Abstract
The survival in freezing temperature for woody plants is exclusively dependent on the perception of coldness and induction of dormancy. CBF/DREB1 transcriptional factors join cold-response conduits and the DAM genes, especially PmDAM6, are well-known regulators of dormancy. Despite the immense importance, little is documented on the association between CBF proteins and the complexity of the promoter region in PmDAM6 with the function of bud dormancy in P. mume. Therefore, this study was based on the cloning of PmDAM6 and six PmCBFs to evaluate their integral roles in the process of bud development. The consistency of expressions in either vegetative or reproductive buds provided a negative control from PmCBFs to PmDAM6 during the onset of dormancy. Besides, PmCBF5 could form heteromeric complexes with PmDAM1 and PmDAM6. PmCBF1, PmCBF3, and PmDAM4 recognized the promoter of PmDAM6 by the alternative binding sites. Therefore, the interactions of these genes formulated the base of an obvious model to respond to the coldness and engendered dormancy release. Findings of this study will further help the unveil the genetic control of bud dormancy and its augmentation in P. mume and may offer an explanation for the vernalization.
Introduction
Formation of a bud for perennial plants is often concomitant with its ability to enter dormant state1 and prevailing cold climates challenge the buds in their capacity to retain the reproductive potential unless favorable conditions arrive2. Therefore, dormancy helps plants to escape bad environmental circumstances and to keep their growth potential alive3. Prunus genus is rich in fruit-bearing species like peach (Prunus persica), plum (Prunus domestica), apricot (Prunus armeniaca), Japanese apricot (Prunus mume), almond (Prunus dulcis) and cherry (Prunus avium). These species are capable of mending their growth habits in accordance with seasonal variations. P. mume has been cultivated in China for over 3000 years. The flower of this tree can bloom in low temperature, earlier than many other species in Prunus. However, the bud induction and floral organ differentiation appeared in the next year. Therefore, it is of immense importance to search out the genetic factors underlying the coldness perception and induction of dormancy4. According to whole genome sequencing analysis, six PmDAM genes were identified, their tandem repeats were distributed in the genome, and six CBF binding sites were found in the upstream of PmDAM genes5. It is supposed that the PmDAMs and their CBF binding sites may be the key factors controlling early dormancy release6.
MADS-box gene family contains transcriptional factors which were found important to have applications in plant organogenesis covering flower organ development, determination of meristematic identity and the vegetative transformation into reproductive phase7. Dorman cy associated MADS-box (DAM) genes play integral roles in specifying the dormancy transitions during the growth curves8–11. In P. persica, the expression profiles of DAM genes are associated with obvious seasonal changes of temperature12,13. As for P. persica and P.avium, the expressions of DAM genes are related to the quantification of the cold response and flowering date manipulation under varying environmental circumstances13–15. PpDAM5 and PpDAM6, showing the expressions closely related to cooling capacity and flowering date14,16, can inhibit peach bud growth at low temperature13. The expressions of PpDAM5 and PpDAM6 were up-regulated during internal dormancy, but down-regulated during dormancy release3,17.
Low temperature significantly affects C-Repeat Binding Factor (CBF), a cold response signal factor isolated by Stockinger18. In Arabidopsis, there are three CBF/DREB1 genes guiding the signal pathway to low temperature response19. CBFs can function to enhance the cold resistance of plants by inducing the downstream genes20,21. Benedict22 transferred the AtCBF1 gene into poplar, comparing it to the wild-type poplar, and found that the cold resistance of the transgenetic poplar was highly enhanced. CBF genes in flowering peach can increase the cold tolerance and can cause dormancy in short-day conditions23. When a peach CBF gene was overexpressed in apple, both non-acclimated and acclimated freezing tolerance was observed and displayed these phenotypes24.
For many woody plants, DAM genes are involved in plant dormancy induction, and CBF genes are related to cold-response pathway. On account of the participation of these genes in supervising the plant growth and development in cold climate, it is necessary to investigate their functions in plant growth and dormancy control. Therefore, studying the interactive influence of both the gene types as determinants of bud activity in P. mume could be a nascent concept inviting plentiful directions for future studies. In this research, we cloned PmDAM6 and six PmCBFs, ascertained their expression profiles in flower bud, flower, leaf bud, and leaf, and tested their roles in the simulated bud dormancy. In addition, multiple interaction experiments were carried out to investigate the protein-protein and protein-DNA associations among PmCBFs and PmDAM6 in controlling bud growth and dormancy. This will invite future studies on molecular perceptive of bud control and cold response conduit.
