Abstract
Adaptive homeostasis enables rapid cellular signaling, leading to transcriptional and translational modifications (Davies, 2016) [1]. The Proteasome is one of the main cellular proteolytic enzymes that plays an essential role in the rapid clearance of oxidatively damaged cellular proteins, and is highly responsive to oxidative stress. Upon exposure to even very low, signaling levels of oxidants, the predominant form of the Proteasome becomes the ATP-independent 20S proteasome that enables rapid clearance of damaged proteins. Subsequently there is also a concurrent upregulation of de novo 20S proteasome synthesis. These cellular adaptations not only ensure effective and efficient removal of damaged proteins, but prepare cells to better cope with future, more severe oxidative insults. Male and female Drosophila melanogaster fruit flies were pretreated with an adaptive amount of an oxidant (10 µM hydrogen peroxide or 0.5 µM paraquat) to assess the changes in proteolytic capacity and the role of the 20S proteasome. Additionally, the adaptive signaling by non-damaging amounts of hydrogen peroxide or paraquat) were used to assess changes in male and female fruit flies, following a subsequent more toxic amount of the two oxidants. Further analysis and detailed results about the adaptive role of the 20S proteasome in multiple D. melanogaster strains can be found in “Sexual Dimorphism in Oxidant-Induced Adaptive Homeostasis in Multiple Wild-Type D. melanogaster Strains” (Pomatto et al., 2018) [2].
Keywords: 20S proteasome, Sexual-dimorphism, Adaptive homeostasis, Proteolysis, D. melanogaster
Specifications Table
| Subject area | Biology, Biochemistry |
| More specific subject area | Drosophila melanogaster, 20S proteasome, adaptive homeostasis |
| Type of data | Table, figure, text |
| How data was acquired | Immunoprecipitation, proteolytic activity assay, survival curves |
| Data format | Analyzed |
| Experimental factors | Flies were either not pretreated (control) or were pretreated with an adaptive amount of an oxidant (0–100 µM hydrogen peroxide or 0–10 µM paraquat). Flies were then subsequently subjected to a semi-lethal amount of the oxidant to assess survival. |
| Experimental features | Flies were either pretreated or not with an adaptive amount of either hydrogen peroxide or paraquat. Afterwards, flies were collected and lysate was either incubated in the absence or presence of the proteasome inhibitor, lactacystin before proteolytic activity was measured. Additionally, lysate was also used for immunoprecipitation was completed. |
| Data source location | University of Southern California, Los Angeles, CA, 90089, USA |
| Data accessibility | The data are supplied with this article |
Value of the data
-
•
Oxidant-induced adaptive increases in proteolytic capacity are dependent upon the 20S proteasome in male and female D. melanogaster.
-
•
Sex-dependent differences in 20S proteasome adaptive responses are oxidant-dependent, with a female-specific response following hydrogen peroxide pretreatment and a male-specific response following paraquat pretreatment.
-
•
Adaptation is a sexually-dimorphic response that is oxidant-dependent across multiple D. melanogaster strains.
1. Data
The 20S proteasome is a crucial mediator for the rapid clearance of damaged proteins. Under homeostatic conditions the majority of the 20S proteasome is sequestered away in the form of the ATP-dependent 26S proteasome, which is comprised of two additional 19S ATP-dependent regulatory caps at either end of the 20S catalytic core [3]. During periods of oxidative stress, the 19S regulatory caps are removed and bound to HSP70, enabling a rapid and immediate pool of 20S proteasome for degradation of oxidized proteins [4], [5]. Earlier work showed that adaptive, non-damaging amounts of an oxidant (hydrogen peroxide) were capable of increasing the synthesis and activity of the 20S proteasome in fruit flies [6], [7] and nematode worms [8]. Here, we present findings to indicate that the adaptive proteolysis in the model organism, D. melanogaster is 20S proteasome-dependent. Males and females of the Oregon-R strain were not pretreated (control, 0 µM) or pretreated with an adaptive amount of an oxidant (10 µM hydrogen peroxide or 0.5 µM paraquat) and lysates were incubated in the absence or presence of 20S-selective inhibitor, lactacystin, to assess whether adaptive increases in proteolytic capacity are dependent on the 20S proteasome. Immunoprecipitation was used to further assess the importance of the 20S proteasome in enabling the adaptive increase in proteolytic capacity following treatment with very low, signaling amounts of hydrogen peroxide. Lastly, we further explored the ubiquity of adaptive homeostasis [1], which enables organisms to quickly activate protective pathways and better cope with stresses, as well as oxidant- and sex-dependent differences in multiple D. melanogaster strains, with further analysis presented in the associated study [2].
