsequently, the leaf primordium converts into a mature leaf through promoting cell division and expansion (Gonzalez et al. 2012). The above-mentioned phases are overlapping and interconnected with impact on final leaf size, which is strictly controlled through spatial–temporal genetic factors (Gonzalez et al. 2012). In barley, the duration of leaf initiation and developmental phases are variable across genotypes and genetically inherited (Kernich et al. 1995; Kirby and Riggs 1978). Gonzalez et al. (2012) reported that the duration of leaf initiation and developmental phases, especially the cell expansion phase, affect final LA. The duration of leaf elongation is considered to be one of the major determinants of grass LA (Chenu et al. 2008; Voorend et al. 2014) that in turn explains the variation in response to environmental cues or across genotypes (Tardieu et al. 2000). Measuring LA during aforementioned developmental times was proposed to be an adequate tool to describe the analysis of kinematic parameters such as cell division, expansion, size and density (Gonzalez et al. 2012).
Quantitative trait loci (QTL) analysis and identification of genes underlying leaf size and shape traits are well advanced in rice. Most of the QTL studies in barley and rice were focused on flag-LA and related traits (Bing et al. 2006; Gyenis et al. 2007; Xue et al. 2008; Zhang et al. 2015). In addition to QTL analyses, several genes controlling flag leaf traits have been identified in rice, such as SEMI-ROLLED LEAF 1 (SRL1), Xiang et al. (2012); SHALLOT-LIKE 1 (SLL1), Zhang et al. (2009); NARROW LEAF 1 (NAL1), Qi et al. (2008); NARROW AND ROLLED LEAF 1 (NRL1), Hu et al. (2010) and NARROW LEAF 7 (NAL7), Fujino et al. (2008). By mutant analysis of these genes, it was found that abnormal leaf shape and size resulted from abnormal cell division and/or expansion. For instance, Jiang et al. (2015) found that leaf length and width were reduced by 50% in nal1-2 and nal1-3 rice mutants as a result of cell division suppression. Aberrant periclinal cell divisions lead to increased cell layers in the leaf epidermis of barley elongation (elo2 and elo5) mutants (Lewis et al. 2009) and Extra cell layers 1 (Xcl1) maize mutant (Kessler et al. 2002). Recently, Jöst et al. (2016) cloned the barley BROAD LEAF 1 (BLF1) gene encoding an INDETERMINATE DOMAIN protein that limits cell divisions as a negative regulator of leaf-width during leaf primordia outgrowth. Barley NARROW LEAFED DWARF1 that encodes a WUSCHEL-RELATED HOMEOBOX 3 (WOX3) regulates the development of the marginal regions in the leaves had been recently cloned by Yoshikawa et al. (2016).
A genome-wide association study (GWAS) was implemented in a nested association mapping in maize to understand the second top leaf (penultimate leaf) architecture at flowering stage (Tian et al. 2011). Allelic variation of the PHOTOPERIOD RESPONSE LOCUS 1 (Ppd-H1) gene was associated with natural variation in leaf size in European winter barley cultivars (Digel et al. 2016). Most of the previous genetic studies focused on the flag leaf or single leaf at a specific stage; but heretofore no genetic study considered the natural variation of LA at different developmental stages using GWAS analysis. Performing such study may provide general implications related to the prevalence of natural genetic variation for LA in cereals.
PHOTOPERIOD RESPONSE LOCUS 1 (Ppd-H1) is one of the central genes controlling heading time in barley where amino acid changes at CCT [CONSTANS, CO-LIKE, TIMING OF CAB1 (TOC1)]-domain separated Ppd-H1 into photoperiod-sensitive (G allele) and ppd-H1 reduced photoperiod sensitivity [T allele; Turner et al. (2005)]. These alleles were used to account for population structure to study the genetic variation of phase duration and plant stature in a worldwide spring barley collection (Alqudah et al. 2014, 2016). Following this approach, main culm LA-blades were collected from the studied accessions at four developmental stages at awn primordium (AP), tipping (TIP), heading (HD) and anther extrusion (AE) stages. The 215 tested accessions were grown under controlled greenhouse (GH) conditions. For GWAS analysis, the 9K gene-based single nucleotide polymorphism (SNP) chip provided a high-resolution genetic map. Here we present the first GWAS study of LA in temperate cereals, showing that a single variant at the Ppd-H1 locus marker 9 (M9) sitting in the CCT-domain had the most significant and consistent effect on LA variation at all studied developmental stages, consistent with Digel et al. (2016). Further GWAS analyses from the two photoperiod groups (i.e. Ppd-H1/ppd-H1) disclosed associations with enriched genomic regions co-locating with putative candidate genes controlling leaf traits known in other crop species and/or novel QTL. Therefore, studying LA is important to explore the influence of allelic variation on the natural genetic variation of LA in barley.
Materials and methods
Plant material and population structure
A diverse spring barley collection of 215 worldwide accessions was used in this study. The accessions had previously been genotyped using high-density 9K SNPs chip from Illumina (Alqudah et al. 2014, 2016). Platform assayed 6355 SNPs that includes eight markers localized in Ppd-H1 gene (BOPA2_12_30870, BOPA2_12_30871, BOPA2_12_30872, BK_12, BK_13, BK_14, BK_15, BK_16) in addition to M9/SNP22 (G/T, Turner et al. (2005), Fig. 1a). Ppd-H1 has been positionally cloned and includes eight exons with pseudo-receiver domain and CCT domain, Turner et al. (2005). Ppd-H1 is the only barley highly confidence gene MLOC_81154.10 (HORVU2Hr1G013400) located in morex_contig_94710 with all above mentioned iSELECT markers at 2H (19.9 cM) based on the recently published high-quality reference genome assembly (Mascher et al. 2017). Population stratification of the germplasm panel was previously published and structured based on M9/SNP22 (G/T) (Alqudah et al. 2014, 2016). Network analysis of nine Ppd-H1 SNP haplotypes was carried out using TCS v1.21 software (http://darwin.uvigo.es/software/tcs.html; Clement (2000) in 215 spring barley accessions (Fig. 1b). To find more information about the accessions’ origins and photoperiod status are present in Table S1.
Fig. 1.