Results
PmCBFs bind to the promoter of PmDAM6
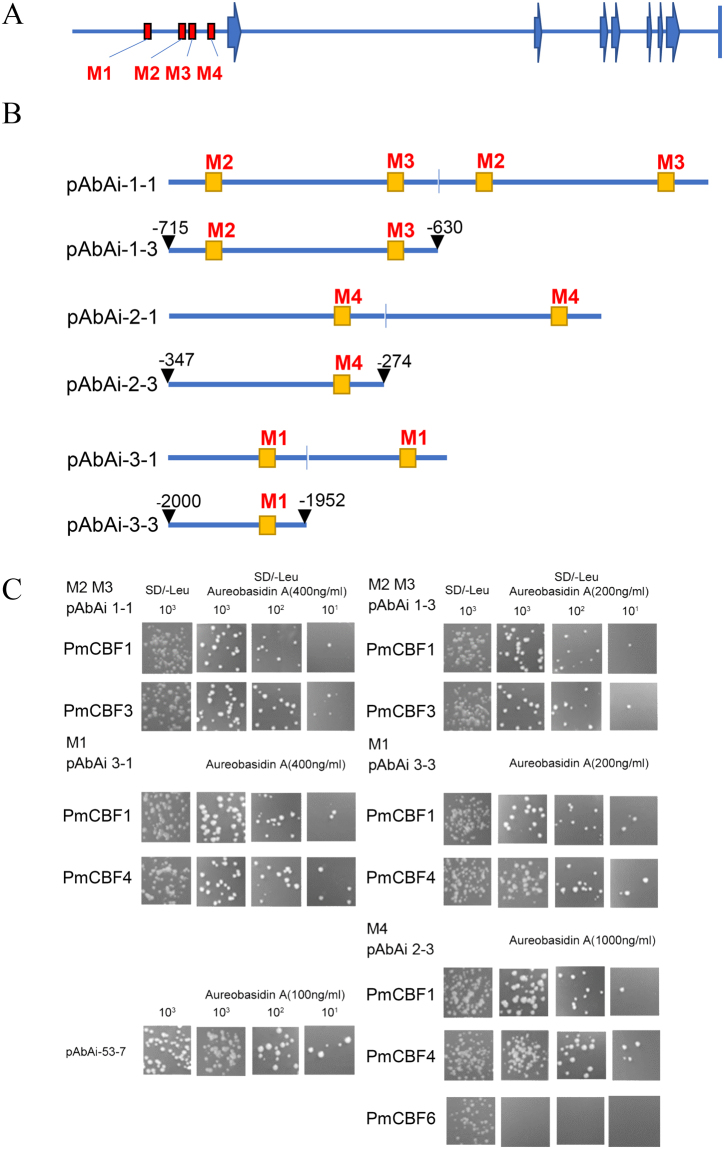
Based on the research of P. mume genome, there exist more binding sites (C-repeat/DRE element) in the promoter of PmDAM6 than those of other PmDAMs. In this research, we obtain the upstream fragment of PmDAM6. Indeed, there are three potential binding sites in the 1 kb region of PmDAM6’s promoter, and four in the 2 kb region. To uncover the role of PmCBFs and PmDAM6 in induction and release of flower bud dormancy, we conducted yeast one hybrid (Y1H) experiments to explore the regulation between six PmCBFs and the promoter of PmDAM6.
As shown in Fig. 1A, the promoter of PmDAM6 (Supplementary Data S2) was separated into four potential binding sites based on the position of CCGAC: M1, −1971; M2, −706; M3, −648; M4, −294. Then, baits (length less than 100 bp) were designed as shown in Fig. 1B. A total of six bait vectors were constructed, M2 and M3 were designed in one bait, which got close to each other, the other two, M1 and M4, were independently performed as baits, then the original sequences of these three baits were duplicated respectively to form other three baits (Supplementary Data S3). Five of these six baits were tested negative to perform the following yeast one hybrid. The corresponding working concentrations of Aureobasidin A (AbA) were as follow: pAbAi-1-1, 400 ng/ml; pAbAi-1-3, 200 ng/ml; pAbAi-2-3, 1000 ng/ml; pAbAi-3-1, 400 ng/ml; pAbAi-3-3, 200 ng/ml. And the pAbAi-2-1 was not considered as a bait because of its normally growing under1000 ng/ml AbA.
Figure 1.
PmCBFs bind the promoter of PmDAM6 in vivo. (A) The genomic structure and promoters of PmDAM6 and the CBF-binding sites in were marked by red blocks. (B) The designed baits in Y1H, the whole promoter was split into three part about 100 bp. (C) The results of Y1H assays between PmCBFs and the baits.
All yeasts with successful transformations showed normal growth on the SD/-Leu solid medium (Fig. 1C). The Y1H assays showed that PmCBF1 and PmCBF3 could associate with baits of pAbAi-2-3, pAbAi-3-1, and pAbAi-3-3; and PmCBF1 and PmCBF4 could bind to baits of pAbAi-2-3, pAbAi-3-1, and pAbAi-3-3. These results suggested that PmCBF1 and PmCBF3 recognized the promoter of PmDAM6 by the binding site M2 and M3, PmCBF1 and PmCBF4 could discern M1 and M4. However, PmCBF2, PmCBF5, and PmCBF6 failed to bind to the four C-repeat/DRE sites of PmDAM6 promoter.
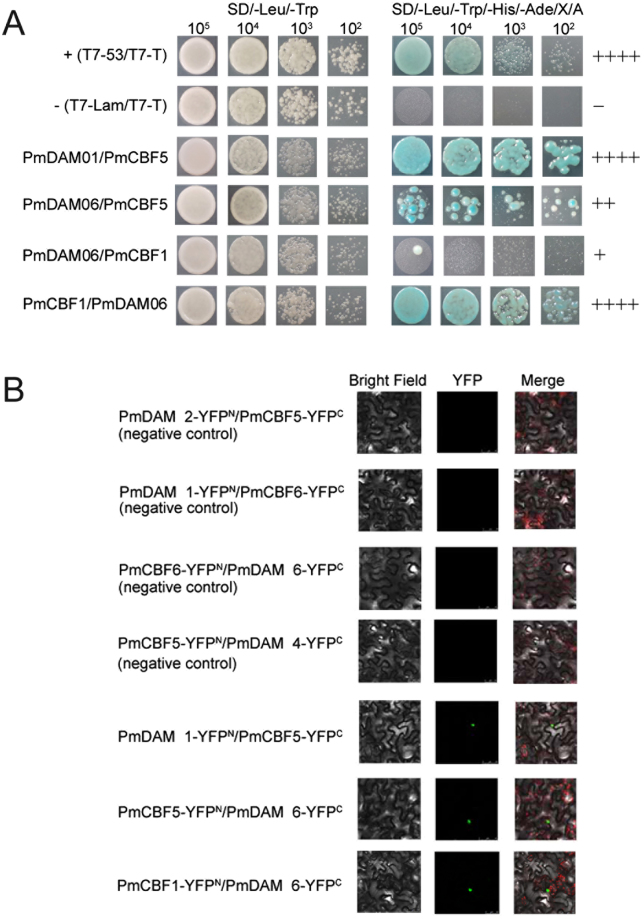
In vivo protein-protein interactions among PmCBFs and PmDAM6
Based on yeast two hybrid (Y2H) and bimolecular fluorescence complementation (BiFC) assay, a further exploration about PmCBFs and PmDAMs was made. According to Yeast two-hybrid experiments, PmCBF5 showed a strong interaction with PmDAM1 and a general interaction with PmDAM6 (Fig. 2A). Moreover, protein-protein interactions among PmDAM6 and PmCBFs were established by BiFC with a yellow fluorescent protein. Fluorescence released by these yellow fluorescent proteins was positioned at nucleus, suggesting the strong inclination among PmCBF5 and these two PmDAMs. PmCBF5 could form heteromeric complexes with PmDAM1 and PmDAM6 (Fig. 2B). As shown in Supplementary Fig. S3, there were no interactions in PmDAM1-YFPN/ YFPC, YFPC/ PmCBF5-YFPN, PmCBF5-YFPC/ YFPN, and YFPN/ PmDAM6-YFPC. In order to exclude the false positives, a member from the same protein family can be chosen to execute a negative control25. Therefore, the interactions of PmDAM2-YFPN/ PmCBF5-YFPC and PmDAM1-YFPN/ PmCBF6-YFPC were used as the negative controls of PmDAM1-YFPN/ PmCBF5-YFPC; the interactions of PmCBF6-YFPN/ PmDAM6-YFPC and PmCBF5-YFPN/ PmDAM4-YFPC were used as the negative controls of PmCBF5-YFPN/ PmDAM6 -YFPC. Fluorescent was not detected the in these negative tests.