2. Experimental design, materials and methods
2.1. D. melanogaster culture
Three common strains of D. melanogaster were utilized: Canton-S, Oregon-R, and w[1118]. All strains were cultured at 25 °C, with 12 h light/dark cycles, on a standard agar/dextrose/corn meal/yeast media [9]. Flies were collected over 48 h from pre-cleared bottles prior to treatments.
2.2. Description of three D. melanogaster strains utilized
Three D. melanogaster strains were utilized: Canton-S (Ca-S), Oregon-R (Or-R), and the mutant reference strain w[1118]. The Canton-S stock was originally collected in Canton, Ohio, and was established and propagated by C.B. Bridges in 1943, due to the strain's low mutation rate [10]. The strain gained increasing usage following its first utilization by S. Benzer in 1967 [11], and since then, is arguably one of the most commonly used wild-type D. melanogaster strains [12], with over 3000 citations referencing the strain on PubMed. Additionally, the Canton-S strain has been previously characterized as a highly oxidant sensitive [13], [14] and short-lived strain [15].
Oregon-R was first collected in Roseburg, Oregon by D.E. Lancefield in 1925 [16]. The strain was utilized for early lifespan [15], [17] and oxidative stress studies [18], including temperature-resistance [19]. The second most commonly used strain, the Oregon-R strain has also been noted as being useful in chemosensory studies [20]. Additionally, over 2000 citations utilize the Oregon-R strain on PubMed.
The mutant reference strain (w[1118]) originated from the Oregon-R strain and contains a spontaneous partial deletion in the white (w) gene, resulting in the development of a white rather than red eye coloration. The strain was first noted by R. Levis (1980) [21], [22] and has since been utilized as a genetic marker and for the generation of isogenic strains [23]. Additionally, it was utilized as the control strain in lifespan studies with the Methuselah long-lived strain [24]. The strain has been cited in over 5000 publications referenced on PubMed.
2.3. D. melanogaster adaptation
24 h prior to treatment, 10 flies were transferred to vials containing 1 mL of 5% sucrose. Upon treatment, flies were transferred to vial with 1 mL of 5% sucrose and low amounts of H2O2 [0–100 µM] or paraquat [0–10 µM], as indicated, for 8 h, and subsequently transferred to vials containing only 1 mL of 5% sucrose for an additional 16 h. Afterwards, flies were transferred to vials containing a toxic dose of H2O2 [4.4 M] or paraquat [30 mM]. Survival was scored every 8 hours, until all flies were dead and quantification (Table 1, Table 2).
Table 1.
Quantification of hydrogen peroxide adaptation curves.