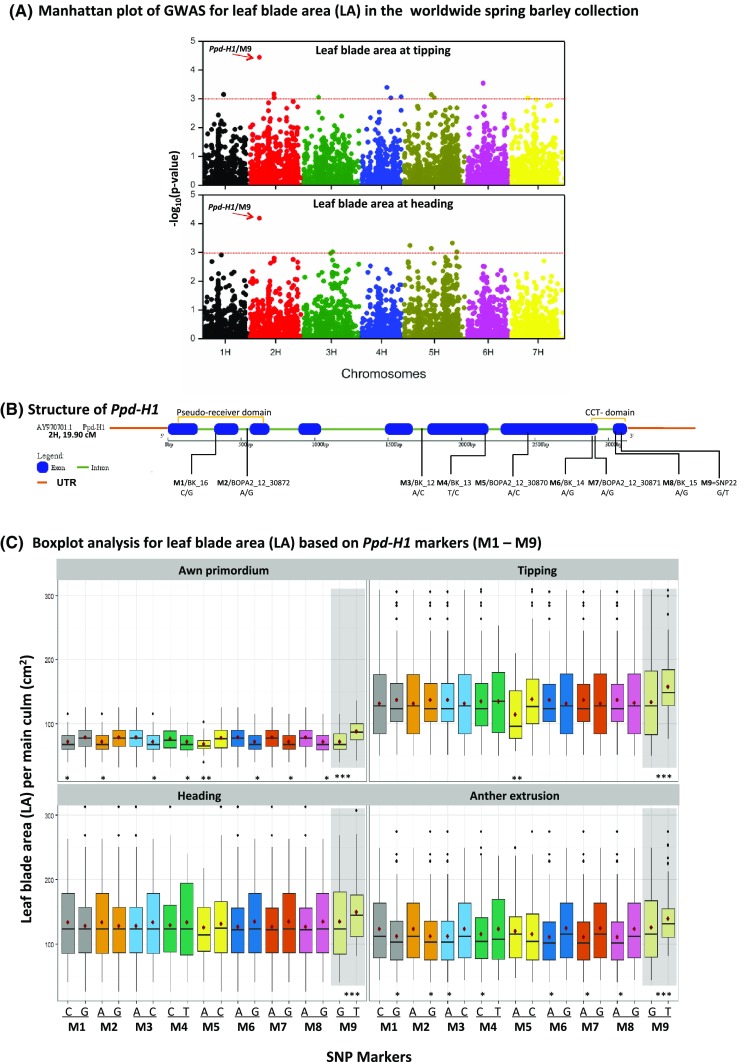
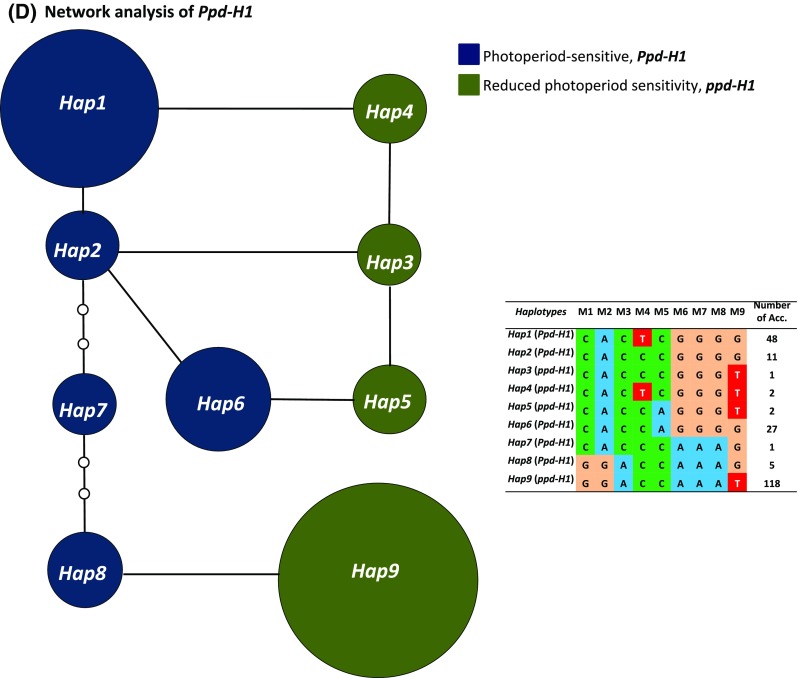
a Manhattan plot of GWAS for leaf blade area at tipping and heading stages; the red-arrow indicates the location of M9 at chromosome 2HS, and the red dotted line denote the threshold significance level, − log10 (P value of 0.001). b Ppd-H1 gene structure. c Box plot analysis of leaf area based on the nine SNPs derived from Ppd-H1. The degree of significance indicated as *P, 0.05; **P, 0.01; ***P, 0.001. Significant differences (P ≤ 0.05) were determined using LSD. Significant differences between the alleles of each marker were calculated at each developmental stage separately. d Network analysis of Ppd-H1 in 215 worldwide spring barley accessions, dark-blue for photoperiod-sensitive haplotypes and dark-green for reduced photoperiod sensitivity. Three biological replicates were used from each accession at each pre-anthesis developmental stage (n = 92 and 123 for photoperiod sensitive and reduced photoperiod sensitivity barley, respectively)
Phenotype data
Thirty plants from each of the 215 spring barley accessions were grown under GH conditions (long-day: LD condition, 16/8 h day/night and ~ 20/~ 16 °C day/night) at the Leibniz Institute of Plant Genetics and Crop Plant Research (IPK) in Gatersleben, Germany (51°49′23″N, 11°17′13″E, altitude 112 m). Details about how the accessions of the population were grown have been previously described (Alqudah et al. 2014). LA of completely unfolded and fully developed leaf blades from the main culm was harvested by hand (all leaves together) at different developmental stages. (AP: awn primordium, Alqudah and Schnurbusch (2014); TIP: tipping, Z49; HD: heading, Z55; AE: anther extrusion, Z65; Hrv: Harvesting, Zadoks et al. (1974). Then the total leaf blade area of the main culm (LA, cm2) was measured by LA-meter (portable Li-COR area meter, Li-3000) as described by Alqudah and Schnurbusch (2015). The data were collected from three randomly selected biological replicates per accession. Summary statistics for LA in each group at different developmental stages were determined by GenStat 16 (GenStat 2014). Analysis of variance (ANOVA) was conducted to compare the differences between accessions, geographic region and groups (Ppd-H1 vs. ppd-H1) using GenStat 16 (GenStat 2014) at probability level P ≤ 0.05. Means were separated according to the Fisher’s least significant difference (LSD) at P ≤ 0.05 levels of probability. Best linear unbiased estimates (BLUEs) was used to estimate each accession’s phenotypic means, which in turn were used in the association analysis while residual maximum likelihood (REML) was used to analyze phenotypic data (GenStat 2014). Broad-sense heritability for LA in each group was calculated across growth times as the ratio between the genetic variance and the phenotypic variance components using GenStat 16 (GenStat 2014).
Association analysis
GWAS of each Ppd-H1 group was performed for LA at each developmental stage using the markers those passing marker-mining tests. Associations between estimated phenotypic traits (BLUEs) and each single marker was calculated using mixed linear model (MLM) in GenStat 16 (GenStat 2014). Eigen-analysis was used as a correction of population structure in each sub-population (Price et al. 2006). The strategy of GWAS analysis, allele mining, allele effect, validation of association [false discovery rate (FDR 0.001)] and creating QTL-association map have been described by Alqudah et al. (2014). Average linkage disequilibrium (LD) for the germplasm panel was previously determined (± 5 cM; Pasam et al. 2012) and used as a confidence interval to determine associated QTL. The Barke × Morex RIL POPSEQ population was used to find the genetic position anchored by physical map positions of highly associated SNP markers (SNPs ≥ FDR) and putative orthologous barley genes (characterized for LA phenotypes in other species) (Mascher et al. 2013). The sequences of highly associated markers (which show a consistent effect on LA, SNPs ≥ FDR) were blasted on the barley genome dataset and gene set (BARLEX; http://apex.ipk-gatersleben.de). The most significant hit was selected to obtain the corresponding genes, transcripts and gene annotation. Functional annotations of the candidate genes were also confirmed using known function in other cereal crops such as rice. Detailed information about these genes, their Genbank accession numbers, and genetic chromosome positions are available in Table S2.
Results
GWAS reveals a specific Ppd-H1 haplotype as major driver for LA variation
GWAS analyses showed several genomic regions associated with LA at TIP and HD stages (Fig. 1a) with the most significant and consistent association signal at Ppd-H1/M9 (Fig. 1a). Based on the physical position of the SNP markers, nine markers (M1–M9) are physically localized within the Ppd-H1 gene (Fig. 1b). M9 was located in the CCT domain and produced one amino acid change [Gly-to-Trp, G/T, Turner et al. (2005)] that defines the ppd-H1 allele (T/M9; reduced photoperiod sensitivity allele). Phenotypic analysis for LA based on the nine Ppd-H1 markers (M1–M9) showed significant differences in LA between the alleles of each marker at the studied developmental stages (Fig. 1c). The results clearly indicated that M9 had the highest effect (***P, 0.001) among the Ppd-H1-derived markers at all developmental stages (Fig. 1c). The accessions carrying the T/M9 allele (reduced photoperiod sensitivity allele) had larger LA than the accessions carrying photoperiod-sensitive allele G/M9 (***P, 0.001; Fig. 1c). To further explore sequence variation at the Ppd-H1 locus, haplotype analysis of the spring barley accessions using these nine SNPs was performed (Fig. 1d). Five haplotypes (Hap1, 2, 6, 7 and 8) were found in 92 accessions carrying sensitive alleles of Ppd-H1. The other four haplotypes (Hap3, 4, 5 and 9) were represented in 123 accessions carrying the insensitive allele of ppd-H1. Notably, the majority of these four insensitive haplotypes, i.e. Hap3, 4, 5 and 9, are mainly from EU (91 accessions) (Fig. 1d). The analyses of the nine markers derived from Ppd-H1 (Fig. 1a, c, d) clearly indicated that the population can be divided into two groups for photoperiod responses based upon M9. Therefore, GWAS analyses were conducted for the two groups separately; accessions carrying the reduced photoperiod sensitivity ppd-H1 allele T/M9 (123 accessions) and a group of 92 accessions carrying the photoperiod-sensitive allele Ppd-H1G/M9 allele [Figure S1 (Alqudah et al. 2014, 2016)].