Figure 2.
Protein-protein interactions between PmCBFs and PmDAMs. (A) Yeast two-hybrid analysis of the protein interactions between PmCBFs and PmDAMs. T7-53/T7-T was positive control, and T7-Lam/T7-T was negative control. The symbol (+) was represented the capacity of the reaction. The more numbers of the symbol (+), the more stronger capacity of the reaction. (B) BiFC analysis of the protein interactions between PmCBFs and PmDAMs. The green fluorescent presented protein position. The red fluorescent showed the chloroplast position.
Crossover of the expressions between PmCBFs and PmDAM6 in flower bud dormancy
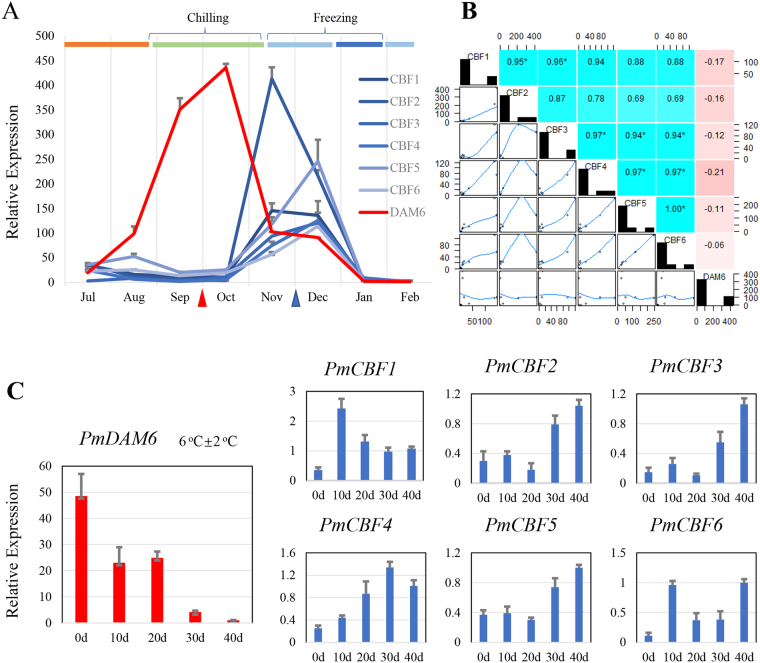
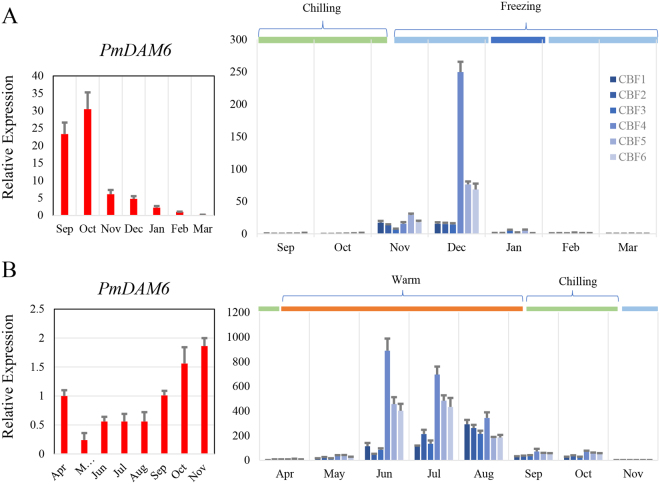
In Beijing, P. mume ‘SanlunYudie’ begins to form buds from June to July, undergoes flower bud differentiation from July to November, steps into dormancy from September to October, retains dormancy from November to January, and initiates dormancy breaking from January to February (Fig. 3A). From July to October, PmDAM6 showed up-regulated in the chilling environment with a temperature under 20 °C and down-regulated in the coldness deepened weather. However, the expression tendencies of six PmCBFs during different periods of flower bud development were basically similar with high positive correlations (Fig. 3B) and were induced vastly under low temperature before freezing. The transcript levels of PmCBFs were low during July-October and January-February, but were high during November-December. The high expression of CBF genes seemed to inhibit the expressions of DAM genes. Together with all expression data, we can conclude that PmDAM6 and PmCBFs function in two temperature regions, respectively. This means PmDAM6 actively respond in the chilling temperature (below 20 oC) and PmCBFs dominate in the freezing zone (below 0 °C), and the regulations in this biological process between these genes are continuous with a suppressive tendency.
Figure 3.