| COHORT 1 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Female | ||||||||
| Genotype | H2O2 | N | Mean (SD) | Median | 90% | Δ Mean % | Δ Median % | (p) |
| Canton-S | 0 µM | 90 | 52 (15) | 56 | 69 | |||
| 10 µM | 90 | 61 (13) | 65 | 78 | 6.87 | 8.26 | 0.0036 | |
| 100 µM | 94 | 60 (14) | 65 | 76 | 6.40 | 8.02 | 0.0047 | |
| Oregon-R | 0 µM | 121 | 60 (15) | 62 | 79 | |||
| 10 µM | 120 | 77 (17) | 82 | 100 | 17.02 | 19.46 | 3.02E−13 | |
| 100 µM | 120 | 74 (14) | 75 | 96 | 15.51 | 16.18 | 2.18E−13 | |
| w[1118] | 0 µM | 96 | 50 (13) | 50 | 70 | |||
| 10 µM | 120 | 62 (13) | 64 | 72 | 10.72 | 11.11 | 0.0002 | |
| 100 µM | 94 | 64 (14) | 64 | 72 | 12.28 | 11.11 | 0.0001 |
| Male | ||||||||
|---|---|---|---|---|---|---|---|---|
| Genotype | H2O2 | N | Mean (SD) | Median | 90% | Δ Mean % | Δ Median % | (p) |
| Canton-S | 0 µM | 81 | 51 (15) | 55 | 67 | |||
| 10 µM | 80 | 51 (15) | 55 | 67 | −0.4108 | 0.000 | 0.9272 | |
| 100 µM | 83 | 52 (15) | 55 | 67 | −0.4643 | 0.000 | 0.9491 | |
| Oregon-R | 0 µM | 80 | 50 (10) | 54 | 61 | |||
| 10 µM | 80 | 50 (10) | 54 | 60 | −0.887 | 0.000 | 0.7749 | |
| 100 µM | 78 | 50 | 54 | 58 | −0.473 | 0.000 | 0.9441 | |
| w[1118] | 0 µM | 119 | 46 (13) | 47 | 64 | |||
| 10 µM | 102 | 46 (14) | 47 | 64 | −0.514 | 0.000 | 0.7111 | |
| 100 µM | 139 | 46 (13) | 47 | 64 | −0.188 | 0.000 | 0.8486 |
| COHORT 2 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Female | ||||||||
| Genotype | H2O2 | N | Mean (SD) | Median | 90% | Δ Mean % | Δ Median % | (p) |
| Canton-S | 0 µM | 95 | 49 (15) | 52 | 63 | |||
| 10 µM | 94 | 59 (13) | 59 | 76 | 8.65 | 6.31 | 0.0031 | |
| 100 µM | 95 | 62 (13) | 61 | 78 | 9.74 | 7.64 | 0.0025 | |
| Oregon-R | 0 µM | 80 | 70 (16) | 73 | 84 | |||
| 10 µM | 81 | 78 (10) | 82 | 98 | 8.041 | 7.775 | 3.92E−05 | |
| 100 µM | 80 | 78 (11) | 82 | 94 | 7.667 | 7.531 | 4.31E−05 | |
| w[1118] | 0 µM | 124 | 41 (16) | 42 | 54 | |||
| 10 µM | 123 | 56 (17) | 55 | 72 | 13.99 | 12.61 | 1.56E−04 | |
| 100 µM | 125 | 58 (17) | 59 | 72 | 14.36 | 15.83 | 2.96E−04 |
| Male | ||||||||
|---|---|---|---|---|---|---|---|---|
| Genotype | H2O2 | N | Mean (SD) | Median | 90% | Δ Mean % | Δ Median % | (p) |
| Canton-S | 0 µM | 84 | 52 (16) | 52 | 62 | |||
| 10 µM | 85 | 49 (17) | 52 | 65 | −1.295 | 0.000 | 0.7179 | |
| 100 µM | 85 | 51 (16) | 52 | 62 | −0.238 | 0.000 | 0.9278 | |
| Oregon-R | 0 µM | 60 | 53 (9) | 54 | 65 | |||
| 10 µM | 60 | 51 (9) | 54 | 60 | −0.749 | 0.000 | 0.6582 | |
| 100 µM | 59 | 48 (10) | 54 | 56 | −2.213 | 0.000 | 0.3709 | |
| w[1118] | 0 µM | 147 | 55 (13) | 58 | 70 | |||
| 10 µM | 136 | 54 (13) | 58 | 70 | −1.071 | 0.000 | 0.3997 | |
| 100 µM | 141 | 55 (12) | 58 | 70 | −0.174 | 0.000 | 0.7161 |
Table 2.
Quantification of Paraquat Adaptation Curves.