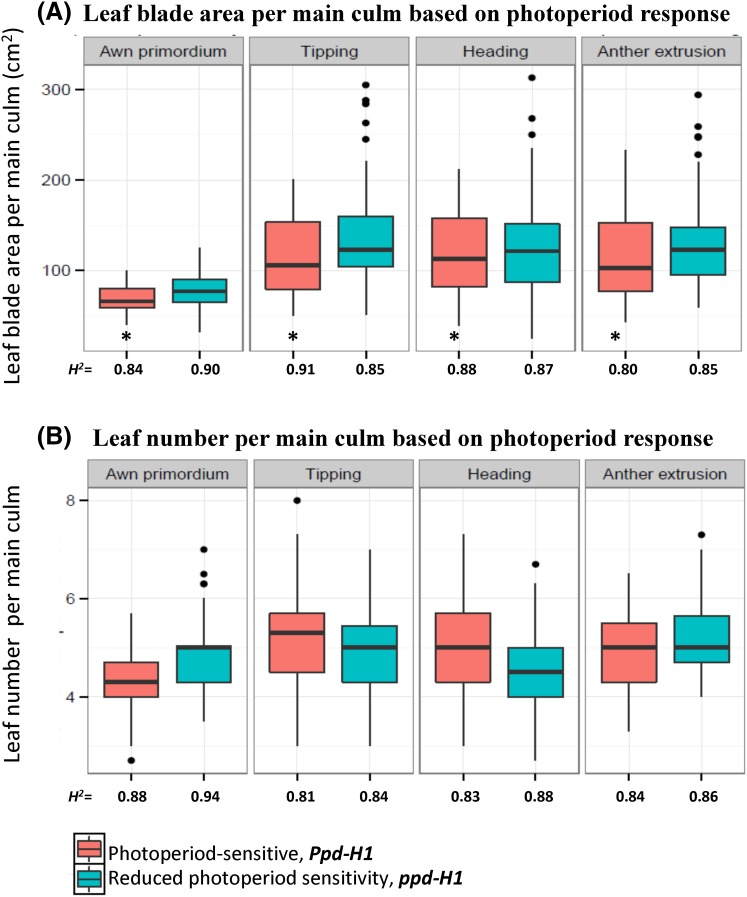
Phenotypic analysis of 215 worldwide spring barley accessions showed that the accessions significantly varied (at P < 0.001) in main culm LA at all developmental stages (Table S3). Interestingly, significant differences in LA among the geographic regions of accessions were found at all developmental stages (Table S3). Photoperiod status of accessions (i.e. Ppd-H1/ppd-H1) was associated with variation in LA at all developmental stages (Table S3). These findings indicated that LA in 215 accessions was influenced by photoperiod status and/or geographical regions. Maximum LA was reached at TIP/HD; thereafter LA decreased especially in photoperiod sensitive accessions (Fig. 2a). Accessions carrying sensitive alleles at Ppd-H1 showed significantly smaller LA than ppd-H1 accessions (at P < 0.05) at all developmental stages (Fig. 2a). For leaf number, there were no significant differences between photoperiod groups (at P < 0.05) at all developmental stages (Fig. 2b), therefore we excluded it from further genetic analyses. The correlation analysis between LA and leaf number per the main culm across all developmental stages showed a moderate positive relationship (Figure S2).
Fig. 2.
Box plot analysis of leaf blade area (a) and leaf number (b) per the main culm based on photoperiod response. n = 92 accessions of photoperiod sensitive (Ppd-H1) and n = 123 accessions with reduced photoperiod sensitivity (ppd-H1). AP awn primordium, Alqudah and Schnurbusch (2014); TIP: tipping, Z49; HD: heading, Z55; AE: anther extrusion, Z65; Hrv: Harvesting, Zadoks et al. (1974). Asterisk denotes leaf area significantly different at P ≤ 0.05 according to LSD between photoperiod groups at the same developmental stage. The degree of significance indicated as *P, 0.05; **P, 0.01; ***P, 0.001
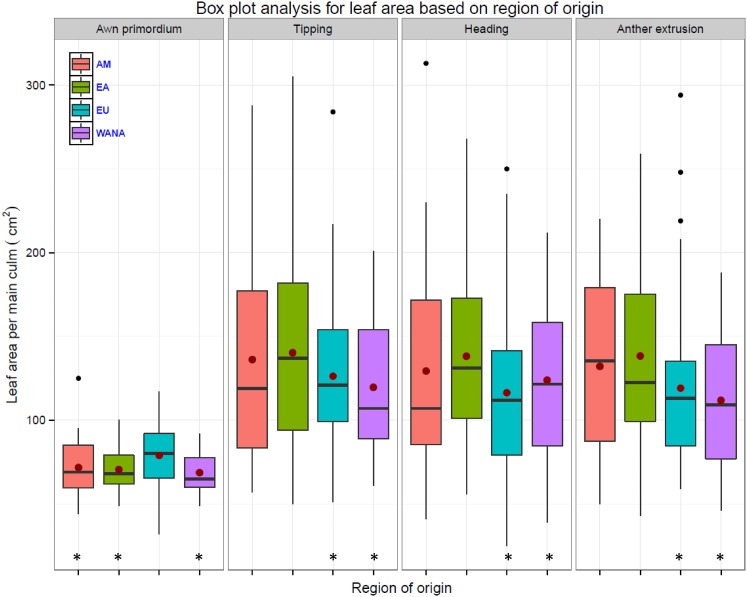
Based on the geographic region of the accessions (including the accessions from both photoperiod groups), European (EU) accessions had significantly larger LA at AP (Fig. 3) followed by accessions from East Asia (EA). There were no significant differences between the regions of the origin in LA within the Ppd-H1 group at all developmental stages (Figure S3a). Whereas the accessions from EA and Americas (AM) had the largest LA in comparison with accessions from EU, West Asia and North Africa (WANA) in the ppd-H1 group (Figure S3b). Leaf number per main culm was not significantly different between the geographic regions of the accessions (Figure S4).
Fig. 3.
Box plot analysis of leaf blade area per the main culm at different developmental stages of 215 spring barley accessions from the different geographical region. Asterisk denotes leaf area significantly different at P ≤ 0.05 according to LSD between geographical region at the same developmental stage. The degree of significance indicated as *P, 0.05; **P, 0.01; ***P, 0.001 (n = 92 and 123 for Ppd-H1 and ppd-H1 barleys, respectively). Number of accessions for WANA = 45, EU = 105, EA = 37 and AM = 28
The genetic variation in LA at the TIP stage between the accessions was explained in the principle component analysis (PCA) using 6355 SNPs. The accessions were almost separated into Ppd-H1 and ppd-H1 spring barleys (Figure S5) confirming the population structure of the collection (Figure S1). The broad-sense heritability values for LA at the developmental stages in each photoperiod group (Ppd-H1 and ppd-H1) were above 0.75 (Fig. 2a), indicating that LA is a highly heritable trait at early- and late-developmental stages and under these growth conditions, which helped us to detect particular QTL for each stage within each photoperiod group.