Expression patterns of PmDAM6 and PmCBFs in flower buds of P. mume. (A) The expression levels of PmCBFs and PmDAM6 in natural environment, the arrow denoted the sampling stages for simulated tests. (B) Correlations between PmCBFs and PmDAM6. The upper half matrix displayed the values of correlation coefficient, stars (*) marked the significant correlation members between two genes. The lower half display the linearity of the expression values. (C) The expression of PmCBFs and PmDAM6 in simulated dormancy under low temperature from the flower bud, the samples were collected in the first stage (red arrow in A). (Warm: higher than 20 °C, Chilling: lower than 20 °C, Freezing: lower than 0 °C).
Expression patterns of PmDAM6 and PmCBFs in different blooming stages and different flower structures
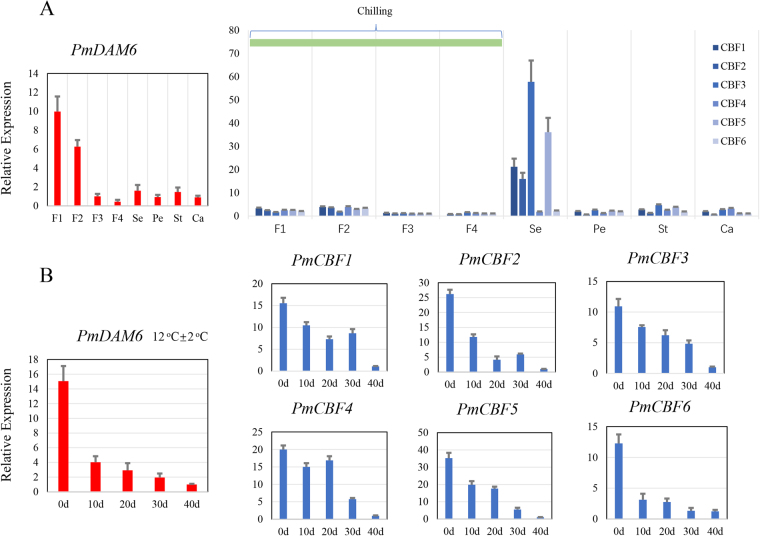
When the temperatures return to chilling, the flower bud started to grow and bloom. To explore the expression patterns of PmDAM6 and PmCBFs genes in the different blooming stages and different floral structures, the blooming process was divided into four stages. On the whole, the expression trends of PmDAM6 also declined from F1 to F4. In addition, PmDAM6 were expressed in all the floral structures, and the expression of PmCBFs kept low levels and got lower in the flower blooming process (Fig. 4A). The expression tendencies of the PmCBFs in the flower blooming stages were basically the same, the transcript levels were relatively high in F1 and F2, but low in F3 and F4. According to the expression patterns of PmCBFs in different flower structures, PmCBFs were divided into two groups. PmCBF1-4 showed high expression specificity in sepals. The other type genes, including PmCBF5-6 were expressed in all of the floral structures without significant differences.
Figure 4.
Expression patterns of PmDAM6 and PmCBFs in bloom process and different flower structures. (A) The flower blooming process was manually divided into four stages according to the size of buds. F1:Small flower bud;F2:Big flower bud;F3:First blooming;F4:Full blooming. The mature flower was disassembled into four parts. Se:Sepal;Pe:Petal;St:Stamen;Ca:Carpel. (B) The expression levels of PmDAM6 and PmCBFs in the simulated dormancy release test, the samples were collected from the second stage (blue arrow in Fig. 3A). (Warm: higher than 20 °C, Chilling: lower than 20 °C, Freezing: lower than 0 °C).
Expression patterns of PmDAM6 and PmCBFs in leaf buds and leaves
The leaf buds experienced a different development process compared to flower bud. They formed in the same period with flower bud, but only enlarged after the flower blooming after dormancy in the cold winter. DAM genes were specifically expressed in leaf buds at different developmental stages. PmDAM1-3 exhibited high expression levels in September, and expression levels of PmDAM4-6 were the highest in October (Fig. 5A). Furthermore, the expression trends of PmDAMs in leaf bud were largely consistent at different development stages and showed significant expressions from September to October which then gradually declined from October to March in the next year. However, the expression levels of PmCBFs were the highest in November and December. Above all, the expression patterns of DAM and CBF genes between leaf bud and flower bud were largely consistent at different development stages.
Figure 5.
Expression patterns of PmDAMs and PmCBFs in leaf buds and mature leaves. (A) Gene expressions in the leaf buds. (B) Gene expressions in the leaves. (Warm: higher than 20 °C, Chilling: lower than 20 °C, Freezing: lower than 0 °C).
The expressions of PmCBFs manifested two types of similar patterns with high levels. The first group (PmCBF1-3) showed peak expressions in August, up-regulated from April to August, and down-regulated from September to November (Fig. 5B). The second group (PmCBF4-6) showed peak expressions in June and July, then declined till November. But all the PmCBFs own high levels from June to August.
Simulated bud dormancy and dormancy release
In order to study the functions of PmCBFs and PmDAMs in the bud dormancy, we detected the expressions of all six PmCBFs and six PmDAMs in the simulated condition of bud dormancy and dormancy release.
One year old branches with flower buds were sampled on October 1, 2015. The growing branches were treated with 6 °C ± 2 °C to induce dormancy. The expressions of PmCBFs were shown in Fig. 3C, and were of two types. One category of PmCBFs, including PmCBF2-5, showed rising expressions, while the other category, containingPmCBF1 and PmCBF6, showed higher expressions in 10-40 days as compared to 0 day, suggesting the low temperature significantly affect the expressions of PmCBFs. However, the expression of PmDAM6 increased in 0–20 days but dropped in the following days. On first day of low temperature exposure, PmDAM6 presented a high expression. During the process of dormancy, PmCBFs accumulated in low temperature, while the expression of PmDAM6 declined.
Warm temperature of spring was simulated at 12 °C ± 2 °C to release the bud dormancy with one year old branches on December 10, 2015. After warm temperature treatment, flower buds turned to expand within 10days, and after 20 days, 95% flower buds showed white petals. As shown in Fig. 4B, the expressions of PmCBFs and PmDAM6 reduced overall. The expressions of PmCBF1, PmCBF3, PmCBF4 and PmCBF5 keep stable, but declined remarkablely during 30–40 days. In contrast, PmCBF2, PmCBF6 and PmDAM6 rapidly declined in 10 days. All results suggested that in the release of dormancy, PmCBFs and PmDAM6 showed a down regulation.