| COHORT 1 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Female | ||||||||
| Genotype | paraquat | N | Mean (SD) | Median | 90% | Δ Mean % | Δ Median % | (p) |
| Canton-S | 0 µM | 80 | 39 (27) | 34 | 68 | |||
| 1 µM | 78 | 38 (26) | 34 | 68 | −0.234 | 0.000 | 0.8835 | |
| 10 µM | 80 | 38 (27) | 33 | 70 | −0.895 | −1.111 | 0.4458 | |
| Oregon-R | 0 µM | 97 | 80 (28) | 80 | 96 | |||
| 1 µM | 100 | 81 (28) | 80 | 96 | 1.083 | 0.000 | 0.8303 | |
| 10 µM | 101 | 80 (27) | 80 | 96 | 1.132 | 0.000 | 0.5338 | |
| w[1118] | 0 µM | 80 | 71 (25) | 74 | 107 | |||
| 1 µM | 79 | 72 (23) | 74 | 107 | 0.885 | 0.000 | 0.2049 | |
| 10 µM | 82 | 71 (25) | 74 | 107 | 0.983 | 0.000 | 0.1371 |
| Male | ||||||||
|---|---|---|---|---|---|---|---|---|
| Genotype | paraquat | N | Mean (SD) | Median | 90% | Δ Mean % | Δ Median % | (p) |
| Canton-S | 0 µM | 200 | 34 (19) | 34 | 40 | |||
| 1 µM | 199 | 44 (17) | 46 | 58 | 9.671 | 11.25 | 2.64E-10 | |
| 10 µM | 202 | 47 (19) | 49 | 64 | 11.96 | 13.22 | 1.54E-11 | |
| Oregon-R | 0 µM | 119 | 49 (11) | 48 | 64 | |||
| 1 µM | 118 | 60 (14) | 64 | 80 | 9.276 | 12.25 | 1.47E-11 | |
| 10 µM | 119 | 61 (19) | 63 | 80 | 10.26 | 11.40 | 1.52E-11 | |
| w[1118] | 0 µM | 139 | 42 (26) | 49 | 75 | |||
| 1 µM | 140 | 56 (26) | 60 | 80 | 8.713 | 7.143 | 8.86E-08 | |
| 10 µM | 140 | 60 (27) | 64 | 88 | 11.48 | 9.154 | 1.15E-08 |
| COHORT 2 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Female | ||||||||
| Genotype | paraquat | N | Mean (SD) | Median | 90% | Δ Mean % | Δ Median % | (p) |
| Canton-S | 0 µM | 90 | 35 (17) | 36 | 66 | |||
| 1 µM | 88 | 35 (18) | 35 | 66 | −0.037 | −0.2375 | 0.8595 | |
| 10 µM | 93 | 34 (18) | 35 | 66 | −0.936 | −1.564 | 0.5770 | |
| Oregon-R | 0 µM | 202 | 117 (27) | 124 | 144 | |||
| 1 µM | 206 | 116 (28) | 124 | 144 | −0.413 | 0.000 | 0.9088 | |
| 10 µM | 200 | 117 (25) | 124 | 144 | −0.588 | 0.000 | 0.9227 | |
| w[1118] | 0 µM | 124 | 75 (27) | 76 | 120 | |||
| 1 µM | 122 | 78 (28) | 82 | 120 | 1.585 | 2.333 | 0.2887 | |
| 10 µM | 120 | 75 (28) | 76 | 120 | −0.404 | 0.000 | 0.7747 |
| Male | ||||||||
|---|---|---|---|---|---|---|---|---|
| Genotype | paraquat | N | Mean (SD) | Median | 90% | Δ Mean % | Δ Median % | (p) |
| Canton-S | 0 µM | 164 | 35 (17) | 33 | 60 | |||
| 1 µM | 160 | 42 (17) | 46 | 60 | 6.963 | 9.332 | 1.15E-06 | |
| 10 µM | 162 | 45 (14) | 49 | 60 | 8.541 | 11.22 | 3.47E-07 | |
| Oregon-R | 0 µM | 220 | 35 (18) | 37 | 48 | |||
| 1 µM | 198 | 49 (18) | 52 | 64 | 12.91 | 14.51 | 1.68E-13 | |
| 10 µM | 200 | 48 (18) | 49 | 64 | 11.11 | 12.88 | 2.17E-12 | |
| w[1118] | 0 µM | 158 | 59 (18) | 58 | 76 | |||
| 1 µM | 164 | 71 (15) | 75 | 84 | 10.00 | 14.47 | 3.76E-10 | |
| 10 µM | 156 | 74 (17) | 79 | 96 | 14.29 | 16.18 | 1.19E-10 |
2.4. Pretreatment with hydrogen peroxide or paraquat
Ten flies were transferred to vials containing 1 mL of 5% sucrose. Upon treatment initiation, flies were transferred to vials containing paraquat [0–10 µM] or H2O2 [0–100 µM] for 8 h before being placed back onto vials with only 1 mL of 5% sucrose for an additional 16 h. Afterwards flies were frozen for down-stream processing.
2.5. Preparation of D. melanogaster
Flies were homogenized in 200 µL proteolysis buffer (50 mM Tris/HCl, 20 mM KCl, 5 mM MgAc, 1 mM DTT, pH 7.5) using an electric pestle. Further lysis was conducted by three ‘freeze-thaw’ cycles, consisting of 5-min intervals on dry ice, followed by incubation in water, and vortexed. Samples were centrifuged for 10,000g for 10 min at 4 °C to remove cuticle fragments. Protein concentration was measured using the Bicinchoninic acid assay (BCA) reducing agent compatible kit (no. 23252, ThermoFisher Scientific).