Natural genetic variation of LA and marker-trait association using GWAS at four developmental stages in two photoperiod groups
A GWAS was performed at AP, TIP, HD, and AE developmental stages for each photoperiod group (Ppd-H1 and ppd-H1) independently using 9K array and mixed models approach (Figure S6). The GWAS analysis showed that most of the significant associations appeared after AP stage, suggesting that there is ample natural phenotypic/genetic variation for LA during late-reproductive phases (after AP to AE).
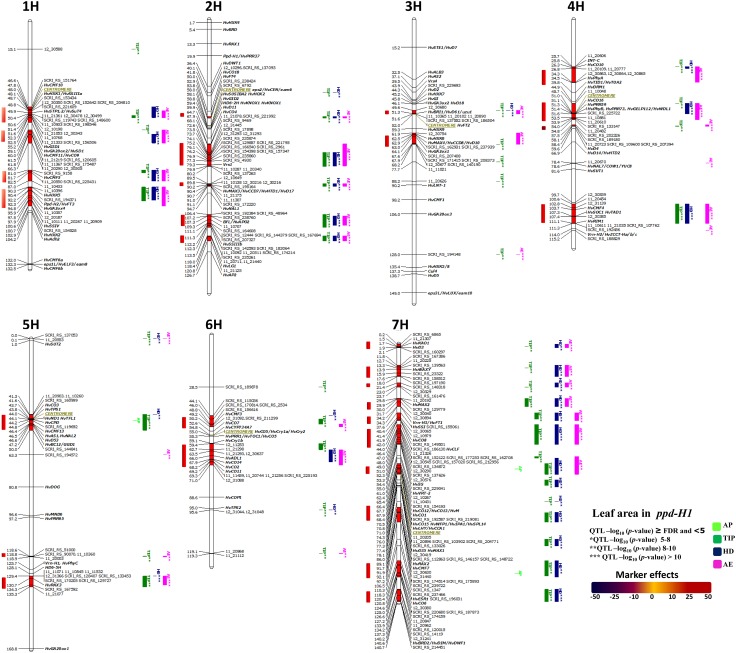
QTL for LA in the Ppd-H1 group
The association analysis in 92 photoperiod sensitive (Ppd-H1) accessions detected four significantly associated chromosomal regions (Figure S6), of which two are without known candidate colocation on 1H, 95.9–97.9 cM and 2H, 50.9–56.4 cM (Fig. 4). These two group-specific QTL show a strong effect on LA variation at different pre-anthesis stages (Fig. 4). The associated markers of the first QTL region (1H) showed contrasting effects of markers within the QTL, indicating that there is allelic variation among the accessions of this group. The second QTL appeared at AP and TIP developmental stages and confirmed the importance of pre-anthesis stages in natural variation of LA. The remaining QTL regions very precisely co-localized with known plant stature and/or heading time genes, such as 1H, 50.4–55.7 cM, which is physically close to TREHALOSE-6-PHOSPHATE SYNTHASE1 (HvTPS1), SUCROSE TRANSPORTER4 (HvSUT4) and GIBBERELLIN INSENSITIVE DWARF1 (HvGID1) genes, and include seven markers, suggesting a role in LA variation. The final association was around the centromeric region of 7H (67.7–71 cM; Fig. 4), including genes CONSTANS 12/13 HvCO 12/HvCO 13/HvM; HvCO1, WEALTHY FARMERS PANICLE1/IDEAL PLANT ARCHITECTURE1/SQUAMOSA PROMOTER BINDING PROTEIN-LIKE14 (HvWFP1/HvIPA1/HvSPL14); LATE ELONGATED HYPOCOTYL/CIRCADIAN CLOCK ASSOCIATED1 (HvLHY/HvCCA1), showed the highest effects on LA variation. The markers in this region with – log10 > FDR showed negative effects (i.e. reduced LA by around − 3 cm2). In addition to these associations, we found two single-marker-trait associations, (2H, 147.3 and 4H, 123.3 cM); however, we could not find more associated markers to these regions most likely due to the limited number of accessions in this group. The region of SIX-ROWED SPIKE 1 (Vrs1) gene (2H, 79.3–80.2 cM) was found to be associated with LA only causing variation at later developmental stages (AE) in this group (Thirulogachandar et al. 2017).
Fig. 4.
Genetically anchored position of highly associated QTL for leaf blade area per the main culm at all barley developmental stages in the photoperiod sensitive (Ppd-H1) group using 9K SNP markers. Bold and italicized gene names indicate genetically anchored positions of known heading time and plant stature genes in the Barke × Morex RILs. Associated chromosomal regions are highlighted with different colors according to developmental stages. Red chromosomal areas indicate the range of significantly associated QTL (within confidence interval ± 5 cM) which are exceeding FDR level of each developmental stage (n = 92 for Ppd-H1 group)
QTL for LA in the ppd-H1 group
The genetic architecture for LA was much more complex and informative in the panel of 123 accessions carrying the less functional ppd-H1 allele. An important feature for this group is that the genetic variation of LA was maximized in stages after TIP (Fig. 5). Moreover, all of the highly associated markers showed positive effects on LA (i.e. increasing LA up to + 6 cm2; Fig. 5). Association analysis in this group showed 34 significantly associated chromosomal regions of which 29 are group-specific (Fig. 5). Seven group-specific QTL were located in the following regions: 2H, 50.0–52.9 and 118–120.8; 4H, 75.5–59.6; 5H, 0.0–0.1 and 118.6–118.9; 6H, 119.1–119.3 and 7H, 21.4–23.7 cM, demonstrating ample allelic diversity for LA in this group.
Fig. 5.
Genetically anchored position of highly associated QTL for leaf blade area per the main culm at all barley developmental stages in the reduced photoperiod sensitivity (ppd-H1) group using 9K SNP markers. Bold and italicized gene names indicate genetically anchored positions of known heading time and plant stature genes in the Barke × Morex RILs. Associated chromosomal regions are highlighted with different colors according to developmental stages. Red chromosomal areas indicate the range of significantly associated QTL (within confidence interval ± 5 cM) which are exceeding FDR level of each developmental stage (n = 123 for ppd-H1 group)
Regions exhibiting strong effects on LA in this group as shown in Fig. 5 span some potential candidate genes involved in strigolactone, brassinosteroid and gibberellin biosynthesis/metabolism, e.g. 2H 89.5–91.1, includes HvMAX3/HvCCD7/HvHTD1/HvD17 (MORE AXILLARY BRANCHES 3/CAROTENOID CLEAVAGE DIOXYGENASE 7/HIGH-TILLERING DWARF 1/DWARF 17); 3H 49.6–51.6 and 61.9–68.2, HvBRI1/UZU1/HvD61 (BRASSINOSTEROID INSENSITIVE1/SEMIBRACHYTIC/DWARF61) and HvHXK6, HvMAX4/CHvCD8/HvD10, HvGA2ox1 (MORE AXILLARY BRANCHES 4/CAROTENOID CLEAVAGE DIOXYGENASE 8/DWARF10, GIBBERELLIN 20 OXIDASE 1), respectively; 5H 129.4–135.3, HvRAX3 (REGULATOR OF AXILLARY MERISTEMS 3); 7H 0.2–2.1, HvKAO1, HvD3 (ENT-KAURENOIC ACID HYDROXYLASE 1, DWARF 3), 11.8–18.0, WAXY, 44.0–55.0 HvCLF (CURLY LEAF) and HvSS1 (SUCROSE SYNTHASE 1), 133.9–140.7, HvBRD2/HvDIM/HvDWF1 (BRASSINOSTEROID DEFICIENT DWARF 2/DIMINUTO/DWARF 1). All of these regions showed highly significant associations (− log10 P value > 8**) at the developmental stages after AP (i.e. TIP, HD and AE stages; Fig. 5).