Discussion
PmCBFs suppress overexpression of PmDAM6 in flower bud dormancy
Acquirement of protective strategies for reproductive organs in plants possesses vital importance for the continuation of life, however, the uncertain environment changes, like low-temperature stress, remains the major hurdle to the success of plants inhabiting the cold regions. Regulatory pathways and transcriptional factors driving these routings are central to the studies about the success of these plants in fluctuating climatic conditions. DAM genes have been shown to be involved in the formation of terminal buds and also intervene growth dynamics in perennial plants like kiwifruit (Actinidia spp.), leafy spurge (Euphorbia esula), raspberry (Rubusidaeus), Populustrichocarpa, and peach17,26–32. In peach, PpDAMs has an association with seasonal dormancy transitions in flower buds26. SVP genes, orthologs of DAM, in Arabidopsis show regulatory effect during floral transitions and play a significant role in specifying the floral meristems33. In the flower bud of Prunus mume, PmDAM6 exhibited a special expression leading to the dormancy under decreasing temperature (Fig. 3A). Moreover, the appearance of high CBF expression levels enhanced the tolerance of coldness in freezing climates and inhibited the expression of PmDAM6. This may lead to a different phenomenon that flowers buds are easier to sense the changes in environment and devise the mechanism of self-protection34,35. Nevertheless, this happens only if plants are shocked by cold. When plants were released from cold weathers, PmDAM6 and PmCBFs just relaxed with all decreasing trends and relatively lower levels (Fig. 4). This must be an integrated result from regulation beyond CBFs and DAMs.
In P. persica, DAMs have shown in vegetative and non-vegetative plant parts development including bud12. Here, the protection mechanism prefers to appear in young tissues facing coldness. Annually sampled leaf buds gave important clues about potent roles of DAM6 and CBF genes in P. mume wherein PmDAM6 showed high expressions in September to October and the CBFs appeared from November to December (Fig. 5A). Besides, MdCBF1, 2 and 4 were discerned in the leaf tissues of apple subjected to cold conditions, and VrCBF exhibited a short expression (1/2 h-2 days) both in young and old leaves, while VrCBF1, 2 and 3 expressed only in younger tissues36. Some reports have indicated a reverse relation between DAM and CBF during growth curves, and dormancy control, wherein FT can be down-regulated by the high expression of DAM30. This may provide an explanation for the growth and dormancy of young tissues.
In the mature leaf aging process, PmDAM6 also showed a negative correlation with PmCBFs especially for PmCBF4-6, though this happened under a warm environment. However, it is confirmed that CBFs function in heat and drought as well as cold response in soybean37. The ectopically expressing PpCBF1 induced aging in leaf and also delayed bud opening in the spring24.
Under artificial control environment, PpDAM5 and PpDAM6 exhibited an upregulation under sustained low temperature and showed a downregulation by warm temperature in peach38. The growth of peach buds are negatively correlated with the expression of PpDAM5 and PpDAM613,39. In addition, the sustained low temperature could satisfy the needs of a chilling requirement in buds to promote the release of dormancy with PpDAM5 and PpDAM6 down regulated16. In P. mume, high expression of PmDAM6 hastened the flower buds into dormancy. With the accumulation of coldness in winter, the expressions of PmCBFs increased, and that of PmDAM6 decreased, indicating that high amount of PmCBFs seemed to depress the expression of PmDAM6. When warm temperature spell in spring, the flower bud successfully released from cold dormancy with both PmCBFs and PmDAM6 declined. All these observations push us to establish the opposite regulations from PmCBFs to PmDAM6
Interactions among PmDAMs and PmCBFs
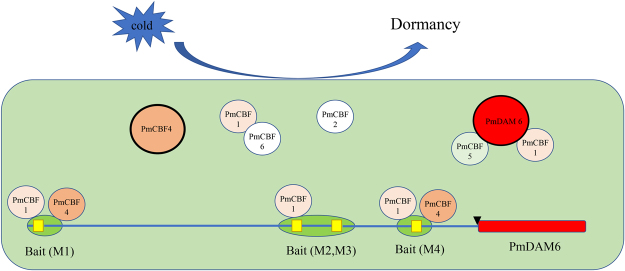
As reported in recent researches, CBF could bind to the promoter of DAM genes based on a fixed motif. For the model plant, Arabidopsis, CBFs recognize DNA sequences with CRT/DRE sites, which cover a core motif of CCGAC. Compared with other herbaceous plant, like oilseed rape, wheat, or rye, the cold response pathway with a member of CBF remains conservative between species suggesting its vital place for the whole pathway. In pear, the interaction between PpCBF and the promoter of PpDAMs give a convincing proof. In Pyrus pyrifolia, the CRT/DRE binding ability between PpCBF and the promoters of PpDAM1and PpDAM3 was verified6. In the genome of P. mume, the conserved binding motif exists in the promoters of PmDAM1, PmDAM4, PmDAM5,and PmDAM6. Particularly, it makes the function of PmDAMs more complex that the PmDAM6 promoter contain more CRT/DRE core sites (Fig. 1). For pear, PpCBF does not bind to the bait, when CGGC was transformed into AAAA, suggesting the regulation of DAM depends on the recognition of this core sequence. In our study, Y1H assays exhibited PmCBF1, PmCBF3 and PmCBF4 indeed interacted with the promoters of PmDAM6 by different sites. But PmCBF3 was not only located in the nucleus but also the chloroplast. Besides, PmCBF5 could form heteromeric complexes with PmDAM1 as well as PmDAM6, suggesting that PmDAMs and PmCBFs might function in the bud dormancy by dimerizations (Fig. 6). Therefore, scrutinizing the expression patterns coupled with dimerizations trends can be a reasonable platform to grasp detailed understanding of protein-protein interactions among PmDAMs and PmCBFs during bud transitions. Though the PmCBFs may have matching functions20, the alternative interaction abilities still provided a possible regulation from chaos. Especially for the PmDAM6, the expression did not get the bottom, when the PmCBFs exaggerated. Conversely, the expression of PmDAM6 kept a relative stabilization, which drove us to hypothesize that the interaction from protein to protein and protein to DNA conducted a feedback loop in the deep dormancy condition (Fig. 6). However, there were still mysteries because of the multiple CRT/DRE core sites that whether the number of binding sites superpose or subduct the function of downstream gene, all the hypotheses need further researches.