2.6. Fluropeptide proteolytic activity assays
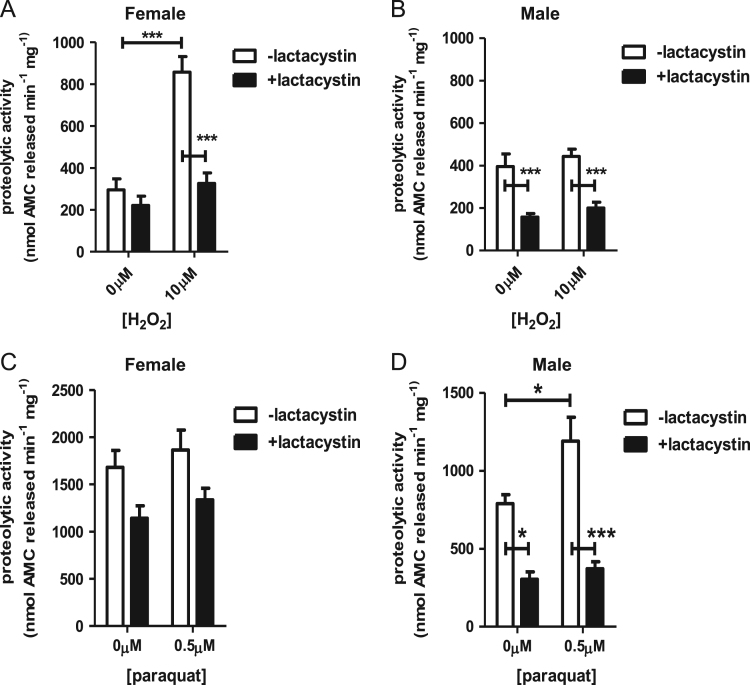
5 µg of whole fly lysate was aliquoted, in triplicate, to 96-well plates. The chymotrypsin-like activity was measured by added 2 µM of the β5-specific substrate, Suc-LLVY-AMC (no. 539141, Calbiochem). Lysate was incubated at 37 °C, and fluorescence readings were recorded every 10 min for 4 h using an excitation/emission of 355 nm/444 nm. Fluorescence units were converted to moles of free 7-amino-4-methylcuomarin (AMC), using an AMC standard curve (no. 164545, Merck), with background subtracted. To measure proteolytic inhibition, 20 µM of the proteasome inhibitor, lactacystin (no. 80052-806, VWR) was added directly to lysate, and incubated on plate shaker for 30 min at 300 rpm, after which, substrate was added (Fig. 1).
Fig. 1.
Proteolysis is largely dependent upon the proteasome (A–D) Male and female progeny of the Or-R strain were either not pretreated or were pretreated with (A,B) 10 µM hydrogen peroxide or (C,D) 0.5 µM paraquat as per Section 2 above. Afterwards, lysates were incubated in the absence (white) or presence (black) of the proteasome selective inhibitor, lactacystin. Subsequently, inhibition of proteolytic capacity was assessed in whole fly lysates by degradation of the fluorogenic peptide, Suc-LLVY-AMC. In the absence of lactacystin, female flies exhibited an adaptive increase in proteolytic capacity following H2O2 pretreatment (Panel A), whereas males exhibited increased proteolytic capacity following paraquat pretreatment (Panel D). In contrast, males did not adapt to H2O2 (Panel B), and females did not adapt to paraquat (Panel C). Importantly, both the adaptive increases in proteolytic capacity induced by H2O2 in females, and by paraquat in males, were blocked in lysates treated with lactacystin (Panels A and D), indicating that the adaptive increases in proteolytic capacity were largely conferred by increased expression of the proteasome. Error bars in all panels denote the standard error of the mean (S.E.M) values. * P<0.05, ** P<0.01, and *** P<0.001, relative to the samples not treated with inhibitor, using one-way ANOVA.