GWAS analysis in the ppd-H1 group identified putative associations of barley LA variation with sugar biosynthetic/metabolism-related candidate genes. One of these associations is located on 5H (0.0–1.0 cM) showing strong effect (− log10 > 10; Fig. 5) of HvSUT2 on LA after AP stage. A second QTL (located on 6H 95.0–95.6 cM) was associated with strong effects at TIP and HD developmental stages and spanned HvTPS2 (Fig. 5). These findings suggest a potential influence of sugar biosynthetic/metabolism related genes on LA at different developmental stages.
Six significant chromosomal regions co-localized with putative heading time genes (heading time-specific associations; Fig. 5). For instance, on 1H, 81.0–87.0 cM including HvCMF5 (CCT MOTIF FAMILY 5); 4H, 25.7–34.5, HvCO10 (CONSTANS 10), HvPhyA (PHYTOCHROM A); 6H, 44.1–50.2 and 59.4–71 including HvCMF3, HvCO14, HvCO2, HvCO11, respectively. These findings indicate the potential role of such genes in barley LA particularly when carrying the ppd-H1 allele.
The GWAS analysis within the ppd-H1 group showed seven strong associated chromosomal regions (− log10 > 10; Fig. 5). Two of these associated regions were located on 1H, 59.1–66.3 and 90.3–100.7 cM including HEXOKINASE 5 (HvHXK5), HvGA20ox2/HvSD1 (GIBBERELLIN 20 OXIDASE 2/SEMIDWARF1) and HvCMF11/HvCO9, and Ppd-H2/HvFT3 (PHOTOPERIOD RESPONSE LOCUS 2/FLOWERING TIME LOCUS 3) and HvGA2ox4. In chromosome 4H we found another association at 100.6–111.3 cM, contains HvCMF4, HvSOC1 (SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1), HvTAD1 (TILLERING AND DWARF 1), HvRIM1 (RICE DWARF VIRUS MULTIPLICATION 1). While the last four associated regions were co-located at 7H, 29.8–34.3 cM includes HvMAX2 and Vrn-H3/HvFT1; 7H, 37.6–42.0 cM includes SOLUBLE STARCH SYNTHASE 1 (HvSSI) and HvCO8; and 7H, 89.1–97.2 cM includes HvRAX2 and HvCMF7; 118.3–127.2 includes HvESR1 (ENHANCER OF SHOOT REGENERATION 1) and HvCO6. Moreover, it is very interesting to note detection of the region close to BFL/HvAPO2 (Barley FLORICAULA/LEAFY/ABERRANT PANICLE ORGANIZATION 2; 2H, 106.4–113.5 cM) and SOLUBLE STARCH SYNTHASE IIIb (HvSSIIIb; 2H, 112.1 cM) as highly associated with LA in this group.
The analysis showed that four strong associations (− log10 > 8; Fig. 5) were found in the centromeric region such as 1H, 46.6–48.8 cM includes HvCMF10 and four sugar-related genes (HvHXK1, HvSSIIIb, HvTPS1 and HvSUT4). The centromeric region of 4H is physically close to HvCO16, HvPRR59, HvPhyB, HvPRR73, and HvGELP112/HvWDL1 (GDSL ESTERASE/LIPASE PROTEIN 112, WILTED DWARF AND LETHAL 1); and 5H, 41.3–50.0 cM includes HvCO3, TREHALOSE-6-PHOSPHATE SYNTHASE 1 (HvTPS1), HvND1/HvTFL1/CSLD (NARROW LEAF AND DWARF 1/TERMINAL FLOWER 1/CURLED LEAF AND DWARF 1), HvCPD (BRASSINOSTEROID C-23 HYDROXYLASE), HvCMF13, HvAS1 (ASPARAGINE SYNTHASE 1), HvD53, HvBC12/GGD1 (BRITTLE CULM12/GIBBERELLIN-DEFICIENT DWARF 1). Finally, the centromeric region of 7H, 65.4–71.2 cM contains HvCO12/HvCO13/HvM, HvCO15, HvWFP1/HvIPA1/HvSPL14, HvLHY/HvCCA1. Due to the strong linkage between genes in the centromeric region, it is not clear which of these genes have an effect on LA. Among these associated chromosomal regions, the SNPs located at 5H 41.3–50.0 cM (Fig. 5) showed the strongest phenotypic effect on LA by increasing LA up to + 6 cm2 (Fig. 5) and occurred exclusively in this group (i.e. ppd-H1).
Discussion
The analysis of LA in our worldwide spring barley collection revealed that there is ample natural variation. Here, we measured LA at different developmental stages for better understanding the underlying genetic factors of LA based on the cumulative main culm LA which is more realistic than individual LA (e.g. flag leaf). Recently, Kebrom and Mullet (2015) highlighted the importance of main shoot leaves as the major source of sugar synthesis and photosynthetic area in sorghum. Utilizing such phenotyping approach under controlled greenhouse conditions and single plants for other cereal crops is therefore important to shed light on the genetic make-up of leaf architecture.
Digel et al. (2016) found that Ppd-H1 controls leaf size in winter barley through controlling duration of cell proliferation and leaf maturation whereas the SNP22 showed the highest effect on leaf size. In the current study, the population structure using two Ppd-H1 alleles (i.e. M9/SNP22 G/T) was effective in uncovering LA variation in barley. The association signals showing many Ppd-H1 group-specific QTL with strong effects on LA variation for instance on 1H, 95.9–97.9 cM; 2H, 50.9–56.4 and 147.3 cM; and finally 4H, 123.3 cM. These are putatively new QTL that appeared during pre-anthesis phases in this panel, demonstrating that advanced genetic analysis for these associations is needed to understand the genetic variation of LA, when Ppd-H1 alleles are functional and fully photoperiod responsive. GID1 was previously shown in rice as soluble receptor mediating GA responses with clear impact on leaf elongation (i.e. the mutant of GID1 produces short leaf length; Ueguchi-Tanaka et al. (2005)), the detected association around GID1 suggests that a mutated allele of HvGID gene might be present in this barley panel.
The accessions carrying the less active allele of Ppd-H1 (i.e. ppd-H1) showed several new group-specific QTL [e.g. (2H, 50.0–52.9 cM and 118–120.8 cM); 4H, 75.5–59.6 cM; (5H, 0.0–0.1 cM and 118.6–118.9 cM); 6H, 119.1–119.3 cM and 7H, 21.4-23.7 cM)] that only appeared here. We were unable to co-locate known candidate genes in these regions, confirming that ppd-H1-carrying accessions exhibited a complex genetic architecture and studying natural genetic variation of LA in this group is worthwhile for further genetic analysis.
Interestingly, one association controlling LA variation in the ppd-H1 group is localized in the region harboring the BFL gene that has not been reported so far in plants. BFL has been implicated to be involved in phase duration and tillering in barley (Alqudah et al. 2014, 2016) and rice (Rao et al. 2008). It was shown that RFL (rice ortholog of BFL) is similarly involved in gibberellin (GA20ox), carotenoid or brassinosteroid biogenesis in rice (Rao et al. 2008). The value of these hormones in leaf development elucidated via their pivotal role in controlling leaf cells proliferation in maize and Arabidopsis (Nakaya et al. 2002; Nelissen et al. 2012) that can postulate the role of BFL gene in barley LA.
In the present study, genomic regions associated with LA were found to span genes involved in the strigolactone biosynthetic pathway such as HvMAX3/HvCCD7/HvHTD1/HvD17; and HvMAX4/CHvCD8/HvD10. The function of these genes is well known in maize as being influential on stem, panicle and root architecture through controlling sequential carotenoid cleavage reactions (Guan et al. 2012). Further studies are needed to evaluate the potential role of these genes in LA.
Similar conclusions can be drawn for gibberellin gene families, which have been associated with LA in the ppd-H1 panel; for instance GA20ox genes (HvGA20ox1, HvGA20ox2/HvSD1, HvGA20ox4) highlighted the importance of these genes for LA. These enzymes are involved in the biosynthesis of active gibberellin forms that in turn may control tillering in barley (Alqudah et al. 2016) and rice plant stature (Lo et al. 2008). In addition to these genes, HvKAO1 and HvBC12/HvGGD1 that are strongly involved in rice plant stature by regulating the GA biosynthesis pathway (Helliwell et al. 2001; Li et al. 2011) were found to be associated with LA in our panel. These observations suggest that accessions carrying the ppd-H1 allele are enriched in genetic variation for GA biosynthesis genes for LA formation. Functional analysis of the proposed link between the GA and LA will maximize our knowledge in leaf development.