Figure 6.
Molecular regulation model of PmDAMs and PmCBFs during flower bud dormancy. PmCBF4 and PmDAM6 could form homodimers, respectively (displayed in larger circles). PmCBF5 could form heteromeric complexes with PmDAM1 and PmDAM6. Meanwhile, PmCBF1 and PmCBF3 recognized the promoter of PmDAM6 by the binding site M2 and M3, PmCBF1 and PmCBF4 could discern M1 and M4.
In our study, we observed the crossover of the expression between PmCBFs and PmDAM6, and provided the evidences that besides multiple recognitions to the promoter of PmDAM6, PmCBFs also could form protein complex with PmDAM6, which act as a center role in dormancy induction. This multiplex regulation might reflect the evolvement of plant towards more rapidly changing environment. Moreover, the expression patterns in vegetative bud and simulated dormancy test of bud convince the negative control in the dormancy formation. We believe this novel research will help direct future discussion on the interactive role of DAM and CBF genes as essential regulators of bud dormancy.
Materials and Methods
Plant Material
P. mume ‘Sanlun Yudie’, an early flowering cultivar, was selected as plant material from the Beijing Forestry University, in Beijing, China (40° 00′ N, 116° 18′ E). These samples were taken from flower bud (from July, 2015 to February, 2016; collected a sample every 30 days; Supplementary Fig. S1), flower (early flowers in three different morphologies, full blooming, sepal, petal, stamen, and pistil, in March, 2016; Supplementary Fig. S2), leaf bud (from September, 2015 to March, 2016; collected a sample every 30 days), leaf (from April, 2016 to November, 2016; collected a sample every 30 days). For the dormancy tests (8 °C for 10 hours during daytime and 4 °C for 14 hours at night) and dormancy releasing tests (14 °C for 10 hours during daytime and 10 °C for 14 hours at night), thirty branches were cut to simulate the tests on October 1, 2015 and December 10, 2015, respectively. In these simulation tests, the flower buds were sampled every 10 days. All samples were immediately immersed in liquid nitrogen and were stored at −80 °C for RNA extraction. Total RNA was extracted by TRIzol reagent (Aidlab, China).
Real-time quantitative PCR
Full length cDNA sequences of six PmDAMs and six PmCBFs (Supplementary Data S1) were amplified through PCR using the primers in Supplementary Table S1. PikoReal real-time PCR system (Thermo Fisher Scientific, Germany) was used to investigate the expressions of PmDAMs and PmCBFs in different organs. The primers of RT-qPCR experiments were shown in Supplementary Table S2 and the experiments were carried out using previous method40. PmPP2A (protein phosphatase 2A) was considered as reference gene41. Three biological replicates were performed to calculate the standard deviation. The correlations between gene expressions were done by spearman method, and significant was analysed with kruskal-wallis test in R.
Gene cloning and Yeast 2 Hybrid assays
Full length cDNA sequences of PmDAMs and PmCBFs were amplified through PCR using specific primers (Supplementary Table S3). These sequences were cloned into pGBKT7 bait vector and pGADT7 prey vector at the EcoRI and BamHI sites, respectively, using an In-Fusion HD Cloning Kit System (Clonetech, USA). The Y2H assays were performed by former methods42. Each interaction analysis was performed three times.
BiFC Assays
Specific primers were used for BiFC assessment (Supplementary Table S4). Full length cDNA sequences of PmDAMs and PmCBFs were cloned, pair-wise, into pCambia1300-YFP-C and pCambia1300-YFP-N to get BiFC constructs. Coexpression analysis was carried out on the leaves of Nicotiana benthamiana according to the procedure stated by Schutze et al.43. Chimeric fluorescence, emitted by fusion proteins, was examined under Leica TCS SP8 Confocal Laser Scanning Platform with YFPs being motivated at 514 nm.
Promoter cloning and Yeast 1 Hybrid assays
The genomic DNA of P. mume was isolated from flower bud using DNAsecure Plant Kit (DP320-02, TianGen, China). 2 kb up-stream promoter sequence of PmDAM6was extracted by PCR using specific primers (Supplementary Table S5) and the plasmid of pMDTM18-T-proDAM6 was obtained by former method42. In 2 kb promoter of PmDAM6, there are four CCGAC sequences called CBF binding site. According these four CBF binding sites, six baits were designed. Three of them were obtained from the original genome, then each of them was duplicated to form the follow three baits. All six fragments were imported to the pAbAi-bait vectors. The special primers related to cloning these fragments were shown in Supplementary Table S6. The plasmids of pGADT7-PmCBFs were obtained in Y2H assays. These plasmids were transformed into the Y1H Gold strains containing pAbAi-bait, respectively, and these tests were screened on SD/-Leu/AbA plates. All transformations and screenings were observed in triplicate. These Y1H assays were executed by a Matchmaker Gold Yeast One-Hybrid System kit (Clontech, America) following its user manual and correlation steps.
Electronic supplementary material
Acknowledgements
The research was supported by the Fundamental Research Funds for the Central Universities (NO. 2016ZCQ02), The research was supported by Ministry of Science and Technology (2013AA102607), National Natural Science Foundation of China (Grant No. 31471906), Special Fund for Beijing Common Construction Project.