2.7. Immunoprecipitation (IP)
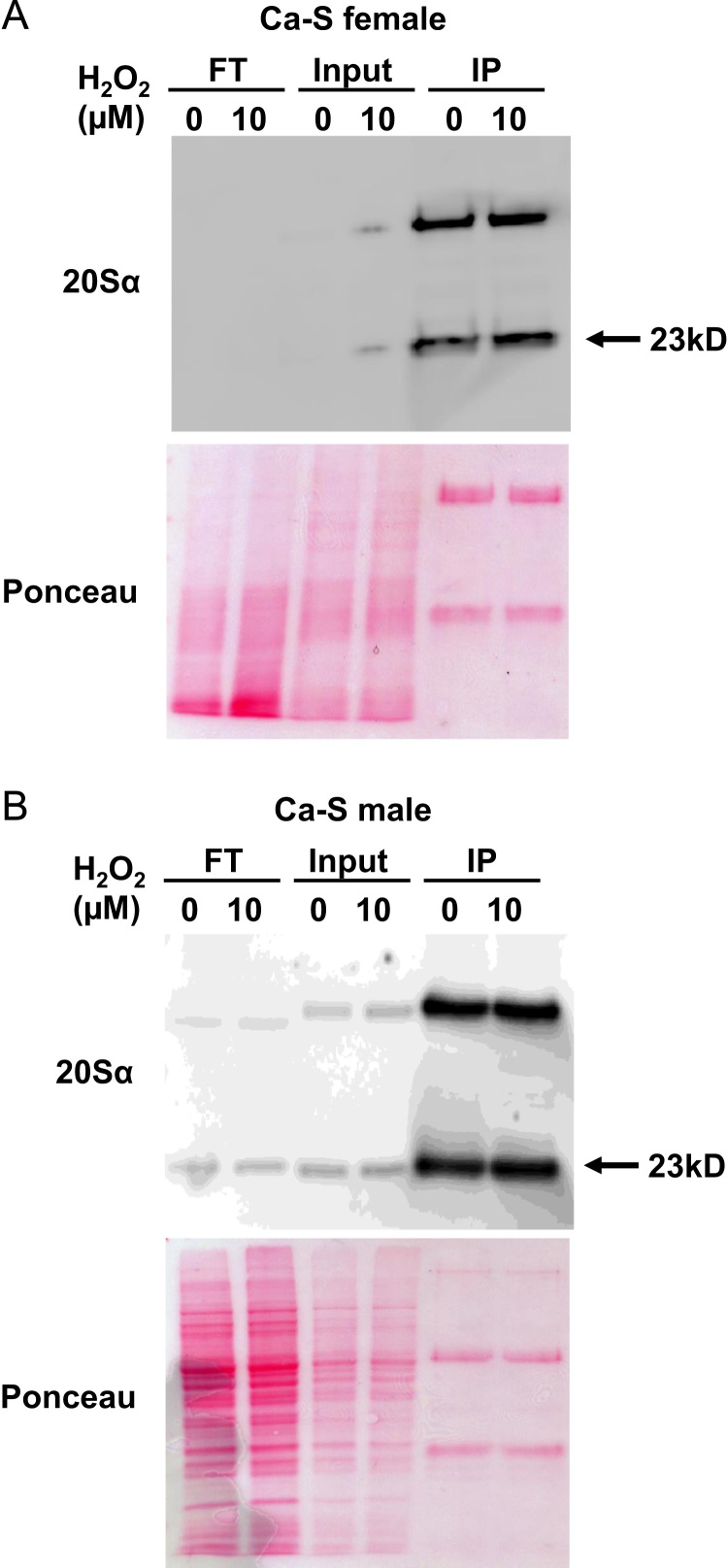
200 flies were homogenized in 400 µL proteolysis buffer (50 mM Tris/HCl, 20 mM KCl, 5 mM MgAc, 1 mM DTT, pH 7.5) using an electric pestle. Further lysis was conducted by three ‘freeze-thaw’ cycles, consisting of 5-min intervals on dry ice, followed by incubation in water, and vortexed. Samples were centrifuged for 10,000g for 10 min at 4 °C to remove cuticle fragments. Protein concentration was measured using the Bicinchoninic acid assay (BCA) reducing agent compatible kit (no. 23252, ThermoFisher Scientific). Each IP sample was normalized to have 400 µg protein in a final volume of 300 µL proteolysis buffer. Next, 20 µL of washed protein G Sepharose 4B beads (no. 10–1242, ThermoFisher Scientific) were added to each sample and placed on an end-over-end shaker for 30 min at 4 °C to pre-clear the lysate. (Antibody binding beads were washed twice by first adding 500 µL 1× PBS, inverting the sample twice to mix, and centrifuging at 2000 rpm for 1 min at 4 °C to pellet the beads, at which point the supernatant was removed). Afterwards, samples were centrifuged at 2000 rpm for 1 min at 4 °C to pellet the beads and the lysate was transferred to fresh tubes, at which point 20 µL of the monoclonal antibody against the α-subunit of the 20S core of D. melanogaster (no. sc-65755, Santa Cruz Biotechnology) was added and samples were rotated at 4 °C, overnight. Next, 40 µL of washed antibody binding beads were added to each IP and rotated at 4 °C, overnight. Samples were centrifuged at 2000 rpm for 2 min at 4 °C. Afterwards, the ‘Flow-through’ (supernatant) was transferred to fresh tubes for down-stream analysis. Beads were washed once with 1×mPER buffer (no. 78501, ThermoFisher Scientific) and three times with ice-cold 1× PBS solution. Samples were rotated for 5 min between washes. Beads were eluted by adding 50 µL of sample buffer containing 5% SDS and heated at 95 °C for 5 min prior to loading on an 10% SDS-PAGE gel (Fig. 2).