Strong connections between rice leaf blade characters and OsBRD2 were found, whereas the leaf phenotype of its mutant becomes more erect, shortened and its overexpression influences plant stature components (Hong et al. 2005). In the current study, we found marker-trait associations in the vicinity of brassinosteroid genes, for instance, HvBRD2/HvDIM/HvDWF1, HvCPD, and HvBRI1/UZU1/HvD61, which are crucial for plant architecture including leaf length and width (Dockter et al. 2014). The leaf phenotype of HvBRI1 mutant (uzu1.a) showed a leaf-unrolling and acute leaf-blade angle as brassinosteroid signaling-deficient. In rice, Brassinosteroid Insensitive 1 (BRI1)-Associated Kinase I (BAK1) has been recently reported that it regulates cell number and enlargement in leaf (Khew et al. 2015). Our GWAS results suggest that there is ample effect of brassinosteroid biosynthetic/metabolism genes on the genetic variation of LA in barley accessions carrying ppd-H1 that have to be validated through further genetic analysis.
Plant stature traits, e.g. bud outgrowth in sorghum, are influenced by LA (Kebrom and Mullet 2015). In Arabidopsis, sucrose prompts leaf cell proliferation and delay the transition to cell expansion via repression of multiple chloroplast-encoded genes and up-regulation of sugar-related genes suggesting that sucrose plays a critical role in the leaf growth (Van Dingenen et al. 2016). Therefore, it might be possible to suggest that sugar-related genes in the accessions carrying-ppd-H1 (i.e. late heading time) play critical role that lead for further stimulation of cell proliferation subsequently larger LA. Further molecular and genetic evidence are essential to validate the proposed link.
Interestingly, several associations were close to genes carrying single CCT [CO, CO-LIKE, TIMING OF CAB1 (TOC1)] and/or b-box domains such as HvCMF and HvCO family genes. Circadian Clocks have a substantial role in plant growth and adaptation, for instance LA of Arabidopsis short-period mutant of TOC1 (toc1-1) showed large LA (Dodd et al. 2005). Postulating a similar role of this gene in the accessions carrying-ppd-H1 allele requires advanced genetic analysis to characterize and understand its role in LA variation.
It was previously shown that the centromeric region of 7H has a strong effect on phase transition (Alqudah et al. 2014) and tillering in this population (Alqudah et al. 2016). Our findings demonstrated that this region is rich in genes that are important for natural variation of different developmental traits. Contrasting effect of highly associated markers in this region had been detected (i.e. reduced LA by − 3 cm2 in Ppd-H1 accessions, while increased LA by + 4.5 cm2 in ppd-H1 accession), indicating that there is allelic variation of at least one gene. Moreover, the region of 7H centromere includes the HvGELP112/HvWDL1 gene, which regulates rice plant stature (Park et al. 2010), suggesting that this gene is potentially a candidate, especially in the ppd-H1 group of accessions.
Conclusion
LA is a complex trait that is regulated by endogenous and environmental factors; however, their interactions are not well understood. The previous lack of information about the natural variation of LA in temperate cereals increased the complexity of this trait for yield improvement. Many putative candidate gene families like CMF-, CO-like genes, sugar-related genes, strigolactones, gibberellin and brassinosteroid biosynthesis genes are proposed to be involved in LA formation. This study gives an overview regarding genetic factors controlling LA that may establish the basis for further work in cereals. More advanced molecular genetic analyses are required to validate the function of the candidate associations.
Author contribution statement
Conceived the project: TS. Designed and performed the experiments: AMA, TS. Analyzed the data: AMA. Network analysis: HMY, AMA. Germplasm Resource and Genotyping: AG. Wrote the paper: AMA, TS with contributions from all co-authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank Annett Beyer for excellent technical assistance. We also thank IPK gardeners for excellent assistance during this work. This study was financially supported by the German Research Council (DFG), Grant number SCHN 768/4-1, the German Federal Ministry of Education and Research (BMBF) GABI-FUTURE Start Program, Grant number 0315071, and IPK core funding to TS.
Compliance with ethical standards
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Electronic supplementary material
The online version of this article (10.1007/s00122-018-3053-2) contains supplementary material, which is available to authorized users.
Contributor Information
Ahmad M. Alqudah, Email: alqudah@ipk-gatersleben.de
Thorsten Schnurbusch, Phone: +49-39482-5826, Email: thor@ipk-gatersleben.de.
References
- Alqudah AM, Schnurbusch T. Awn primordium to tipping is the most decisive developmental phase for spikelet survival in barley. Funct Plant Biol. 2014;41:424–436. doi: 10.1071/FP13248. [DOI] [PubMed] [Google Scholar]
- Alqudah A, Schnurbusch T. Barley leaf area and leaf growth rates are maximized during the pre-anthesis phase. Agronomy. 2015;5:107–129. doi: 10.3390/agronomy5020107. [DOI] [Google Scholar]
- Alqudah AM, Sharma R, Pasam RK, Graner A, Kilian B, Schnurbusch T. Genetic dissection of photoperiod response based on GWAS of pre-anthesis phase duration in spring barley. PLoS One. 2014;9:e113120. doi: 10.1371/journal.pone.0113120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alqudah AM, Koppolu R, Wolde GM, Graner A, Schnurbusch T. The genetic architecture of barley plant stature. Front Genet. 2016;7:117. doi: 10.3389/fgene.2016.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bing Y, Wei-Ya X, Li-Jun L, Yong-Zhong X. QTL analysis for flag leaf characteristics and their relationships with yield and yield traits in rice. Acta Genet Sin. 2006;33:824–832. doi: 10.1016/S0379-4172(06)60116-9. [DOI] [PubMed] [Google Scholar]
- Chen W, Xu Z, Zhang L. Physiological bases of super high yield breeding in rice. Shenyang: Liaoning Science and Technology Publishing Company; 1995. [Google Scholar]
- Chenu K, Chapman SC, Hammer GL, McLean G, Salah HBH, Tardieu F. Short-term responses of leaf growth rate to water deficit scale up to whole-plant and crop levels: an integrated modelling approach in maize. Plant Cell Environ. 2008;31:378–391. doi: 10.1111/j.1365-3040.2007.01772.x. [DOI] [PubMed] [Google Scholar]
- Clement M. TCS: a computer program to estimate gene genealogies. Mol Ecol. 2000;9:1657–1660. doi: 10.1046/j.1365-294x.2000.01020.x. [DOI] [PubMed] [Google Scholar]