Author Contributions
K.Z. and Q.Z. designed the experiments; Y.Z. completed the experiments; K.Z. and Y.Z. wrote the manuscript; S.A. improved the manuscript. K.Z., Y.Z., X.Y., X.X., Y.H., Y.L. and L.S. analyzed the data. All authors read and approved the final manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-22537-w.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rohde A, Bhalerao RP. Plant dormancy in the perennial context. Trends in Plant Science. 2007;12:217. doi: 10.1016/j.tplants.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 2.Díaz-Riquelme J, Grimplet J, Martínez-Zapater JM, Carmona MJ. Transcriptome variation along bud development in grapevine (Vitis vinifera L.) BMC Plant Biology. 2012;12:181. doi: 10.1186/1471-2229-12-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamane H. Regulation of Bud Dormancy and Bud Break in Japanese Apricot (Prunus mume Siebold & Zucc.) and Peach [Prunus persica (L.) Batsch]: A Summary of Recent Studies. Journal- Japanese Society for Horticultural Science. 2014;83:187–202. doi: 10.2503/jjshs1.CH-Rev4. [DOI] [Google Scholar]
- 4.Campoy JA, Ruiz D, Egea J. Dormancy in temperate fruit trees in a global warming context: A review. Scientia Horticulturae. 2011;130:357–372. doi: 10.1016/j.scienta.2011.07.011. [DOI] [Google Scholar]
- 5.Zhang Q, et al. The genome of Prunus mume. Nat Commun. 2012;3:1318. doi: 10.1038/ncomms2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niu Q, et al. Dormancy-associated MADS-box genes and microRNAs jointly control dormancy transition in pear (Pyrus pyrifoliawhite pear group) flower bud. Journal of experimental botany. 2015;67:239. doi: 10.1093/jxb/erv454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Messenguy F, Dubois E. Role of MADS box proteins and their cofactors in combinatorial control of gene expression and cell development. Gene. 2003;316:1–21. doi: 10.1016/S0378-1119(03)00747-9. [DOI] [PubMed] [Google Scholar]
- 8.Leseberg CH, Li A, Kang H, Duvall M, Mao L. Genome-wide analysis of the MADS-box gene family in Populus trichocarpa. Gene. 2006;378:84–94. doi: 10.1016/j.gene.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 9.Campbell M, Segear E, Beers L, Knauber D, Suttle J. Dormancy in potato tuber meristems: chemically induced cessation in dormancy matches the natural process based on transcript profiles. Functional & Integrative Genomics. 2008;8:317–328. doi: 10.1007/s10142-008-0079-6. [DOI] [PubMed] [Google Scholar]
- 10.Díazriquelme J, Lijavetzky D, Martínezzapater JM, Carmona MJ. Genome-wide analysis of MIKCC-type MADS box genes in grapevine. Plant Physiology. 2009;149:354–369. doi: 10.1104/pp.108.131052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horvath D. Common mechanisms regulate flowering and dormancy. Plant Science. 2009;177:523–531. doi: 10.1016/j.plantsci.2009.09.002. [DOI] [Google Scholar]
- 12.Li, Z., Reighard, G. L., Abbott, A. G. & Bielenberg, D. G. Dormancy-associated MADS genes from the EVG locus of peach [Prunus persica (L.) Batsch] have distinct seasonal and photoperiodic expression patterns. 60, 3521–3530 (2009). [DOI] [PMC free article] [PubMed]
- 13.Jiménez S, Li Z, Reighard GL, Bielenberg DG. Identification of genes associated with growth cessation and bud dormancy entrance using a dormancy-incapable tree mutant. BMC Plant Biology. 2010;10:25. doi: 10.1186/1471-2229-10-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan S, et al. Mapping quantitative trait loci associated with chilling requirement, heat requirement and bloom date in peach (Prunus persica) The New phytologist. 2010;185:917–930. doi: 10.1111/j.1469-8137.2009.03119.x. [DOI] [PubMed] [Google Scholar]
- 15.Castède S, et al. Mapping of Candidate Genes Involved in Bud Dormancy and Flowering Time in Sweet Cherry (Prunus avium) Plos One. 2015;10:e0143250. doi: 10.1371/journal.pone.0143250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leida C, Conesa A, Llácer G, Badenes ML, Ríos G. Histone modifications and expression of DAM6 gene in peach are modulated during bud dormancy release in a cultivar-dependent manner. New Phytologist. 2012;193:67–80. doi: 10.1111/j.1469-8137.2011.03863.x. [DOI] [PubMed] [Google Scholar]
- 17.Yamane, H., Kashiwa, Y., Ooka, T., Tao, R. & Yonemori, K. Suppression Subtractive Hybridization and Differential Screening Reveals Endodormancy-associated Expression of an SVP/AGL24-type MADS-box Gene in Lateral Vegetative Buds of Japanese Apricot. Journal of the American Society for Horticultural Science American Society for Horticultural Science133 (2008).