Fig. 2.
Immunoprecipitation of the proteasome Female and male progeny of the Ca-S strain were either untreated, or were pretreated with 10 µM H2O2 as per Section 2, above. Afterwards, whole fly lysates underwent immunoprecipitation (IP) against the 20S proteasome α subunit D. melanogaster specific monoclonal antibody, with a small portion (not utilized in the IP) reserved for the input. Panel A shows a Western blot (upper gel) of flow-through (FT), input, and IP against the 20Sα subunit for female flies, with a Ponceau stained lower gel, and Panel B reports the results of the same procedures for male flies. Ponceau staining, which is a reversible method to detect protein bands on PVDF membranes, as an assessment of protein loading between treatment groups. The amount of 20S proteasome α subunit present in each lysate was assessed by three stages on the western blot: flow-through (FT), input, and IP against the 20Sα subunit. The flow-through (FT) was to assess the IP efficiency: a means to measure how much of the 20Sα subunit was not pulled out from the lysate, with the lower the density, indicating higher IP efficiency by the antibody. The input assessed how much 20Sα subunit was present in lysate collected following no pretreatment (0 µM H2O2) or pretreatment (10 µM H2O2). The IP was the amount of the 20S proteasome α subunit detected when the monoclonal antibody against the 20S α subunit was added to the lysate to ‘pull out’ the 20S proteasome and enable detection of protein amount.
2.8. Western blots
10 µg of whole fly lysate was run on a 4–15% gradient SDS-PAGE gel (no. 4568084, Bio-Rad) for 1 h at 100 V before being transferred at 4 °C to a PVDF membrane (no. 1620177XTU, Bio-Rad). The goat polyclonal anti-Actin-HRP antibody, conjugated to horseradish peroxidase (1:1000 dilution, no sc-1616, Santa Cruz Biotechnology) was used as the protein loading control. Monoclonal antibody against the α-subunit of the 20S core of D. melanogaster was used (1:100 dilution, no. sc-65755, Santa Cruz Biotechnology) (Fig. 2).
Acknowledgements
This work was supported by NSF Grant [DGE-1418060] to LCDP, NIH/NIA Grants [AG011833 and R56AG049629] to JT, and NIH/NIA Grant [AG052374] and NIH/NIEHS Grant [ES003598] to KJAD.