- Digel B, Tavakol E, Verderio G, Tondelli A, Xu X, Cattivelli L, Rossini L, von Korff M. Photoperiod-H1 (Ppd-H1) controls leaf size. Plant Physiol. 2016;172:405–415. doi: 10.1104/pp.16.00977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockter C, Gruszka D, Braumann I, Druka A, Druka I, Franckowiak J, Gough SP, Janeczko A, Kurowska M, Lundqvist J, Lundqvist U, Marzec M, Matyszczak I, Muller AH, Oklestkova J, Schulz B, Zakhrabekova S, Hansson M. Induced variations in brassinosteroid genes define barley height and sturdiness, and expand the green revolution genetic toolkit. Plant Physiol. 2014;166:1912–1927. doi: 10.1104/pp.114.250738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd AN, Salathia N, Hall A, Kevei E, Toth R, Nagy F, Hibberd JM, Millar AJ, Webb AAR. Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science. 2005;309:630–633. doi: 10.1126/science.1115581. [DOI] [PubMed] [Google Scholar]
- Donald CM. The breeding of crop ideotypes. Euphytica. 1968;17:385–403. doi: 10.1007/BF00056241. [DOI] [Google Scholar]
- Driever SM, Lawson T, Andralojc PJ, Raines CA, Parry MAJ. Natural variation in photosynthetic capacity, growth, and yield in 64 field-grown wheat genotypes. J Exp Bot. 2014;65(17):4959–4973. doi: 10.1093/jxb/eru253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers JB. Sugar as a key component of the shoot branching regulation network. Plant Cell Environ. 2015;38:1455–1456. doi: 10.1111/pce.12519. [DOI] [PubMed] [Google Scholar]
- Fujino K, Matsuda Y, Ozawa K, Nishimura T, Koshiba T, Fraaije M, Sekiguchi H. NARROW LEAF 7 controls leaf shape mediated by auxin in rice. Mol Genet Genom. 2008;279:499–507. doi: 10.1007/s00438-008-0328-3. [DOI] [PubMed] [Google Scholar]
- GenStat . VSN. GenStat for Windows. 16. UK: VSN International Hemel Hempstead; 2014. [Google Scholar]
- Gonzalez N, Vanhaeren H, Inzé D. Leaf size control: complex coordination of cell division and expansion. Trends Plant Sci. 2012;17:332–340. doi: 10.1016/j.tplants.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Guan JC, Koch KE, Suzuki M, Wu S, Latshaw S, Petruff T, Goulet C, Klee HJ, McCarty DR. Diverse roles of strigolactone signaling in maize architecture and the uncoupling of a branching-specific subnetwork. Plant Physiol. 2012;160:1303–1317. doi: 10.1104/pp.112.204503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyenis L, Yun SJ, Smith KP, Steffenson BJ, Bossolini E, Sanguineti MC, Muehlbauer GJ. Genetic architecture of quantitative trait loci associated with morphological and agronomic trait differences in a wild by cultivated barley cross. Genome Natl Res Counc Canada. 2007;50:714–723. doi: 10.1139/G07-054. [DOI] [PubMed] [Google Scholar]
- Helliwell CA, Chandler PM, Poole A, Dennis ES, Peacock WJ. The CYP88A cytochrome P450, ent-kaurenoic acid oxidase, catalyzes three steps of the gibberellin biosynthesis pathway. Proc Natl Acad Sci. 2001;98:2065–2070. doi: 10.1073/pnas.98.4.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Z, Ueguchi-Tanaka M, Fujioka S, Takatsuto S, Yoshida S, Hasegawa Y, Ashikari M, Kitano H, Matsuoka M. The rice brassinosteroid-deficient dwarf2 mutant, defective in the rice homolog of Arabidopsis DIMINUTO/DWARF1, is rescued by the endogenously accumulated alternative bioactive brassinosteroid, dolichosterone. Plant Cell. 2005;17:2243–2254. doi: 10.1105/tpc.105.030973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Zhu L, Zeng D, Gao Z, Guo L, Fang Y, Zhang G, Dong G, Yan M, Liu J, Qian Q. Identification and characterization of NARROW AND ROLLED LEAF 1, a novel gene regulating leaf morphology and plant architecture in rice. Plant Mol Biol. 2010;73:283–292. doi: 10.1007/s11103-010-9614-7. [DOI] [PubMed] [Google Scholar]
- Jennings PR. Plant type as a rice breeding objective. Crop Sci. 1964;4:13–15. doi: 10.2135/cropsci1964.0011183X000400010005x. [DOI] [Google Scholar]
- Jiang D, Fang J, Lou L, Zhao J, Yuan S, Yin L, Sun W, Peng L, Guo B, Li X. Characterization of a null allelic mutant of the rice NAL1 gene reveals its role in regulating cell division. PLoS One. 2015;10:e0118169. doi: 10.1371/journal.pone.0118169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jöst M, Hensel G, Kappel C, Druka A, Sicard A, Hohmann U, Beier S, Himmelbach A, Waugh R, Kumlehn J, Stein N, Lenhard M. The INDETERMINATE DOMAIN protein BROAD LEAF1 limits barley leaf width by restricting lateral proliferation. Curr Biol. 2016;26:903–909. doi: 10.1016/j.cub.2016.01.047. [DOI] [PubMed] [Google Scholar]
- Kebrom TH, Mullet JE. Photosynthetic leaf area modulates tiller bud outgrowth in sorghum. Plant Cell Environ. 2015;38:1471–1478. doi: 10.1111/pce.12500. [DOI] [PubMed] [Google Scholar]
- Kernich GC, Halloran GM, Flood RG. Variation in developmental patterns of wild barley H spontaneum and cultivated barley. Euphytica. 1995;82:105–115. doi: 10.1007/BF00027056. [DOI] [Google Scholar]
- Kessler S, Seiki S, Sinha N. Xcl1 causes delayed oblique periclinal cell divisions in developing maize leaves, leading to cellular differentiation by lineage instead of position. Development. 2002;129:1859–1869. doi: 10.1242/dev.129.8.1859. [DOI] [PubMed] [Google Scholar]
- Khew C-Y, Teo C-J, Chan W-S, Wong H-L, Namasivayam P, Ho C-L. Brassinosteroid insensitive 1-associated kinase 1 (OsI-BAK1) is associated with grain filling and leaf development in rice. J Plant Physiol. 2015;182:23–32. doi: 10.1016/j.jplph.2015.05.003. [DOI] [PubMed] [Google Scholar]
- Kirby E, Appleyard M. Cereal development guide. Stoneleigh: NAC Cereal Unit; 1987. [Google Scholar]
- Kirby EJM, Riggs TJ. Developmental consequences of 2-row and 6-row ear type in spring barley. 2. Shoot apex, leaf and tiller development. J Agric Sci. 1978;91:207–216. doi: 10.1017/S002185960005677X. [DOI] [Google Scholar]
- Lewis D, Bacic A, Chandler PM, Newbigin EJ. Aberrant cell expansion in the elongation mutants of barley. Plant Cell Physiol. 2009;50:554–571. doi: 10.1093/pcp/pcp015. [DOI] [PubMed] [Google Scholar]
- Li J, Jiang J, Qian Q, Xu Y, Zhang C, Xiao J, Du C, Luo W, Zou G, Chen M, Huang Y, Feng Y, Cheng Z, Yuan M, Chong K. Mutation of rice BC12/GDD1, which encodes a kinesin-like protein that binds to a GA biosynthesis gene promoter, leads to dwarfism with impaired cell elongation. Plant Cell Online. 2011;23:628–640. doi: 10.1105/tpc.110.081901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo S-F, Yang S-Y, Chen K-T, Hsing Y-I, Zeevaart JAD, Chen L-J, Yu S-M. A novel class of gibberellin 2-oxidases control semidwarfism, tillering, and root development in rice. Plant Cell Online. 2008;20:2603–2618. doi: 10.1105/tpc.108.060913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascher M, Muehlbauer GJ, Rokhsar DS, Chapman J, Schmutz J, Barry K, Munoz-Amatriain M, Close TJ, Wise RP, Schulman AH, Himmelbach A, Mayer KF, Scholz U, Poland JA, Stein N, Waugh R. Anchoring and ordering NGS contig assemblies by population sequencing (POPSEQ) Plant J Cell Mol Biol. 2013;76:718–727. doi: 10.1111/tpj.12319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascher M, Gundlach H, Himmelbach A, Beier S, Twardziok SO, Wicker T, Radchuk V, Dockter C, Hedley PE, Russell J, Bayer M, Ramsay L, Liu H, Haberer G, Zhang XQ, Zhang Q, Barrero RA, Li L, Taudien S, Groth M, Felder M, Hastie A, Simkova H, Stankova H, Vrana J, Chan S, Munoz-Amatriain M, Ounit R, Wanamaker S, Bolser D, Colmsee C, Schmutzer T, Aliyeva-Schnorr L, Grasso S, Tanskanen J, Chailyan A, Sampath D, Heavens D, Clissold L, Cao S, Chapman B, Dai F, Han Y, Li H, Li X, Lin C, McCooke JK, Tan C, Wang P, Wang S, Yin S, Zhou G, Poland JA, Bellgard MI, Borisjuk L, Houben A, Dolezel J, Ayling S, Lonardi S, Kersey P, Langridge P, Muehlbauer GJ, Clark MD, Caccamo M, Schulman AH, Mayer KFX, Platzer M, Close TJ, Scholz U, Hansson M, Zhang G, Braumann I, Spannagl M, Li C, Waugh R, Stein N. A chromosome conformation capture ordered sequence of the barley genome. Nature. 2017;544:427–433. doi: 10.1038/nature22043. [DOI] [PubMed] [Google Scholar]