- 18.Stockinger EJ, Gilmour SJ, Thomashow MF. Arabidopsis thaliana CBF1 Encodes an AP2 Domain-Containing Transcriptional Activator that Binds to the C-Repeat/DRE, a Cis-Acting DNA Regulatory Element that Stimulates Transcription in Response to Low Temperature and Water Deficit. Proceedings of the National Academy of Sciences. 1997;94:1035–1040. doi: 10.1073/pnas.94.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chinnusamy V, Zhu J, Zhu JK. Cold stress regulation of gene expression in plants. Trends in Plant Science. 2007;12:444–451. doi: 10.1016/j.tplants.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Novillo F, Alonso JM, Ecker JR, Salinas J. CBF2/DREB1C is a negative regulator of CBF1/DREB1B and CBF3/DREB1A expression and plays a central role in stress tolerance in Arabidopsis. P Natl Acad Sci USA. 2004;101:3985–3990. doi: 10.1073/pnas.0303029101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Novillo F, Medina J, Salinas J. Arabidopsis CBF1 and CBF3 have a different function than CBF2 in cold acclimation and define different gene classes in the CBF regulon. Proc Natl Acad Sci USA. 2007;104:21002–21007. doi: 10.1073/pnas.0705639105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benedict C, et al. The CBF1-dependent low temperature signalling pathway, regulon and increase in freeze tolerance are conserved in Populus spp. Plant Cell & Environment. 2006;29:1259–1272. doi: 10.1111/j.1365-3040.2006.01505.x. [DOI] [PubMed] [Google Scholar]
- 23.Wisniewski M, Norelli J, Bassett C, Artlip T, Macarisin D. Ectopic expression of a novel peach (Prunus persica) CBF transcription factor in apple (Malus × domestica) results in short-day induced dormancy and increased cold hardiness. Planta. 2011;233:971–983. doi: 10.1007/s00425-011-1358-3. [DOI] [PubMed] [Google Scholar]
- 24.Wisniewski M, Norelli J, Artlip T. Overexpression of a peach CBF gene in apple: a model for understanding the integration of growth, dormancy, and cold hardiness in woody plants. Front Plant Sci. 2015;6:85. doi: 10.3389/fpls.2015.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kudla J, Bock R. Lighting the Way to Protein-Protein Interactions: Recommendations on Best Practices for Bimolecular Fluorescence Complementation Analyses. The Plant Cell. 2016;28:1002–1008. doi: 10.1105/tpc.16.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bielenberg DG, et al. Sequencing and annotation of the evergrowing locus in peach [Prunus persica (L.) Batsch] reveals a cluster of six MADS-box transcription factors as candidate genes for regulation of terminal bud formation. Tree Genetics & Genomes. 2008;4:495–507. doi: 10.1007/s11295-007-0126-9. [DOI] [Google Scholar]
- 27.Mazzitelli L. Co-ordinated gene expression during phases of dormancy release in raspberry (Rubus idaeus L.) buds. Journal of Experimental Botany. 2007;58:1035–1045. doi: 10.1093/jxb/erl266. [DOI] [PubMed] [Google Scholar]
- 28.Sasaki R, et al. Functional and expressional analyses of PmDAM genes associated with endodormancy in Japanese apricot. Plant Physiology. 2011;157:485–497. doi: 10.1104/pp.111.181982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu RM, et al. Conservation and divergence of four kiwifruit SVP-like MADS-box genes suggest distinct roles in kiwifruit bud dormancy and flowering. J Exp Bot. 2012;63:797–807. doi: 10.1093/jxb/err304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horvath DP, Sung S, Kim D, Chao W, Anderson J. Characterization, expression and function of DORMANCY ASSOCIATED MADS-BOX genes from leafy spurge. Plant molecular biology. 2010;73:169. doi: 10.1007/s11103-009-9596-5. [DOI] [PubMed] [Google Scholar]
- 31.Li ZM, et al. PtSVP, an SVP homolog from trifoliate orange (Poncirus trifoliata L. Raf.), shows seasonal periodicity of meristem determination and affects flower development in transgenic Arabidopsis and tobacco plants. Plant Molecular Biology. 2010;74:129–142. doi: 10.1007/s11103-010-9660-1. [DOI] [PubMed] [Google Scholar]
- 32.T R, et al. A molecular timetable for apical bud formation and dormancy induction in poplar. Plant Cell. 2007;19:2370–2390. doi: 10.1105/tpc.107.052811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gregis V, et al. Identification of pathways directly regulated by SHORT VEGETATIVE PHASE during vegetative and reproductive development in Arabidopsis. Genome Biology. 2013;14:R56–R56. doi: 10.1186/gb-2013-14-6-r56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee JY, Lee DH. Use of serial analysis of gene expression technology to reveal changes in gene expression in Arabidopsis pollen undergoing cold stress. Plant Physiol. 2003;132:517–529. doi: 10.1104/pp.103.020511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kreps JA, et al. Transcriptome changes for Arabidopsis in response to salt, osmotic, and cold stress. Plant Physiol. 2002;130:2129–2141. doi: 10.1104/pp.008532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao HG, Siddiqua MS, Nassuth A. Three grape CBF/DREB1 genes respond to low temperature, drought and abscisic acid. Plant Cell & Environment. 2006;29:1410–1421. doi: 10.1111/j.1365-3040.2006.01524.x. [DOI] [PubMed] [Google Scholar]
- 37.Kidokoro S, et al. Soybean DREB1/CBF-type transcription factors function in heat and drought as well as cold stress-responsive gene expression. Plant J. 2015;81:505–518. doi: 10.1111/tpj.12746. [DOI] [PubMed] [Google Scholar]
- 38.Yamane H, Ooka T, Jotatsu H, Sasaki R, Tao R. Expression analysis of PpDAM5 and PpDAM6 during flower bud development in peach (Prunus persica) Scientia Horticulturae. 2011;129:844–848. doi: 10.1016/j.scienta.2011.05.013. [DOI] [Google Scholar]
- 39.Yamane, H. et al. Expressional regulation of PpDAM5 and PpDAM6, peach (Prunus persica) dormancy-associated MADS-box genes, by low temperature and dormancy-breaking reagent treatment. 62, 3481–3488 (2011). [DOI] [PMC free article] [PubMed]
- 40.Zhou, Y. et al. Genome-wide identification, characterization and expression analysis of the TCP gene family in Prunus mume. Frontiers in Plant Science7, 10.3389/fpls.2016.01301 (2016). [DOI] [PMC free article] [PubMed]
- 41.Wang T, Hao R, Pan H, Cheng T, Zhang Q. Selection of Suitable Reference Genes for Quantitative Real-time Polymerase Chain Reaction in Prunus mume during Flowering Stages and under Different Abiotic Stress Conditions. Journal of the American Society for Horticultural Science American Society for Horticultural Science. 2014;139:113–122. [Google Scholar]
- 42.Zhou Y, et al. SEP-class genes in Prunus mume and their likely role in floral organ development. BMC Plant Biology. 2017;17:10. doi: 10.1186/s12870-016-0954-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schütze K, Harter K, Chaban C. Bimolecular fluorescence complementation (BiFC) to study protein-protein interactions in living plant cells. Methods Mol Biol. 2009;479:189–202. doi: 10.1007/978-1-59745-289-2_12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.