References
- 1.Davies K.J. Adaptive homeostasis. Mol. Asp. Med. 2016;49:1–7. doi: 10.1016/j.mam.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pomatto L.C., Wong S., Tower J., Davies K.J. Sexual dimorphism in oxidant-induced adaptive homeostasis in multiple wild-type D. melanogaster strains. Archives of biochemistry and biophysics, 2017;636:57–60. doi: 10.1016/j.abb.2017.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raynes R., Pomatto L.C., Davies K.J. Degradation of oxidized proteins by the proteasome: distinguishing between the 20S, 26S, and immunoproteasome proteolytic pathways. Mol. Asp. Med. 2016;50:41–55. doi: 10.1016/j.mam.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grune T. HSP70 mediates dissociation and reassociation of the 26S proteasome during adaptation to oxidative stress. Free Radic. Biol. Med. 2011;51(7):1355–1364. doi: 10.1016/j.freeradbiomed.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reeg S. The molecular chaperone Hsp70 promotes the proteolytic removal of oxidatively damaged proteins by the proteasome. Free Radic. Biol. Med. 2016;99:153–166. doi: 10.1016/j.freeradbiomed.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pickering A.M. A conserved role for the 20S proteasome and Nrf2 transcription factor in oxidative stress adaptation in mammals, Caenorhabditis elegans and Drosophila melanogaster. J. Exp. Biol. 2013;216(4):543–553. doi: 10.1242/jeb.074757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pomatto L.C. The age-and sex-specific decline of the 20s proteasome and the Nrf2/CncC signal transduction pathway in adaption and resistance to oxidative stress in Drosophila melanogaster. Aging (Albany NY) 2017;9(4):1153. doi: 10.18632/aging.101218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raynes R. Aging and SKN-1-dependent loss of 20S proteasome adaptation to oxidative stress in C. elegans. J. Gerontol. Ser. A: Biomed. Sci. Med. Sci. 2016;72(2):143–151. doi: 10.1093/gerona/glw093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ren C., Finkel S.E., Tower J. Conditional inhibition of autophagy genes in adult Drosophila impairs immunity without compromising longevity. Exp. Gerontol. 2009;44(3):228–235. doi: 10.1016/j.exger.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stern C., Schaeffer E.W. On primary attributes of Alleles in Drosophila Melanogaster. Proc. Natl. Acad. Sci. USA. 1943;29(11):351–361. doi: 10.1073/pnas.29.11.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benzer S. Behavioral mutants of Drosophila isolated by countercurrent distribution. Proc. Natl. Acad. Sci. USA. 1967;58(3):1112–1119. doi: 10.1073/pnas.58.3.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colomb J., Brembs B. Sub-strains of Drosophila Canton-S differ markedly in their locomotor behavior. F1000Research. 2014;3:176. doi: 10.12688/f1000research.4263.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Girardot F., Monnier V., Tricoire H. Genome wide analysis of common and specific stress responses in adult Drosophila melanogaster. BMC Genom. 2004;5(1):74. doi: 10.1186/1471-2164-5-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strycharz J.P. Resistance in the highly DDT-resistant 91-R strain of Drosophila melanogaster involves decreased penetration, increased metabolism, and direct excretion. Pestic. Biochem. Physiol. 2013;107(2):207–217. [Google Scholar]
- 15.Ganetzky B., Flanagan J.R. On the relationship between senescence and age-related changes in two wild-type strains of Drosophila melanogaster. Exp. Gerontol. 1978;13(3–4):189–196. doi: 10.1016/0531-5565(78)90012-8. [DOI] [PubMed] [Google Scholar]
- 16.Lindsley D.L. Genetic variations of Drosophila melanogaster. Carnegie Inst. Wash. Publ. 1968;627 [Google Scholar]
- 17.Lints F.A. Unexplained variations in life span of the Oregon-R strain of Drosophila melanogaster over a four-year period. Exp. Gerontol. 1989;24(3):265–271. doi: 10.1016/0531-5565(89)90017-x. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y., Salmon A.B., Harshman L.G. A cost of reproduction: oxidative stress susceptibility is associated with increased egg production in Drosophila melanogaster. Exp. Gerontol. 2001;36(8):1349–1359. doi: 10.1016/s0531-5565(01)00095-x. [DOI] [PubMed] [Google Scholar]
- 19.Miquel J. Effects of temperature on the life span, vitality and fine structure of Drosophila melanogaster. Mech. Ageing Dev. 1976;5(Supplement C):347–370. doi: 10.1016/0047-6374(76)90034-8. [DOI] [PubMed] [Google Scholar]
- 20.Jones W.D. Two chemosensory receptors together mediate carbon dioxide detection in Drosophila. Nature. 2007;445(7123):86. doi: 10.1038/nature05466. [DOI] [PubMed] [Google Scholar]
- 21.Bingham P.M. The regulation of white locus expression: a dominant mutant allele at the white locus of Drosophila melanogaster. Genetics. 1980;95(2):341–353. doi: 10.1093/genetics/95.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hazelrigg T., Levis R., Rubin G.M. Transformation of white locus DNA in Drosophila: dosage compensation, zeste interaction, and position effects. Cell. 1984;36(2):469–481. doi: 10.1016/0092-8674(84)90240-x. [DOI] [PubMed] [Google Scholar]
- 23.Tollefsbol T.O. Vol. 371. Springer Science & Business Media; 2007. (Biological Aging: Methods and Protocols). [Google Scholar]
- 24.Lin Y.-J., Seroude L., Benzer S. Extended life-span and stress resistance in the Drosophila mutant methuselah. Science. 1998;282(5390):943. doi: 10.1126/science.282.5390.943. [DOI] [PubMed] [Google Scholar]