- Nakaya M, Tsukaya H, Murakami N, Kato M. Brassinosteroids control the proliferation of leaf cells of Arabidopsis thaliana. Plant Cell Physiol. 2002;43:239–244. doi: 10.1093/pcp/pcf024. [DOI] [PubMed] [Google Scholar]
- Nelissen H, Rymen B, Jikumaru Y, Demuynck K, Van Lijsebettens M, Kamiya Y, Inzé D, Beemster Gerrit TS. A local maximum in gibberellin levels regulates maize leaf growth by spatial control of cell division. Curr Biol. 2012;22:1183–1187. doi: 10.1016/j.cub.2012.04.065. [DOI] [PubMed] [Google Scholar]
- Park J-J, Jin P, Yoon J, Yang J-I, Jeong H, Ranathunge K, Schreiber L, Franke R, Lee I-J, An G. Mutation in wilted dwarf and lethal 1 (WDL1) causes abnormal cuticle formation and rapid water loss in rice. Plant Mol Biol. 2010;74:91–103. doi: 10.1007/s11103-010-9656-x. [DOI] [PubMed] [Google Scholar]
- Pasam RK, Sharma R, Malosetti M, van Eeuwijk FA, Haseneyer G, Kilian B, Graner A. Genome-wide association studies for agronomical traits in a world wide spring barley collection. BMC Plant Biol. 2012;12:16. doi: 10.1186/1471-2229-12-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38:904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- Qi J, Qian Q, Bu Q, Li S, Chen Q, Sun J, Liang W, Zhou Y, Chu C, Li X, Ren F, Palme K, Zhao B, Chen J, Chen M, Li C. Mutation of the rice narrow leaf1 gene, which encodes a novel protein, affects vein patterning and polar auxin transport. Plant Physiol. 2008;147:1947–1959. doi: 10.1104/pp.108.118778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao NN, Prasad K, Kumar PR, Vijayraghavan U. Distinct regulatory role for RFL, the rice LFY homolog, in determining flowering time and plant architecture. Proc Natl Acad Sci USA. 2008;105:3646–3651. doi: 10.1073/pnas.0709059105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharman BC. Leaf and bud initiation in the Gramineae. Bot Gaz. 1945;106:269–289. doi: 10.1086/335298. [DOI] [Google Scholar]
- Tao Z. Effects of some photosynthetic organs on milking and grain yield of barley. Barley Sci. 1999;1:007. [Google Scholar]
- Tardieu F, Reymond M, Hamard P, Granier C, Muller B. Spatial distributions of expansion rate, cell division rate and cell size in maize leaves: a synthesis of the effects of soil water status, evaporative demand and temperature. J Exp Bot. 2000;51:1505–1514. doi: 10.1093/jexbot/51.350.1505. [DOI] [PubMed] [Google Scholar]
- Thirulogachandar V, Alqudah AM, Koppolu R, Rutten T, Graner A, Hensel G, Kumlehn J, Bräutigam A, Sreenivasulu N, Schnurbusch T, Kuhlmann M. Leaf primordium size specifies leaf width and vein number among row-type classes in barley. Plant J. 2017;91:601–612. doi: 10.1111/tpj.13590. [DOI] [PubMed] [Google Scholar]
- Tian F, Bradbury PJ, Brown PJ, Hung H, Sun Q, Flint-Garcia S, Rocheford TR, McMullen MD, Holland JB, Buckler ES. Genome-wide association study of leaf architecture in the maize nested association mapping population. Nat Genet. 2011;43:159–162. doi: 10.1038/ng.746. [DOI] [PubMed] [Google Scholar]
- Turner A, Beales J, Faure S, Dunford RP, Laurie DA. The pseudo-response regulator Ppd-H1 provides adaptation to photoperiod in barley. Science. 2005;310:1031–1034. doi: 10.1126/science.1117619. [DOI] [PubMed] [Google Scholar]
- Ueguchi-Tanaka M, Ashikari M, Nakajima M, Itoh H, Katoh E, Kobayashi M, T-y Chow, Y-iC Hsing, Kitano H, Yamaguchi I, Matsuoka M. GIBBERELLIN INSENSITIVE DWARF1 encodes a soluble receptor for gibberellin. Nature. 2005;437:693–698. doi: 10.1038/nature04028. [DOI] [PubMed] [Google Scholar]
- Van Dingenen J, De Milde L, Vermeersch M, Maleux K, De Rycke R, De Bruyne M, Storme V, Gonzalez N, Dhondt S, Inzé D. Chloroplasts are central players in sugar-induced leaf growth. Plant Physiol. 2016;171:590–605. doi: 10.1104/pp.15.01669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorend W, Lootens P, Nelissen H, Roldán-Ruiz I, Inzé D, Muylle H. LEAF-E: a tool to analyze grass leaf growth using function fitting. Plant Methods. 2014;10:37. doi: 10.1186/1746-4811-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Zhou G, Yu H, Yu S. Fine mapping a major QTL for flag leaf size and yield-related traits in rice. Theor Appl Genet. 2011;123:1319–1330. doi: 10.1007/s00122-011-1669-6. [DOI] [PubMed] [Google Scholar]
- Xiang J-J, Zhang G-H, Qian Q, Xue H-W. SEMI-ROLLED LEAF1 encodes a putative glycosylphosphatidylinositol-anchored protein and modulates rice leaf rolling by regulating the formation of bulliform cells. Plant Physiol. 2012;159:1488–1500. doi: 10.1104/pp.112.199968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue D-W, M-c Chen, M-x Zhou, Chen S, Mao Y, G-p Zhang. QTL analysis of flag leaf in barley (Hordeum vulgare L.) for morphological traits and chlorophyll content. J Zhejiang Univ Sci B. 2008;9:938–943. doi: 10.1631/jzus.B0820105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Lu D. Genetic analysis on morpho-physiological traits of barley flag leaf. Sci Agric Sin. 1991;24:20–26. [Google Scholar]
- Yoshikawa T, Tanaka S-Y, Masumoto Y, Nobori N, Ishii H, Hibara K-I, Itoh J-I, Tanisaka T, Taketa S. Barley < i>NARROW LEAFED DWARF1</i> encoding a WUSCHEL-RELATED HOMEOBOX 3 (WOX3) regulates the marginal development of lateral organs. Breed Sci. 2016;66:416–424. doi: 10.1270/jsbbs.16019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadoks JC, Chang TT, Konzak CF. A decimal code for the growth stages of cereals. Weed Res. 1974;14:415–421. doi: 10.1111/j.1365-3180.1974.tb01084.x. [DOI] [Google Scholar]
- Zhang G-H, Xu Q, Zhu X-D, Qian Q, Xue H-W. SHALLOT-LIKE1 Is a KANADI transcription factor that modulates rice leaf rolling by regulating leaf abaxial cell development. Plant Cell Online. 2009;21:719–735. doi: 10.1105/tpc.108.061457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Ye W, Ren D, Tian P, Peng Y, Gao Y, Ruan B, Wang L, Zhang G, Guo L, Qian Q, Gao Z. Genetic analysis of flag leaf size and candidate genes determination of a major QTL for flag leaf width in rice. Rice. 2015;8:2. doi: 10.1186/s12284-014-0039-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.